Low-valent vanadium catecholate clusters†
Emma
Scales
a,
Lorenzo
Sorace
b,
Andrea
Dei
b,
Andrea
Caneschi
b,
Christopher A.
Muryn
a,
David
Collison
a and
Eric J. L.
McInnes
*a
aSchool of Chemistry, The University of Manchester, Manchester, UK M13 9PL. E-mail: eric.mcinnes@manchester.ac.uk; Fax: +44-(0)161-275-4616; Tel: +44-(0)161-275-4619
bDipartimento di Chimica, Universita di Firenze, Sesto Fiorentino, Italy
First published on 26th May 2010
Abstract
A simple synthetic route to low-valent vanadium catecholate clusters, via solvothermal reaction of [V(acac)3] with catechols in alcohols, is presented. The syntheses and X-ray structures of [VIII6O(dbcat)4(OMe)8(MeOH)2] (1; which has the Lindqvist structure), [VIII6O2(dbcat)2(dbsq)2(acac)2(PhCO2)4(EtO)2] (2; which could alternatively be formulated as [VIII4VIV2O2(dbcat)4(acac)2(PhCO2)4(EtO)2]) and [VIV2(dbcat)4(EtOH)2] (3) are reported (dbcat2− = 3,5-tBu2-catecholate; dbsq− = 3,5-tBu2-semiquinone). The X-ray structures of the primary products of the reactions of 1 and 3 with atmospheric oxygen, [V6O3(“dbcat”)4(OMe)8] (4) and [V2O2(“dbcat”)4] (5), are also reported—the assignments of redox states for these four-electron oxidised species are more ambiguous.
Vanadium catechol chemistry is very rich given the redox active nature of both metal ion and ligand.1–3 It was recognized early on that catecholates could stabilize low metal-ion oxidation states (exploited by nature in VIII sequestering tunichromes)2 and early studies on simple tris-chelates1 addressed their complicated redox chemistry and ambiguity in electronic structure. The low-valent species are reducing, and reactions with oxygen give Pierpont's mixed-valence ligand dimer3 [(VVO)(dbsq)(dbcat)]2 (dbcat2− = 3,5-tBu2-catecholate; dbsq− = 3,5-tBu2-semiquinone), which has received renewed attention since its identification as the catalyst resting state in very efficient vanadium-based catechol dioxygenation catalysis.4 Shilov et al.'s discovery that VII catecholates efficiently reduce dinitrogen in protic media,5 due to the probably tetrametallic nature of the as yet unidentified catalyst, gives further impetus to low valent vanadium catecholate research. Despite this very few oligomeric examples are known.6 Given that solvothermal methods, well established in polyoxovanadate synthesis,7 have proved useful in preparing large VIII clusters8 particularly in reducing solvents such as alcohols,8,9 we have now begun to explore such reactions to prepare low valent vanadium catecholates. Here we report that this is a viable route, with the product very sensitive to small changes in reaction conditions: we report the isolation of two types of hexavanadium tetracatecholates, one of which has the Lindqvist-type structure, and dimetallic tetracatecholates where the latter is a reduced form of Pierpont's dimer. We also report the isolation of the products of reaction of two of these species with atmospheric oxygen.
A simple solvothermal reaction of [VIII(acac)3] (acac− = acetylacetonate) with dbcatH2 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in MeOH at 150 °C under anaerobic conditions gives dark brown crystals of [V6O(dbcat)4(OMe)8(MeOH)2] (1) on cooling (60%).‡ The EtOH version of this material can be made equivalently, but the crystallography is much poorer. 1 crystallises in P
2) in MeOH at 150 °C under anaerobic conditions gives dark brown crystals of [V6O(dbcat)4(OMe)8(MeOH)2] (1) on cooling (60%).‡ The EtOH version of this material can be made equivalently, but the crystallography is much poorer. 1 crystallises in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and the molecules lie on an inversion centre (Fig. 1). The core consists of six vanadium ions defining an octahedron, centred on a μ6-oxide, O1. Eight μ2-methoxides bridge V1 and symmetry equivalent (s.e.) to the remaining four metal ions, spanning eight of the edges of the {V6} octahedron. The hexacoordinations of V1,1A are completed by terminal MeOH. Four doubly deprotonated dbcat ligands lie in the equatorial plane defined by V2,3,2A,3A, each chelating one metal ion and bridging to a second via the 1-oxygen, the 2-position being trans to O1. Redox assignment for 1 is straightforward: bond valence sum (BVS) analysis gives all metal ions as VIII (mean 2.94; Table S1†). Charge balance then requires all the dbcat ligands to be in their catecholate (dbcat2−) form; this is consistent with their C–O and C–C(ind) [between the C(O) atoms] bond lengths.10 Hence, 1 is formulated as [VIII6O(dbcat)4(OMe)8(MeOH)2], and this is confirmed by its magnetic properties which can be fit to a spin Hamiltonian involving only VIII (s = 1) ions (Fig. S1†). The {VIII6O19} core of 1 is a stabilised form of an all VIII Lindqvist ion (Fig. S2†). Lindqvist structures incorporating vanadium(III) are extremely rare.11
, and the molecules lie on an inversion centre (Fig. 1). The core consists of six vanadium ions defining an octahedron, centred on a μ6-oxide, O1. Eight μ2-methoxides bridge V1 and symmetry equivalent (s.e.) to the remaining four metal ions, spanning eight of the edges of the {V6} octahedron. The hexacoordinations of V1,1A are completed by terminal MeOH. Four doubly deprotonated dbcat ligands lie in the equatorial plane defined by V2,3,2A,3A, each chelating one metal ion and bridging to a second via the 1-oxygen, the 2-position being trans to O1. Redox assignment for 1 is straightforward: bond valence sum (BVS) analysis gives all metal ions as VIII (mean 2.94; Table S1†). Charge balance then requires all the dbcat ligands to be in their catecholate (dbcat2−) form; this is consistent with their C–O and C–C(ind) [between the C(O) atoms] bond lengths.10 Hence, 1 is formulated as [VIII6O(dbcat)4(OMe)8(MeOH)2], and this is confirmed by its magnetic properties which can be fit to a spin Hamiltonian involving only VIII (s = 1) ions (Fig. S1†). The {VIII6O19} core of 1 is a stabilised form of an all VIII Lindqvist ion (Fig. S2†). Lindqvist structures incorporating vanadium(III) are extremely rare.11
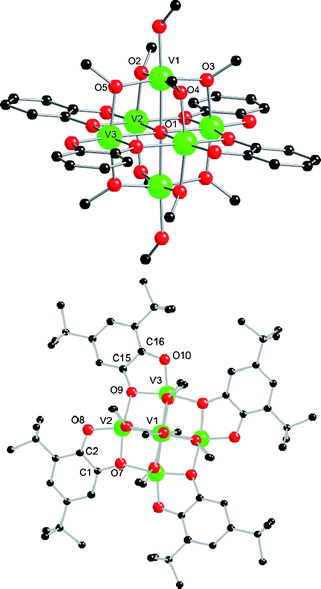 | ||
| Fig. 1 Crystal structure of 1 viewed perpendicular to (top; tBu groups removed for clarity) and down (bottom) the V1⋯V1A vector. Scheme: V (green), O (red), C (black), H omitted for clarity. Selected bond lengths (Å): V–O1 2.1614(7)–2.2096(8), V1–O6(ROH) 2.053(2), V–O(Me) 1.9635(19)–1.994(2), V–O(cat) 1.9055(18)–2.0420(19), C–O(cat) 1.356(3)–1.374(3), C–C(ind) 1.411(4) and 1.417(4). | ||
If an excess of PhCO2H is added as a co-ligand to the reaction that gives 1, a different hexametallic tetracatecholate is isolated: solvothermal reaction of [V(acac)3], dbcatH2 and PhCO2H (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in EtOH at 150 °C for 12 h gives dark blue/black needles of [V6O2(dbcat)4(acac)2(PhCO2)4(EtO)2] (2; Fig. 2) directly on slow cooling (62%).‡2 is centrosymmetric and contains three unique vanadium ions. The six vanadium ions form an edge-sharing bitetrahedral core. V1,1A,2A,3 are connected via a μ4-oxide (O1). V3 is bridged to V1 and V1A by two benzoates; the coordination at V3 is completed by a chelating diketonate and a μ-alkoxide (O7A) bridging to V2A. V2 (and s.e.) is chelated by two catecholates which also bridge to V1 and V1A (via O3 and O5A, respectively); the coordination geometry at V2,2A is trigonal prismatic. BVS analysis unambiguously assigns V1 and V3 as VIII (sum 3.01 and 3.05, respectively; Table S1†) but V2 is ambiguous giving intermediate values between 3+ and 4+. This leaves two charge-balanced possibilities: [VIII6O2(dbcat)2(dbsq)2(acac)2(PhCO2)4(EtO)2] or [VIII4VIV2O2(dbcat)4(acac)2(PhCO2)4(EtO)2]. The “dbcat” C–C(ind) distances are significantly different for the two unique ligands [C1–C2 1.343(14) and C15–C16 1.428(15) Å] which could indicate a difference in redox state [C–C(ind) is longer for semiquinones than catecholates, typically ca. 1.44 cf. 1.40 Å, respectively].10 From magnetic measurements, χT(T) plateaus above 100 K up to room temperature at a value of 4.1 emu K mol−1 (Fig. S3†). This could be consistent with {VIII4VIV2} (calculated 4.3 emu K mol−1 for g = 1.9) or {VIII6(dbsq)2} if the semiquinone spins are strongly antiferromagnetically coupled to the coordinated metal ions (V2,2A) to give two effective s = ½ centres. This is the case in [VIII(sq)3] species.1
2) in EtOH at 150 °C for 12 h gives dark blue/black needles of [V6O2(dbcat)4(acac)2(PhCO2)4(EtO)2] (2; Fig. 2) directly on slow cooling (62%).‡2 is centrosymmetric and contains three unique vanadium ions. The six vanadium ions form an edge-sharing bitetrahedral core. V1,1A,2A,3 are connected via a μ4-oxide (O1). V3 is bridged to V1 and V1A by two benzoates; the coordination at V3 is completed by a chelating diketonate and a μ-alkoxide (O7A) bridging to V2A. V2 (and s.e.) is chelated by two catecholates which also bridge to V1 and V1A (via O3 and O5A, respectively); the coordination geometry at V2,2A is trigonal prismatic. BVS analysis unambiguously assigns V1 and V3 as VIII (sum 3.01 and 3.05, respectively; Table S1†) but V2 is ambiguous giving intermediate values between 3+ and 4+. This leaves two charge-balanced possibilities: [VIII6O2(dbcat)2(dbsq)2(acac)2(PhCO2)4(EtO)2] or [VIII4VIV2O2(dbcat)4(acac)2(PhCO2)4(EtO)2]. The “dbcat” C–C(ind) distances are significantly different for the two unique ligands [C1–C2 1.343(14) and C15–C16 1.428(15) Å] which could indicate a difference in redox state [C–C(ind) is longer for semiquinones than catecholates, typically ca. 1.44 cf. 1.40 Å, respectively].10 From magnetic measurements, χT(T) plateaus above 100 K up to room temperature at a value of 4.1 emu K mol−1 (Fig. S3†). This could be consistent with {VIII4VIV2} (calculated 4.3 emu K mol−1 for g = 1.9) or {VIII6(dbsq)2} if the semiquinone spins are strongly antiferromagnetically coupled to the coordinated metal ions (V2,2A) to give two effective s = ½ centres. This is the case in [VIII(sq)3] species.1
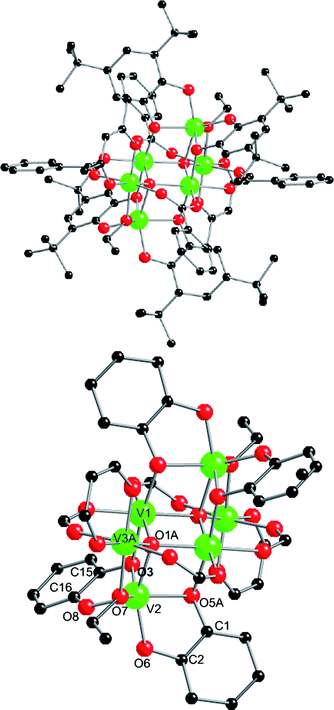 | ||
| Fig. 2 Top: crystal structure of 2. Bottom: core with tBu, Ph and Me groups removed for clarity. Selected bond lengths (Å): V–O1 1.993(6)–2.047(7), V–O(R) 1.930(7) and 2.010(7), V–O(acac) 1.937(7) and 1.972(7), V–O(benzoate) 1.997(7)–2.033(7). V–O(cat) distances: V2–O3,O8 2.036(6) and 1.863(7); V2–O5A,O6 2.034(7) and 1.896(6). Internal dbcat distances: C15,16–O3,O8 1.368(12) and 1.354(12); C1,2–O5,O6 1.393(12) and 1.402(12); C1–C2 1.343(14); C15–C16 1.428(15). | ||
If a lower quantity of carboxylic acid is used in this reaction, a much smaller species results: reaction of [V(acac)3], dbcatH2 and PhCO2H (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in EtOH at 150 °C followed by standing for several days gives blue/black crystals of [V2(dbcat)4(EtOH)2]·EtOH (3; 40%; Fig. 3).‡ Although benzoate is not incorporated in the product, the reaction fails in the absence of PhCO2H. 3 crystallises in P
1) in EtOH at 150 °C followed by standing for several days gives blue/black crystals of [V2(dbcat)4(EtOH)2]·EtOH (3; 40%; Fig. 3).‡ Although benzoate is not incorporated in the product, the reaction fails in the absence of PhCO2H. 3 crystallises in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) : the two vanadium ions are each chelated by two catecholates, one of which also bridges (via O1,5) to the other metal ion. The coordination at each vanadium is completed by a terminal alcohol. BVS analysis gives both vanadium ions as VIV (valence sums 3.82 and 3.75, Table S1†) and metric parameters of the ligands are typical of catecholates, giving a charge balanced formula of [VIV2(dbcat)4(EtOH)2]. This product is intriguing as it appears to be a four-electron reduced form of Pierpont's [VV2O2(dbcat)2(dbsq)2] dimer where alcohols have replaced the terminal oxides.
: the two vanadium ions are each chelated by two catecholates, one of which also bridges (via O1,5) to the other metal ion. The coordination at each vanadium is completed by a terminal alcohol. BVS analysis gives both vanadium ions as VIV (valence sums 3.82 and 3.75, Table S1†) and metric parameters of the ligands are typical of catecholates, giving a charge balanced formula of [VIV2(dbcat)4(EtOH)2]. This product is intriguing as it appears to be a four-electron reduced form of Pierpont's [VV2O2(dbcat)2(dbsq)2] dimer where alcohols have replaced the terminal oxides.
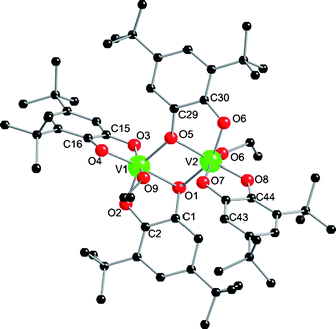 | ||
| Fig. 3 Crystal structure of 3. Selected bond lengths (Å): V–O(H)R 2.0815(13) and 2.0442(14) Å; V1,2–O1,5 1.9823(12)–2.0809(12); other V–O(cat) 1.8293(13)–1.9337(12); C–O range 1.353(2)–1.369(2) Å. C–C(ind) range 1.403(2)–1.408(2). | ||
Complexes 1–3 are all highly air sensitive. This is not surprising given the low valency; Pierpont's dimer itself is formed on aerial oxidation of [VIII(dbsq)3] and the dimer reacts further with O2 to ultimately give V2O5 with liberation of 3,5-tBu2-benzoquinone3 [dioxygenation catalysis is observed in the presence of excess dbcatH2].4 For 1 and 3 we have managed to isolate the products of reaction with atmospheric O2.
1 rapidly oxidizes to a dark blue material on exposure to air, both in the solid state and in solution. This blue material is difficult to crystallize, but on occasion we have isolated poor quality crystals from THF solution, revealing these to be [V6O3(“dbcat”)4(OMe)8] (4; Fig. 4).‡ Mass spectra confirm 4 as the species in solution. Better quality crystals of the same species can be isolated from reaction of [V(acac)3], dbcatH2 and Mg(OH)2 in MeOH at 150 °C, crystallising in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) as [V6O3(“dbcat”)4(OMe)8]·1.4MeOH·H2O with two half-molecules per asymmetric unit. There is one major difference from the structure of 1: the apical vanadium ions now have terminal oxides [V1–O6 1.598(4) Å, υV=O 986 cm−1], replacing the coordinated MeOH in 1. The redox assignment for 4 is not clear-cut. The apical vanadium ions (V1,1A) have clearly oxidised and BVS assigns them as VIV (valence sum +4.10, Table S1†) with V2,2A (+3.22) and V3,3A (+3.10) as VIII. This would require two “dbcat” ligands to be in the semiquinone state to charge balance; [VIII4VIV2O3(dbcat)2(dbsq)2(OMe)8]. However, there are no significant differences in the C–O(cat) [average 1.359 cf. 1.360 Å] or C–C(ind) [1.388(8) cf. 1.397(8) Å] lengths for the two crystallographically unique ligands in a molecule to support a localised mixed valence description for the ligands. This does not preclude a delocalised description, and this could be favoured by the planar {V4(“dbcat”)4} structure. Such delocalisation could also include the metal ions [{VIII(dbsq)}2+ ↔ {VIV(dbcat)}2+] which could be the cause of the slightly high BVS sum for V2,2A.
as [V6O3(“dbcat”)4(OMe)8]·1.4MeOH·H2O with two half-molecules per asymmetric unit. There is one major difference from the structure of 1: the apical vanadium ions now have terminal oxides [V1–O6 1.598(4) Å, υV=O 986 cm−1], replacing the coordinated MeOH in 1. The redox assignment for 4 is not clear-cut. The apical vanadium ions (V1,1A) have clearly oxidised and BVS assigns them as VIV (valence sum +4.10, Table S1†) with V2,2A (+3.22) and V3,3A (+3.10) as VIII. This would require two “dbcat” ligands to be in the semiquinone state to charge balance; [VIII4VIV2O3(dbcat)2(dbsq)2(OMe)8]. However, there are no significant differences in the C–O(cat) [average 1.359 cf. 1.360 Å] or C–C(ind) [1.388(8) cf. 1.397(8) Å] lengths for the two crystallographically unique ligands in a molecule to support a localised mixed valence description for the ligands. This does not preclude a delocalised description, and this could be favoured by the planar {V4(“dbcat”)4} structure. Such delocalisation could also include the metal ions [{VIII(dbsq)}2+ ↔ {VIV(dbcat)}2+] which could be the cause of the slightly high BVS sum for V2,2A.
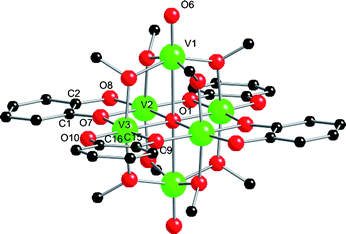 | ||
| Fig. 4 Structure of one of the unique molecules in the crystal structure of 4. Selected bond lengths (Å): V–O1 2.0848(12)–2.4650(12), V1–O6 1.598(4), V–O(Me) 1.974(4)–1.997(4), V–O(cat) 1.858(4)–2.012(4), C–O(cat) 1.346(7)–1.372(6), C–C(ind) 1.388(4) and 1.397(4). | ||
The magnetic properties of 4 do not clarify this issue, but do confirm that V1,1A are VIV rather than VV (Fig. S4†). Monitoring the aerial oxidation of 1 to 4 by UV-Vis (CH2Cl2; Fig. S5†)12 shows the collapse of a single peak at 468 nm (ε = 9 × 103 M−1 cm−1) with growth of broad bands at 578 and 734 nm (16 × 103 M−1 cm−1). Broad bands between 700–800 nm are a common feature of many dbsq− complexes,13 but are not observed in e.g. [VIV(dbcat)3]2−.1 This supports the oxidation of some of the ligands to dbsq−.
The primary oxidation product of the dimetallic 3 can also be isolated: when the filtrate from the synthesis of 3 is left to evaporate slowly in air dark crystals of [V2O2(“dbcat”)4]·3EtOH (5) form (14%, based on initial reagent amounts in the synthesis of 3).‡ The two coordinated alcohols of 3 have been replaced by terminal oxides (O5) (Fig. 5). Although we have been unable to obtain satisfactory elemental analyses for 5, its formula is unambiguous from the X-ray structure. 5 is an isomer of Pierpont's dimer, the only structural difference being the location of the oxo-group relative to the bridging ligand, being trans to O3 in 5 and cis in Pierpont's complex. Again, assignment of redox states in the oxidation product is not straightforward. BVS analysis gives the metal ions as VV (valence sum 5.02, Table S1†). Charge balance then requires [VV2O2(dbcat)2(dbsq)2], which is equivalent to Pierpont's dimer.3 However, there is no significant difference between the metric parameters of the chelating and bridging “dbcat” ligands in the structure of 5, all being in the range expected for catecholate. To date we have been unable to obtain reliable magnetic data for 5 due to magnetic impurities (presumably vanadium oxides).
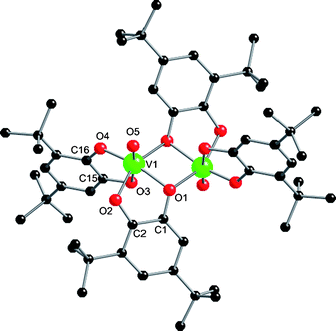 | ||
| Fig. 5 Crystal structure of 5. Selected bond lengths (Å): V1–O5 1.591(3). Bridging ligand: C1–O1 1.375(4), C2–O2 1.353(5), C1–C2 1.389(5). Terminal ligand: C15–O3 1.372(4), C16–O4 1.357(4), C15–C16 1.399(5). | ||
Conclusions
This work shows that high-nuclearity, low-valent vanadium catecholates are readily accessible via solvothermal routes. Given the redox active nature of metal and ligand this chemistry is rich and very sensitive to, for example, subtle changes in stoichiometry. The highly reducing nature of the complexes may lead to useful reactivity, and we are currently attempting to extend the chemistry to VII with a view to exploring Shilov's observations on nitrogen fixation. 1 and 3 (the complexes for which we have isolated the primary oxidation products) both undergo four-electron oxidation and addition of two oxygen atoms in air, to 4 and 5, respectively. While it seems likely that this process involves oxidation of both metal ion and catecholate, unambiguous assignment of the charge distribution in the oxidised species requires further detailed physical studies—these will be reported later.Acknowledgements
We thank the EPSRC and the EC (“MagMaNet” NoE, and a Marie Curie Host Fellowship for Young Researchers award to E. S.) for funding and Daresbury Laboratories for access to synchrotron X-ray facilities.Notes and references
- (a) R. M. Buchanan, H. H. Downs, W. B. Shorthill, C. G. Pierpont, S. L. Kessel and D. N. Hendrickson, J. Am. Chem. Soc., 1978, 100, 4318 CrossRef CAS; (b) S. R. Cooper, Y.-B. Koh and K. N. Raymond, J. Am. Chem. Soc., 1982, 104, 5092 CrossRef CAS; (c) P. J. Bosserman and D. T. Sawyer, Inorg. Chem., 1982, 21, 1545 CrossRef CAS; (d) M. E. Cass, N. R. Gordon and C. G. Pierpont, Inorg. Chem., 1986, 25, 3962 CrossRef CAS.
- See for example: (a) A. R. Bulls, C. G. Pippin, F. E. Hahn and K. N. Raymond, J. Am. Chem. Soc., 1990, 112, 2627 CrossRef CAS; (b) C. L. Simpson and C. G. Pierpont, Inorg. Chem., 1992, 31, 4308 CrossRef CAS.
- M. E. Cass, D. L. Greene, R. M. Buchanon and C. G. Pierpont, J. Am. Chem. Soc., 1983, 105, 2680 CrossRef CAS.
- C.-X. Yin and R. G. Finke, J. Am. Chem. Soc., 2005, 127, 13988 CrossRef CAS; C.-X. Yin, Y. Sasaki and R. G. Finke, Inorg. Chem., 2005, 44, 8521 CrossRef CAS.
- (a) S. A. Isalva, L. A. Nikonova and A. E. Shilov, Nouv. J. Chim., 1981, 5, 21 Search PubMed; (b) A. F. Shestakov and A. E. Shilov, Kinet. Catal., 2001, 42, 653 CrossRef CAS.
- (a) S. Lee, K. Nakanishi, M. Y. Chiang, R. B. Frankel and K. Spartalian, J. Chem. Soc., Chem. Commun., 1988, 785 RSC; (b) M. Mazzanti, C. Floriani, A. Chiesi-Villa and C. Guastini, J. Chem. Soc., Dalton Trans., 1989, 1793 RSC; (c) N. P. Luneva, S. A. Mironova, A. E. Shilov, M. Y. Antipin and Y. T. Struchkov, Angew. Chem., Int. Ed. Engl., 1993, 32, 1178 CrossRef.
- See for example: M. I. Khan and J. Zubieta, Prog. Inorg. Chem., 1995, 43, 1 Search PubMed.
- R. H. Laye, M. Murrie, S. Ochsenbein, A. R. Bell, S. J. Teat, J. Raftery, H. U. Güdel and E. J. L. McInnes, Chem.–Eur. J., 2003, 9, 6215 CrossRef CAS; R. H. Laye, Q. Wei, P. V. Mason, M. Shanmugan, S. J. Teat, E. K. Brechin, D. Collison and E. J. L. McInnes, J. Am. Chem. Soc., 2006, 128, 9020 CrossRef CAS; I. S. Tidmarsh, R. H. Laye, P. R. Brearley, M. Shanmugam, E. C. Sañudo, L. Sorace, A. Caneschi and E. J. L. McInnes, Chem.–Eur. J., 2007, 13, 6329 CrossRef CAS; R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968 CrossRef CAS; S. Khanra, M. Kloth, H. Mansaray, C. A. Muryn, F. Tuna, E. C. Sañudo, M. Helliwell, E. J. L. McInnes and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2007, 46, 5568 CrossRef CAS.
- R. H. Laye and E. J. L. McInnes, Eur. J. Inorg. Chem., 2004, 2811 CrossRef CAS.
- C. G. Pierpont and R. M. Buchanan, Coord. Chem. Rev., 1981, 38, 45 CrossRef CAS; C. G. Pierpont and C. W. Lange, Prog. Inorg. Chem., 1994, 41, 331 CAS; C. G. Pierpont, Coord. Chem. Rev., 2001, 219–221, 415 CrossRef CAS; P. Chaudhuri, C. N. Verani, E. Bill, E. Bothe, T. Weyermuller and K. Wieghardt, J. Am. Chem. Soc., 2001, 123, 2213 CrossRef CAS.
- C. Aronica, G. Chastanet, E. Zueva, S. A. Borshch, J. M. Clemente-Juan and D. Luneau, J. Am. Chem. Soc., 2008, 130, 2365 CrossRef CAS; L. J. Batchelor, R. Shaw, S. J. Markey, M. Helliwell and E. J. L. McInnes, Chem.–Eur. J., 2010, 16, 5554 CAS.
- We have also monitored the cyclic voltammetry response of 1 as a function of time, see Fig S6†.
- See for example: M. W. Lynch, D. N. Hendrickson, B. J. Fitzgerald and C. G. Pierpont, J. Am. Chem. Soc., 1984, 106, 2041 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: BVS results for 1–5; polyhedral view of 1; χ(T), χT(T) for 1, 2 {also M(H)} and 4; UV/Vis for 1 → 4; cyclic voltammogram for 1; cif files for 1–5. CCDC reference numbers 762899–762903. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00142b |
| ‡ Synthesis: all manipulations were conducted under anaerobic conditions. The reactions were conducted under solvothermal conditions in a sealed Teflon-lined autoclave, heated to 150 °C for 12 h, then cooled to room temperature at 0.05 °C min−1. 1: [V(acac)3] (0.25 g) and dbcatH2 (0.166 g) in MeOH (7.5 ml) were treated as above. Brown plates of [V6O(dbcat)4(MeO)8(MeOH)2)]·4MeOH formed directly on cooling (61%). Elemental analysis calcd. (%) for C70H128O23V6: C 51.16, H 7.85, V 18.58; found: C 51.68, H 7.42, V 18.27. Selected IR data (KBr; ν/cm−1: 1465, 1243, 988. UV-Vis (CH2Cl2): 468 nm (ε = 8981 M−1 cm−1). 4: brown crystals of 1 oxidise on exposure to air to give a blue/black powder of [V6O3(“dbcat”)4(MeO)8] which can occasionally be recrystallised from THF. MALDI-MS (dithranol matrix in CH2Cl2): m/z 1483 (M+). Elemental analysis calcd. (%) for C64H104O19V6: C 51.80, H 7.07, V 20.61; found: C 49.75, H 7.14, V 20.30. Selected IR data (KBr; ν/cm−1: 1465, 1240, 986. UV-Vis [CH2Cl2; ν/nm (ε/M−1 cm−1)] 578 (15946), 734 (15554). Crystals of 4·1.4MeOH·H2O can be obtained from solvothermal reaction of [V(acac)3] (0.25 g), dbcatH2 (0.159 g) and Mg(OH)2 (0.042 g, 0.718 mmol) in EtOH (low yield). 2: [V(acac)3] (0.25 g), dbcatH2 (0.159 g) and PhCO2H (0.175 g) in EtOH (7.5 ml) were treated as above. Black needles of [V6O2(dbcat)4(acac)2(PhCO2)4(EtO)2] form directly on cooling, with more isolated on concentration of the filtrate (62%). Elemental analysis calcd. (%) for V6C98H134O24: C 58.80, H 6.75, V 15.27; found: C 58.68, H 6.48, V 14.89. 3: [V(acac)3] (0.25 g), dbcatH2 (0.318 g), PhCO2H (0.044 g) and EtOH (7.5 ml) were treated as above. Blue/black crystals of [V2(dbcat)4(EtOH)2]·EtOH formed from the resultant dark blue/black solution after several days (40%). Elemental analysis calcd. (%) for C62H98O11V2: C 66.41, H 8.81, V 9.08; found: C 66.20, H 8.44, V 9.47. 5: the filtrate from the above reaction was left to evaporate in air, forming blue/black crystals of [V2O2(“dbcat”)4]·3EtOH after several hours (14%). Elemental analysis calcd. (%) for C62H95O13V2: C 64.57, H 8.56, V 8.83; found: C 61.23, H 7.26, V 13.23. We were unable to obtain a good analysis for 5. 1: C70H128O23V6, M = 1643.36, triclinic, a = 11.232(2), b = 13.693(3), c = 14.106(3) Å, α = 96.27(3), β = 91.40(3), γ = 90.45(3)°, U = 2155.9(7) Å3, T = 150 K, space group P 4: C67H120O24V6, M = 1545.99, triclinic, a = 13.520(3), b = 18.220(4), c = 19.290(4) Å, α = 89.59(3), β = 74.53(3), γ = 82.69(3)°, U = 4540.6(7) Å3, T = 100 K, space group P 2: C98H124O24V6, M = 1991.61, monoclinic, a = 11.486(4), b = 28.417(11), c = 14.966(5) Å, β = 100.286(9)°, U = 4806(3) Å3, T = 100 K, space group P21/n, Z = 2, μ = 0.631 mm−1, 8118 reflections measured, 4254 unique (Rint = 0.1527). Final R1 = 0.0778, GOF = 0.836, wR2 [I > 2σ(I)] = 0.1614. 3: C62H98O11V2, M = 1121.28, triclinic, a = 11.2421(8), b = 14.0373(10), c = 21.6847(16) Å, α = 105.9570(16), β = 93.7700(10), γ = 95.8850(10)°, U = 3257.0(4) Å3, T = 150 K, space group P 5: C62H98O13V2, M = 1153.28, monoclinic, a = 13.7603(12), b = 11.8807(10), c = 20.3294(18) Å, β = 95.249(2)°, U = 3309.6(5) Å3, T = 100 K, space group P21/c, Z = 2, μ = 0.338 mm−1, 12901 reflections measured, 4046 unique (Rint = 0.0572). Final R1 = 0.0613, GOF = 0.985, wR2 [I > 2σ(I)] = 0.1712. |
| This journal is © The Royal Society of Chemistry 2010 |
