Modifying MOFs: new chemistry, new materials
Seth M.
Cohen
*
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, California, USA. E-mail: scohen@ucsd.edu; Fax: +1-858-822-5598; Tel: +1-858-822-5596
First published on 12th May 2010
Abstract
The postsynthetic modification (PSM) of metal–organic frameworks (MOFs) has grown substantially in the past few years. In this minireview, recent progress in the area of PSM is highlighted, with an emphasis on several recent advancements. The scope and limitations of PSM are described and future prospects are discussed.
 Seth M. Cohen | Seth M. Cohen obtained a Bachelor of Science degree in Chemistry and a Bachelor of Arts degree in Political Science at Stanford University. He obtained his PhD at the University of California, Berkeley in inorganic chemistry with Prof. Kenneth N. Raymond. He performed postdoctoral research in the laboratory of Prof. Stephen J. Lippard at the Massachusetts Institute of Technology. In 2001, he joined the University of California, San Diego in the Department of Chemistry and Biochemistry, where he is currently an Associate Professor. His research interests are primarily in the areas of metal–organic frameworks and medicinal bioinorganic chemistry. |
Introduction
In the past two decades, research around the class of materials best known as metal–organic frameworks (MOFs) or porous coordination polymers (PCPs) has grown to an unprecedented level of activity, as described in a recent thematic issue of ChemSocRev.1 The high porosity, stability, and well-defined crystalline structures of these hybrid inorganic–organic materials have made them a topic of interest for organic and inorganic chemists, as well as materials scientists. Among many recent developments in the study of MOFs, the postsynthetic modification (PSM) of these materials has become both an active area of research and an important tool for exploiting and expanding their unique properties. In this short review, a personal perspective on the motivation for, and significant accomplishments in, the area of PSM of MOFs will be described. The content provided here is sufficient to familiarize the reader with the basics of PSM, but is in no way a comprehensive review of the subject; the reader is referred to a recent review of PSM for more details.2What is postsynthetic modification (PSM)?
The term PSM was derived from the posttranslational modification of proteins,3 whereby chemical functionality is introduced by chemical modification of an intact polypeptide. Just as is found in Nature with posttranslational modification, PSM of MOFs may serve to introduce chemical functionality that is not readily attainable from the constituent building blocks (i.e. amino acids for polypeptides; organic ligands and metal ions for MOFs). PSM is the process of chemically modifying a MOF solid directly; that is, performing heterogeneous reactions on the intact, crystalline material. The ability to perform PSM on the organic components of MOFs was predicted by Robson and Hoskins in 1990 and was demonstrated in 1999 and 2000 by the groups of Lee and Kim, respectively.4–6 PSM represents an alternative to the conventional introduction of chemical functionality in MOFs. Typically, the MOF precursors will contain any desired functional groups, which upon synthesis of the MOF (typically under solvothermal conditions), results in the desired functional groups being incorporated into the lattice (Fig. 1). In contrast, by using PSM the MOF is synthesized under conventional solvothermal conditions, after which reagents are introduced to chemically modify the intact structure. For example, the amine-bearing IRMOF-3 (IRMOF = isoreticular metal–organic framework), which is comprised of Zn(II) clusters and 2-amino-1,4-benzenedicarboxylate ligands (NH2-BDC), can readily undergo PSM, as shown at the bottom of Fig. 1. IRMOF-3 can be modified with anhydrides to generate amide-bearing IRMOFs with different substituents.7,8 Some advantages of PSM include: inclusion of a wide variety of functional groups, not limited by solvothermal synthesis; facile isolation of the modified MOFs (e.g. heterogeneous reaction); and incorporation of multiple functional units into a single MOF. Although different kinds of PSM on MOFs have been described, for the purpose of this discussion, only covalent PSM of the organic components of a MOF will be considered.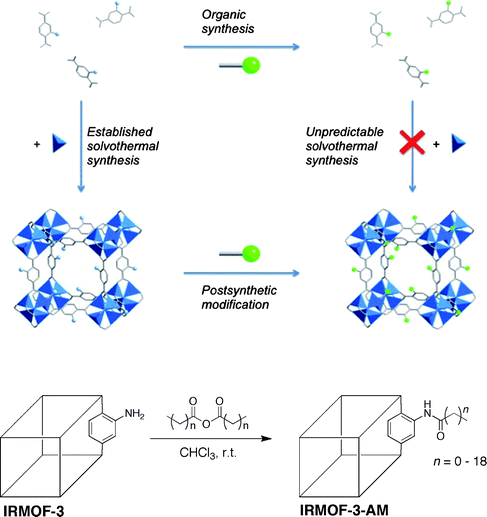 | ||
| Fig. 1 Top: illustration comparing PSM modification of MOFs with conventional incorporation of functional groups embedded in the ligand precursor. The MOF depicted is IRMOF-3 (isoreticular metal–organic framework), which is a cubic lattice comprised of NH2-BDC ligands (represented in stick form) and Zn4O clusters (zinc ions represented by blue tetrahedra). Bottom: scheme of a simple PSM reaction on IRMOF-3. The MOF is represented as a line drawing with only one modification site shown for clarity. | ||
Requirements for PSM
PSM on several different MOF structures using a variety of chemical reagents is a budding topic in the chemical literature (Fig. 2).2 In order to successfully achieve these transformations, each of these systems had several features in common. Requirements for achieving PSM include:1. The MOF must be sufficiently porous to allow access of all required reagents to the interior of the lattice (unless only surface modification is desired, vide infra).
2. The MOF must possess an available functional group that can undergo a chemical transformation.
3. The MOF must be stable to the reaction conditions (e.g. solvent, caustic reagents, temperature).
4. The MOF must be stable to any by-products produced by the reaction conditions (e.g. acids, radicals).
To date, PSM is limited by these parameters and so both the choice of MOF (e.g. suitable porosity, chemical stability) and choice of reaction (e.g. suitable solvents, chemical by-products) will govern the scope of transformations that can be realized by PSM.
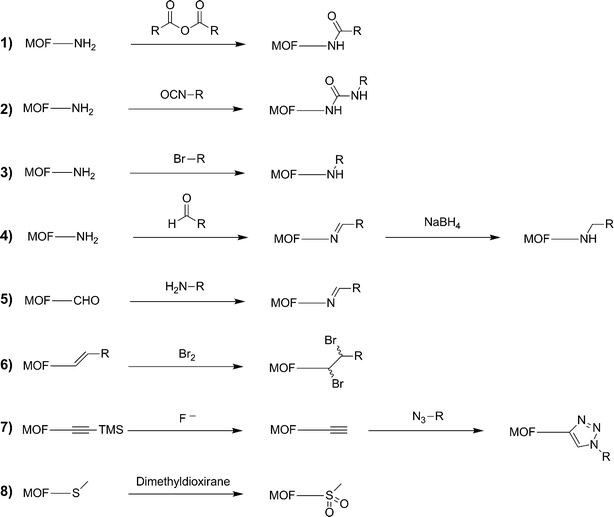 | ||
| Fig. 2 List of organic PSM reactions that have been reported on MOFs. | ||
Recent advancements
The past year has produced a number of excellent advancements in the area of PSM of MOFs. These recent reports demonstrate varying aspects of PSM, from capturing transient organic species to achieving a greater level of control over the PSM process. In this section, we describe three advancements in PSM, each of which highlights a different aspect of the use of PSM with MOFs.PSM for modulating reaction outcomes
PSM of MOFs is inherently a heterogeneous reaction environment. Liquid solvent and dissolved reagents flow along the surface and through the pores of the MOF modifying the components of the crystalline solid. Because of the rigid structure of the MOF and confined channels within the lattice, one might expect that these heterogeneous PSM reactions might have different product outcomes when compared with their solution phase, homogenous counterparts. Indeed, this has been demonstrated in a recent study and shows that MOFs may be a medium for inducing different, and perhaps desirable, product distributions from chemical reactions.To date, the most clear example showing the difference between a solution and a MOF-based chemical reaction has been reported by Jones and Bauer.9 In their report, a cubic MOF constructed of trans-4,4′-stilbene dicarboxylate (SDC) having the formula Zn4O(SDC)3 (Fig. 3) was prepared and treated with Br2 at room temperature for 48 h. Under these conditions, the SDC ligands within the framework were converted to the dibromide adduct (Fig. 2, entry 6). Most importantly, the sole product of the MOF reaction with Br2 was the meso-stereoisomer. This is in striking contrast to the homogenous reaction of the ligand with Br2 in CH2Cl2, which resulted in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of meso- to rac-isomers. The stereoselectivity of the MOF reaction is rationalized as follows: the bromonium intermediate is accessible for back-side attack by bromide, but carbon–carbon bond rotation is inhibited because the ligand is immobilized as part of the MOF framework (Fig. 3).9 The restricted bond rotation allows for only the formation of the meso-product. This study definitively shows that PSM reactions within the MOF lattice can influence the stereochemical outcome of a reaction. These findings suggest that MOFs may become interesting platforms for generating new reaction outcomes and obtaining a better understanding of reaction mechanisms. Indeed, another recent report has shown that an unstable organic intermediate could be isolated and structurally characterized within the matrix of a MOF;10 a commentary on this fascinating piece of work can be found elsewhere.11
1 ratio of meso- to rac-isomers. The stereoselectivity of the MOF reaction is rationalized as follows: the bromonium intermediate is accessible for back-side attack by bromide, but carbon–carbon bond rotation is inhibited because the ligand is immobilized as part of the MOF framework (Fig. 3).9 The restricted bond rotation allows for only the formation of the meso-product. This study definitively shows that PSM reactions within the MOF lattice can influence the stereochemical outcome of a reaction. These findings suggest that MOFs may become interesting platforms for generating new reaction outcomes and obtaining a better understanding of reaction mechanisms. Indeed, another recent report has shown that an unstable organic intermediate could be isolated and structurally characterized within the matrix of a MOF;10 a commentary on this fascinating piece of work can be found elsewhere.11
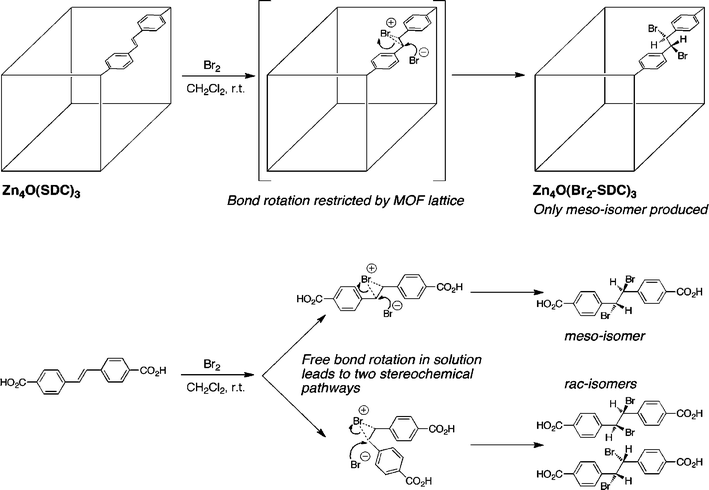 | ||
| Fig. 3 The stereochemical outcome of a bromination reaction is different with PSM on a MOF (top) than with the same ligand in solution (bottom). Restricted bond rotation within the MOF leads to a single stereoisomer. | ||
Spatial control of PSM
Another important issue in the realm of PSM is the degree and distribution of conversion in these heterogeneous reactions. In some cases, PSM is nearly quantitative and hence modification is essentially uniform throughout the crystalline lattice.7,8 However, when conversions are substantially less than complete (either due to inherent reaction limitations or by design), spatial control over where modification occurs within a MOF crystal is particularly challenging.Hupp and Nguyen et al. have reported a clever solution to this problem that allows for PSM to be applied in such a way that the surface and interior of the MOF structure can be distinguished.12 In their study, a MOF was constructed from two different ligands (Fig. 4); one of these ligands contained a trimethylsilyl-protected (TMS) acetylene group suitable for modification. The acetylene group can undergo ‘click’ chemistry with an azide upon removal of the TMS group (Fig. 2, entry 7) with a fluoride source. In order to achieve selective functionalization, the MOF crystals were first soaked in chloroform and then exposed to an aqueous solution of KF. The aqueous solution will not permeate the interior of the chloroform-saturated MOF due to the low partition coefficient of KF in chloroform, resulting in deprotection of only the surface TMS-acetylene groups. The MOF is then subjected to ‘click’ conditions and only the surface-exposed, deprotected acetylene groups are available to undergo the reaction. Subsequent deprotection of the interior TMS-acetylene groups with TEAF in THF, allowed for transformation of the interior acetylene groups with a different azide, giving a MOF with a ‘core–shell’ modification pattern.12 This study clearly showed, for the first time, control over the spatial distribution of PSM. The availability of such core–shell MOFs will likely have a substantial impact on the application of MOFs in many areas, including their use in biotechnology and biomedicine.
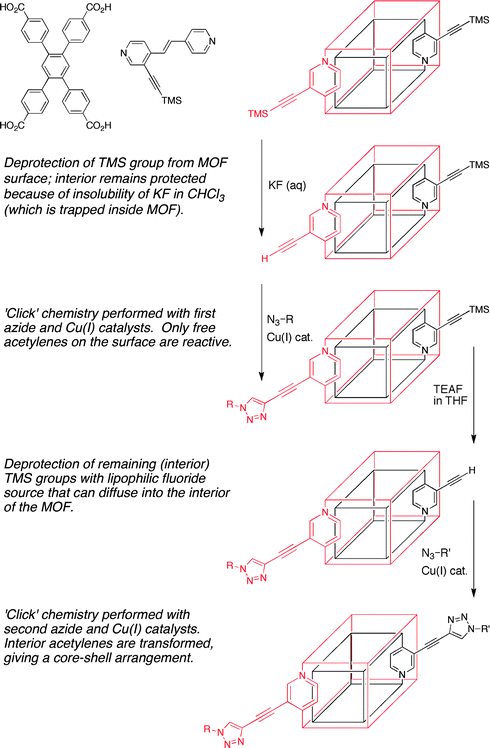 | ||
| Fig. 4 Selective functionalization of the exterior (red) and interior (black) of a MOF crystal. The ligands used to prepare this MOF are shown in the upper left. | ||
PSM for biomedical applications of MOFs
The use of MOFs as materials for biotechnology applications has garnered increasing interest in the past several years. Although still in its infancy, PSM has made its way into this important and relatively unexplored application of MOFs. The groups of Férey and Lin have made some of the most substantial contributions in developing MOFs, and more specifically in the development of nanocrystalline MOFs (NMOFs), as drug delivery13,14 and medical imaging vehicles.15,16 A series of reports have described the development of NMOFs with both luminescent and magnetic resonance imaging capabilities. Most recently, Lin et al. reported an important step toward combining diagnostic and therapeutic NMOFs (to obtain so-called ‘theranostic’ particles), which would incorporate imaging capability and drug delivery into a single platform.17 As described below, this advancement was achieved, in large part, using PSM.MIL-101(Fe) (MIL = Material Institut Lavoisier),18,19 comprised of Fe(III) clusters and a mixture of 1,4-benzenedicarboxylate and NH2-BDC ligands, was formulated into NMOFs for use as biocompatible particles. Specifically, the MIL-101(Fe) material was selected for its very high porosity and low toxicity. The NH2-BDC ligand of MIL-101(Fe) was used so that dye (imaging) or drug (therapeutic) molecules could be attached to the NMOFs via PSM. Alkylation of the NH2-BDC ligands (Fig. 2, entry 3) with an organic fluorophore (Br-BODIPY) was used to integrate an imaging component. Similarly, acylation (Fig. 2, entry 1) with a cisplatin prodrug (ESCP = c,c,t-[PtCl2(NH3)2(OEt)(O2CCH2CH2CO2H)]) was used to provide a therapeutic payload (Fig. 5). The fluorescence of the BODIPY dye was quenched by the Fe(III) centers of the NMOF. The quenching allowed for degradation of the NMOF components to be monitored by emergence of BODIPY emission upon release from the particle. The NMOFs showed a t1/2 of ∼2.5 h in PBS buffer at 37 °C; however, encapsulation of the particles in silica (Fig. 5) increased this t1/2 to ∼16 h.17 The dye-loaded particles were shown to be taken up by HT-29 human colon adenocarcinoma cells. Treatment of the same cell line with the ESCP-NMOFs resulted in cytotoxicity quite comparable to known platinum drugs.17 Overall, this study showed the potential of NMOFs in biomedicine and how PSM will play a central role in their development.
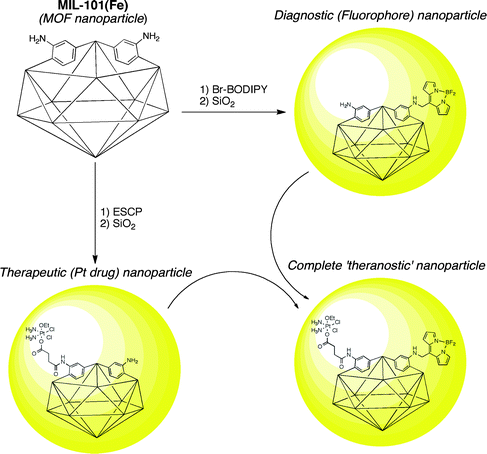 | ||
| Fig. 5 Preparation of fluorophore- and drug-loaded NMOFs using PSM. Combining the two approaches could lead to a complete ‘theranostic’ NMOF. Encapsulation in silica is represented by the yellow spheres. | ||
Concluding remarks
PSM is rapidly growing as a technique for advancing the chemistry of MOFs along several fronts. Recent examples presented here show how PSM has uncovered new reactivity in organic transformations, has been applied selectively to the surface of a MOF crystal, and has turned MOF nanocrystals into viable theranostics. It is clear that PSM will continue to provide new discoveries in the realm of MOF chemistry. As stated earlier, the term PSM was suggested as an analogy to the posttranslational modification of proteins. Looking forward to the future of PSM, perhaps a better analogy for PSM is by comparison to the rapidly expanding area of ‘bioorthogonal’ chemistry.20 Bioorthogonal chemistry is an area of research where new chemical reagents and reactions have been developed for selective modification of biomolecules in vitro and in vivo. Bioorthogonal chemistry can be thought of as a type of ‘synthetic posttranslational modification’ (although it is applicable to biomolecules other than polypeptides). As mentioned above, PSM of MOFs is constrained by limitations defined by the structure and stability of the MOF. Similarly, bioorthogonal chemistry must navigate the challenges summarized in Table 1.| Bioorthogonal chemistry | Postsynthetic modification | |
|---|---|---|
| Solvent limitations | Water, buffer, biological media | Non-caustic solvents |
| Reaction conditions | Mild, no toxic by-products | Mild, no degrading (strongly acidic) by-products |
| Introduction of chemical ‘handle’ | Reaction must occur with native functional groups or a synthetic ‘handle’ must be readily introduced by chemical biology | Reaction must proceed with MOF ‘as is’ or otherwise a ‘handle’ compatible with solvothermal synthesis must be provided |
| Reaction selectivity | Reagents must be highly selective to avoid reactivity with native functional groups | Reagents must be selective for groups installed on the MOF framework |
In essence, the challenges facing bioorthogonal chemistry parallel those in the PSM of MOFs. While the chemical biologist tries to modify a select biomolecule without denaturing the substrate or killing the cell, the MOF chemist tries to modify the framework without degrading the porous, crystalline structure of the MOF. However, the chemical biologist is largely confined by the limits of the biological medium, while the MOF chemist has the opportunity to broaden the scope of chemical reactions to be used for PSM with the ongoing development of more chemically robust and porous MOFs (e.g. MILs, ZIFs).19,21 The opportunities and significance of PSM on MOFs are only just now being revealed, and certainly many exciting discoveries will be reported in the near future.
The author would like to thank the University of California, San Diego, the National Science Foundation (CHE-0546531; instrumentation grants CHE-9709183, CHE-0116662, and CHE-0741968, MOF synthesis), and the Department of Energy (DE-FG02-08ER46519, gas sorption by MOFs) for support of research projects related to the PSM of MOFs.
Notes and references
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC
.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC
.
- C. T. Walsh, S. Garneau-Tsodikova and G. J. Gatto, Angew. Chem., Int. Ed., 2005, 44, 7342–7372 CrossRef CAS
.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS
.
- Y.-H. Kiang, G. B. Gardner, S. Lee, Z. Xu and E. B. Lobkovsky, J. Am. Chem. Soc., 1999, 121, 8204–8215 CrossRef CAS
.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS
.
- K. K. Tanabe, Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2008, 130, 8508–8517 CrossRef CAS
.
- Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368–12369 CrossRef CAS
.
- S. C. Jones and C. A. Bauer, J. Am. Chem. Soc., 2009, 131, 12516–12517 CrossRef CAS
.
- T. Kawamichi, T. Haneda, M. Kawano and M. Fujita, Nature, 2009, 461, 633–635 CrossRef CAS
.
- S. M. Cohen, Nature, 2009, 461, 602–603 CrossRef CAS
.
- T. Gadzikwa, O. K. Farha, C. D. Malliakas, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2009, 131, 13613–13615 CrossRef CAS
.
- P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Ferey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS
.
- P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS
.
- K. M. L. Taylor, A. Jin and W. Lin, Angew. Chem., Int. Ed., 2008, 47, 7722–7725 CrossRef CAS
.
- K. M. L. Taylor, W. J. Rieter and W. Lin, J. Am. Chem. Soc., 2008, 130, 14358–14359 CrossRef CAS
.
- K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS
.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS
.
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191–214 RSC
.
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef CAS
.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. D. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2010 |
