Cyclodextrin functionalized polymers as drug delivery systems
Jiawen
Zhou
and
Helmut
Ritter
*
Heinrich-Heine-Universität Düsseldor, Institut für Organische Chemie und Makromolekulare Chemie, Lehrstuhl II, Universitätsstraße 1, 40225, Düsseldorf, Germany. E-mail: h.ritter@uni-duesseldorf.de; Fax: +49 211-8115840; Tel: +49 211-8114760
First published on 3rd September 2010
Abstract
Cyclodextrins belong to the most important and promising macrocyclic hosts, since they are inexpensive, water-soluble natural products, non-toxic, readily functionalized and commercially available. Cyclodextrin chemistry creates lots of research areas. The present review focuses on investigations of cyclodextrins in polymer chemistry and their applications in drug delivery systems. Click reactions as a possibility to obtain polymer chains with covalently attached cyclodextrins are described, and examples for the application of such polymers in drug delivery are shown.
 Jiawen Zhou | Jiawen Zhou was born in 1982 in Zhejiang, China. She received her diploma in chemistry in 2007 from the Heinrich-Heine University of Düsseldorf in Germany. She is currently working on her PhD thesis which is focused on ring-opening polymerization of lactones in the research group of Prof. Dr H. Ritter in the institute for organic and macromolecular chemistry at the Heinrich-Heine University of Düsseldorf. |
 Helmut Ritter | Helmut Ritter obtained his degree in chemistry at the University of Marburg in 1972. He received the doctoral degree in 1975 from the University of Mainz, where he worked with Prof. Ringsdorf on “Synthesis of polymeric drugs”. In 1976 he moved to Bayer AG in Krefeld-Uerdingen and worked in the Central Research & Development Department on pigment modification with polymers, wood protection with oligomeric fungicides and new materials for membrane technology. Prof. Ritter joined the University of Wuppertal in 1982, where he received his habilitation in organic chemistry and polymer chemistry in 1989. He joined the institute of organic chemistry of the University of Mainz in February. Since October 2001 he is a Professor at the University of Düsseldorf, institute of organic and macromolecular chemistry. |
1. Introduction
Enzymes have fascinated scientists since their discovery. Recognition of an enzyme and a substrate was first described by Emil Fischer as the well-known lock and key principle.1One aim in the organic chemistry has been the synthesis of molecules that mimic the active centers of enzymes and promote catalysis. List et al. showed that L-proline catalyzes the direct aldol reaction, similarly to the aldolase of type I.2 In the search of new challenge, chemists have changed their view from focusing on a single molecule to molecular assemblies in the past decades. For example the Mn(II)–sodium dodecyl sulfate complex mimicking the active group of peroxidase was studied.3
Through the discovery of macrocycles such as crown ethers, calixarenens and curcurbiturils, the chemistry of molecular recognition started by Cram,4 Pedersen,4 and Lehn5 has been developed, and various supramolecular architectures have been constructed. New components were studied for materials, as well as mimics for biological structural formation and functions. Whereas Cram et al. developed host–guest chemistry, Lehn proposed the concept of supramolecular chemistry as “molecule beyond the molecule”. Originally supramolecular chemistry was defined in terms of the non-covalent interaction between a “host” and a “guest” molecule. These reversible non-covalent interactions can be electrostatic Coulomb, dipole–dipole, van der Waals, solvophobic, hydrophobic, or hydrogen bonding interactions. Selectivity of recognition increases as hosts and guests fit well together. Intelligent molecular systems can be created based on host–guest recognition, which can self-organize and show different behaviors than non-organized crystalline matters. These supramolecular structures can be used to develop molecular systems with specific functions, such as molecular machines stimulus-responsive artificial muscles,6,7 intelligent drug carriers,8 stimuli-responsive hydrogels9 or surfaces with photo-switchable polarities.10 Easton et al. investigate in cyclodextrin modified compounds acting as molecular machines. An interesting example is the trans-6A-deoxy-6A-(N-methylcinnamido)-β-cyclodextrin and the cis isomer function as a machine, with the alkene moiety serving as a photochemical on/off switch.11,12
Cyclodextrins belong to the most important and promising macrocyclic hosts since they are inexpensive, water-soluble natural products, non-toxic, readily functionalized and commercially available. They are able to recognize the thickness, polarity, and chirality of monomeric and polymeric guest molecules.
Since their discovery in 1891, cyclodextrins (CDs) have entranced many scientists. Their captivating structure has attracted not only chemists but also physicists, biologists, engineers, and many others, in attempts to take advantage of the unique properties.
2. Structure and properties of cyclodextrins
Cyclodextrins belong to the family of cyclic oligomers composed of α-(1→4)-linked D-glucopyranose units in 4C1 chair conformation (Fig. 1). They were discovered by Villers,13 identified by Schardinger,14 and systematically investigated by Freudenberg15 and Cramer.16 Using CD glucosyltransferases (CGTases), cyclodextrins can be produced by enzymatic degradation of starch, already on an industrial scale. Using specific precipitation agents n-octanol, toluene, and cyclohexadec-8-en-1-one for α-, β-, and γ-CDs, respectively, they can be isolated from the reaction mixture with high purities.17 Higher oligomers need to be isolated by chromatographic methods, because of the missing precipitation agents available. Cyclomaltopentaose, containing five glucopyranose units, has been chemically synthesized in small quantities.18 In addition, even the structure of a CD with 26 glucopyranose units is known.19 This review mainly focuses on α-, β-, and γ-CDs as hosts because of their ready availability.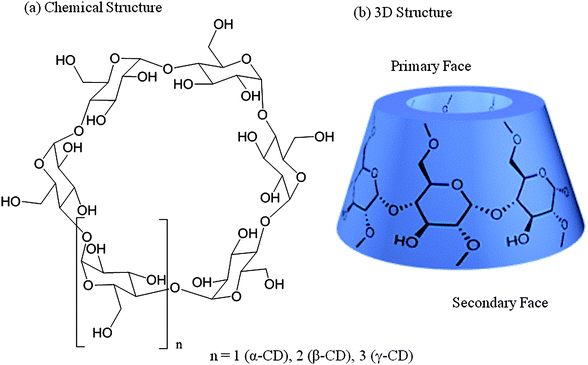 | ||
| Fig. 1 Structures of cyclodextrins.19 | ||
As shown by X-ray and neutron beam crystallography, α-, β-, and γ-CDs are shaped like hollow truncated cones (Fig. 1).19 The primary C-atoms from position 6 of the glucopyranose ring are located at the narrow side (primary face), while those located at the wider entrance are secondary C-atoms from positions 2 and 3, and therefore, less prone to chemical transformation (secondary face). Due to the lack of rotation around the bonds connecting the glucopyranose units, no conformational isomers are possible, unlike the calixarenes.20 The non-reduction character of CDs makes them behave as polyols. Modifications as substitution of different positions of CD were intensively investigated.21 The central cavity is relatively hydrophobic, whereas the outer surface shows a hydrophilic behavior. The inner diameter of the cavity in unmodified CDs ranges from about 4.7 to 8.3 Å, depending on the ring-size (see Table 1), and is about 8 Å in depth. These dimensions allow for the inclusion of several guest molecule types bearing appropriate size, to form inclusion complexes. Some properties of guest molecules change through the formation of inclusion complexes, e.g. the solubility and reactivity. This phenomenon constitutes the basis of most CD applications.
In the present article, we review how supramolecular chemistry of CD can be combined with polymer chemistry.
3. Cyclodextrin attached on polymers
Cyclodextrins can be used to complex hydrophobic monomers and polymers to make them water-soluble.23–30 Synthesizing polymers attached to CD is a consistent challenge for chemists.3.1. Click chemistry and cyclodextrin
Click reactions in cyclodextrin chemistry have been studied in recent years.31–37 Stimuli-responsive Janus-type copolymers based on β-CD derivatives were synthesized via click chemistry.38 We investigated the synthesis of cyclodextrin monomethacrylate via click reaction (Scheme 1).35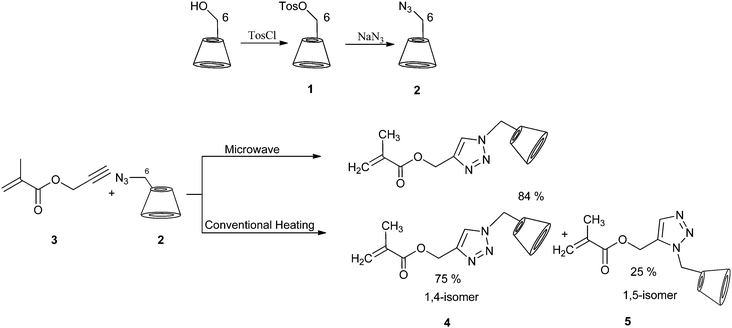 | ||
| Scheme 1 Synthetic route of CD-azide and monomethacrylate CD 4 and 5. Adapted with permission from ref. 35. Copyright 2008 American Chemical Society. | ||
Kinetics of the click reaction was studied under conventional heating and microwave conditions. From the two possible regioisomers, we discovered that an isomer mixture was obtained, when the reaction was carried out under conventional conditions, whereas reactions performed under microwave irradiation proceeded regioselectively. Furthermore, the reaction rate was easily increased under microwave conditions. The reaction was completed after 30 minutes, and the monomethacrylate cyclodextrin 4 polymerized (Scheme 2).
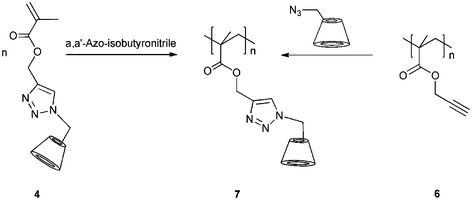 | ||
| Scheme 2 Synthesis of poly(methacrylate cyclodextrin) 7 on two reaction route. Adapted with permission from ref. 35. Copyright 2008 American Chemical Society. | ||
Due to the bulky CD ring, only water-soluble oligomers were obtained 7 (Mn = 1.30 × 10 4 g mol−1 and PD = 1.4). Therefore, a polymer analogous coupling of 6I-azido-6I-deoxycyclomaltoheptaose 2 onto poly(propargyl methacrylate) 6 was carried out. We used classical, free-radical-initiated polymerization on dioxane, and the obtained polymer (Mn = 1.04 × 105 g mol−1 and PD = 1.6) was completely soluble in DMF indicating that no cross-linking took place. This clearly demonstrates the preparation of pure monofunctional CD-methacrylate via click chemistry. The size of the polymers was investigated by DLS measurements in DMF solution (Fig. 2).
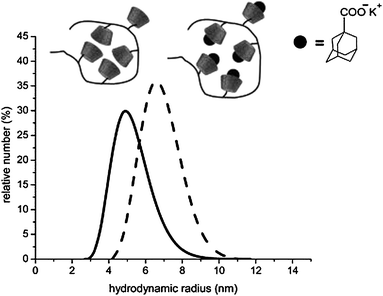 | ||
Fig. 2 Hydrodynamic volume of poly(methacrylate cyclodextrin) 7 in DMF solution before (—) and after (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) complexation with adamantly carboxylates (polymer concentration = 10 g L−1, 25 °C). Reprinted with permission from ref. 35. Copyright 2008 American Chemical Society. ) complexation with adamantly carboxylates (polymer concentration = 10 g L−1, 25 °C). Reprinted with permission from ref. 35. Copyright 2008 American Chemical Society. | ||
The CDs attached on polymers covalently can complex guest molecules. In our investigations, we used adamantyl carboxylate as guest molecules to obtain inclusion complexes. Compared to the polymer, the hydrodynamic radius of the complex slightly increased, due to the electrostatic repulsion between the carboxylate groups and reduction of H-bonds interaction between the dimerized CD rings.
The resulting polymer has potential applications in supramolecular chemistry and drug delivery.
3.2. Copolymers with covalently attached cyclodextrins
In recent investigations, we radically copolymerized monomethacrylate CD with N-isopropylacrylamide (NIPAAM) using different initiators under various reaction conditions, respectively (Scheme 3).39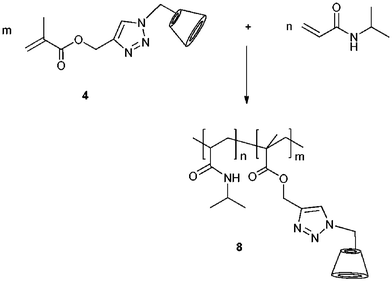 | ||
| Scheme 3 Synthetic pathway of NIPAAM–CD copolymer 8. Adapted with permission from Springer & Business Media.39 | ||
The copolymers were characterized by 1H NMR, turbidity and dynamic light scattering measurements. The thermosensitive properties of aqueous solutions of copolymers were analyzed by monitoring the changes of transparency as a function of temperature. N-Isopropylacrylamide containing copolymers (CD-monomer/NIPAAM = 1/20) 8 show identical LCST transitions at 36 °C, which are significantly higher than that of poly(NIPAAM) homopolymer at 32 °C. The increasing values are caused by the influence of hydrophilic CD-components in the copolymers, which leads to a better water-solubility of copolymers in comparison to a homopolymer of NIPAAM. In addition, the inclusion-effect of suitable guest molecules into the cavity of polymer attached CD ring on the thermo-sensitivity and coil dimensions of corresponding aqueous polymer solutions were investigated. The cloud points of the studied copolymers decreased with increasing hydrophobicity, which corresponds to the general assumptions of Taylor and Cerankowski.40 The characteristic increase or decrease of the LCST in comparison to the LCST value of pure copolymer is clearly a result of the complexation of the hydrophobic or hydrophilic guests in the CD cavity. To prove these phenomena, we used a relatively amphiphilic guest molecule, adamantyl carboxylate (AdC) and the more hydrophobic polymerizable guest adamantyl methylacrylamide (AdM) for complexation. Compared to the starting copolymer, the LCST of complexed copolymer after addition of AdC increased. The inclusion of the guest's hydrophobic part into the CD cavity causes an increase of the hydrophilic properties of the polymer. The free carboxylic group of AdC is located preferentially in the water phase, therefore, a slightly increased solubility of that complex in water can be explained. This leads to higher LCST values of 53 °C for (8 + AdC) complex. The complexation of the adamantyl component of AdM in the CD's cavity leads to a supramolecular structure bearing a guest, in which the more hydrophobic methyl methacryl group is located in the water phase. This causes a decrease of the LCST compared to the uncomplexed polymer. In addition, the dependence of the hydrodynamic volume on the amount of CD-monomers in the copolymer was studied (Fig. 3). The copolymer with high CD-monomer content shows a smaller coil size than the one with lower CD-monomer amount. Because of the strong hydrogen bond interactions between the CD moieties on copolymer chain, some intramolecular interactions can be formed.
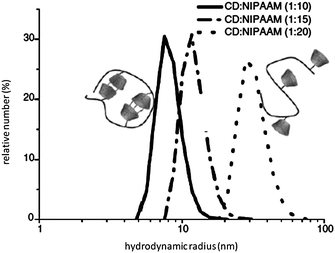 | ||
Fig. 3 Hydrodynamic volumes of polymer 8 (CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIPAAM = 1 NIPAAM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), 8a (CD 20), 8a (CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIPAAM = 1 NIPAAM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), and 8b (CD 10), and 8b (CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NIPAAM = 1 NIPAAM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) in aqueous solution (10 g L−1, 25 °C). Reprinted with permission from ref. 39. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. 15) in aqueous solution (10 g L−1, 25 °C). Reprinted with permission from ref. 39. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
4. Drug delivery systems
Drug delivery is the method of administering a pharmaceutical compound to achieve a therapeutic effect in humans or animals.41 Current research areas of drug delivery include the development of targeted delivery, in which the drug is only active in the target area of the body and sustained release formulations, in which the drug is released over a period of time in a controlled manner from a formulation.42 Types of sustained release formulations are for example liposomes,43 drug loaded, biodegradable microspheres44 or drug polymer conjugates.45 The investigations of chemistry researchers concentrate on substances, which can load and release drugs in the target area.464.1. Polymeric drugs
A fascinating area in polymer science is the investigation of polymers in biomaterials and in medicine.47 Polymers may be pharmacologically active, if they are used as carriers for drugs. Due to their intrinsic properties, they may influence body distribution, excretion or cell absorption of the pharmaca they carry. For example smart nanofibers of block copolymers with thermal-sensitive surface carrying materials for drug and gene delivery are of great interest.48Ringsdorf et al. have well investigated polymeric drugs in the early seventies of the last century. Via a spacer group the drugs were covalently attached to the hydrophilic polymer backbone.49
There are various scientific articles describing the advantages of drugs included in cyclodextrins, e.g. increased solubility, enhanced bioavailability and improved stability.50 The complexation of anti-HIV drug in CD is an interesting example.44
Among the modified nucleobases, 5-fluorouracil (5-FU) is one of the frequently used chemotherapeutic agents, since the biological and tumor-inhibitory properties were described (Fig. 4).51 5-FU is the active metabolite of 5-fluorocytosine, the prodrug being converted by the bacterial and fungal cytosine deaminase. The role of the intestinal flora in the fluorocytosine conversion process was postulated, although the human cells lack this enzyme.52 The non-toxic 5-fluorocytosine derivates could also be used in intestinal cancer therapy.
Adamantyl groups are known to form strong inclusion complexes not only with β-CD but also with cyclodextrin-containing polymers. In addition to the stable inclusion complexes, adamantyl moieties can also facilitate the partition into the lipid cell membrane, as a result of increased lipophilicity.
In order to develop new potential anticancer agents, we have investigated the synthesis of the 5-fluorocytosine 10 analogue bearing an adamantyl moiety, and its non-covalent inclusion complexation with CD covalently attached to a polymer (Scheme 4).53
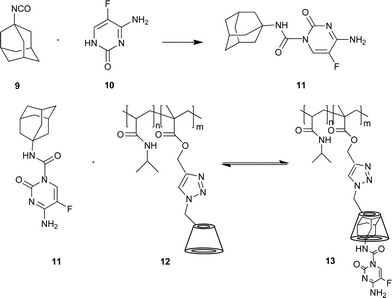 | ||
| Scheme 4 Synthesis of the adamantyl-modified 5-fluorocytosine 11 and the non-covalent attaching of the antitumor agent 11 to the polymer 12. Reprinted with permission from ref. 53. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
The adamantyl-modified antitumor agent 11 was combined with water soluble copolymers 12 bearing covalently attached CD. DLS, UV/VIS and cloud point measurements for the cyclodextrin-containing copolymer were performed. The copolymer was complexed with an equivalent ratio of fluorocytosine-derivate.
The changes of turbidity as a function of temperature were monitored in order to investigate the thermosensitive properties of aqueous solutions of the cyclodextrin-bearing copolymer 12 and the supramolecular polymer network containing the drug analogue 13 (Fig. 5). At 30 °C the cyclodextrin-containing copolymer shows a turbidity point, which correlates to the LCST of poly(NIPAAM). The cloud point increases up to 36 °C, by the formation of inclusion complexes of the copolymer with the synthesized compound 13.
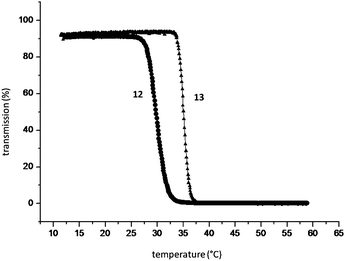 | ||
| Fig. 5 Turbidity analysis of aqueous solutions of the CD-containing polymer 12 and measurement of the supramolecular complexed polymer 13. Reprinted with permission from ref. 53. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
In order to release the drug to achieve drug activity, the supramolecular polymer system 13 was treated with α-amylase from Aspergillus oryzae in water, as an example. As predicted, CDs were enzymatic hydrolyzed to glucose and maltooligosaccharides, whereas the drug 11 was released. This delivery process was spectrophotometrically proved. Thus, the complexation and releasing of an antitumor agent on a polymer chain were observed with the described system.
4.2. Chiral recognition by use of cyclodextrins
An important subject in pharmaceutical, biological, medical systems is the chiral recognition of hosts. CDs are chiral, and the first stereoselective inclusion of a chiral polymer chain with CDs was obtained by Tonelli et al., who showed that only natural isotactic poly(3-hydroxybutyrate) can be included in α-CD cavities, whereas the atactic one cannot be included.54 In 2007, Yui et al. published the enantioselective complexation of isotactic polylactides with CD rings.55 In the case of a free radical polymerization of a CD complexed racemic N-methacryloyl-phenylalaninmethylester, we showed that the D-enantiomer polymerizes preferentially, whereas the L-enantiomer tends to accumulate in the solution.56Very recently, we investigated the enantioselective inclusion of chiral side groups of synthetic polymers with CD. We showed that a chiral recognition of the polymeric bound amino acid with CD takes place.57
We chose D- and L-tryptophane (D- and L-Trp) as a chiral model compound since it is an essential amino acid implemented in proteins with an aromatic moiety, and therefore suitable to build inclusion complexes with RAMEB–CD.58–60
N-Acryloyl-D-tryptophan 15D and N-acryloyl-L-tryptophan 15L were synthesized under Schotten–Baumann conditions by using acryloyl chloride and radically copolymerized with NIPAAM in different molar ratios (Scheme 5). The formation of inclusive complexes between RAMEB–CD with either 15D or 15L Trp aromatic side groups was analyzed by 2D ROESY-spectroscopy and UV-measurements. The uncomplexed aqueous solutions of the copolymers exhibited identical UV absorptions. In contrast, the copolymers bearing the L-enantiomers led to an increase of the UV absorption, whereas in the case of the attached D-amino acid a decreased absorption was observed.
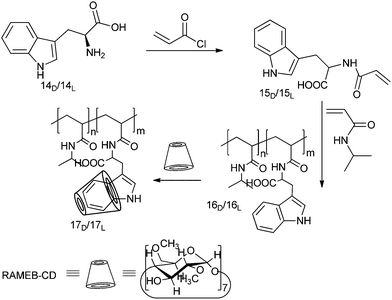 | ||
| Scheme 5 Synthetic route to the chiral pH- and temperature-responsive copolymers 16D and 16L, and their corresponding supramolecular systems 17D and 17L with randomly 1,8-methylated β-CD. Reprinted with permission from ref. 57. Copyright 2010 American Chemical Society. | ||
Further analyses with DLS and turbidity methods confirmed the preferred complexation of RAMEB–CD with the D-enantiomer (Fig. 6). These findings can generate a new model for chirality identification of side groups in polymer matrixes, which can potentially offer great opportunities, for example in drug development.
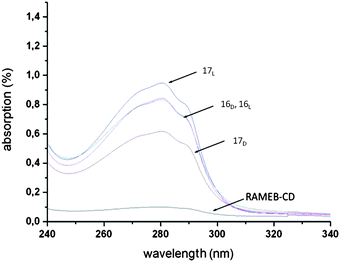 | ||
Fig. 6 UV-spectrum of the RAMEB–CD, copolymer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 16D,L and 17D,L in aqueous solution (0.5 mg mL−1). Reprinted with permission from ref. 57. Copyright 2010 American Chemical Society. 20) 16D,L and 17D,L in aqueous solution (0.5 mg mL−1). Reprinted with permission from ref. 57. Copyright 2010 American Chemical Society. | ||
4.3. Cyclodextrin based nanocarriers
Nanoparticles have attracted great attention as potential carriers for controlled and targeted delivery of drugs.45 Zhang and Ma have investigated in versatile nanocarriers for drug delivery.61 While the hydrophilic outer shell, which comprises hydrophilic polymers, provides the colloidal stability, the hydrophobic inner core serves as a nanocontainer for hydrophobic drugs. Harada, Kataoka, Kabanov and co-workers showed in their pioneering works that the interactions between core forming segments of block copolymers can be electrostatic.62 Ma et al. synthesized a block copolymer with polyaspartamide block that contained ethylenediamine (EDA) units (PEG-b-PEDA) 18.63 β-CD was then covalently linked to PEG-b-PEDA to obtain PEG-b-PCD 19 (Scheme 6). The cyclodextrin conjugated block acts as the host segment, which is able to form inclusion complexes with hydrophobic substances, while the hydrophilic segment can provide the resultant, stable assemblies with stability. The formation of polymeric assemblies mediated by the host–guest interactions between PEG-b-PCD and hydrophobic substances was initially investigated by using pyrene as a guest molecule. The schematic illustration of the formation of several host–guest assemblies are shown in Fig. 7.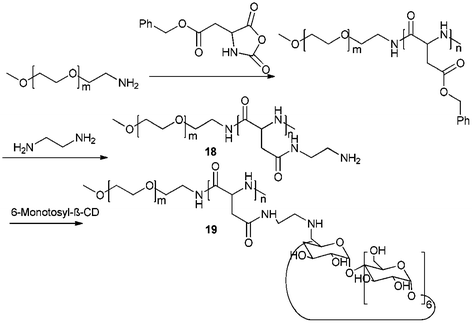 | ||
| Scheme 6 Synthetic pathway of the nanocarriers PEG-b-PCD 19. Reprinted with permission from ref. 61. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
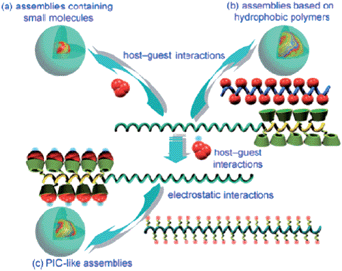 | ||
| Fig. 7 Schematic illustration of the formation of various host–guest assemblies: (a) small hydrophobic molecules mediated assemblies, (b) assemblies formed in the presence of ahydrophobic polymer, and (c) PIC-like assemblies. Reprinted with permission from ref. 61. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
PEG-b-PCD assemblies that contained pyrene were analyzed by atomic force microscopy, DLS measurements and TEM images. DLS measurements showed a mean diameter of 27.3 nm, and the electron microscopy images revealed the mean assembly size to be about 20.0 nm. A similar phenomenon of simultaneous encapsulation and assembly formation was also obtained for PEG-b-PCD in the presence of other hydrophobic compounds, such as indomethacin (IND), a non-steroidal anti-inflammatory drug and coumarin 102. A preliminary in vitro release study was carried out, which demonstrated the chemical-stimulated release behavior of assemblies based on PEG-b-PCD and IND. In the presence of adamantine carboxylic acid, a competitive guest molecule with a higher complexation constant than the one of IND, the drug release rate was accelerated.
Durand et al. have investigated in nanosized contrast agents, which have potential in magnetic resonance molecular imaging applications for clinical diagnosis.63 They studied nanoparticles composed of non-covalent adduct between a gadolinum complex, a polymer of β-CD (p-β-CD: Mw = 1.5 × 106 g mol−1) and a dextran grafted with alkyl chains (MD). The cyclic polyaminocarboxylate template DOA3 (1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid) and adamantine were connected through an acetamide spacer (Scheme 7).
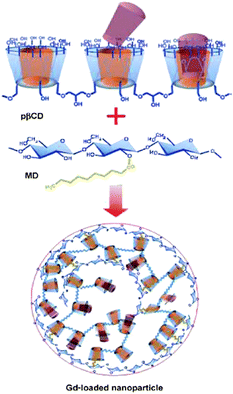 | ||
| Scheme 7 Synthesis pathway of the Gd(III)-loaded nanoparticle. Reprinted with permission from ref. 63. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Based on a “lock-key” recognition process in which the hydrophobic alkyl chains of MD and the adamantly moieties of macrocyclic Gd(III) chelates were included in the cavities of p-β-CD, these supramolecular assemblies can spontaneously be formed under mild conditions in a one-step procedure. These supramolecular adducts indicated a diameter of about 200 nm. Using quasi-elastic light scattering (QELS), the size of the nanoparticles in solution and their homogeneity (polydispersity) were determined after their preparation.
Those two examples show the high potentials of nanoparticles combined with cyclodextrins as nanocarriers for drug delivery.
5. Conclusion-outlook
Recent progresses in construction of polymers with covalently attached CD have inspired interesting development of supramolecular materials for drug delivery.Because of its excellent complexation ability, CD is an important host in drug delivery systems. In some cases CD showed enantioselectivity in the formation of inclusion complexes with guests bearing chiral groups.
The research of CD-based supramolecular biomaterials for drug delivery is an emerging area, which still faces many challenges in future studies. Although various successful studies have been demonstrated, however, material designs are expected to show a better balance of higher functions and performances. Intensive biocompatibility and biodegradability studies are required for the supramolecular drug delivery systems.
References
- E. Fischer, Ber. Dtsch. Chem. Ges., 1894, 27, 2985 CrossRef CAS.
- B. List, R. A. Lerner and C. F. Barbaras III, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS.
- L.-H. Chen, L.-Z. Liu and H.-X. Shen, Anal. Chim. Acta, 2003, 480, 143 CrossRef CAS.
- (a) D. J. Cram, J. Inclusion Phenom., 1988, 6, 397 CrossRef CAS; (b) C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 2495 CrossRef CAS; (c) C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017 CrossRef CAS.
- J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89 CrossRef.
- C. Dietrich-Buchecker, M. C. Jimenez-Molero, V. Sartor and J. P. Sauvage, Pure Appl. Chem., 2003, 75, 1383 CrossRef CAS.
- D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 174 CrossRef CAS.
- C. Moon, Y. M. Kwon, W. K. Lee, Y. J. Park and V. C. Yang, J. Controlled Release, 2007, 124, 43 CrossRef CAS.
- Y. Chen, X.-H. Pang and C.-M. Dong, Adv. Funct. Mater., 2010, 20, 579 CrossRef CAS.
- J. Berna, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Perez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704 CrossRef CAS.
- C. J. Easton, S. F. Lincoln, L. Bar and H. Onagi, Chem.–Eur. J., 2003, 10, 3120.
- R. J. Coulston, H. Onagi, S. F. Lincoln and C. J. Easton, J. Am. Chem. Soc., 2006, 128, 14750 CrossRef CAS.
- A. Villers, C. R. Acad. Sci., 1891, 112, 536 Search PubMed.
- F. Schardinger, Eur. Food Res. Technol., 1903, 6, 865 CrossRef CAS.
- L. Freudenberg and R. Jakobi, Justus Liebigs Ann. Chem., 1935, 518, 102 CrossRef.
- K. Freudenberg and F. Cramer, Chem. Ber., 1950, 83, 296 CrossRef CAS.
- G. Wenz, Angew. Chem., Int. Ed. Engl., 1994, 19, 344.
- T. Nakagawa, K. Ueno, M. Kashiw and J. Watanabe, Tetrahedron Lett., 1994, 35, 1921 CrossRef CAS.
- W. Saenger, J. Jacob, K. Gessler, T. Steiner, D. Hoffmann, H. Sanbe, K. Koiumi, S. M. Smith and T. Takaha, Chem. Rev., 1998, 98, 1787 CrossRef CAS.
- A. F. D. de Namor, R. M. Cleverley and M. L. Zapata-Ormachea, Chem. Rev., 1998, 98, 2495 CrossRef.
- A. R. Khan, P. Forgo, K. J. Stine and V. T. D'Souza, Chem. Rev., 1977, 98, 1998.
- G. Wenz, Inclusion Polymers, Springer Verlag, 1st edn, 2009 Search PubMed.
- O. Kretschmann, C. Steffens and H. Ritter, Angew. Chem., Int. Ed., 2007, 46, 2708 CrossRef CAS.
- C. Koopmans and H. Ritter, J. Am. Chem. Soc., 2007, 129, 3502 CrossRef CAS.
- O. Kretschmann, S. W. Choi, M. Miyauchi, I. Tomatsu, A. Harada and H. Ritter, Angew. Chem., Int. Ed., 2006, 45, 4361 CrossRef.
- H. Köllisch, C. Barner-Kowollik and H. Ritter, Macromol. Rapid Commun., 2006, 27, 848 CrossRef.
- S. Schmitz and H. Ritter, Angew. Chem., Int. Ed., 2005, 44, 5658 CrossRef CAS.
- K. Ohga, Y. Takashima, H. Takahashi, Y. Kawaguchi, H. Yamaguchi and A. Harada, Macromolecules, 2005, 38, 5897 CrossRef CAS.
- A. Harada, A. Hashidzume and Y. Takashima, Adv. Polym. Sci., 2006, 201, 1 CAS.
- G. Wenz, Angew. Chem., Int. Ed. Engl., 1994, 33, 803 CrossRef.
- F. Perez-Balderas, M. Ortega-Munoz, J. Morales-Sanfrutos, F. Hernandez-Mateo, F. G. Calvo-Flores, J. A. Calvo-Asin, J. Isac-Garcia and F. Santoyo-Gonzalez, Org. Lett., 2003, 5, 1951 CrossRef CAS.
- M. Ortega-Munoz, J. Morales-Sanfrutos, F. Perez-Balderas, F. Hernandez-Mateo, M. D. Giron-Gonzalez, N. Sevillano-Tripero, R. Salto-Gonzalez and F. Santoyo-Gonzalez, Org. Biomol. Chem., 2007, 5, 2291 RSC.
- T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS.
- R. Hoogenboom, B. C. Moore and U. S. Schubert, Chem. Commun., 2006, 4010 RSC.
- M. Munteanu, S. W. Choi and H. Ritter, Macromolecules, 2008, 41, 9619 CrossRef CAS.
- L. Barr, S. Lincoln and C. J. Easton, Supramol. Chem., 2005, 17, 547 CrossRef CAS.
- S. Sriniva Sachari and T. M. Reineke, Biomaterials, 2009, 30, 928 CrossRef.
- Z. Ge, J. Xue, J. Hu, Y. Zhang and S. Liu, Soft Matter, 2009, 5, 3932 RSC.
- S. W. Choi, M. Munteanu and H. Ritter, J. Polym. Res., 2009, 16, 389 Search PubMed.
- L. D. Taylor and L. D. Cerankowski, J. Polym. Sci., Polym. Chem. Ed., 1975, 13, 2551 CrossRef.
- (a) M. S. Wang, S. Bddapati and M. R. Sierks, Biochem. Biophys. Res. Commun., 2009, 386, 526 CrossRef CAS; (b) Y. Meissner and A. Lamprecht, J. Pharm. Sci., 2008, 97, 2878 CrossRef CAS.
- (a) J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000 CrossRef CAS; (b) F. Ferrari, M. Sorrenti, S. Rossi, L. Catenacci, G. Sandri, M. C. Bonferoni, C. B. Caramella and G. Bettinetti, Pharm. Dev. Technol., 2008, 13, 65 CrossRef CAS.
- (a) B. McCormack and G. Gregoriadis, Int. J. Pharm., 1998, 162, 59 CrossRef CAS; (b) B. McCormack and G. J. Gregoriadis, J. Drug Targeting, 1994, 2, 449 Search PubMed; (c) B. McCormack and G. Gregoriadis, Int. J. Pharm., 1994, 112, 249 CrossRef CAS.
- J. S. Torne and P. R. Vavia, J. Inclusion Phenom. Macrocyclic Chem., 2006, 56, 253 CrossRef CAS.
- (a) S. Pandya, J. S. Mansuri and P. Patel, Pharm. Rev., 2008, 6(2) Search PubMed; (b) S. S. Banerjee and D. H. Chen, Cyclodextrin conjugated magnetic colloidal nanoparticles as a nanocarrier for targeted anticancer drug delivery, Nanotechnology, 2008, 19 Search PubMed; (c) A. L. Nielsen, K. Steffensen and K. L. Larsen, Colloids Surf., B, 2009, 73, 267 CrossRef CAS; (d) A. Vyas, S. Saraf and S. Saraf, J. Inclusion Phenom. Macrocyclic Chem., 2008, 62, 23 CrossRef CAS.
- T. Loftsson, Pharmazie, 1998, 53, 733 CAS.
- D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581 CrossRef CAS.
- G. D. Fu, L. Q. Xu and F. Yao, ACS Appl. Mater. Interfaces, 2009, 1, 239 Search PubMed.
- (a) H. L. Gros, H. Ringsdorf and H. Schupp, Angew. Chem., Int. Ed. Engl., 1981, 20, 305 CrossRef; (b) H. Ringsdorf, J. Polym. Sci., 1975, 51, 135 Search PubMed.
- (a) M. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875 CrossRef CAS; (b) T. Loftsson and M. Brewester, J. Pharm. Sci., 1996, 85, 1017 CrossRef CAS; (c) K. Connors, Chem. Rev., 1997, 97, 1325 CrossRef CAS; (d) H. Gonzales, S. J. Hwang and M. E. Davis, Bioconjugate Chem., 1999, 10, 1068 CrossRef CAS; (e) M. E. Davis and M. E. Brewster, Nat. Rev. Drug Discovery, 2004, 3, 1023 CrossRef CAS; (f) H. H. Sigurdusson, E. Knudsen, T. Loftsson, N. Leeves, J. F. Sigurjonsdottir and M. Masson, J. Inclusion Phenom. Macrocyclic Chem., 2003, 44, 169; (g) A. Lutka, Acta Pol. Pharm., 2002, 59, 45 CAS; (h) T. Higashi, F. Hirayama, S. Yamashita, S. Misumi, H. Arima and K. Uekama, Int. J. Pharm., 2009, 374, 26 CrossRef CAS.
- (a) R. Duchinsky, E. Pleven and C. Heidelberg, J. Am. Chem. Soc., 1957, 79, 4559 CAS; (b) C. Heidelberg, N. K. Chaudhuri, P. B. Danneberg, D. Mooren, L. Griesbach, R. Duchinsky, R. J. Schnitzer and C. Heidelberg, Nature, 1957, 197, 663 CrossRef.
- M. C. Malet-Martino, R. Martino, M. de Forni, A. Andremont, O. Hartmann and J. P. Armand, Infection, 1991, 19, 178 CrossRef CAS.
- A. Maciollek, M. Munteanu and H. Ritter, Macromol. Chem. Phys., 2010, 211, 245 CrossRef CAS.
- X. Shuai, F. E. Porbeni, M. Wie, T. Bullions and A. E. Tonelli, Macromolecules, 2002, 35, 3778 CrossRef CAS.
- Y. Ohya, S. Takamido, K. Nagahama, T. Ooya, R. Katoono and N. Yui, Macromolecules, 2007, 40, 18.
- S. Schwarz-Barac, H. Ritter and D. Schollmeyer, Macromol. Rapid Commun., 2003, 24, 325 CAS.
- S. Gingter, E. Bezdushna and H. Ritter, Macromolecules, 2010, 43, 3128 CrossRef CAS.
- A. Harada and M. Kamachi, Nature, 1992, 356, 25 CrossRef.
- A. Harada and M. Kamachi, Macromolecules, 1990, 23, 2821 CrossRef CAS.
- D. Taura, Y. Tanigudi, A. Hashidzume and A. Harada, Macromol. Rapid Commun., 2009, 30, 1741 CrossRef CAS.
- J. Zhang and P. X. Ma, Angew. Chem., Int. Ed., 2009, 48, 964 CrossRef CAS.
- (a) A. Harada and K. Kataoka, Macromolecules, 1995, 28, 5294 CrossRef CAS; (b) A. V. Kabanov, T. K. Bronich, V. A. Kabanov, K. Yu and A. Eisenberg, Macromolecules, 1996, 29, 6797 CrossRef CAS.
- E. Battistini, E. Gianoliu, R. Gref, P. Couvreur, S. Fuzerova, M. Othman, S. Aime, B. Badet and P. Durand, Chem.–Eur. J., 2008, 14, 4551 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |