Synthesis of silica-polymer hybrids by combination of RAFT polymerization and azide-alkyne cycloaddition ‘click’ reactions†
Youke
Huang
a,
Tengteng
Hou
a,
Xiangqian
Cao
a,
Sébastien
Perrier
b and
Youliang
Zhao
*a
aKey Lab of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: ylzhao@suda.edu.cn; Fax: +86-512-65882045; Tel: +86-512-65882045
bKey Centre for Polymers & Colloids, School of Chemistry, the University of Sydney, NSW 2006, Australia
First published on 31st August 2010
Abstract
An original strategy is presented to synthesize highly pure multiblock copolymers (up to tetrablock) of various monomers tethered to silica particles, by combining RAFT polymerization and click chemistry. Two approaches were compared that follow this strategy. In a first approach, Z-azide-functionalized polymers were prepared via a RAFT process and then tethered to silica particles via a direct click reaction. This approach led to well-defined grafted polymers with polydispersity indices lower than their “as-prepared” precursors, due to lack of dead chains. In a second approach, a one-pot method was employed, using clickable RAFT agents to perform RAFT polymerization and click reaction simultaneously. This route afforded grafted polymers with polydispersity typically less than 1.25, whilst the chain length of grafted polymers was usually shorter than that of free polymers formed in solution, due to shielding effect and heterogeneous reaction condition. A series of well-defined homopolymers, di-, tri- and tetrablock copolymers could be efficiently grafted onto silica particles, and the grafting density was usually ranged between 0.017 and 0.085 chains nm−2, evident from GPC, IR, elemental and thermogravimetric analyses. The one-pot approach seems more promising than the incremental route, since it is a one step reaction that still maintains controllability over surface modification.
Introduction
The construction of polymer-related materials with controlled compositions, topologies and functionalities has been an enduring focus in polymer science. The fast development and progress in “living”/controlled radical polymerization and highly efficient organic reaction such as click reaction enable synthesis of more complex functional materials.1–23 Controlled radical polymerization (CRP) approaches such as iniferter-mediated polymerization,1 nitroxide-mediated polymerization,2 atom transfer radical polymerization,3,4 and reversible addition-fragmentation chain transfer (RAFT) polymerization5–9 have been developed to synthesize well-defined polymers with controlled molecular weight, low polydispersity, and variable functionality. Click reactions such as azide-alkyne cycloadditions, Diels–Alder reactions, and thiol-ene/yne/bromide reactions which emerged in the past decade are a versatile and promising tool in macromolecular engineering owing to their high efficiency, variable environments and relatively mild conditions.6–15 At present, the combination of click reaction and CRP techniques to construct functional materials is an important tendency in polymer and materials science, which has been applied to synthesize many types of complex polymer-related materials such as functional block, star, branched and graft polymers, bioconjugates and hybrid materials.16–23Meanwhile, the surface modification of solid substrates such as inorganic particles, carbon and cross-linked resins with polymeric chains to form functional core-shell hybrid materials has attracted continuous attention due to their variable interfacial, mechanical and thermal properties and potential applications in optics, electronics, engineering and biosciences.23–43 Among them, much attention has been paid to the synthesis and properties of silica-polymer hybrids due to their unique properties derived from the chemical resistance, mechanical stability, and variable particle sizes and specific surface area of silica particles and versatile physico-chemical properties of surface-tethered polymeric chains.23–29 Various polymerization approaches, especially surface-initiated controlled radical polymerization (SI-CRP), click reaction and their combination, have been used to create these hybrid materials. So far, some types of macromolecular architectures such as homopolymer, diblock copolymer, branched polymer and V-shaped polymer have been grafted onto substrate surfaces by SI-CRP, however, the surface-tethered polymers typically comprise chemical compositions less than three, and examples of multicomponent polymers such as tri- and tetrablock copolymer-grafted silica particles are very scarce.37 It is still a challenge to synthesize silica-polymer hybrids with relatively high grafting density and well-defined block copolymers with multiple components.
Generally, the surface modification based on SI-CRP techniques can be performed by both “grafting to” and “grafting from” approaches, and both of them have advantages and limitations.28 The grafting to approach can be straightforwardly used to graft well-defined prefabricated homopolymers and block copolymers to a substrate surface, however, the grafting densities obtained are relatively low due to steric repulsions between polymeric chains and decreased reaction efficiency. The grafting from approach allows high grafting density and accurate control over composition and architecture, however, the structural defects of the tethered polymeric chains such as the presence of undesired grafted chains are usually unavoidable. During SI-CRP via the grafting from approach, the concentration of polymer radicals on the substrate surface is relatively high, the termination and irreversible transfer are liable to produce dead chains, and sometimes polymeric chains with lower chemical compositions remain on the substrate surface due to lowered reinitiation activity during chain extension polymerization. For example, Ranjan and Brittain synthesized silica nanoparticles grafted with PSt by combination of RAFT polymerization and click chemistry via the grafting from approach, and then the resultant silica-PSt hybrid was used as a macro RAFT agent to mediate chain extension polymerization of methyl acrylate.18 Although a high grafting density of up to 0.22 chains nm−2 could be achieved, the chain extension polymerization proceeded very poorly, and the low reinitiation efficiency of about 40% illustrated the grafted chains were indeed mixtures of PSt and PSt-b-PMA diblock copolymers.18 Recently, they also successfully developed a one-pot method based on tandem RAFT polymerization and click reaction to synthesize silica nanoparticles grafted with PSt, which could afford a high grafting density of up to 0.51–0.70 chains nm−2.16 The tandem approach had significant advantages over “grafting to” and “grafting from” approaches in terms of labor, time and cost, so represented a more promising method for synthesizing inorganic–organic hybrid materials.16 However, the example of the tandem approach to silica-polymer hybrids is very limited, so it is very important to explore the possibility of synthesizing block copolymer grafted silica particles via this method.
Although CRP techniques enable functionality at the chain end of the polymers, it's almost impossible to achieve 100% chain-end functionality after polymerization due to some side reactions such as termination and irreversible transfer.44 This problem can be partly addressed by combining advantages of solid-phase synthesis and a Z-group approach based on RAFT polymerization. In previous studies, two basic approaches have been developed to this end: one was to perform the Z-supported RAFT graft approach in which the RAFT process was directly conducted on the surface of solid supports,30–36 another was to utilize the combinatorial approach based on RAFT polymerization and coupling reactions37 in which the Z-functionalized polymers were synthesized by a RAFT process followed by a coupling reaction to perform the graft reaction. Both ways using the grafting to approach could afford better-defined silica-polymer hybrids, and the resultant Z-supported polymers were almost 100% living, evident from highly efficient chain extension polymerization of a second monomer to prepare silica-supported block copolymers.34,37 However, the above methods still possess some limitations and need further improvement. The Z-supported RAFT graft polymerization was quite difficult to generalize for the synthesis of tri- and tetrablock copolymer-grafted silica since the formed free and grafted polymers had quite different molecular weights whilst a free polymer was usually necessary to restrict some side reactions.30–37 The combination of the RAFT process and hydroxyl-alkoxysilane coupling reaction could afford silica particles grafted with well-defined multiblock copolymers, however, the alkoxysilane functionality was sensitive to moisture and high temperature, thus the RAFT process for the synthesis of Z-functionalized polymers should be performed under vigorously anhydrous conditions at a relatively low temperature such as 60 °C, otherwise the efficiency of coupling reaction would be significantly reduced due to partly lost alkoxysilane functionality.
The combinational approach comprising RAFT polymerization and coupling reactions is very promising for the synthesis of solid supports grafted with well-defined multiblock copolymers. The efficient, robust and facile click reaction tolerates moisture and various functionalities,10,12,15 and the versatile RAFT process enables use of macro chain transfer agents with terminal azide or alkyne functionality, so their combination is expected to enable the synthesis of silica-polymer hybrids with relatively high grafting density under relatively moderate conditions. In this continuous study, the azide-alkyne addition reaction10 was chosen instead of previous hydroxyl-alkoxysilane coupling reactions37 to improve the reaction conditions. Incremental and tandem RAFT polymerization and click reaction were utilized to synthesize silica particles grafted with various polymeric chains. The reaction conditions were optimized, and homopolymer and multiblock copolymer grafted silica particles were prepared from highly pure polymer blocks.
Linking multiblock copolymers to the surfaces of solid substrates has great importance for both fundamental studies and applications. In addition to applications in inorganic–organic hybrid materials, surface-tethered multiblock copolymers usually exhibit different physico-chemical properties such as melting point, glass transition temperature and microphase-separated structures, from those of their corresponding linear analogs. This study presents a versatile approach to prepare block copolymer attached to silica particles. In addition, the purity of multiblock copolymers may affect their self-assembly behaviors, thus the ability to synthesize highly pure block copolymers is of great importance. This work presents a facile route that enables the synthesis of highly pure block copolymers via grafting reaction, followed by de-grafting reaction by aminolysis.
Experimental
Materials
All solvents, monomers, and other chemicals were purchased from Aldrich unless otherwise stated. Silica gel (Qingdao Haiyang Chemical Co., Ltd) had an average particle size of 10 μm, BET specific surface area of 297.1 m2 g−1, and average pore size of 11.5 nm. 4-(Chloromethyl)phenyltrimethoxysilane (95%) was purchased from ABCR GmbH & Co. KG, Germany. 3-(Methoxycarbonylphenylmethylsulfanylthiocarbonylsulfanyl)propionic acid (MPPA),33 3-azide-1-propanol,45 and solketal acrylate (SA)46 were synthesized according to literature methods. Synthesis of alkyne-functionalized silica particles (Si-alkyne, with an alkyne loading of 0.433 mmol g−1) and S-azidepropoxycarbonylethyl S′-methoxycarbonylphenylmethyl trithiocarbonate (AMP) is described in the ESI.† 2,2′-Azobis(isobutyronitrile) (AIBN) was recrystallized twice from ethanol. Methyl acrylate (MA, 99%), butyl acrylate (BA, 99%), tert-butyl acrylate (tBA, 98%), N,N-dimethylacrylamide (DMA, 99%), N-acrylomorpholine (NAM, 97%), methyl methacrylate (MMA, 99%), and styrene (St, 99%) were passed through a basic alumina column to remove the inhibitor. N-Isopropylacrylamide (NIPAM, 97%) was recrystallized twice from mixtures of hexane and toluene. Other chemicals were of analytical grade and used as received.General procedure for RAFT polymerization
In a typical polymerization, St (18.7 g, 180 mmol), AMP (0.372 g, 0.90 mmol), and AIBN (14.8 mg, 0.090 mmol) were added to a glass tube with a magnetic stirring bar, and toluene was added until the total volume was 60 mL. The tube was sealed with a rubber septum, and the contents cooled with ice-water bath were degassed with nitrogen for 20 min. The tube was subsequently immersed in an oil bath preheated to 60 °C. After 18 h, the polymerization was stopped by cooling the tube in ice water. Azide-functionalized PSt (5.29 g, 26.3% of conversion) was obtained by concentration and precipitation into cold methanol. The molecular weight and polydispersity of PSt-N3 obtained by GPC were Mn = 5830, PDI = 1.10.General procedure for chain extension polymerization
In a typical run, PSt (Mn = 5830, PDI = 1.10, 1.17 g, 0.20 mmol), SA (3.72 g, 20.0 mmol), and AIBN (3.3 mg, 0.020 mmol) were added to a glass tube with a magnetic stirring bar, and dioxane was added until the total volume was 10.0 mL. The tube was sealed with a rubber septum, and the contents were degassed with nitrogen for 15 min. The polymerization was performed at 60 °C for 18 h and then stopped by cooling the tube in ice water. The diblock copolymer (2.95 g) was recovered by precipitation into hexane, and monomer conversion was determined to be 47.8% by gravimetry. The molecular weight and polydispersity of PSt-b-PSA-N3 determined by GPC were Mn = 13800 and PDI = 1.12.Synthesis of silica-polymer hybrids by an incremental method
In a typical run, PSt-N3 (Mn = 5830, PDI = 1.10, 1.23 g, 0.21 mmol), Si-alkyne (0.528 g, 0.21 mmol), CuSO4 (1.7 mg, 10.6 μmol), sodium ascorbate (NaAsco, 4.2 mg, 21.2 μmol), and toluene (12.3 mL) were added to a round flask, and the mixtures were degassed with nitrogen for 15 min. The reaction was conducted with stirring at 90 °C for 18 h. The resultant silica-polystyrene hybrid (SiO2-g-PSt) was filtered and washed thoroughly with toluene and THF. After drying at 60 °C under vacuum, 0.557 g of SiO2-g-PSt was obtained. The hybrid sample was then subjected to aminolysis and TGA measurement. GPC analysis: grafted PSt, Mn(g) = 5640, PDI(g) = 1.07. The weight (Gr = 23.6%) and molar (Gp = 41.8 μmol g−1) grafting ratios were determined by TGA using eqn (1) and (2),34,35,47 where Gr and Gp are the weight and molar ratio of grafted polymer to solid support, WSi-polymer,T and WSiO2,T are the residual weight percent of silica-polymer hybrid and flash silica at temperature of T, and Mn,GPC(g) is molecular weight of grafted polymer.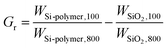 | (1) |
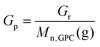 | (2) |
Synthesis of silica-polymer hybrids by one-pot method
In a typical reaction, St (6.75 g, 64.8 mmol), AMP (89.2 mg, 0.216 mmol), Si-alkyne (0.25 g, 0.108 mmol), CuSO4 (1.7 mg, 10.6 μmol), sodium ascorbate (NaAsco, 4.2 mg, 21.2 μmol), and toluene (14.0 mL) were added to a round flask, and the mixtures were degassed with nitrogen for 15 min. The reaction was carried out with stirring at 60 °C for 20 h and then cooled down to room temperature. The reaction mixtures were concentrated and precipitated into methanol, and monomer conversion was obtained to be 21.8% by gravimetry. The resultant mixtures were dissolved in 20 mL of THF, filtered and washed thoroughly with toluene and THF. The solid sample was collected and dried under vacuum until a constant weight was achieved, and 0.30 g of SiO2-g-PSt was obtained. The hybrid sample was subjected to aminolysis and TGA measurement. GPC analyses: grafted PSt, Mn(g) = 5620, PDI(g) = 1.12; free PSt, Mn(f) = 8060, PDI(g) = 1.10. TGA analyses: Gr = 21.5%, Gp = 38.3 μmol g−1.General procedure to cleave grafted chains from the substrate surface
In a typical run, 200 mg of silica-polymer hybrid sample, 4.0 mL of THF, and 20.0 μL (0.212 mmol) of methyl methanethiosulfonate were added to a glass tube. The solution was degassed with nitrogen for 5 min, and then 14.0 μL (0.106 mmol) of degassed n-hexylamine was injected into the mixture. After stirring at room temperature overnight, the solution was filtered off, and the recovered polymer was subjected to GPC analysis.Characterization
The number-average molecular weight (Mn) and polydispersity (PDI) of polymer samples were measured on a Waters 150-C gel permeation chromatography equipped with three Ultrastyragel columns with 10 μm bead size at 35 °C. THF was used as an eluent at a flow rate of 1.0 mL min−1, and PSt samples were calibrated with PSt standard samples; other samples were calibrated using PMMA standard samples. Their effective molecular weight ranges were 100–10 000 for Styragel HT2, 500–30 000 for Styragel HT3, and 5000–600 000 for Styragel HT4. The pore sizes are 50, 100, and 1000 nm for Styragels HT2, HT3, and HT4, respectively. 1H NMR (400 MHz) and 13C (100 MHz) spectra were recorded on a Varian UNITY INOVA 400 spectrometer at 25 °C using CDCl3 as a solvent. Fourier transform infrared (FT-IR) spectra were recorded on a Perkin-Elmer 2000 spectrometer using KBr disks. Thermogravimetric analyses (TGA) were carried out using a TA Instruments TGA 2050 thermogravimetric analyzer from room temperature to 800 °C at a rate of 10 °C min−1 under nitrogen. C, H, N and S were determined by combustion followed by chromatographic separation and thermal conductivity detection using a Carlo-Erba EA 1110CHNO-S Elemental Analyzer. Chlorine analysis was conducted using the Schoniger Oxygen Flask combustion method followed by the relevant titration. Time-of-Flight Mass Spectrometer (TOF MS) with an Electron Impact (EI) Ionization Source was recorded on a Micromass Mass Spectrometer.Results and discussion
The coupling reaction between hydroxyl and alkoxysilane was previously adopted to synthesize silica-polymer hybrids. Although the coupling reaction was very efficient, rigorously anhydrous conditions were indispensable when performing the RAFT polymerization, thus the reaction conditions needed further improvement.37 To this end, the azide-alkyne cycloaddition reaction was used instead in this study since both the RAFT polymerization and click reaction permitted the presence of moisture and variable temperature. S-Azidepropoxycarbonylethyl S′-methoxycarbonylphenylmethyltrithiocarbonate (AMP) was synthesized by esterification reaction between 3-azide-1-propanol and MPPA, and alkyne-functionalized silica particles (Si-alkyne) were prepared by two step reactions: (i) introduction of the benzylchloride functionality to the surface by reacting silica particles with 4-(chloromethyl)phenyltrimethoxysilane; (ii) attaching the alkyne functionality to substrate surface by coupling reaction between silica-supported benzylchloride and propynol. Elemental analysis revealed a loading in active sites of 0.433 mmol of alkyne/g of silica, corresponding to a grafting density of 0.877 molecules of alkyne/nm2. Silica particles grafted with homopolymers and block copolymers were synthesized by the incremental or one-pot method, and the grafted polymers were recovered by aminolysis and then subjected to GPC analysis.Synthesis of azide-functionalized macro CTAs
The terminal azide-functionalized polymers were synthesized by the RAFT process using AMP as the original RAFT chain transfer agent (CTA, Scheme 1), and the polymerization results are listed in Tables S1 and S2 (see the ESI†). First, RAFT polymerization of vinyl monomers such as methyl methacrylate (MMA), styrene (St), and N-isopropylacrylamide (NIPAM) mediated by AMP was conducted in toluene or dioxane at 60 °C, and azide-functionalized homopolymers such as PMMA, PSt and PNIPAM were obtained. The molecular weight of PMMA-N3 was higher than theoretical value, and the polydispersity was 1.43, which was similar to our previous finding.34,35,37,48 The molecular weight of other homopolymers was very close to the calculated value, with polydispersity around 1.1, corresponding to the controlled nature of RAFT polymerization.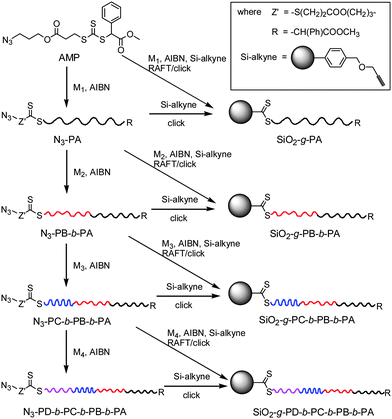 | ||
| Scheme 1 Synthesis of silica-polymer hybrids by incremental and tandem RAFT polymerization and click reaction. | ||
Azide-functionalized PSt was then used as a macro CTA to mediate chain extension polymerization of other monomers such as N-acrylomorpholine (NAM), NIPAM, solketal acrylate (SA), and tert-butyl acrylate (tBA), and well-defined diblock copolymers with controlled molecular weight and polydispersity less than 1.2 were obtained. On this basis, some tri- and tetrablock copolymers with controlled molecular weight, relatively low polydispersity (1.1 < Mw/Mn < 1.25) and terminal azide functionality were also synthesized by successive chain extension polymerization using various macro CTAs.
Synthesis of silica-polymer hybrids via the incremental method
Azide-functionalized macro CTAs were prefabricated by the aforementioned RAFT process, and then a successive copper(I)-catalyzed azide-alkyne cycloaddition10 was performed to achieve silica-polymer hybrids. To further optimize reaction conditions, click reaction between azide-functionalized PSt (Mn = 5830, PDI = 1.10) and Si-alkyne was conducted, and effects of some important influence factors such as reaction temperature, time and feed ratio on click reaction were investigated. In addition, dependence of grafting ratio on molecular weight of PSt-N3 was also studied.The click reaction ([PSt-N3]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0
[Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0
[CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20
[NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WPSt-N3/Vtoluene = 0.10 g mL−1) was initially conducted in toluene at various temperatures for 18 h, and the effects of reaction temperature on weight grafting ratio are shown in Fig. 1. Although most of the azide-alkyne cycloaddition reaction in solution could be efficiently performed at ambient temperature, the click reaction conducted at 30 °C for 18 h in this study only led to 10.8 wt% of polymeric chains grafted onto the surface of silica particles, which may be ascribed to less efficiency originating from heterophase reaction environments. The weight grafting ratio (Gr) was liable to increase with increasing temperature, and a maximum value of 24 wt% could be obtained when the reaction proceeded between 90 and 110 °C, indicating the efficiency of click reaction was strongly dependent on reaction temperature. With increasing temperature, the mobility of polymeric chains in solution was accelerated, polymeric chains were more easily to diffuse to the surface of solid substrate, thus the reactivity of azide functionality in the chain end of PSt-N3 was promoted, leading to significantly increased grafting ratio. When the temperature was beyond 90 °C, only limited increase in Gr values with enhanced temperature was observed, indicating most of the reactive sites on substrate surface had been shielded by grafted chains, and steric repulsions between polymeric chains became more pronounced, preventing the diffusion of azide-functionalized polymers to the surface for further reaction.
2, WPSt-N3/Vtoluene = 0.10 g mL−1) was initially conducted in toluene at various temperatures for 18 h, and the effects of reaction temperature on weight grafting ratio are shown in Fig. 1. Although most of the azide-alkyne cycloaddition reaction in solution could be efficiently performed at ambient temperature, the click reaction conducted at 30 °C for 18 h in this study only led to 10.8 wt% of polymeric chains grafted onto the surface of silica particles, which may be ascribed to less efficiency originating from heterophase reaction environments. The weight grafting ratio (Gr) was liable to increase with increasing temperature, and a maximum value of 24 wt% could be obtained when the reaction proceeded between 90 and 110 °C, indicating the efficiency of click reaction was strongly dependent on reaction temperature. With increasing temperature, the mobility of polymeric chains in solution was accelerated, polymeric chains were more easily to diffuse to the surface of solid substrate, thus the reactivity of azide functionality in the chain end of PSt-N3 was promoted, leading to significantly increased grafting ratio. When the temperature was beyond 90 °C, only limited increase in Gr values with enhanced temperature was observed, indicating most of the reactive sites on substrate surface had been shielded by grafted chains, and steric repulsions between polymeric chains became more pronounced, preventing the diffusion of azide-functionalized polymers to the surface for further reaction.
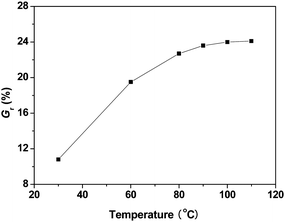 | ||
| Fig. 1 The dependence of the weight grafting ratio on reaction temperature. | ||
The reaction ([PSt-N3]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0
[Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0
[CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20
[NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WPSt-N3/Vtoluene = 0.10 g mL−1) was then performed at 90 °C, and dependence of weight grafting ratio on reaction time is depicted in Fig. 2. The reaction could be classified into three stages. In the first stage (0–9 h), there were no clear steric repulsions between polymeric chains due to relatively low grafting density, and the azide-alkyne cycloaddition proceeded smoothly, thus the Gr value almost exhibited linear increase with increasing reaction time. In the second stage (9–18 h), the reaction became increasing difficult due to relatively high grafting density, and the steric repulsion between polymeric chains partly prevented the azide functionality in PSt chains from approaching the substrate surface, so the Gr value was liable to slowly increase with extended time. In the last stage (more then 18 h), the substrate surface was wholly covered by grafted chains, and the azide and alkyne groups could not further approach each other any more, thus the Gr value tended to be constant and independent from reaction time. In Fig. 2, a maximum Gr value could almost be obtained in 18 h for reaction conducted at 90 °C. When the click reaction was performed at temperatures ranging between 80 and 110 °C, the time to reach a maximum weight grafting ratio was observed to be in the range of 15–25 h.
2, WPSt-N3/Vtoluene = 0.10 g mL−1) was then performed at 90 °C, and dependence of weight grafting ratio on reaction time is depicted in Fig. 2. The reaction could be classified into three stages. In the first stage (0–9 h), there were no clear steric repulsions between polymeric chains due to relatively low grafting density, and the azide-alkyne cycloaddition proceeded smoothly, thus the Gr value almost exhibited linear increase with increasing reaction time. In the second stage (9–18 h), the reaction became increasing difficult due to relatively high grafting density, and the steric repulsion between polymeric chains partly prevented the azide functionality in PSt chains from approaching the substrate surface, so the Gr value was liable to slowly increase with extended time. In the last stage (more then 18 h), the substrate surface was wholly covered by grafted chains, and the azide and alkyne groups could not further approach each other any more, thus the Gr value tended to be constant and independent from reaction time. In Fig. 2, a maximum Gr value could almost be obtained in 18 h for reaction conducted at 90 °C. When the click reaction was performed at temperatures ranging between 80 and 110 °C, the time to reach a maximum weight grafting ratio was observed to be in the range of 15–25 h.
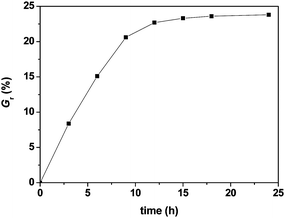 | ||
| Fig. 2 Effects of reaction time on weight grafting ratio. | ||
The reaction ([Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0
[CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20
[NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WPSt-N3/Vtoluene = 0.10 g mL−1) was also conducted in toluene at 90 °C for 18 h to investigate effects of azide/alkyne feed ratio on graft reaction. Fig. 3 depicts the effects of PSt-N3 to Si-alkyne feed ratio on weight grafting ratio and molar percent of azide-functionalized polymer participated in the click reaction (Ge). When the [PSt-N3]0/[Si-alkyne]0 ratio increased from 0.5 to 10, the weight grafting ratio increased from 15.2% to 32.4%, however, the molar percent of reacted PSt-N3 gradually decreased from 12.0% to 1.28%. The Gr value significantly increased with an increase in PSt-N3 amount when the feed ratio was less than 2, indicating the remarkably increased probability of a cycloaddition reaction with raised feed ratio in this region. When [PSt-N3]0/[Si-alkyne]0 ratio was beyond 2, however, only limited increase in weight grafting ratio was noted even if large excess of PSt-N3 was used, suggesting the presence of prominent steric repulsion. These results indicated relatively high grafting ratio could be achieved at the cost of more unreacted azide-functionalized polymers, and the grafting density could reach 0.113 chains nm−2 as 10-fold of PSt-N3 was used for the click reaction.
2, WPSt-N3/Vtoluene = 0.10 g mL−1) was also conducted in toluene at 90 °C for 18 h to investigate effects of azide/alkyne feed ratio on graft reaction. Fig. 3 depicts the effects of PSt-N3 to Si-alkyne feed ratio on weight grafting ratio and molar percent of azide-functionalized polymer participated in the click reaction (Ge). When the [PSt-N3]0/[Si-alkyne]0 ratio increased from 0.5 to 10, the weight grafting ratio increased from 15.2% to 32.4%, however, the molar percent of reacted PSt-N3 gradually decreased from 12.0% to 1.28%. The Gr value significantly increased with an increase in PSt-N3 amount when the feed ratio was less than 2, indicating the remarkably increased probability of a cycloaddition reaction with raised feed ratio in this region. When [PSt-N3]0/[Si-alkyne]0 ratio was beyond 2, however, only limited increase in weight grafting ratio was noted even if large excess of PSt-N3 was used, suggesting the presence of prominent steric repulsion. These results indicated relatively high grafting ratio could be achieved at the cost of more unreacted azide-functionalized polymers, and the grafting density could reach 0.113 chains nm−2 as 10-fold of PSt-N3 was used for the click reaction.
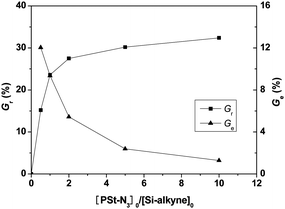 | ||
| Fig. 3 The effects of PSt-N3 to Si-alkyne feed ratio on weight grafting ratio and molar percent of reacted PSt-N3 (Ge). | ||
Finally, the click reaction ([PSt-N3]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0
[Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0
[CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20
[NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, WPSt-N3/Vtoluene = 0.10 g mL−1) using PSt-N3 with various molecular weights was conducted at 90 °C for 18 h, and the results are shown in Fig. 4. When the molecular weight of PSt-N3 increased from 5830 to 48900 g mol−1, the weight grafting ratio decreased from 23.6% to 13.9%, and the molar grafting ratio significantly decreased from 40.5 to 2.84 μmol g−1. Enhanced steric hindrance and reduced end-group reactivity could account for this phenomenon. On one hand, the increased molecular weight of azide-functionalized polymer tended to reduce the reactivity of the azide functionality due to incremental degree of curling polymeric chain and embedded end group resulting from more complex configuration. On the other hand, with increasing chain length, the steric repulsions became more pronounced, the azide functionality was therefore more difficult to approach the alkyne group to perform the cycloaddition reaction.
2, WPSt-N3/Vtoluene = 0.10 g mL−1) using PSt-N3 with various molecular weights was conducted at 90 °C for 18 h, and the results are shown in Fig. 4. When the molecular weight of PSt-N3 increased from 5830 to 48900 g mol−1, the weight grafting ratio decreased from 23.6% to 13.9%, and the molar grafting ratio significantly decreased from 40.5 to 2.84 μmol g−1. Enhanced steric hindrance and reduced end-group reactivity could account for this phenomenon. On one hand, the increased molecular weight of azide-functionalized polymer tended to reduce the reactivity of the azide functionality due to incremental degree of curling polymeric chain and embedded end group resulting from more complex configuration. On the other hand, with increasing chain length, the steric repulsions became more pronounced, the azide functionality was therefore more difficult to approach the alkyne group to perform the cycloaddition reaction.
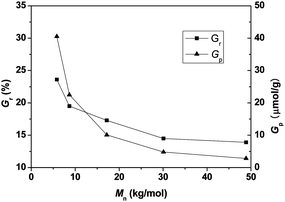 | ||
| Fig. 4 Effects of molecular weight of PSt-N3 on weight and molar grafting ratios. Mn and PDI of various PS–N3 samples were 5830, 1.10; 8670, 1.12; 17200, 1.19; 30100, 1.21; and 48900, 1.23. | ||
The above results illustrate that PSt grafted silica can be efficiently prepared by click reaction under optimized conditions. This incremental method is a versatile approach to the synthesis of silica-polymer hybrids, and it could be generalized to synthesize silica particles grafted with various polymers especially block copolymers. To illustrate this, a series of azide-functionalized homopolymers, di-, tri-, and tetrablock copolymers synthesized by the aforementioned RAFT process were then reacted with alkyne-functionalized silica particles. Factors such as chain length and rigidity of polymeric chains, types of solvents, temperature, reaction time and feed ratios of polymer-N3 to Si-alkyne may affect the grafting reaction. In this study, the click reaction was conducted under fixed conditions (Table 1).
| run | graft polymerb | M n(a)c | PDI(a)c | M n/gd | PDI/gd | G r (%)e | G p/μmol g−1f |
|---|---|---|---|---|---|---|---|
a Reaction conditions: [polymer-N3]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0 [Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0 [CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20 [NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Wpolymer-N3/Vtoluene = 0.10 g mL−1, in toluene at 90 °C for 18 h.
b Polymers obtained by RAFT polymerization.
c Molecular weight and polydispersity of the “as-prepared” azide-functionalized polymers.
d Molecular weight and polydispersity of grafted polymers obtained by GPC.
e Weight grafting ratio determined by TGA.
f Apparent molar grafting ratio calculated by the equation Gp = Gr/Mn(g). 2, Wpolymer-N3/Vtoluene = 0.10 g mL−1, in toluene at 90 °C for 18 h.
b Polymers obtained by RAFT polymerization.
c Molecular weight and polydispersity of the “as-prepared” azide-functionalized polymers.
d Molecular weight and polydispersity of grafted polymers obtained by GPC.
e Weight grafting ratio determined by TGA.
f Apparent molar grafting ratio calculated by the equation Gp = Gr/Mn(g).
|
|||||||
| 1 | PMMA | 8700 | 1.43 | 8640 | 1.36 | 27.5 | 31.8 |
| 2 | PSt | 5830 | 1.10 | 5640 | 1.07 | 23.6 | 41.8 |
| 3 | PNIPAM | 8760 | 1.12 | 8720 | 1.10 | 28.8 | 33.0 |
| 4 | PSt-b-PNAM | 12200 | 1.10 | 11800 | 1.09 | 31.2 | 26.4 |
| 5 | PSt-b-PNIPAM | 9360 | 1.15 | 9520 | 1.11 | 28.3 | 29.7 |
| 6 | PSt-b-PSA | 13800 | 1.12 | 13600 | 1.08 | 28.4 | 20.9 |
| 7 | PSt-b-PtBA | 14800 | 1.10 | 14500 | 1.09 | 28.9 | 19.9 |
| 8 | PSt-b-PNAM-b-PNIPAM | 17400 | 1.17 | 17500 | 1.14 | 25.4 | 14.5 |
| 9 | PSt-b-PNAM-b-PDMA | 16900 | 1.23 | 16600 | 1.18 | 27.8 | 16.7 |
| 10 | PSt-b-PSA-b-PMA | 18300 | 1.11 | 18500 | 1.06 | 27.1 | 14.6 |
| 11 | PSt-b-PNAM-b-PNIPAM-b-PtBA | 24800 | 1.20 | 24000 | 1.12 | 22.5 | 9.38 |
| 12 | PSt-b-PNAM-b-PDMA-b-PtBA | 25500 | 1.22 | 25200 | 1.16 | 21.1 | 8.37 |
The grafted polymers and their “as-prepared” precursors had quite similar molecular weights, whilst the polydispersity indices of grafted polymers were usually slightly lower than those of their precursors. This phenomenon is consistent with our previous study and could be explained by lack of dead polymeric chains.37 During the graft reaction using Z-functionalized macro CTAs, only living polymeric chains comprising both the azide functionality and the reactive thiocarbonyl-thio moiety could be covalently attached to the surface of flash silica during click reaction. Consequently, any dead chains were removed by filtration and washing during the purification process, and the resultant Z-supported polymers with thiocarbonyl-thio moiety at the surface were living, evident from highly efficient chain extension polymerization of a second monomer.41,44 As a comparison, the GPC traces of typical grafted samples up to tetrablock copolymer such as PSt, PSt-b-PSA, PSt-b-PSA-b-PMA, and PSt-b-PNAM-b-PNIPAM-b-PtBA and their “as-prepared” precursors are shown in Fig. 5. The tailing and shoulder were usually noted in GPC traces of azide-functionalized polymers, corresponding to side reactions such as termination and irreversible transfer in solution. No shoulder and significant tailing were observed in GPC traces of grafted polymers, which was in accordance with the theoretical expectation. All the grafted polymers lacked dead chains, and they could be easily de-grafted from the substrate surface by some ways such as aminolysis and etching, thus the incremental method could also be used to synthesize well-defined highly pure block copolymers. In Table 1, all the homopolymers and di-, tri-, and tetrablock block copolymers de-grafted from the substrate surface possessed low polydispersity (1.07 < Mw/Mn < 1.18) except PMMA.
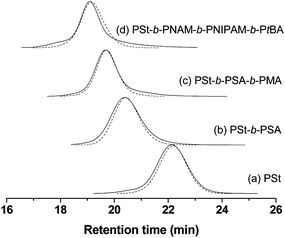 | ||
Fig. 5 GPC traces of grafted polymers (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) and their “as-prepared” precursors ( ) and their “as-prepared” precursors (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ). ). | ||
The resultant silica-polymer hybrids were characterized by IR, elemental analysis and TGA. In the IR spectra, a strong and broad absorption corresponding to the stretching vibration of Si–O bond of silica particles was noted at about 1080 cm−1, and typical absorptions corresponding to characteristic groups in various grafted chains were observed at 1736 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PMMA, PBA and PSA), 1600 (νC
O, PMMA, PBA and PSA), 1600 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, PSt), 699 (C–H out of plane bending, PSt), 1645 (νC
C, PSt), 699 (C–H out of plane bending, PSt), 1645 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PNAM), 1654 (νC
O, PNAM), 1654 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PNIPAM), 1634 (νC
O, PNIPAM), 1634 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PDMA), 1740 (νC
O, PDMA), 1740 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PMA) and 1729 cm−1 (νC
O, PMA) and 1729 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, PtBA), respectively.
O, PtBA), respectively.
In Table 1, the weight (Gr) and molar (Gp) grafting ratios of polymeric chains on substrate surface were determined by TGA. The TGA curves of flash silica, Si-alkyne and typical silica-polymer hybrids are listed in Fig. 6. On this basis, the Gr and Gp values were calculated using eqn (1) and (2), respectively. When the molecular weight of grafted polymers increased from 5640 to 25200 g mol−1, the weight grafting ratio varied between 21.1% and 31.2%, and the molar grafting ratio was decreased from 41.8 to 8.37 μmol g−1. Fig. S2† depicts the dependence of molar grafting ratio on molecular weight of grafted chains to demonstrate their mutual relationship. The molar grafting ratio decreases with increasing grafted chain length, indicating that the molecular weight is the most important factor to influence the grafting density during the click reaction conducted under the settled condition. In some cases, a different grafting ratio is notable for reaction using distinct grafted polymers with similar chain length, and similar grafting ratio is also observed for reaction using grafted polymers with different chain length, which may be ascribed to dissimilar modality and rigidity of the polymeric chains.37
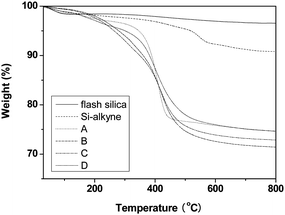 | ||
| Fig. 6 TGA curves of flash silica, Si-alkyne, and typical silica-polymer hybrids obtained by click reaction: A, SiO2-g-PSt; B, SiO2-g-PSA-b-PSt; C, SiO2-g-PMA-b-PSA-b-PSt; D, SiO2-g-PtBA-b-PNIPAM-b-PNAM-b-PSt. | ||
Taking into account the specific surface area of 297.1 m2 g−1, the grafting density was deduced to be in the range of 0.017–0.085 chains nm−2, which was similar to that obtained using hydroxyl-alkoxysilane coupling reaction.37 Although the combinatorial approach involving click reaction could not afford significantly increased grafting density, it is more promising in synthesis of inorganic-polymer hybrids due to improved reaction conditions such as tolerance to moisture and variable temperature. Thus, a series of well-defined homopolymers and block copolymers up to tetra-composition could be efficiently grafted onto the surface of silica particles. It should be mentioned that it is still a challenge to achieve high grafting density when using click reactions, which could be attributed to low efficiency resulting from significant steric hindrance and heterophase reaction environments, both typical limitations of the grafting to approach.
Synthesis of silica-polymer hybrids via one-pot method
The aforementioned incremental method was shown to efficiently synthesize Z-supported “living” polymers, although it is more time consuming due to stepwise reactions. More recently, Ranjan and Brittain synthesized PSt grafted silica nanoparticles via tandem RAFT polymerization and click chemistry.16 The one-pot method had advantages of reduced labour, time and cost, and it is more promising as compared with the incremental method. To demonstrate the versatility of this tandem approach, simultaneous RAFT polymerization and click reaction was attempted to synthesize silica-polymer hybrids, in which AMP and macro CTAs were used as RAFT mediators and raw materials for click reaction as well.The simultaneous RAFT polymerization and click reaction was potentially applicable to synthesize silica particles grafted with other polymeric chains. Theoretically, homopolymer grafted silica could be obtained when AMP was used as a RAFT agent, and block copolymers with (n + 1) block(s) could be achieved when polymer with n segment(s) (n = 1, 2, 3) was used as a clickable macro RAFT agent, thus the tandem reaction permitted the synthesis of multiblock copolymer grafted silica particles. To prove this concept, RAFT polymerization of some vinyl monomers such as MA, NAM, DMA, NIPAM and SA using azide-functionalized AMP, PSt, PSt-b-PSA, PSt-b-PNAM, PSt-b-PNAM-b-PDMA or PSt-b-PNAM-b-PNIPAM as mediators and click reaction was carried out via the one-pot method. The tandem reaction ([CTA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0
[Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0
[AIBN]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0
[CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20
[NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [M]0 = 3.0 mol L−1) was performed in dioxane or toluene at 60 °C for 20 h, and the results are listed in Table 2.
2, [M]0 = 3.0 mol L−1) was performed in dioxane or toluene at 60 °C for 20 h, and the results are listed in Table 2.
| Run | CTA | M | [M]0/[CTA]0 | M n(th)b | M n(f)c | PDI (f)c | M n/gd | PDI/gd | G r (%)e | G p/μmol g−1f |
|---|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions: [CTA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Si-alkyne]0 [Si-alkyne]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN]0 [AIBN]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuSO4]0 [CuSO4]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NaAsco]0 = 20 [NaAsco]0 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [M]0 = 3.0 mol L−1, in dioxane (runs 3 to 7) or toluene (other runs) at 60 °C for 20 h. Macro CTAs (Mn, PDI): PSt-N3 (5360, 1.08), PSt-b-PSA-N3 (12400, 1.12), PSt-b-PNAM-N3 (10200, 1.11), PSt-b-PNAM-b-PNIPAM-N3 (15600, 1.13), and PSt-b-PNAM-b-PDMA-N3 (14800, 1.19).
b Theoretically calculated molecular weight.
c Molecular weight and polydispersity of free polymers.
d Molecular weight and polydispersity of grafted polymers.
e Weight grafting ratio determined by TGA.
f Apparent molar grafting ratio. 2, [M]0 = 3.0 mol L−1, in dioxane (runs 3 to 7) or toluene (other runs) at 60 °C for 20 h. Macro CTAs (Mn, PDI): PSt-N3 (5360, 1.08), PSt-b-PSA-N3 (12400, 1.12), PSt-b-PNAM-N3 (10200, 1.11), PSt-b-PNAM-b-PNIPAM-N3 (15600, 1.13), and PSt-b-PNAM-b-PDMA-N3 (14800, 1.19).
b Theoretically calculated molecular weight.
c Molecular weight and polydispersity of free polymers.
d Molecular weight and polydispersity of grafted polymers.
e Weight grafting ratio determined by TGA.
f Apparent molar grafting ratio.
|
||||||||||
| 1 | AMP | St | 300 | 7510 | 8060 | 1.10 | 5620 | 1.12 | 21.5 | 38.3 |
| 2 | AMP | MA | 150 | 12900 | 13500 | 1.15 | 9440 | 1.16 | 28.8 | 30.5 |
| 3 | AMP | NAM | 100 | 12200 | 12800 | 1.11 | 7660 | 1.17 | 23.2 | 30.3 |
| 4 | AMP | DMA | 150 | 15000 | 15600 | 1.15 | 9140 | 1.18 | 25.6 | 28.0 |
| 5 | PSt | NAM | 150 | 16200 | 15900 | 1.16 | 11200 | 1.16 | 30.5 | 27.2 |
| 6 | PSt | NIPAM | 150 | 14200 | 14800 | 1.17 | 10800 | 1.15 | 29.1 | 26.9 |
| 7 | PSt | SA | 100 | 15500 | 14200 | 1.21 | 10500 | 1.22 | 28.8 | 27.4 |
| 8 | PSt | MA | 150 | 13200 | 12900 | 1.19 | 9560 | 1.20 | 27.3 | 28.6 |
| 9 | PSt-b-PSA | MA | 200 | 21900 | 20500 | 1.18 | 16400 | 1.23 | 26.2 | 16.0 |
| 10 | PSt-b-PNAM | MA | 200 | 18800 | 19600 | 1.13 | 14800 | 1.15 | 27.6 | 18.6 |
| 11 | PSt-b-PNAM-b-PNIPAM | MA | 200 | 23300 | 25200 | 1.19 | 19700 | 1.21 | 24.3 | 12.3 |
| 12 | PSt-b-PNAM-b-PDMA | MA | 200 | 22500 | 24100 | 1.21 | 18600 | 1.25 | 24.9 | 13.4 |
It is noteworthy that not all of the living material is made of the targeted block copolymers, since some low-component impurities such as living homo and diblock copolymers may remain in the triblock copolymer sample; this is integrally part of the RAFT mechanism. The chain length of grafted polymers was typically shorter than that of free polymers formed in solution due to significant shielding effect and heterophase reaction conditions. Fig. 7 depicts GPC traces of typical free and grafted polymer samples such as PSt, PSt-b-PNAM, PSt-b-PNAM-b-PMA and PSt-b-PNAM-b-PNIPAM-b-PMA and their corresponding macro RAFT agents. Shoulder and tailing corresponding to dead chains originating from side reactions were observed in GPC traces of free polymers, which became more notable for block copolymers obtained by chain extension polymerization. As compared with GPC traces of clickable macro CTAs, the GPC traces of the resultant grafted block copolymers were completely shifted towards higher molecular weight values, without noticeable tailing and shoulder, and the polydispersity indices remained low (1.12 < Mw/Mn < 1.25), suggesting that well-defined homopolymers, di-, tri- and tetrablock copolymers grafted onto silica particles could be obtained by simultaneous RAFT and click reactions.
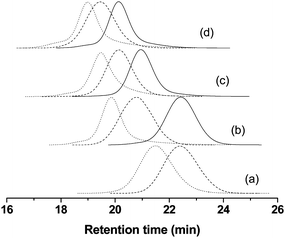 | ||
Fig. 7 GPC traces of macro CTA (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ), grafted polymer ( ), grafted polymer (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) and free polymer (⋯). The free and grafted polymers produced via one-pot method were PSt (a), PSt-b-PNAM (b), PSt-b-PNAM-b-PMA (c) and PSt-b-PNAM-b-PNIPAM-b-PMA (d), and chain transfer agents were AMP (a), PSt (b), PSt-b-PNAM (c) and PSt-b-PNAM-b-PNIPAM (d), respectively. ) and free polymer (⋯). The free and grafted polymers produced via one-pot method were PSt (a), PSt-b-PNAM (b), PSt-b-PNAM-b-PMA (c) and PSt-b-PNAM-b-PNIPAM-b-PMA (d), and chain transfer agents were AMP (a), PSt (b), PSt-b-PNAM (c) and PSt-b-PNAM-b-PNIPAM (d), respectively. | ||
Theoretically, clicking and polymerizing simultaneously would result in a complex reaction behavior, and the overall reaction may be dependent on many different parameters such as molecular weights and types of grafted polymers, feed ratios, reaction time and temperature. For instance, when the molecular weight of grafted polymers was changed between 5620 and 19700 g mol−1, the molar grafting ratio determined by TGA ranged between 12.3 and 38.3 μmol g−1 (Table 2), suggesting only 2.8–8.8% of alkyne functionality on substrate surface could efficiently participated in the azide-alkyne cycloaddition reaction even if one-fold excess of azide-functionalized RAFT agents were used. It is therefore remarkable that the interference between click reaction and RAFT polymerization is not very significant in this study, since the molecular weight of the grafted block copolymers increases noticeably whilst the polydispersity indices remained low. Although the tandem reaction proceeded at the low temperature of 60 °C, the apparent grafting density of 0.025–0.078 chains nm−2 was close to that obtained by the incremental method (Fig. S2†). Thus, the click reaction and RAFT process show a good compatibility, and can be used to synthesize silica particles grafted with well-defined polymers. The grafting density obtained via the one-pot method is similar to that achieved by the incremental method, thus it may prove a more promising approach for the synthesis of polymer modified solid supports.
Conclusions
Combinatorial approaches based on the combination of RAFT polymerization and azide-alkyne cycloaddition reactions were used to synthesize homopolymer and multiblock copolymer grafted silica particles. Azide-functionalized polymers were synthesized by a RAFT process using AMP as an original RAFT agent. The incremental method comprised RAFT polymerization to synthesize various macro CTAs and a subsequent click reaction between azide-functionalized polymers and alkyne-functionalized silica to prepare silica-polymer hybrids, which could afford very well-defined grafted polymers up to tetrablock copolymers with controlled compositions and low polydispersity (Mw/Mn < 1.18). When the grafted polymeric chains had molecular weight varying between 5640 and 25200 g mol−1, the grafting density of 0.017–0.085 chains nm−2 could be achieved by the incremental method. The one-pot method involved simultaneous RAFT polymerization and click reaction using clickable azide-functionalized RAFT agents. Under optimized conditions, the tandem reaction could afford silica particles grafted with well-defined homopolymers, di-, tri-, and tetrablock copolymers with polydispersity less than 1.25 and grafting density of 0.025–0.078 chains nm−2 dependent on chain lengths of grafted polymers. IR, TGA and GPC analyses indicated both methods could efficiently afford the target silica-polymer hybrids. The tethering of polymeric chains to particles via the thiocarbonyl thio group is a simple yet effective technique to obtain highly pure homopolymer and multiblock copolymer grafted to silica particles. Indeed, the polymeric chains tethered to the silica particles have all the characteristic of living chains, and they can be further used for the synthesis of block copolymer grafted silica particles.37 This combinatorial approach is also a very versatile route to the synthesis of highly pure multiblock copolymers which could be de-grafted from the substrate surface by aminolysis.Acknowledgements
The financial support of this work by the National Natural Science Foundation of China (Grants 20844001 and 20874067) is gratefully acknowledged.Notes and references
- T. Otsu, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2121 CrossRef CAS.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- M. Ouchi, T. Terashima and M. Sawamoto, Chem. Rev., 2009, 109, 4963 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379 CrossRef CAS.
- S. Perrier and P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5347 CrossRef CAS.
- L. Barner, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Macromol. Rapid Commun., 2007, 28, 539 CrossRef CAS.
- A. E. Smith, X. W. Xu and C. L. McCormick, Prog. Polym. Sci., 2010, 35, 45 CrossRef CAS.
- C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Q. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2008, 29, 952 CrossRef CAS.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620 CrossRef CAS.
- C. R. Becer, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900 CrossRef CAS.
- J. Xu, L. Tao, C. Boyer, A. B. Lowe and T. P. Davis, Macromolecules, 2010, 43, 20 CrossRef CAS.
- B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1 CrossRef CAS.
- R. Ranjan and W. J. Brittain, Macromol. Rapid Commun., 2007, 28, 2084 CrossRef CAS.
- R. Ranjan and W. J. Brittain, Macromolecules, 2007, 40, 6217 CrossRef CAS.
- R. Ranjan and W. J. Brittain, Macromol. Rapid Commun., 2008, 29, 1104 CrossRef CAS.
- Y. Li and B. C. Benicewicz, Macromolecules, 2008, 41, 7986 CrossRef CAS.
- S. R. Gondi, A. P. Vogt and B. S. Sumerlin, Macromolecules, 2007, 40, 474 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2010, 1, 17 RSC.
- A. J. Inglis, M. H. Stenzel and C. Barner-Kowollik, Macromol. Rapid Commun., 2009, 30, 1792 CrossRef CAS.
- F. Santoyo-Gonzalez and F. Hernandez-Mateo, Chem. Soc. Rev., 2009, 38, 3449 RSC.
- G. J. de Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef.
- G. Kickelbick, Prog. Polym. Sci., 2003, 28, 83 CrossRef CAS.
- B. Radhakrishnan, R. Ranjan and W. J. Brittain, Soft Matter, 2006, 2, 386 RSC.
- Y. Tsujii, K. Ohno, S. Yamamoto, A. Goto and T. Fukuda, Adv. Polym. Sci., 2006, 197, 1 CAS.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437 CrossRef CAS.
- M. H. Stenzel, Macromol. Rapid Commun., 2009, 30, 1603 CrossRef CAS.
- D. H. Nguyen and P. Vana, Polym. Adv. Technol., 2006, 17, 625 CrossRef CAS.
- R. Rotzoll and P. Vana, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7656 CrossRef CAS.
- M. H. Stenzel, L. Zhang and W. T. S. Huck, Macromol. Rapid Commun., 2006, 27, 1121 CrossRef CAS.
- S. Perrier, P. Takolpuckdee and C. A. Mars, Macromolecules, 2005, 38, 6770 CrossRef CAS.
- Y. L. Zhao and S. Perrier, Macromolecules, 2006, 39, 8603 CrossRef CAS.
- Y. L. Zhao and S. Perrier, Macromolecules, 2007, 40, 9116 CrossRef CAS.
- D. H. Nguyen, M. R. Wood, Y. L. Zhao, S. Perrier and P. Vana, Macromolecules, 2008, 41, 7071 CrossRef CAS.
- Y. K. Huang, Q. Liu, X. D. Zhou, S. Perrier and Y. L. Zhao, Macromolecules, 2009, 42, 5509 CrossRef CAS.
- M. M. Titirici and B. Sellergren, Chem. Mater., 2006, 18, 1773 CrossRef CAS.
- K. Ohno, T. Morinaga, K. Koh, Y. Tsujii and T. Fukuda, Macromolecules, 2005, 38, 2137 CrossRef CAS.
- T. Wu, Y. F. Zhang, X. F. Wang and S. Y. Liu, Chem. Mater., 2008, 20, 101 CrossRef CAS.
- C. Y. Hong, Y. Z. You and C. Y. Pan, Chem. Mater., 2005, 17, 2247 CrossRef CAS.
- H. Kong, C. Gao and D. Y. Yan, J. Am. Chem. Soc., 2004, 126, 412 CrossRef CAS.
- C. Z. Li and B. C. Benicewicz, Macromolecules, 2005, 38, 5929 CrossRef CAS.
- Handbook of Radical Polymerization, ed.K. Matyjaszewski and T. P. Davis, Wiley, Hoboken, NJ, 2002 Search PubMed.
- V. Ladmiral, T. M. Legge, Y. L. Zhao and S. Perrier, Macromolecules, 2008, 41, 6728 CrossRef CAS.
- N. A. A. Rossi, Y. Q. Zou, M. D. Scott and J. N. Kizhakkedathu, Macromolecules, 2008, 41, 5272 CrossRef CAS.
- T. Y. Guo, P. Liu, J. W. Zhu, M. D. Song and B. H. Zhang, Biomacromolecules, 2006, 7, 1196 CrossRef CAS.
- Y. L. Zhao and S. Perrier, Chem. Commun., 2007, 4294 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of AMP and Si-alkyne, polymerization results for synthesis of azide-functionalized polymers, NMR spectra of AMP, and TGA curves of typical silica-polymer hybrids obtained by the tandem reaction. See DOI: 10.1039/c0py00165a |
| This journal is © The Royal Society of Chemistry 2010 |
