Selenium-containing block copolymers and their oxidation-responsive aggregates
Ning
Ma
a,
Ying
Li
a,
Huifeng
Ren
a,
Huaping
Xu
*a,
Zhibo
Li
b and
Xi
Zhang
*a
aKey Lab of Organic Optoelectronics and Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084, People's Republic of China. E-mail: xuhuaping@mail.tsinghua.edu.cn; xi@mail.tsinghua.edu.cn; Fax: +86-10-62792406
bState Key Laboratory of Polymer Physics and Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, People's Republic of China
First published on 8th July 2010
Abstract
A selenium-containing amphiphilic block copolymer (PEG-PUSe-PEG) with a hydrophobic polyselenide block and two hydrophilic poly(ethylene glycol) (PEG) blocks was synthesized via polymerization of toluene diisocyanate (TDI) with monoselenide-containing diols and subsequent termination with PEG monomethylether. PEG-PUSe-PEG is able to self-assemble in aqueous solution to form block copolymer aggregates. Interestingly, it was found that the aggregates have a good oxidation-responsiveness and undergo a structural dissociation in a mild oxidative environment (such as 0.1% H2O2 v/v) due to the unique sensitivity of selenide groups in presence of oxidants. Compared with the sulfide analogue PEG-PUS-PEG, PEG-PUSe-PEG is more sensitive to oxidants. It is anticipated that selenium-containing block copolymer aggregates may find application in the field of drug delivery systems.
Introduction
Selenium-containing polymers have been mainly used as optoelectronic or semiconducting materials and catalysts.1–14 However, they have not been widely studied due to the limited solubility and poor stability. Our group has reported the introduction of the diselenide group into the core of the dendritic structures to improve the stability and realized the high efficient glutathione peroxidase activity.15,16 Recently, by the introduction of long alkyl chain in the polymer backbone and subsequent termination with long poly(ethylene glycol) chain, we have successfully synthesized a block copolymer containing diselenide with good solubility and stability.17One of the unique properties of the selenium compounds is redox responsiveness.18 They can be used as dual responsive materials because they respond to both oxidants and reductants. Compared with other redox responsive materials, such as polysulfides,19–21 the redox responsiveness of selenium-containing polymers may be more sensitive and faster. Considering that inflammatory cells often exhibit a more oxidative atmosphere intra-cellularly than the healthy ones,22,23 it is significant to develop oxidation-responsive drug vehicles which can disassemble and release the loaded drugs in an oxidative media.24–28 In this regard, selenium-containing polymers could be promising candidates.
Herein, we describe an approach for preparing monoselenide-containing amphiphilic block copolymers (denoted as PEG-PUSe-PEG) by the condensation of dihydroxylalkyl selenide and slightly excess diisocyanate, followed by a termination of poly(ethylene glycol) monomethylether. As shown in Scheme 1, PEG-PUSe-PEG is able to form micellar aggregates by dissolving in DMF and adding water dropwise. In addition, the aggregates can be disassembled upon addition of oxidants because the amphiphilic polyselenide block copolymer become hydrophilic after oxidation, therefore providing potential materials for preparation of controlled loading and release systems.
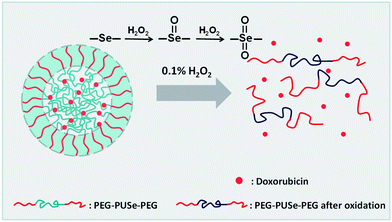 | ||
| Scheme 1 A schematic of oxidation-responsive disassembly of selenium-containing block copolymer micelles. | ||
Experimental
Materials
Poly(ethylene glycol) (PEG) monomethylether (Mw = 1900) and 11-bromoundecanol were purchased from Alfa Aesar. Doxorubicin (Hydrochloride, 99.7%) was a product of Zhongshuo Pharmaceutical Co. Ltd., Beijing. Diphenyldiselenide was from Acros. Selenium powder, sodium borohydride, 2, 4-toluene diisocyanate, 30% H2O2, anhydrous sodium sulfate (Na2SO4) and other organic solvents and chemicals used in this work were analytical grade products from Beijing Chemical Reagent Company. PEG was dried in vacuum at 60 °C for 4 h before use. Tetrahydrofuran (THF) was dried by CaH2 to remove the moisture and oxidative impurity. The solvents and chemicals were used as received unless stated.Synthesis of di-(1-hydroxylundecyl) selenide (1)
Sodium hydroselenide (NaHSe) was prepared through the reaction between Se powder and sodium borohydride in water. 0.15 g (4.0 mmol) sodium borohydride was dissolved in 2 mL of deionized water, and then 0.16 g (2.0 mmol) Se powder was added. The Se powder was dissolved with the generation of H2 and a colorless solution was obtained within 5 min. After the flask was sealed with a rubber plug, a solution of 1.0 g (4.0 mmol) 11-bromoundecanol in 10 mL anhydrous THF was injected into it under Ar flow. The reaction was performed at 50 °C for 12 h and the obtained solution was diluted with 100 mL of CH2Cl2 and dried with anhydrous Na2SO4. Then the product was purified by column chromatography with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CH2Cl2 and ethyl acetate as eluent, and white powder was obtained with a yield of 52%. 1H-NMR (300 MHz, CDCl3) δ (ppm): 3.64 (4H, t, HOCH2), 2.55 (4H, t, SeCH2), 1.71–1.20 (36H, m, HOCH2(CH2)9CH2Se); 77Se-NMR (600 MHz, CDCl3, diphenyldiselenide as reference (δ = 464.1 ppm)) δ (ppm): 161.4; ESI-MS: calculated 421.56, found 421.36.
1 mixture of CH2Cl2 and ethyl acetate as eluent, and white powder was obtained with a yield of 52%. 1H-NMR (300 MHz, CDCl3) δ (ppm): 3.64 (4H, t, HOCH2), 2.55 (4H, t, SeCH2), 1.71–1.20 (36H, m, HOCH2(CH2)9CH2Se); 77Se-NMR (600 MHz, CDCl3, diphenyldiselenide as reference (δ = 464.1 ppm)) δ (ppm): 161.4; ESI-MS: calculated 421.56, found 421.36.
Synthesis of PEG-PUSe-PEG block copolymer (2)
0.52 g (1.25 mmol) di-(1-hydroxylundecyl) selenide was first dissolved in 5 mL anhydrous THF in a 20 mL flask and sealed with a rubber plug, and the flask was then degassed with Ar for 20 min. A solution of 0.19 mL (1.35 mmol) 2,4-toluene diisocyanate (TDI) in 2 mL anhydrous THF was injected into the flask under Ar flow. The system was moved to an oil bath at 50 °C and allowed to react for 12 h with stirring. 0.16 g (0.085 mmol) PEG monomethylether dissolved in 2 mL anhydrous THF was then injected into the flask under Ar flow. The reaction was carried out for another 12 h. The solvent was removed and the solid residue was washed three times by deionized water and acetone. 0.45 g white powder of PEG-PUSe-PEG block copolymer was obtained after vacuum drying. 1H-NMR (300 MHz, CDCl3) δ (ppm): 7.04 (3H, b, aromatic H), 4.15 (4H, b, NHCOOCH2), 3.66 (8H, b, OCH2CH2 of PEG), 2.57 (4H, b, SeCH2), 2.19 (4H, b, NHCOOCH2CH2), 1.94–1.19 (32H, b, NHCOOCH2CH2(CH2)8CH2Se); Mn(NMR) = 4.90 × 104, Mw(GPC) = 1.95 × 104, Mw/Mn(GPC) = 2.12.Synthesis of di-(1-hydroxylundecyl) sulfide (3)
0.16 g (2.0 mmol) sodium sulfide (Na2S) was dissolved in 5 mL of deionized water and the solution was bubbled with Ar for 20 min. The flask was sealed with a rubber plug and a solution of 1.0 g (4 mmol) 11-bromoundecanol in 10 mL anhydrous THF was injected into it under Ar flow. The reaction was performed at 50 °C for 12 h and the obtained solution was diluted with 100 mL of CH2Cl2 and dried with anhydrous Na2SO4. Then the product was purified by column chromatography with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CH2Cl2 and ethyl acetate as eluent, and white powder was obtained with a yield of 55%. 1H-NMR (300 MHz, CDCl3) δ (ppm): 3.64 (4H, t, HOCH2), 2.50 (4H, t, SCH2), 1.70–1.18 (36H, m, HOCH2(CH2)9CH2S); ESI-MS: calculated 374.66, found (+ Br−) 454.74.
1 mixture of CH2Cl2 and ethyl acetate as eluent, and white powder was obtained with a yield of 55%. 1H-NMR (300 MHz, CDCl3) δ (ppm): 3.64 (4H, t, HOCH2), 2.50 (4H, t, SCH2), 1.70–1.18 (36H, m, HOCH2(CH2)9CH2S); ESI-MS: calculated 374.66, found (+ Br−) 454.74.
Synthesis of PEG-PUS-PEG block copolymer (4)
The PEG-PUS-PEG block copolymer was prepared using a similar procedure to PEG-PUSe-PEG, in which 0.47 g (1.25 mmol) di-(1-hydroxylundecyl) sulfide, 0.19 mL (1.35 mmol) 2,4-toluene diisocyanate (TDI), and 0.15 g (0.080 mmol) PEG monomethylether were used. 0.42 g white powder of PEG-PUS-PEG block copolymer was obtained. 1H-NMR (300MHz, DMSO-d6)δ (ppm): 7.05 (3H, b, aromatic H), 4.03 (4H, b, NHCOOCH2), 3.50 (8H, b, OCH2CH2 of PEG), 2.45 (4H, b, SCH2), 2.10 (4H, b, NHCOOCH2CH2), 1.67–1.09 (32H, b, NHCOOCH2CH2(CH2)8CH2S); Mn(NMR) = 2.08 × 104, Mw(GPC) = 1.08 × 104, Mw/Mn(GPC) = 1.58.Oxidation of di-(1-hydroxylundecyl) selenide
To further study the structure of PEG-PUSe-PEG copolymers after oxidation in 0.1% H2O2, di-(1-hydroxylundecyl) selenide was used as a model molecule to investigate the oxidation process. 0.042 g di-(1-hydroxylundecyl) selenide was dissolved in 5 mL THF and mixed with a 10 mL 0.1% H2O2 aqueous solution. The reaction was performed at room temperature (∼20 °C) for 5 h under stirring and the solution turned turbid and white precipitates were formed. The product was obtained by filtration and washed three times by deionized water. 0.035 g white powder was obtained after vacuum drying. 1H-NMR (300 MHz, CDCl3) δ (ppm): 3.64 (4H, t, HOCH2), 3.33 (4H, t, CH2SeO2), 2.04 (4H, t, CH2CH2SeO2), 1.71–1.20 (32H, m, HOCH2 (CH2)8CH2CH2SeO2); 77Se-NMR (600 MHz, CDCl3, diphenyldiselenide as reference (δ = 464.1 ppm)) δ (ppm): 1022.5; ESI-MS: calculated 453.55, found 453.33.Instruments and methods
The 1H-NMR and 77Se-NMR spectra were recorded on a JEOL JNM-ECA 300 (300 MHz) and a JEOL JNM-ECA 600 (600 MHz) spectrometer, respectively. ESI-mass spectroscopy measurement was carried out on a PE Sciex API 3000 spectrometer. Gel permeation chromatography (GPC) measurement was performed on a Waters 515 apparatus using polystyrene as a standard and THF as an eluent. The size distribution of the aggregates was analyzed by a Malvern 3000HS Zetasizer using a monochromatic coherent He–Ne laser (633 nm) as the light source and a detector that detected the scattered light at an angle of 90°. Transmission electron microscopy (TEM) images were obtained from a JEM-2010 Microscope with an accelerating voltage of 120 kV and the sample was stained by 0.2% phosphotungstic acid hydrate before observation. Cryo-TEM samples were prepared in a controlled environment vitrification system (CEVS) at 28 °C. The vitrified samples were then stored in liquid nitrogen until they were transferred to a cryogenic sample holder (Gatan 626) and examined by a JEM 2200FS TEM (200 kV) at about −174 °C. The phase contrast was enhanced by underfocus. Fluorescence was measured with a Hitachi F-7000 spectrofluorometer. X-Ray photoelectron spectroscopy (XPS) measurements were performed on an ESCA Lab220i-XL equipment with Si wafer as the substrates. Fourier transform infrared (FT-IR) spectra were obtained on a Bruker IFS 66v/s spectrometer using KBr substrates.Preparation of block copolymer aggregates
A 1 mL solution of 10 mg mL−1 block copolymer in DMF was added into 15 mL of deionized water under sonication, followed by dialysis against deionized water. After 72 h, the volume of the solution was increased to 20 mL with the addition of deionized water to obtain an aggregate solution with a concentration of 0.5 mg mL−1 for further experiments. To prepare Doxorubicin (Dox) loaded micelles, the block copolymer and Dox were both dissolved in DMF at a concentration of 10 mg mL−1. Consequently the solution was dropped into deionized water and dialyzed through the procedure mentioned above. It should be noted that the obtained micellar solution was dialyzed against deionized water until the water outside the dialysis tube exhibited negligible fluorescence emission of Dox. This dialysis ensures the removal of Dox molecules that failed to be entrapped by the polymer micelles. The final loading capacity of Dox is ∼0.055 mg mL−1, as estimated by fluorescence spectra.Characterization of PEG-PUSe-PEG after oxidation
After oxidation, the product was collected by freeze drying, and a slightly grey powder was obtained. 1H-NMR (600 MHz, CDCl3) δ (ppm): 7.04 (3H, b, aromatic H), 4.14 (4H, b, NHCOOCH2), 3.64 (8H, b, OCH2CH2 of PEG), 3.34 (4H, b, SeO2CH2), 2.19 (4H, b, NHCOOCH2CH2), 2.00 (4H, b, SeO2CH2CH2), 1.95–1.19 (28H, b, NHCOOCH2CH2(CH2)7CH2CH2SeO2); 77Se-NMR (600 MHz, CDCl3, diphenyldiselenide as reference (δ = 464.1 ppm)) δ (ppm): 1025.1.Oxidation-responsive release of Dox from the aggregates
For the release experiments, 3 mL of Dox-loaded micellar solution was kept in a dialysis tube and then placed into 30 mL of 0.1% H2O2 solution. 1 mL of the solution inside the dialysis tube was taken every hour to perform fluorescence measurements and dropped back after fluorescence measurements. The percentage of Dox released was estimated by comparing the remaining fluorescence with the original fluorescence.Results and discussion
As shown in Fig. 1, the PEG-PUSe-PEG copolymer (2) has been prepared by the condensation of selenide-containing diols (1) and slight excess TDI as well as the termination with PEG monomethylether. Normally the synthesis of selenium-containing polymers suffers from the low solubility of the generated polymers. Here, in our system a long alkyl chain was used to ensure a better solubility of the whole block copolymer. Therefore the condensation of (1) and TDI was successfully performed and PEG chains were successfully attached to the condensed PUSe block. The molecular weight of the copolymer PEG-PUSe-PEG (2) was determined by both the NMR method and GPC measurements. For NMR method, the molecular weight can be calculated according to the integral ratio of the peaks at 4.15 ppm (NHCOOCH2) and 3.66 ppm (OCH2CH2 of PEG) and the known molecular weight of PEG blocks, which provides a result of ∼4.90 × 104 g mol−1. Moreover, the polydispersity index of the block copolymer by GPC measurements is 2.12.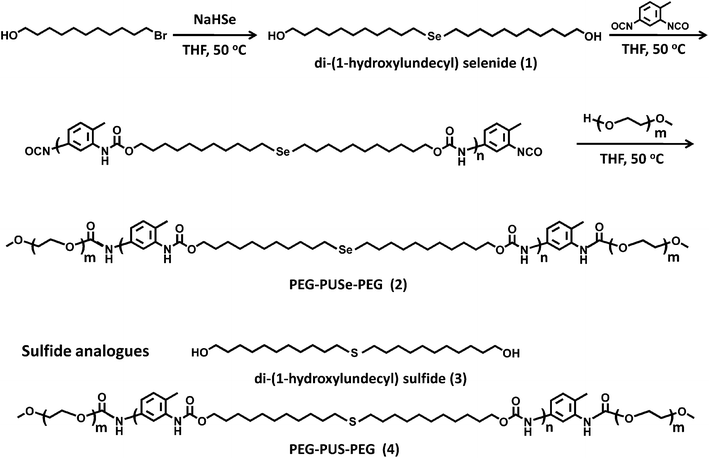 | ||
| Fig. 1 Synthetic procedure for the selenide block copolymers and their sulfide analogues. | ||
PEG-PUSe-PEG (2) is an amphiphilic block copolymer with one hydrophobic selenium-containing polyurethane block and two hydrophilic PEG blocks, which can self-assemble in aqueous solution. To study the self-assembly behavior of the block copolymer, the critical aggregate concentration (CAC) of PEG-PUSe-PEG was measured by the fluorescent probe method using pyrene as the probe.29 In the experiments, 5 μL of 5 × 10−3 mg mL−1 pyrene solution in acetone was added to PEG-PUSe-PEG aqueous solutions with different concentrations and the solutions were sonicated for 10 min before fluorescence emission measurements. As shown in Fig. 2a, the CAC is ∼1.4 × 10−4 mg mL−1. The CAC is determined as the concentration when pyrene exhibited an apparent decrease in the I1/I3 ratio with an increasing concentration of the block copolymer. Such a decrease normally indicates the aggregation of block copolymers. The size of the PEG-PUSe-PEG aggregates was determined by DLS measurements, which gave a result of about 71 nm in average diameter, as shown in Fig. 2b. The morphology of the PEG-PUSe-PEG aggregates was observed by TEM. It is shown that the PEG-PUSe-PEG block copolymer self-assembled into spherical aggregates in water with a diameter ranging from about 50 nm to 80 nm, which agrees well with the DLS results (Fig. 3a). The specific structure of the PEG-PUSe-PEG aggregates was further investigated with cryo-TEM. The image in Fig. 3b clearly shows that the PEG-PUSe-PEG block copolymer in aqueous solution has an aggregating structure with a solid core, which is generally considered to be a micellar structure. It is noteworthy that the micelles were quite stable in an ambient environment and could maintain their structures for at least one month.
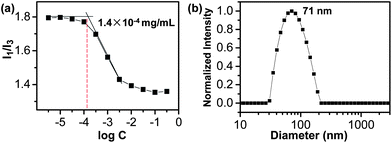 | ||
| Fig. 2 The CAC determinations of PEG-PUSe-PEG block copolymer (a) and DLS results for the aggregates (b). | ||
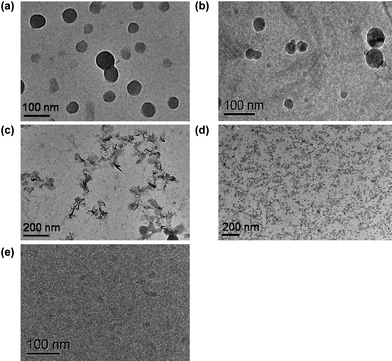 | ||
| Fig. 3 TEM (a) and cryo-TEM (b) images of PEG-PUSe-PEG copolymer micelles; TEM images of the aggregates after 2 h (c) and 5 h (d) oxidation in 0.1% H2O2 solution; cryo-TEM image of the oxidized aggregates after 5 h (e). | ||
Since the hydrophobic selenide groups intend to form hydrophilic selenoxide or selenone groups in an oxidative environment, we employ 0.1% H2O2 (v/v) as the oxidant to study the oxidation-responsiveness of the selenide block copolymer aggregates. In experiments, an appropriate amount of H2O2 was added into the micellar solution to obtain a final concentration of 0.1% v/v and the solution was used for further investigation after a certain period of time. From the TEM images shown in Fig. 3c and 3d, the micellar structure of PEG-PUSe-PEG copolymer was converted to irregular aggregates after two hours of oxidation and these irregular aggregates were further decomposed into tiny aggregates of several nanometres in 0.1% H2O2 aqueous solution for another three hours. Moreover, the cryo-TEM results also prove that no obvious aggregates were observed in the solution after oxidation in 0.1% H2O2 for 5 h, indicating that the micellar structures have been destroyed by the oxidation stimuli (Fig. 3e).
In order to illustrate the structural change in the selenide block copolymer upon the oxidation, the oxidized residue was analyzed by XPS. The solution before and after the addition of the oxidant was dropped onto the surface of Si wafers and dried under a reduced pressure to perform the XPS measurements. As shown in Fig. 4a, the binding energy of Se 3d5 shifts from 55.9 eV to 59.5 eV, suggesting a higher valency of selenium which is quite close to selenone groups.301H-NMR analysis of the oxidized products shows that the oxidation only induced the change of chemical shift of the methylenes next to the selenium atoms from 2.57 ppm to 3.34 ppm, indicating that the methylene protons next to selenium atom were located in a more polar chemical environment after oxidation in H2O2. These results also indicate that the polymeric structure was maintained during the oxidation experiments. 77Se-NMR results also prove that a new peak appeared at 1025.1 ppm, in the chemical shifting range of selenone and replaced the one at 161.4 ppm seen prior to oxidation. To further investigate the oxidation procedure, di-(1-hydroxylundecyl) selenide (1) was used as a model molecule to characterize the oxidized product. The oxidation of (1) is described at the experimental part and the ESI-MS data show that the molecular weight of the selenide diol (1) increases from 421.36 to 453.33 after oxidation, indicating that two oxygen atoms have been introduced, thus providing a selenone structure. As shown in the FT-IR spectra of oxidized selenide diol (1) (Fig. 4b), the strong characteristic absorption bands appear at 904 and 880 cm−1, corresponding to asymmetric and symmetric stretching vibration of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se
Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups,31 respectively, which clearly demonstrates the formation of selenones upon oxidation in 0.1% H2O2. The oxidized selenide diol has chemical shifts of 3.33 ppm in 1H-NMR spectra (SeO2CH2) and 1022.5 ppm in 77Se-NMR spectra, which agrees very well with the 1H-NMR and 77Se-NMR results of oxidized selenide block copolymer (3.34 ppm and 1025.1 ppm, respectively). Herein it can be concluded that the oxidation induces the formation of selenone groups, which has greatly changed the amphiphilicity of the selenium-containing block copolymer and enhanced its solubility in water, thus resulting in the oxidation-responsive disassembly of the formed aggregates.
O groups,31 respectively, which clearly demonstrates the formation of selenones upon oxidation in 0.1% H2O2. The oxidized selenide diol has chemical shifts of 3.33 ppm in 1H-NMR spectra (SeO2CH2) and 1022.5 ppm in 77Se-NMR spectra, which agrees very well with the 1H-NMR and 77Se-NMR results of oxidized selenide block copolymer (3.34 ppm and 1025.1 ppm, respectively). Herein it can be concluded that the oxidation induces the formation of selenone groups, which has greatly changed the amphiphilicity of the selenium-containing block copolymer and enhanced its solubility in water, thus resulting in the oxidation-responsive disassembly of the formed aggregates.
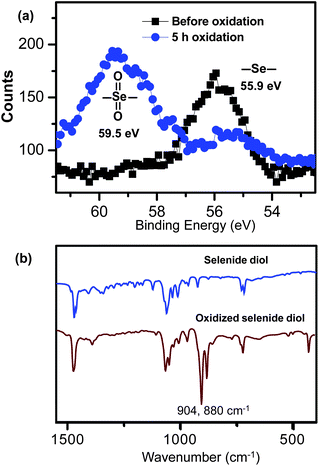 | ||
| Fig. 4 XPS analysis of the selenide polymer aggregates before and after oxidation (a) and FT-IR studies on di-(1-hydroxylundecyl) selenide before and after oxidation in 0.1% H2O2 (b), the strong characteristic absorption bands appearing at 904 and 880 cm−1 after oxidation correspond to asymmetric and symmetric stretching vibration of the selenone groups. | ||
For comparison, a polysulfide analogue of PEG-PUSe-PEG (2), PEG-PUS-PEG (4) was prepared through the similar procedure. The aggregates of the sulfide block copolymer PEG-PUS-PEG (4) were also observed as spherical aggregates by TEM. Interestingly, the PEG-PUS-PEG aggregates don't exhibit apparent morphological changes upon oxidation in 0.1% H2O2 for 5 h, which is quite different from their selenide analogue, as shown in Fig. 5a and 5b. XPS was employed to follow the oxidation process of sulfide block copolymer, and it is found that only ∼30% of sulfide groups have been converted to a relative low oxidized state (binding energy 166.0 eV) after 5 h, which corresponds to sulfoxide group, shown in Fig. 5c. The sulfide groups undergo a high conversion to sulfone groups with an electron binding energy of 168.5 eV after an oxidation as long as 24 h (Fig. 5d). These results indicate that the polysulfide block copolymer has a much lower rate of conversion upon mild oxidations than the selenide analogue, which can almost complete the conversion from selenide to selenone structures within only 5 h in same oxidative conditions, as shown in Fig. 4a.
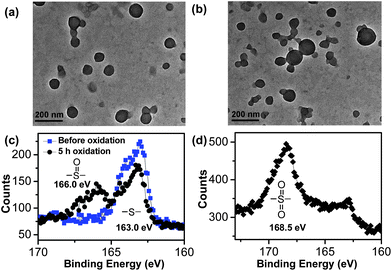 | ||
| Fig. 5 TEM images of PEG-PUS-PEG aggregates before (a) and after an oxidation in 0.1% H2O2 for 5 h (b), and XPS studies of the PEG-PUS-PEG copolymers and the products upon oxidation in 5 h (c) and 24 h (d). | ||
Due to the good oxidation-responsiveness of the selenium-containing block copolymer, it is possible to use the PEG-PUSe-PEG micelles to incorporate some functional species, especially drugs. In this work, we choose Doxorubicin, a fluorescent anticancer drug, as a model drug to investigate the oxidation-responsive release properties from the polyselenide micelles for the convenience of fluorescence detection. Through the process mentioned in the experimental section, Dox-loaded micelles with a loading concentration of 0.055 mg mL−1 were obtained, as estimated by fluorescence measurements. The release behavior of Dox was monitored by the decreasing fluorescence of the solution in the dialysis tube and the release percentage of Dox was calculated by comparing the fluorescence of the obtained solution and original solution. As shown in Fig. 6, Dox molecules can be released in a controllable manner upon oxidation in 0.1% H2O2 and sustain a continuous release during a ten-hour period. After an oxidation for 10 h, the release reaches equilibrium and the final release percentage of Dox is ∼72%. It should be noted that 0.1% H2O2 solution is a quite mild oxidative environment while the polyselenide block copolymer micelles can still show a good response to it. Moreover, polysulfide block copolymer micelles were also employed to incorporate Dox and the oxidation-responsive release behavior was studied. It is shown that the PEG-PUS-PEG micelles exhibit a slower release rate and a lower release percentage of Dox (∼41%), as compared with the PEG-PUSe-PEG copolymer, which may be related to their lower oxidation sensitivity. In addition, according to the results of additional oxidation and release experiments, there is no obvious change on the releasing behavior of Dox when 0.1 M PBS buffer was used instead of deionized water as the aqueous media. These results demonstrate that an oxidation-responsive drug release is realized by using polyselenide block copolymer micelles as the stimuli-responsive vehicles. It should be pointed out that the amount of selenium (14 μg mL−1) we used is very close to the appropriate concentration range of selenium-containing compounds used as drugs.32 A detailed study of the toxicity of PEG-PUSe-PEG is under way.
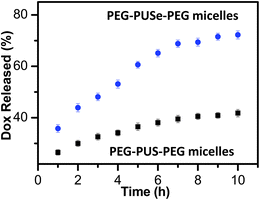 | ||
| Fig. 6 Oxidation-responsive release studies of Dox from the polyselenide block copolymer micelles and their polysulfide analogues. | ||
Conclusions
In conclusion, an amphiphilic block copolymer with oxidation-responsive selenide moieties was synthesized. The aggregates formed from the selenium-containing block copolymer can undergo a structural disassembly and a controlled release of loaded cargos in a mild oxidative environment. The good sensitivity to the oxidation stimuli can be attributed to the highly active selenide groups in presence of oxidants, which is more sensitive than their sulfide analogues. Considering its good oxidation-responsiveness and mild operating conditions, this selenium-containing block copolymer is expected to be further employed as a smart drug vehicle for loading and release of drugs in physiological environments.Acknowledgements
This work was financially supported by the National Basic Research Program (2007CB808000), National Natural Science Foundation of China (20904028, 50973051, 20974059), Tsinghua University Initiative Scientific Research Program (2009THZ02-2), the NSFC-DFG joint grant (TRR 61) and China Postdoctoral Science Foundation (20080440362).Notes and references
- Y. Okamoto, T. Yano and R. Homsany, Ann. N. Y. Acad. Sci., 1972, 192, 60 CrossRef CAS.
- W. H. H. Günther and M. N. Salzman, Ann. N. Y. Acad. Sci., 1972, 192, 25 CrossRef CAS.
- H. Kroll and E. F. Bolton, J. Appl. Polym. Sci., 1970, 14, 2319 CrossRef CAS.
- D. J. Sandman, M. Rubner and L. Samuelson, J. Chem. Soc., Chem. Commun., 1982, 1133 RSC.
- R. Sugimoto, K. Yoshino, S. Inoue and K. Tsukagoshi, Jpn. J. Appl. Phys., 1985, 24, L425 CrossRef.
- T.-T. Ong, S.-C. Ng and H. S. O. Chan, Polymer, 2003, 44, 5597 CrossRef CAS.
- A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC.
- A. L. Braga, D. S. Lüdtke, F. Vargas and R. C. Braga, Synlett, 2006, 1453 CrossRef CAS.
- D. M. Freudendahl, S. Santoro, S. A. Shahzad, C. Santi and T. Wirth, Angew. Chem., Int. Ed., 2009, 48, 8409 CrossRef.
- R. Michels, M. Kato and W. Heitz, Makromol. Chem., 1976, 177, 2311 CrossRef CAS.
- K. C. Nicolaou, J. Pastor, S. Barluenga and N. Winssinger, Chem. Commun., 1998, 1947 RSC.
- J. T. W. Kan and P. H. Toy, J. Sulfur Chem., 2005, 26, 509 Search PubMed.
- J. T. Rotruck, A. L. Pope, H. E. Ganther, A. B. Swanson, D. G. Hafeman and W. G. Hoekstra, Science, 1973, 179, 588 CrossRef CAS.
- G. Mugesh, W.-W. du Mont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS.
- X. Zhang, H. Xu, Z. Dong, Y. Wang, J. Liu and J. Shen, J. Am. Chem. Soc., 2004, 126, 10556 CrossRef CAS.
- H. Xu, J. Gao, Y. Wang, Z. Wang, M. Smet, W. Dehaen and X. Zhang, Chem. Commun., 2006, 796 RSC.
- N. Ma, Y. Li, H. Xu, Z. Wang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 442 CrossRef CAS.
- D. Liotta, Organoselenium Chemistry, John Wiley & Sons, 1987 Search PubMed.
- A. Napoli, M. Valentini, N. Tirelli, M. Muller and J. A. Hubbell, Nat. Mater., 2004, 3, 183 CrossRef CAS.
- A. Rehor, J. A. Hubbell and N. Tirelli, Langmuir, 2005, 21, 411 CrossRef CAS.
- G. Kilcher, G. Duckhan and N. Tirelli, Langmuir, 2007, 23, 12309 CrossRef CAS.
- L. M. Coussens and Z. Werb, Nature, 2002, 420, 860 CrossRef CAS.
- J. S. Valentine and E. B. Gralla, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8178 CrossRef CAS.
- J. Rodriguez-Hernandez, F. Checot, Y. Gnanou and S. Lecommandoux, Prog. Polym. Sci., 2005, 30, 691 CrossRef CAS.
- C. J. F. Rijcken, O. Soga, W. E. Hennink and C. F. van Nostrum, J. Controlled Release, 2007, 120, 131 CrossRef CAS.
- O. Onaca, R. Enea, D. W. Hughes and W. Meier, Macromol. Biosci., 2009, 9, 129 CrossRef CAS.
- Y. Wang, H. Xu and X. Zhang, Adv. Mater., 2009, 21, 2849 CrossRef CAS.
- H. Fu, D. M. Policarpio, J. D. Batteas and D. E. Bergbreiter, Polym. Chem., 2010 10.1039/c0py00060d.
- T. Cao, P. Munk, C. Ramireddy, Z. Tuzar and S. E. Webber, Macromolecules, 1991, 24, 6300 CrossRef CAS.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-Ray Photoelectron Spectra, Appendix B. Chemical State Tables, Perkin-Elmer, Physical Electronics Division, 1992 Search PubMed.
- G. Socrates, Infrared Characteristic Group Frequencies, John Wiley & Sons, 1980, ch. 16: Sulfur and Selenium Compounds Search PubMed.
- H. Xu and K. Huang, Selenium: Its Chemistry, Biochemistry and Application in Life Science, Huazhong Univ. Sci. Tech. Press, 1994, (in Chinese) Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
