Redox-controlled ‘smart’ polyacrylamide solubility†
Hui
Fu
,
Danielle M.
Policarpio
,
James D.
Batteas
* and
David E.
Bergbreiter
*
Department of Chemistry, Texas A&M University, P.O. Box 30012, College Station, Texas 77842-3012, USA. E-mail: batteas@chem.tamu.edu; bergbreiter@tamu.edu
First published on 24th March 2010
Abstract
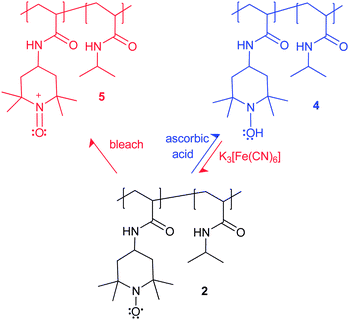
“Smart” acrylamide copolymers containing N-isopropyl and 4-N-amino-2,2,6,6-tetramethylpiperidin-1-oxyl-4-yl (TEMPO) groups undergo reversible redox behavior leading to LCST changes. Oxidation (NaOCl/H2O) or reduction (ascorbic acid) changes the copolymer's LCST from 18 °C to 35–40 °C.
Responsive polymers that change their physical properties in response to a chemical or physical stimulus are of broad interest in chemistry and materials science.1–3 There is both an interest in understanding the molecular interactions that underlie a responsive polymer's property changes as well as an interest in developing new responsive polymers that can be used in applications in areas as diverse as drug delivery and sensors. Responsive behavior typically involves physical changes in a polymer's properties. Changes in a polymer's solubility—changes that are often exhibited by polymers that have lower critical solution temperature (LCST) behavior—are examples of such physical changes. While many synthetic and natural polymers exhibit LCSTs, poly(N-isopropylacrylamide) (PNIPAM) and its copolymers are perhaps the most common examples of materials with LCSTs.4,5 Here we describe PNIPAM copolymers whose solubility is controlled by reversible oxidation/reduction chemistry.
Several examples of polymers with redox responsiveness have been reported. This includes polymers with disulfide bonds that form polymersomes which assemble to protect biomolecules in the extracellular environment but are later reduced and disassemble after endocytosis.6 Similarly, diselenide-containing amphiphiles that assemble or disassemble in a redox sensitive manner have also been reported.7 Polymers other than redox-sensitive conjugated polymers are less common but poly-(N-isopropylacrylamide) copolymers containing pendent ferrocenyl groups have been reported.8,9 While the ferrocenyl group in these monomers interfered with the radical polymerization and led to relatively low molecular weight copolymers, the ferrocene groups in the ca. 2–3 kDa polymer could still be oxidized to ferrocenium ions. This oxidation increased the LCST of a copolymer containing ca. 9 mol% ferrocenyl groups by ca. 7 °C (from ca. 26 °C to 33 °C (at 10% scattering)).8
A nitroxyl group like tetramethylpiperidinoxyl can readily be oxidized or reduced under mild conditions. This process wherein TEMPO is reversibly oxidized and reduced underlies the use of TEMPO as an organocatalyst for oxidations in organic synthesis.10 This chemistry (shown for TEMPO in eqn (1)) has also been used by Nishide and Oyaizu to design nitroxyl-containing polymers that are useful as new flexible battery materials.11
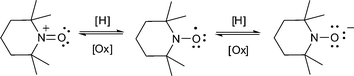 | (1) |
Our earlier work showed that subtle changes in PNIPAM's microstructure significantly affect PNIPAM's LCST and solubility.12–14 These studies suggested to us that incorporating a TEMPO group like that shown in eqn (1) into a PNIPAM copolymer would lead to polymers whose LCSTs could change significantly on oxidation/reduction of this TEMPO group.
Incorporation of nitroxyl groups into a PNIPAM copolymer to generate PNIPAM-derivatives with redox-sensitive LCSTs proved to be possible using the electrophilic poly(N-acryloxysuccinimide) (PNASI) polymers originally developed by Ringsdorf.15 We previously used PNASI as a starting material in the synthesis of libraries of poly(N-alkylacylamides) from mixtures of amines.13 As shown in Scheme 1, we were able to prepare a poly(N-alkylacrylamide) copolymer 2 containing a mixture of N-isopropyl and N-4-tetramethylpiperidinoxyl groups from PNASI, 1, using a mixture of 4-amino-TEMPO and isopropylamine. This synthesis avoids the complications one would expect if the TEMPO group was directly incorporated as a TEMPO-containing monomer during a radical polymerization. In addition, the synthesis of 2 from 1 has some analytical advantages. Specifically, while the paramagnetic nitroxyl-containing copolymer 2 could not be analyzed by 1H NMR spectroscopy, we can readily analyze polymer 3 prepared from the same PNASI precursor. 4-Amino-TEMPO and 4-amino-2,2,6,6-tetramethylpiperidine are likely to have very similar reactivity toward PNASI. Thus, to estimate the mole fraction of TEMPO in 2, a second reaction of the same sample of PNASI was used to form polymer 3 (Scheme 1) using the same ratio of N-isopropylamine and 4-amino-2,2,6,6-tetramethylpiperidine. In a reaction using a 1 : 9 ratio of the piperidinyl amine and isopropylamine the product copolymer 3 was analyzed by 1H NMR spectroscopy which showed the ratio of isopropyl/piperidinyl groups was 14 : 86 (ca. 1 : 6). Polymers 2 and 3 from this same reaction were also analyzed by GPC. GPC analysis of the copolymers 2 and 3 showed that these polymers had a Mn of 178 and 171 kDa, respectively, with PDIs of 1.76 and 1.97, respectively. Copolymers with other ratios of N-isopropyl/N-4-amino-TEMPO had similar behavior. For example, a sample of 2 prepared using an 85 : 15 starting ratio of isopropylamine/4-amino-TEMPO had an LCST of 21.7 °C at 10% clouding vs. a 23.1 °C LCST observed when a 90 : 10 starting ratio of isopropylamine/4-amino-TEMPO was used.
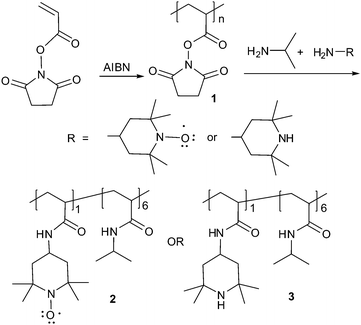 | ||
| Scheme 1 Synthesis of redox-active PNIPAM-co-TEMPO and structurally analogous diamagnetic copolymers from PNASI. | ||
The LCSTs of the copolymers 2 and 3 were then analyzed using a digital melting point apparatus that was used in a cold room.16 A 10 mg mL−1 aqueous solution of 2 had a LCST of 23.1 °C at 10% clouding while solution of 3 with the same concentration did not show cloudiness even at 100 °C. In the case of copolymer 2, addition of 10 µL of 0.8 M L-ascorbic acid to 1 mL of a 10 mg mL−1 solution of 2 caused 2's LCST to become indistinct. Addition of a kosmotropic salt (Na2SO4) addressed this problem. Specifically, when 0.1 M Na2SO4 was added to the solution of 2 and L-ascorbic acid, LCSTs could be easily observed. This modest concentration of Na2SO4 affects the LCST of 2 alone, lowering it 4.8 °C to 18.3 °C. All subsequent analyses of LCSTs were carried out in the presence of 0.1 M Na2SO4. Separate LCST analyses using a PNIPAM sample (Mn = 322 kDa, PDI = 1.06 by GPC) showed that this low concentration of Na2SO4 also lowers PNIPAM's LCST by similar amount (4.3 °C).
The polymer 2 was analyzed by cyclic voltammetry to confirm that it would undergo the desired redox behavior. As expected, 2 had electrochemical behavior analogous to that reported for TEMPO itself. TEMPO has a redox potential of 0.63 V vs. Ag/AgCl17 while 2 has a redox potential of 0.66 V vs. Ag/AgCl (a cyclic voltammogram is provided in the ESI†). Polymer 2 was also characterized by ESR spectroscopy and its spectrum had the characteristic 3-line spectrum of a nitroxyl group (the ESR spectrum is provided in the ESI†). Polymers like 2 containing ca. 0.1 mol% of TEMPO groups as well as other TEMPO containing polymers studied previously have similar redox behavior and ESR spectra.18,19
Reduction of the nitroxyl-containing polymer 2 with L-ascorbic acid will form the hydroxylamine-containing polymer 4 and oxidation will form the oxidation product 5 (Scheme 2). As expected, this redox chemistry affects the LCST of solutions that initially contained 10 mg mL−1 of 2. This is illustrated in Fig. 1 where a 14 °C change in the solution's LCST behavior is seen as increasing amounts of L-ascorbic acid are added to 2 to form the reduced polymer 4. Reoxidation of the hydroxylamine-containing polymer 4 by K3[Fe(CN)6] largely reverses the changes of the LCST seen on reduction (Fig. 2). Addition of 48 mM K3[Fe(CN)6] to a solution containing 4 led to a significant 10 °C decrease in polymer 4's LCST. However, reoxidation with K3[Fe(CN)6] did not completely reverse the effects of reduction to regenerate a solution with 2's LCST.
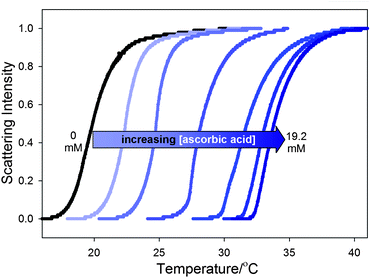 | ||
| Fig. 1 Clouding curves for a 10 mg mL−1 sample of polymer 2 with 0.1 M Na2SO4 in solutions that contain increasing amounts of L-ascorbic acid. The L-ascorbic acid concentration varies from left to right with concentrations of 0, 1.6, 3.2, 6.4, 9.6, 14.4, and 19.2 mM. Added Na2SO4 (0.1 M) was used to enhance detectability of the samples' LCST. | ||
![Clouding curves for a 10 mg mL−1 sample of polymer 2 with 19.6 mM l-ascorbic acid and 0.1 M Na2SO4 in solutions that contain increasing amounts of K3[Fe(CN)6]. The K3[Fe(CN)6] concentration varies from right to left with concentrations of 0, 4, 12, 20, 24, 28, 32, 40, and 48 mM.](/image/article/2010/PY/c0py00060d/c0py00060d-f2.gif) | ||
| Fig. 2 Clouding curves for a 10 mg mL−1 sample of polymer 2 with 19.6 mM L-ascorbic acid and 0.1 M Na2SO4 in solutions that contain increasing amounts of K3[Fe(CN)6]. The K3[Fe(CN)6] concentration varies from right to left with concentrations of 0, 4, 12, 20, 24, 28, 32, 40, and 48 mM. | ||
Control experiments show that the changes in LCSTs are complicated by the fact that these additives as solutes can also affect a polymer's LCST. In control experiments, we examined the effects of L-ascorbic acid, K3[Fe(CN)6], and a mixture of L-ascorbic acid and K3[Fe(CN)6] on the LCST of PNIPAM both in the presence of and in the absence of 0.1 M Na2SO4. These experiments with PNIPAM alone showed that addition of 20 mM of L-ascorbic acid led to a small ca. 0.2 °C decrease in PNIPAM's LCST. Addition of 48 mM K3[Fe(CN)6] decreased PNIPAM's LCST by 1.7 °C. A mixture containing both L-ascorbic acid and K3[Fe(CN)6] at these concentrations led to a further decrease in PNIPAM's LCST from 31.9 °C to 29.4 °C. LCST analyses of PNIPAM in the presence of 0.1 M Na2SO4 and these additives led also to decreases in the LCST of the PNIPAM/0.1 M Na2SO4 solution with the 1 to 2 °C changes in LCST that were essentially the same as those seen with these additives and PNIPAM in the absence of Na2SO4. Clouding curves for these experiments are provided in ESI†.
Oxidation of 2 to form 5 can be accomplished with household bleach. This oxidation process converts the nitroxyl groups of 2 to oxoammonium groups and also produces a change in LCST as shown in Fig. 3. In these studies with bleach, distinct clouding curves could be observed without the addition of Na2SO4. Increasing amounts of added bleach gradually changed the clouding curves to higher temperatures. With the addition of 30 µL household bleach straight out of bottle, the clouding temperature at 10% clouding intensity increased from 23.1 to 39.3 °C. Reversal of this oxidation process was less successful. We believe this could be because the NaOCl solution irreversibly covalently modifies the secondary amides present in polymer 2. Experiments in which 30 uL of bleach were added to a 0.1 mg mL−1 solution of PNIPAM alone led to a decrease in PNIPAM's LCST from 31.4 to 29.2 °C (see ESI† for LCST curves). The reversibility of these oxidations is further complicated because the reducing agent (L-ascorbic acid) used to reduce 5 back to 2 will not just form 2 but can form 4 as well (vide infra).
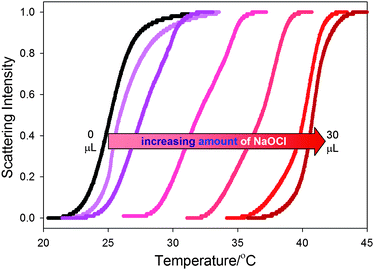 | ||
| Fig. 3 Clouding curves for a 10 mg mL−1 sample of polymer 2 in solutions that contain increasing amounts of bleach. The amount of added bleach varies from left to right with the additions of 0, 5, 10, 15, 20, 25, and 30 µL of bleach, respectively. | ||
Conclusions
Our studies show that incorporation of 5–10 mol% redox active spin labels in a polyacrylamide containing 90–95 mol% N-isopropyl groups leads to materials that exhibit redox sensitive LCST behavior. The observed LCSTs of these materials are shown to vary from ca. 18 to 39 °C, a variation that could be useful both in the design of redox-sensitive materials for drug delivery applications in vivo and in the synthesis of surfaces with redox-mediated wettability.Acknowledgements
Support for this research by the Robert A. Welch Foundation (DEB, Grant A-0639), the National Science Foundation (DMR-0907233), and the National Science Foundation REU program (grant CHE-0755207, DP summer support) is gratefully acknowledged.Notes and references
- H. G. Boerner, H. Kuehnle and J. Hentschel, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1 CrossRef CAS.
- I. Tokarev, M. Motornov and S. Minko, J. Mater. Chem., 2009, 19, 6932 RSC.
- M. W. Urban, Prog. Polym. Sci., 2009, 34, 679 CrossRef CAS.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163 CrossRef CAS.
- I. C. Barker, J. M. G. Cowie, T. N. Huckerby, D. A. Shaw, I. Soutar and L. Swanson, Macromolecules, 2003, 36, 7765 CrossRef CAS.
- S. Cerritelli, D. Velluto and J. A. Hubbell, Biomacromolecules, 2007, 8, 1966 CrossRef CAS.
- N. Ma, Y. Li, H. Xu, Z. Wang and X. Zhang, J. Am. Chem. Soc., 2010, 132, 442 CrossRef CAS.
- F. Zuo, C. Luo, X. Ding, Z. Zheng, X. Cheng and Y. Peng, Supramol. Chem., 2008, 20, 559 CrossRef CAS.
- N. Kuramoto and Y. Shishido, Polymer, 1998, 39, 669 CrossRef CAS.
- T. Vogler and A. Studer, Synthesis, 2008, 1979 CAS.
- H. Nishide and K. Oyaizu, Science, 2008, 319, 737 CrossRef CAS.
- D. E. Bergbreiter, R. Hughes, J. Besinaiz, C. Li and P. Osburn, J. Am. Chem. Soc., 2003, 125, 8244 CrossRef CAS.
- D. E. Bergbreiter, N. A. Aviles-Ramos and D. Ortiz-Acosta, J. Comb. Chem., 2007, 9, 609 CrossRef CAS.
- S. Furyk, Y. Zhang, D. Ortiz-Acosta, P. S. Cremer and D. E. Bergbreiter, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1492 CrossRef CAS.
- H. G. Batz, G. Franzmann and H. Ringsdorf, Angew. Chem., Int. Ed. Engl., 1972, 11, 1103 CAS.
- D. E. Bergbreiter and H. Fu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 186 CrossRef CAS.
- H. Nishide, S. Iwasa, Y.-J. Pu, T. Suga, K. Nakahara and M. Satoh, Electrochim. Acta, 2004, 50, 827 CrossRef CAS.
- F. M. Winnik, M. F. Ottaviani, S. H. Bossmann, M. Garcia-Garibay and N. J. Turro, Macromolecules, 1992, 25, 6007 CrossRef CAS.
- K. Oyaizu, Y. Ando, H. Konishi and H. Nishide, J. Am. Chem. Soc., 2008, 130, 14459 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional clouding curves for control experiments. See DOI: 10.1039/c0py00060d |
| This journal is © The Royal Society of Chemistry 2010 |