Self-exploding capsules
Bruno G.
De Geest
*a,
Stefaan
De Koker
b,
Jo
Demeester
c,
Stefaan C.
De Smedt
c and
Wim E.
Hennink
d
aLaboratory of Pharmaceutical Technology, Department of Pharmaceutics, Ghent University, Ghent, Belgium. E-mail: Br.DeGeest@ugent.be
bLaboratory of Molecular Immunology, Department of Molecular Biomedical Research, Ghent University, Ghent, Belgium
cLaboratory of General Biochemistry and Physical Pharmacy, Department of Pharmaceutics, Ghent University, Belgium
dDepartment of Pharmaceutics, Utrecht University, Utrecht, The Netherlands
First published on 24th December 2009
Abstract
Self-exploding microcapsules are core-shell capsules that consist of a degradable microgel core surrounded by a semi-permeable membrane. When the microgel core degrades, the swelling pressure increases and finally the rupture of the surrounding membrane leads to release of the encapsulated species. This type of capsule could find applications in fields such as e.g. drug delivery, tissue engineering and microfluidics.
Introduction
The advent of biotechnology has offered the possibility to produce a large variety of macromolecular drugs such as peptides, proteins and nucleic acids such as DNA and small interfering RNA (siRNA).1 These molecules however are usually not stable in the gastrointestinal tract, are poorly absorbed and therefore cannot be administered using common oral dosage forms. Therefore such drug molecules are almost exclusively delivered by parenteral (i.e. injection/needle based) administration, where drug molecules are injected directly in the bloodstream (i.e. intravenous injection) or within a specific tissue (e.g. subcutaneous, intramuscular, etc…) being the target site of the drug molecules or from which they can diffuse into the bloodstream and/or reach other tissues.Drug delivery scientists have been challenged with numerous questions concerning the spatially and temporary controlled delivery of drug molecules.2,3 For example, to avoid side effects, some drugs have to be targeted to specific cells or specific cellular compartments, which might be achieved by formulating them in tiny nano- or microscale particles coated with specific ligands.4,5 In addition, many drug molecules have a short half-life and limited stability in the extracellular matrix, thus requiring repeated administrations in order to maintain effective levels at the target site. This problem is tackled by developing controlled release systems that encapsulate drug molecules and gradually release them by diffusion, matrix degradation or a combination of both.6 In other cases where e.g. tolerance is induced at constant drug levels, a more pulsatile delivery of the drug at pre-programmed time intervals is required instead. Another case is vaccination, where multiple booster injections are commonly necessary in order to induce protective immunity.7 With this in mind, drug delivery scientists have been working towards the development of single shot vaccine formulations where one single administration mimics multiple booster injections by releasing the antigen in a pulsatile manner at different time intervals. Microcapsules that can release their payload after a pre-programmed time or upon application of a specific stimulus could be a promising solution to this purpose. Several systems have been reported based on enzymatic degradable membranes or remote capsules opening by laser light, magnetic field or ultrasound. A recent concept are the self-exploding capsules which can explode and release their content without the requirement of an external source or stimulus.8–12
The concept of self-exploding capsules
Self-exploding capsules consist of a degradable core surrounded by a semi-permeable shell. When the microgel core degrades, the swelling pressure increases and until the swelling pressure exceeds the tensile strength of the membrane causing the membrane to rupture and the capsule to release its payload. In this concept the degradation rate of the core governs the time point of explosion and thus the release of encapsulated material. By injecting a mixture of antigen-loaded self-exploding microcapsules with different microgel core degradation rates and thus different rupturing times, separated antigen release pulses can be obtained, allowing one to replace multiple conventional booster injection by one single shot (Fig. 1B). | ||
| Fig. 1 (A) A schematic representation of the concept ‘self-exploding capsules’. A drug-loaded hydrogel core is surrounded by a semi-permeable membrane. Degradation of the microgel core leads to a decrease in cross-links (black dots) and consequently an increase in swelling pressure. When the swelling pressure exceeds a critical value, the membrane explodes and encapsulated drugs are released. (B) A schematic representation of the simultaneous administration of multiple populations of capsules, each exploding at a different time point, leading to pulsed drug release. | ||
Core structure of degradable dextran
Degradable cross-linked dextran
Hydrogels can be obtained by a variety of methods involving ionic complexation, stereo complexation or covalent coupling.13 The use of covalent reactions to cross-link hydrophilic polymers, forming stable three dimensional hydrogels, offers the advantage of tight control over the hydrogel properties by choosing the cross-link density of the polymer network. Moreover, when using degradable cross-links one is also able to tailor the degradation rate of the network. Dextran-hydroxyethyl methacrylate (dex-HEMA) was introduced by the Hennink group and is based on dextran, a natural polysaccharide produced by bacteria, modified with methacrylate groups (see Fig. 2 for the reaction scheme).14–16 These methacrylate groups can undergo radical polymerisation to form a three dimensional polyHEMA sub-network connecting the dextran chains (Fig. 3). Degradability is provided by incorporation of a carbonate ester between the dextran backbone and the pending methacrylate groups (Fig. 3). This is achieved by activating HEMA with 1,1′-carbonyldiimidazole (CDI) followed by conjugation to dextran. Carbonate esters can hydrolyze in water under physiological conditions (i.e. 37 °C and pH 7.4) making them well suited for controlled drug release in a physiological relevant framework. | ||
| Fig. 2 The reaction scheme for the two-step synthesis the synthesis of dex-HEMA, by CDI (1,1′-carbonydiimidazole) activation of HEMA (hydroxyethyl methacrylate) followed by grafting onto dextran. | ||
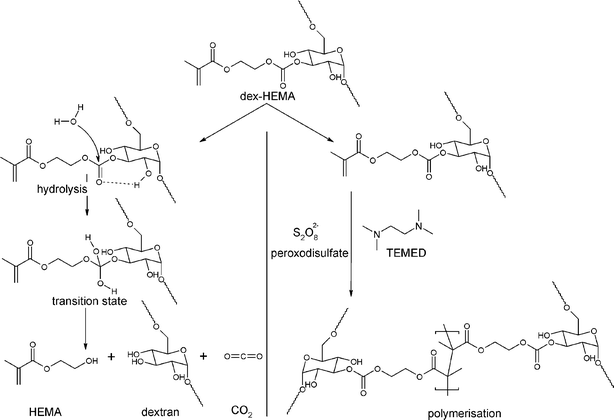 | ||
| Fig. 3 Reaction scheme of the hydrolysis and polymerisation of dex-HEMA. Hydrolysis takes place under physiological conditions at the carbonate esters which link the methacrylate moieties to the dextran backbone. Polymerisation occurs through radical polymerisation of the pending methacrylate moieties. | ||
It was elaborately reported by the Hennink group that swelling, degradation and release properties of dex-HEMA hydrogels could be tightly controlled by varying the degree of substitution (i.e. the number of methacrylate groups per 100 glucopyranose units in dextran) and by varying the water content of the hydrogels.17 Furthermore, it has been reported that a wide variety of proteins can be successfully incorporated and released in a well controlled fashion from these hydrogels.15
Fabrication of spherical dextran microgels
Dextran has the attractive property that most proteins have a higher affinity for an aqueous dextran phase compared to an aqueous poly(ethylene glycol) phase. Moreover, at elevated concentrations, dextran and poly(ethylene glycol) do not mix and form a so-called water-in-water emulsion. This allows the preparation of dextran microspheres in all aqueous conditions without the use of potentially hazardous organic solvents.23–25Fig. 4A schematically depicts how dex-HEMA microgels with varying size distributions are obtained. An aqueous dextran phase is slowly introduced into a stirring PEG solution. By varying the stirring speed of the PEG solution one is able to influence the size distribution of the obtained dex-HEMA/PEG emulsion. Simple addition of a water soluble initiator/catalyst system such as KPS/TEMED leads to radical polymerisation and the formation of solid microgels. Fig. 4B shows different size distribution curves, as measured by laser diffraction, of dex-HEMA microgels with varying diameter obtained using the above mentioned procedure.
 | ||
| Fig. 4 (A) A schematic representation of the preparation of polydisperse dex-HEMA microgels by an all aqueous dex-HEMA/PEG emulsification procedure. (B) Size distribution (as measured by laser diffraction) curves of dex-HEMA microgels obtained by varying the kinetic energy put into the emulsification process. | ||
Fig. 5A shows a photograph of the droplet generating microfluidic devices which were prepared by PDMS based soft lithography methods leading to the formation of microscopic channels between a glass cover slide and a PDMS slab which was previously casted on a patterned silicon wafer. The microfluidic emulsification process is schematically shown in Fig. 5C. Aqueous PEG exhibited insufficient shear force to act as continuous phase and it was not possible to form dex-HEMA droplets through microfluidic emulsification of an aqueous dex-HEMA phase into an aqueous PEG phase. Therefore mineral oil enriched with ABIL EM-90 as surfactant was used as an organic continuous phase. Note that without addition of surfactant droplet formation was not possible (Fig. 5C). As shown in Fig. 5C monodisperse droplets are successfully generated and by increasing the pressure on the aqueous phase the droplet generation frequency increases and the formed droplets move downwards in a necklace-like pattern. When the droplets leave the microfluidic chip they are collected and irradiated by UV light, leading to the formation of solid microgels which can then be transferred into an aqueous medium by washing with water miscible organic solvents like acetone, methanol or ethanol. As shown in Fig. 5B highly monodisperse microgels can be obtained is this way. Larger microgels with diameters of several hundreds of micron can also be prepared using a tubing based microfluidic device.29
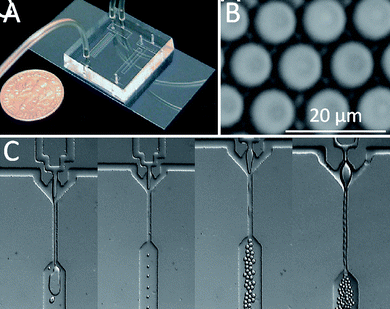 | ||
| Fig. 5 (A) Photograph of a PDMS based microfluidic chip used to fabricate monodisperse microgels. (B) Monodisperse dex-HEMA microgels produced by microfluidic emulsification. (C) Stereo microscopy image of the in-line droplet generating geometries in absence of surfactant (left pane) and at different pressures of the aqueous dex-HEMA stream (second to fourth panes). | ||
Shell structure of self-exploding capsules
The shell surrounding the microgels is deposited onto the microgel surface through electrostatic interaction. Initially lipids were chosen for this purpose because lipid bilayer membranes are ubiquitous in nature and known for their selective permeability while their mechanical properties are in the range of the forces which can be generated by degradable dex-HEMA microgels.30,31 As spontaneous adsorption of a lipid bilayer membrane onto a microgel surface was not likely to occur, a first requirement was to provide the microgels with a net surface charge in order to allow electrostatic interaction as driving force for lipid adsorption. Therefore dex-HEMA was copolymerised with methacrylic acid, yielding anionic dex-HEMA-MAA microgels, and dimethyl aminoethyl methacrylate, yielding cationic dex-HEMA-DMAEMA microgels, respectively.32,33Lipid coated self-exploding capsules
Lipid coating was performed by incubating the charged microgels in a dispersion of oppositely charged liposomes. Through electrostatic interaction these liposomes adsorbed onto the surface of the microgels and spread forming a lipid bilayer. This process is schematically shown in Fig. 6, and lipid bilayer formation on solid colloidal substrates has extensively been investigated by pioneering work of the groups of Needham34,35 and Pennefather.36–38 More recent work of Mornet39 and Troutier40–42 have investigated the influence of the process parameters and lipid constituents on the formation of colloidal supported lipid bialyers. | ||
| Fig. 6 A schematic representation of liposome adsorption and spreading onto a spherical template leading to the formation of a lipid bilayer coating surrounding the template. | ||
As lipid coating, mixtures of respectively SOPC:DOTAP and SOPC:DOPA were used to coat respectively dex-HEMA-MAA and dex-HEMA-DMAEMA microgels. SOPC is a lipid bearing a net neutral charge while DOTAP is a cationic and DOPA an anionic lipid. Confocal and scanning electron microscopy (Fig. 7A–B) clearly demonstrated a successful lipid coating onto the microgel surface. The behaviour of the lipid coated microgels upon degradation of the microgels core was assessed by confocal microscopy by adding a drop of alkaline solution to a drop of microcapsule suspension. As mentioned above, dex-HEMA hydrogels degrade through hydrolysis of the carbonate ester bond which links the polymerized methacrylates to the dextran backbone. Van Dijk-Wolthuis et al. reported that the reaction kinetics of the hydrolysis reaction are pH dependent, exhibiting a minimal reaction rate at pH values between 4 and 5 while the reaction is strongly accelerated at more acidic and alkaline conditions.15,17 Whereas degradation at physiological pH varies between days and weeks, degradation in 0.1 M NaOH only takes a few seconds to minutes. As shown in Fig. 7C, upon increasing the pH to highly alkaline, the microcapsules start to swell and inflated bubbles appear on their surface. Finally, the entire capsule membrane explodes.
 | ||
| Fig. 7 (A) Confocal microscopy image of dex-HEMA-MAA microgels coated with a SOPC:DOTAP lipid membrane. The microgels were stained green fluorescent by incorporation of FITC-dextran (70 kDa) while the lipid membrane was stained red fluorescent by incorporation of 0.5 mol% rhodamine-DOPE. (B) Corresponding scanning electron microscopy image. (C) Series of confocal microscopy snapshots taken at different time points after addition of a alkaline solution to SOPC:DOTAP coated dex-HEMA-MAA microgels. The microgels were stained red fluorescent by incorporation of TRITC-dextran (158 kDa) and the lipid membrane was stained green fluorescent by incorporation of 0.5 mol% BODIPY-FL. | ||
These experiments gave a proof-of-concept that the swelling pressure of a degrading microgel could trigger the explosion of a surrounding membrane. However, through the formation of the inflated bubbles, we could also observe that it was not a single lipid bilayer deposited onto the microgel surface, but rather a patchwork of lipid multilayers. This was confirmed by X-ray reflectometry experiments on the same lipid mixtures deposited on a polysaccharide coated silicon substrate. For this reason, i.e. lack of control over the exact composition of the microcapsule coating, and because long term stability of lipid membranes under physiological conditions is questionable, we sought for alternative coating strategies to provide dex-HEMA microgels with an adequate membrane.
Polyelectrolyte multilayer coated microgels
Polyelectrolyte multilayer coating has emerged as a versatile coating strategy for an almost unlimited variety of surfaces in a broad field of applications.43 Pioneered by the work of Decher on planar surfaces in the beginning of the nineties,44–48 this technique is based on the sequential adsorption of charged species onto an oppositely charged substrate using electrostatic interactions as driving force. This approach was extended onto colloidal substrates in the late nineties by the work of Sukhorukov et al.49Fig. 8 schematically illustrates this method for coating colloids. Starting from e.g. a positively charged substrate, a polyanion is adsorbed followed by several washing/centrifugation steps to remove non-adsorbed polyelectrolytes. Subsequently a next layer of a polycation is applied and the whole procedure is repeated until a desired number of layers is deposited. This allows one to tailor the characteristics of the coating onto the nanoscale by varying the coating thickness as well as the composition. | ||
| Fig. 8 A schematic representation of layer-by-layer (LbL) polyelectrolyte multilayer coating of colloidal substrates. | ||
Taking into account the possibility to provide dex-HEMA microgels with a net surface charge through polymerisation with a charged methacrylate it is evident that LbL coating of dex-HEMA could be a viable strategy for obtaining self-exploding capsules with a tight control over the physicochemical and mechanical properties of the coating.
Van Tomme et al. have investigated the influence of the co-monomer, i.e. MAA and DMAEMA, on the degradation kinetics of the corresponding dex-HEMA microgels.50 It was found that dex-HEMA-DMAEMA microgels exhibited degradation times, ranging from a few weeks to months while dex-HEMA-MAA microgels degraded much more slowly. We reasoned that for our application the faster degradation kinetics of dex-HEMA-DMAEMA microgels would be favourable. Therefore, in all following experiments we used dex-HEMA-DMAEMA microgels and for simplicity of presentation, we will denote them in this review as dex-HEMA microgels.
Self-exploding polyelectrolyte capsules – the proof of concept
The proof of concept was realized by coating dex-HEMA microgels with an average diameter of approximately 5 μm with 3 alternating layers of sodium polystyrene sulfonate (PSS) and polyallylamine hydrochloride (PAH).51,52Fig. 9 shows confocal microscopy and scanning electron microscopy images of the microgels before and after coating. Green fluorescent dextran (i.e. FITC-dextran) was incorporated as a model drug and appears to be homogenously distributed throughout the volume of the microgels. Scanning electron microscopy reveals a smooth surface of uncoated dex-HEMA microgels. After coating with (PSS/PAH)3 a distinct red fluorescent (due to rhodamine labelled PAH) ring surrounding the microgels appears on the confocal microscopy image while scanning electron microscopy reveals a rough vermiculate surface. This peculiar roughness is most likely due to the differential drying between the microgel template and the polyelectrolyte membrane. Similar findings have been reported previously by McAloney et al. on planar multilayer films using atomic force microscopy.53,54 | ||
| Fig. 9 Confocal (A & C) and scanning electron (B & D) microscopy images of uncoated (A & B) and (PSS/PAH)3 coated (C & D) dex-HEMA microgels. Green fluorescent dextran (i.e. FITC-dextran was incorporated as model drug and PAH was red fluorescent labelled with rhodamine. The inset in pane C shows the molecular structure of PSS and PAH. | ||
As degradation of the microgel core varies from days to weeks, depending on the cross-link density of the microgel core it is virtually impossible to monitor the degradation behaviour of the capsules in real-time by confocal microscopy under physiological conditions (i.e. pH 7.4 and 37 °C). To circumvent this limitation, the capsules were pre-heated for few minutes at 80 °C in aqueous medium buffered at pH 9 to accelerate the hydrolysis reaction through which dex-HEMA hydrogels degrade. Subsequently the capsules were moved back to room temperature under the confocal microscope, still in aqueous medium at pH 9. This alkaline pH was chosen because polyelectrolyte membranes consisting of PSS/PAH are known to exhibit a pH dependent permeability.55,56 At a pH above 8 the membrane is impermeable for macromolecules with a molecular weight above 20 kDa, while at lower pH the membrane appears to be permeable. Thus, when operating at a pH of 9, the capsule membrane is in its ‘closed’ state and the degradation products of the dex-HEMA microgels, which are 19 kDa dextran, should remain entrapped within the capsule membrane and therefore contribute to the increase in osmotic pressure within the capsule. Fig. 10A–D shows a series of confocal microscopy snapshots taken at regular time intervals after the pre-heating step. The capsule swells until at a certain moment the capsule ruptures and releases its payload, leaving behind a ruptured empty ‘bag’.
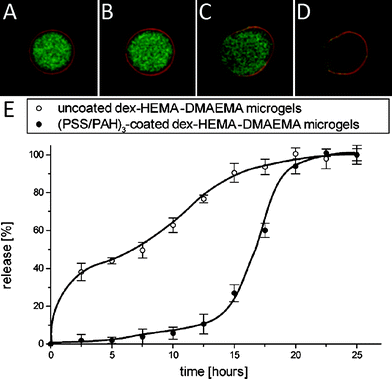 | ||
| Fig. 10 Snapshots (A–D) of (PSS/PAH)3 coated dex-HEMA microgels showing the rupturing of the polyelectrolyte membrane. The time interval between the snapshots is 15 min. The microgel core was labelled green fluorescent by incorporation of FITC-dextran while the coating was labelled red fluorescent using RITC labelled PAH. (E) Cumulative release curves of 158 kDa TRITC-dextran from uncoated dex-HEMA (○) and (PSS/PAH)3 coated dex-HEMA microgel (●). The data points are interconnected with a cubic B-spline. The experiments were run in duplicate. | ||
The graph in Fig. 10E shows the cumulative release curves, in a carbonate buffer (pH 9; 37 °C) as release medium, of 158 kDa TRITC-dextran as model drug from both uncoated and (PSS/PAH)3 coated dex-HEMA microgels. The uncoated microgels exhibit a burst release followed by a sustained release. On the contrary, the (PSS/PAH)3 coated microgels only release few content during the early stage of incubation and release their entire payload in a pulsatile fashion after approximately 15 h. These data give the proof-of-principle that the swelling pressure of a degradable microgel core can rupture a surrounding polyelectrolyte membrane causing a pulsatile release of its payload.
Bio-polymer coated microgels under physiological conditions
As PSS and PAH are not biodegradable nor are they commonly used biocompatible polymers in drug delivery, further focus was put on the use of so-called bio-polyelectrolytes. In a series of experiments a wide range of polypeptides and polysaccharides was evaluated as component for multilayer coating of dex-HEMA microgels. Several of these biopolymers such as hyaluronic acid, poly-L-ornithine, alginate and chitosan exhibited too high a viscosity and/or too low a charge density to allow, at least in our hands, successful LbL coating of dex-HEMA microgels without aggregation to occur or they resulted merely in the formation of a blurred coating when visualized by confocal microscopy.On the contrary, successful LbL coating of dex-HEMA microgels was achieved when using poly-L-arginine (pARG) as polycation in combination with respectively chondroitin sulfate (CHON), poly-L-aspartic (pASP) acid, poly-L-glutamic acid (pGLU) and dextran sulfate (DEXS).57 Subsequently we evaluated the behaviour of the microcapsules upon incubation over prolonged times at physiological conditions. When verified that uncoated microgels were completely degraded, the corresponding LbL coated microgels were visualized by confocal microscopy. These images, shown in Fig. 11, clearly point out a different behaviour of the microcapsules depending on the type of polyanion used in the multilayer coating. Capsules containing chondroitin sulfate, poly-L-aspartic acid or poly-L-glutamic acid (moderate Mw) did all explode. Capsules containing poly-L-glutamic acid (high Mw) partly exploded, partly remained intact while still encapsulating their payload. Capsules containing dextran sulfate did not explode at all but lost their payload, most likely due to increased permeability of this coating during degradation of the microgel core.
 | ||
| Fig. 11 Confocal images of dex-HEMA microgels (fluorescently labelled with 150 kDa FITC-dextran) coated with (A) (CHON/pARG)4, (B) (pGLUhigh/pARG)4, and (C) (DEXS/pARG)4 after degradation of the microgel core. The insets in the upper right corner show the microcapsules prior to degradation. In (A), all microcapsules were broken and released their contents. In (B), both broken as well as intact (still filled with 150 kDa FITC-dextran) microcapsules were observed. The capsules in (C) remained intact, but had released their contents by diffusion through the bio-polyelectrolyte coating. In all cases, the pARG was fluorescently labelled with RITC. | ||
Subsequently we were interested in the release behaviour of these microcapsules. As capsules based on chondroitin sulfate, poly-L-glutamic acid (moderate Mw) and poly-L-aspartic acid were all shown to explode upon incubation under physiological conditions they were loaded with FITC-labelled bovine serum albumin (BSA) as model protein drug and incubated in phosphate buffer at 37 °C. Samples were withdrawn at regular time intervals and measured for their fluorescence. Fig. 12 shows a typical cumulative release profile obtained in these experiments. Instead of the desired pulsatile release profile a rather sustained release is observed.
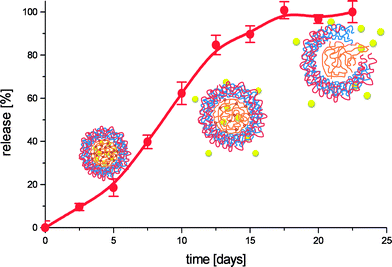 | ||
| Fig. 12 Cumulative release profile of FITC-dextran (150 kDa) from (pGLU/pARG)4 coated dex-HEMA microgels, incubated at physiological conditions. The schematic representation show the hypnotized behaviour of the microcapsules as function of degradation time. | ||
To investigate the reason for this behaviour FITC-BSA loaded and empty capsules were put under the confocal microscope at different time points during degradation. It was observed that upon degradation, and thus swelling of the microgel core, the LbL membrane stretched and premature leakage of the microcapsules' payload took place. This was confirmed when probing the permeability of empty capsules at different degradation times using FITC-dextran. When non-degraded capsules showed to be impermeable to FITC-dextran with a Mw of 70 kDa, they became permeable during microgels core degradation for FITC-dextran with a Mw up to 500 kDa. These findings make it reasonable to assume that permeability of the LbL membrane during degradation of the microgel core is an important issue, hampering the encapsulated compounds to be released in a pulsatile way.
Towards pulsed release
In order to achieve pulsed release from self-exploding microcapsules under physiological conditions, an important strategy was to prevent the above mentioned premature release of the microcapsules' payload through the membrane prior to microcapsule explosion. For this purpose two strategies were pursued. First, we fabricated larger dex-HEMA microgels with diameters above 100 μm. This would reduce the surface/volume ratio and would thus reduce the available surface through which premature release could occur. Secondly, we made use of fluorescent nanoparticles (i.e. green fluorescent 50 nm carboxylated latex beads) as model drug. While soluble macromolecules, such as dextran and BSA, were able to diffuse through the microcapsule membrane, we expected the membrane mesh size to be small enough to prevent outwards diffusion of nanoparticles. Note that the use of nanoparticles is not that far away from reality as several vaccine formulations are based on particulate antigen (i.e. Hepatitis B vaccine composed of virus-like particles)58 which have dimensions of several tens of nanometres.As the polyelectrolyte pair we opted for dextran sulfate/poly-L-arginine, as in other research on hollow CaCO3 templated polyelectrolyte capsules we elaborately studied the biocompatibility and tissue reaction in vitro and in vivo of this polyelectrolyte pair with promising results.59,60 In a previous paragraph we described that dex-HEMA microgels coated with (DEXS/pARG)4 were not able to explode upon degradation of the microgel core.57 However, these microgels had a mean diameter of 5 μm. Laplace's law explains that for a given membrane thickness (implying a fixed tensile strength), the membrane tension will increase equally with the radius when a given internal pressure is applied. Therefore we expected that 150 μm capsules made a reasonable chance to explode upon degradation of the microgel core.
Fig. 13A shows a series of confocal microscopy images recorded at different time points after addition of a drop of alkaline solution to a drop of capsule suspension. Gradually, the capsules start to swell, explode and release their payload. In a subsequent experiment the capsules were incubated at physiological conditions for 30 days, to ensure complete microgel core degradation, and the result was visualized by confocal (Fig. 13B) and scanning electron (Fig. 13C) microscopy. On these images no intact capsules are observed as only debris of broken capsules is visible. Clearly, these capsules also explode upon incubation at physiological conditions.
 | ||
| Fig. 13 (A) Confocal microscopy snapshots of exploding capsules triggered by the addition of sodium hydroxide. (B) Confocal microscopy images of microcapsules after incubation in physiological conditions (i.e. pH 7.4 and 37 °C for 30 days). (C) A SEM image of an exploded microcapsule. (D) Cumulative release curves upon incubation at physiological conditions of 50 nm green fluorescent latex beads from exploding microcapsules fabricated from dex–HEMA with a DS of 2.5 (red curve) and 5 (blue curve). | ||
As the next step in our research we evaluated the fate of these self-exploding capsules in vivo. Therefore the capsules were injected subcutaneously in mice and tissue sections were taken at different time points and visualized by confocal microscopy. As shown in Fig. 14, one day post injection, the capsules are distributed through the tissue and are obviously still intact. Two weeks post injection, the capsules are mostly intact, although several of them appear deformed. This is likely to be due to the pressure of the surrounding tissue onto the capsules whose rigidity strongly decreases upon degradation of the microgel core. One month post injection, most of the capsules are no longer intact and have released their payload. Debris of the LbL membrane appear scattered through the tissue. Confocal images recorded at higher magnifications gave an indication that this debris is mostly located inside phagocyting cells. This hypothesis is further confirmed when taking into account the hematoxylin and eosin (H & E) staining in Fig. 14. These images show that the capsule membrane becomes gradually infiltrated with inflammatory cells. Furthermore, the injection spot becomes surrounded by several layers of fibroblasts which is a common foreign body response.61 Similar observations were reported by De Koker et al. on subcutaneously injected hollow 3 μm sized DEXS/pARG capsules in mice.62 Also in this experimental set-up, the injected volume was infiltrated by inflammatory cells and gradually surrounded by fibroblasts. Further it was shown that upon cellular uptake, the capsules became largely deformed and finally digested.
 | ||
| Fig. 14 (Left column) Fluorescence microscopy images of paraffin sections taken at several time points after subcutaneous injection of microcapsules. The images are an overlay of the DIC channel, green fluorescence channel (nanoparticles) and red fluorescence channel (LbL coating). (Right column) H&E staining of tissue sections taken 14 days after subcutaneous injection of microcapsules. | ||
It is important to note that when comparing the tissue reaction of subcutaneous injected self-exploding capsules to subcutaneous injected alum, which is the classic adjuvant used in vaccine formulations, a similar degree of inflammation is observed (for images see De Koker et al.62). This allows us to speculate that the induced inflammatory response can be considered as relatively moderate, thus indicating potential use of the self-exploding capsules as vaccine delivery system.
However, it also has to be noted that, in order to obtain pulsed release in vivo, further optimization of both core and shell structure is still required. Firstly, the core material of the self-exploding capsules should be optimized in order to exhibit less swelling of the capsules during core degradation prior to explosion, but which dramatically increase their swelling pressure when turning from gel into liquid upon total degradation. This would enhance the integrity of the capsules within the surrounding tissue upon subcutaneous administration. Secondly, the LbL coating should be modified in order to withstand infiltration by inflammatory cells, as premature rupturing caused by digestion of the shell would also lead to premature release of encapsulated payload.
Encapsulation and release of nano-and microparticles
In an attempt to minimize premature release through the capsule membrane prior to explosion, an alternative strategy was to reinforce the polyelectrolyte membrane. For this purpose a cross-linkable polycation was applied. Diazoresin (DAR) can be covalently coupled to sulfonate groups upon irradiation with light, as shown in Fig. 15A, forming a tight cross-linked membrane. This approach has been successfully applied by Caruso and coworkers63 and Zhu and McShane64 to enhance the mechanical strength and reduce the permeability of hollow polyelectrolyte capsules.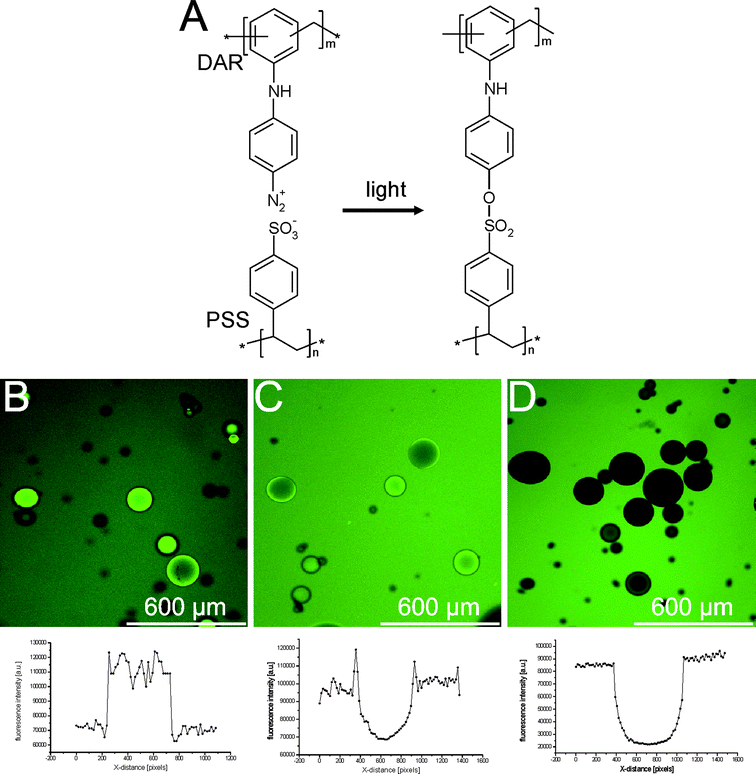 | ||
| Fig. 15 (A) Molecular structure of the cross-linked PSS/DAR membrane. (B–D) Confocal microscopy images and fluorescence intensity profiles of (B) bare dex-HEMA microgels, (C) dex-HEMA microgels coated with (PSS/PAH)4, and (D) dex-HEMA microgels coated with (PSS/DAR)2 incubated in a 1 mg ml−1 solution of 20 kDa FITC-dex. | ||
A substantial decrease in permeability was also observed when a LbL coating based on PSS/DAR was deposited onto dex-HEMA microgels.65Fig. 15B–D show confocal microscopy images of (B) uncoated dex-HEMA microgels, (C) (PSS/PAH)4 coated dex-HEMA microgels and (D) (PSS/DAR)2 coated dex-HEMA microgels, incubated in a solution of 20 kDa FITC-dextran. This fluorescent probe strongly accumulates inside uncoated microgels, likely to be due to charge interaction between the cationic DMAEMA groups of the microgels and the anionic FITC groups. Dex-HEMA microgels coated with (PSS/PAH)4 accumulated less fluorescence, but are still permeable to the fluorescent probe. Importantly, dex-HEMA microgels coated with only 2 bilayers PSS/DAR became completely impermeable to the 20 kDa FITC-dextran, as is evident from plotting the fluorescence intensity profiles across a capsule section.
When the (PSS/DAR)2 coated dex-HEMA microgels were transferred to the confocal microscope and tested for their ability to explode, a remarkable behaviour was observed. Upon addition of an alkaline solution, the capsules started to swell, similarly as previously described self-exploding capsules. However, at the moment of explosion, the capsules torn apart and literally ejected their payload, being 200 nm green fluorescent latex beads, into the surrounding medium. The unique property of these capsules is revealed when measuring the distance travelled by the ejected latex beads as a function of time. As shown in Fig. 16, the latex beads travel a distance of 400 μm in 40 s. When comparing this to the distance which could be travelled by merely Brownian motion, a 800 fold increase in speed is calculated.
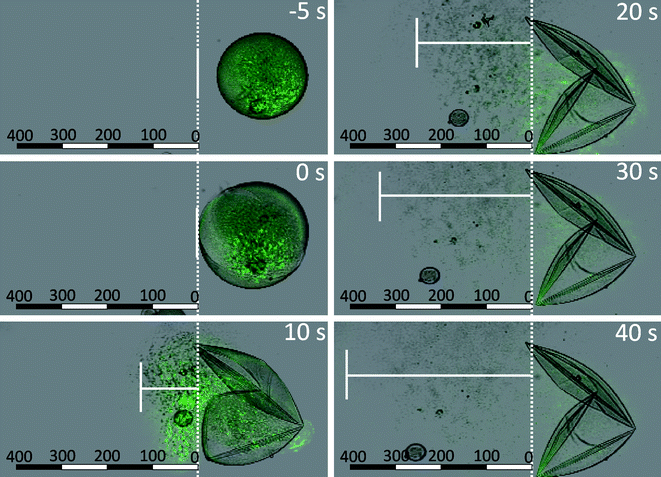 | ||
| Fig. 16 Confocal microscopy snapshots taken at regular time intervals (overlay of green fluorescence channel and transmission channel) of (PSS/DAR)2 coated microgels containing FITC-NP during the degradation of the microgel core triggered by the addition of sodium hydroxide. The microcapsule explodes 10 s after addition of sodium hydroxide, and subsequently the edge of the propagating front of released nanoparticles is marked by the vertical white line. The units of the scale bar are μm. | ||
While commonly released species only spread slowly into the surrounding medium, this type of exploding capsule could propel species, allowing them to travel relatively large distances over a short time. The biocompatibility and degradability of the PSS/DAR system is of course questionable, impairing their applicability for in vivo drug delivery applications. However, other applications could be envisioned as well, e.g. in the field of microfluidics where due to the inherent laminar flow, ejected nanoparticles could overcome certain transport barriers. For such applications it could be important to have control over the direction in which the payload is released. This issue was recently tackled by Bedard et al. who functionalized (PSS/DAR)4 dex-HEMA microgels with gold nanoparticles and subsequently dissolved the microgel core at alkaline pH, without rupturing the LbL coating.66 As gold nanoparticles can be remotely heated by IR laser irradiation, it was demonstrated that by positioning the laser beam at a specific location onto the capsule surface the gold nanoparticles could be locally heated. This induced the creation of a small opening in the LbL coating through which the encapsulated payload was released in a radial direction, driven by the osmotic pressure of the degraded dex-HEMA microgels which was still retained in the capsules.
The concept of ejecting particulate matter from self-exploding capsules was further explored by encapsulating hollow polyelectrolyte capsules in self-exploding capsules.67 Hollow polyelectrolyte capsules are fabricated by LbL coating of a sacrificial template followed by the dissolution of this template.10,11,68–71 In our research we used, approximately 3 μm sized, CaCO3 microparticles which are synthesized by mixing equimolar amounts of CaCl2 and Na2CO3 both dissolved in water in the presence of FITC-dextran as model drug (Fig. 17A). The precipitated CaCO3 microparticles are highly porous and can encapsulate relative large amount of macromolecules.72–74 Subsequently these microparticles are coated with 2 bilayers DEXS/pARG and incorporated in dex-HEMA microgels. These microgels are in turn coated with 4 bilayers DEXS/pARG. A confocal microscopy images of the resulting multi-compartment capsules is depicted in Fig. 17B and shows the small LbL capsules being scattered through the larger LbL coated microgel. Explosion of the capsules could again be induced by addition of alkaline medium or by incubation at physiological conditions for several weeks. Scanning electron microscopy images of exploded capsules are depicted in Fig. 17C–D. These images correspond to the release of LbL coated CaCO3 microparticles (Fig. 17C) and the release of hollow DEXS/pARG capsules(Fig. 17D). The latter is achieved by treating, prior to microgel degradation, the multi-compartment capsules with an aqueous EDTA solution. EDTA complexes calcium and leads to the dissolution of the CaCO3 core template, yielding hollow capsules.
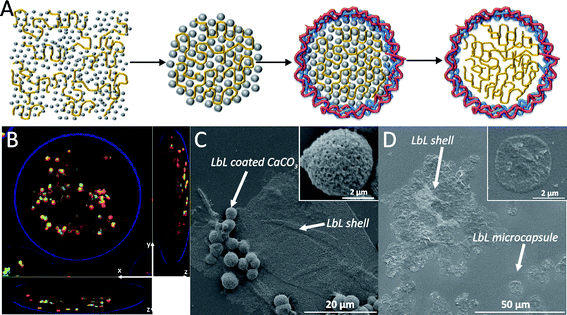 | ||
| Fig. 17 (A) A schematic representation of the synthesis of hollow macromolecule loaded polyelectrolyte capsules. (B) A confocal microscopy image of a gel bead [with an outer (DEXS/pARG)4 shell loaded with LbL microcapsules containing FITC-dextran. The x, y, and z axis are indicated on the images. The outer LbL shell was assigned as fluorescent blue, the membrane of the LbL microcapsules is in red while the LbL microcapsules' interior is in green due to the FITC-dextran. (C and D) SEM images of remnants of the outer LbL shell and released (DEXS/pARG)2 coated CaCO3 core templates (C) and hollow LbL microcapsules (D). The insets in (C) and (D) are magnified images of the released particles. | ||
The rationale behind this research is again situated in the field of vaccine delivery. Several groups,75,76 as well as our own group, are evaluating the use of hollow polyelectrolyte capsules as vaccine delivery vehicles. Due to their size of several microns they are selectively taken up by phagocyting cells such as dendritic cells, which are professional antigen presenting cells. As polyelectrolyte capsules can encapsulate large amounts of antigen under mild process conditions with a capsule surface that can further be tailored with additional ligands, they hold promise for antigen delivery. In combination with self-exploding capsules, a formulation could be developed that, as mentioned in the introduction of this review, could cover multiple booster injections with a single shot injection.
Conclusions
In this review we have given an overview of our research on self-exploding capsules. The main rationale was the potential use of such capsules for single shot vaccination. Whereas multiple injections (i.e. the so called booster injections) are commonly required to generate sufficient immunity, a single shot vaccine, comprising of different populations of capsules each exploding after a different time intervals, could eliminate the need for booster injections.Starting from the abstract concept of a degradable microgel core surrounded by a semi-permeable membrane, the topic was developed step-by-step. Biodegradable methacrylated dextran (dex-HEMA) copolymerised with charged methacrylates was chosen as core material. Spherical microgels could be obtained via emulsification and electrostatic interactions were used as driving force to provide the microgels with a semi-permeable membrane. The proof-of-concept that the swelling pressure of a degradable microgel core could rupture a surrounding membrane was demonstrated using both lipids and polyelectrolytes as coating constituents. Further optimisation lead to the use of microgels with diameters above 100 μm, coatings composed of polysaccharides or polypeptides and the use of nanoparticles as a model particulate antigen. Preliminary in vivo studies involving subcutaneous injection of self-exploding capsules in mice, yielded promising results showing a moderate inflammation and release of the capsules' payload. However, the polyelectrolyte shell of the microcapsules tested was prone to degradation and infiltration by inflammatory cells, which may result in premature release and uptake of their payload, hampering release in a pulsatile fashion. As a result, shell stability and resistance to cellular infiltration are challenges that will need to be addressed in order to control microcapsule explosion and payload release in vivo.
Finally, we also described the concept of self-exploding capsules that could potently eject particulate matter such as nano/micro-particles and even microcapsules. Such ejecting capsules could, upon explosion, propel their payload with an approximately 800 fold velocity increase compared to Brownian motion. For these capsules, applications in the field of tissue engineering and microfluidics could be envisioned as well.
Acknowledgements
BGDG acknowledges the FWO Vlaanderen for a postdoctoral scholarship.Notes and references
- R. Langer and D. A. Tirrell, Nature, 2004, 428, 487–492 CrossRef CAS.
- J. T. Santini, M. J. Cima and R. Langer, Nature, 1999, 397, 335–338 CrossRef CAS.
- A. C. R. Grayson, I. S. Choi, B. M. Tyler, P. P. Wang, H. Brem, M. J. Cima and R. Langer, Nat. Mater., 2003, 2, 767–772 CrossRef CAS.
- D. A. LaVan, T. McGuire and R. Langer, Nat. Biotechnol., 2003, 21, 1184–1191 CrossRef.
- D. A. LaVan, D. M. Lynn and R. Langer, Nat. Rev. Drug Discovery, 2002, 1, 77–84 CrossRef CAS.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- D. T. O'Hagan and N. M. Valiante, Nat. Rev. Drug Discovery, 2003, 2, 727–735 CrossRef CAS.
- M. Bedard, B. G. De Geest, A. Skirtach, H. Moehwald and B. G. Sukhorukov, Adv. Colloid Interface Sci. DOI:10.1016/J.CIS.2009.07.007.
- M. F. Bedard, B. G. De Geest, H. Moehwald, G. B. Sukhorukov and A. G. Skirtach, Soft Matter, 2009, 5, 3927–3931 RSC.
- B. G. De Geest, G. B. Sukhorukov and H. Mohwald, Expert Opin. Drug Delivery, 2009, 6, 613–624 Search PubMed.
- B. G. De Geest, S. De Koker, G. B. Sukhorukov, O. Kreft, W. J. Parak, A. G. Skirtach, J. Demeester, S. C. De Smedt and W. E. Hennink, Soft Matter, 2009, 5, 282–291 RSC.
- B. G. De Geest, N. N. Sanders, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2007, 36, 636–649 RSC.
- W. E. Hennink and C. F. van Nostrum, Adv. Drug Delivery Rev., 2002, 54, 13–36 CrossRef CAS.
- W. N. E. Vandijkwolthuis, O. Franssen, H. Talsma, M. J. Vansteenbergen, J. J. K. Vandenbosch and W. E. Hennink, Macromolecules, 1995, 28, 6317–6322 CrossRef CAS.
- W. N. E. van Dijk Wolthuis, J. A. M. Hoogeboom, M. J. vanSteenbergen, S. K. Y. Tsang and W. E. Hennink, Macromolecules, 1997, 30, 4639–4645 CrossRef CAS.
- W. N. E. van Dijk Wolthuis, S. K. Y. Tsang, J. J. KettenesvandenBosch and W. E. Hennink, Polymer, 1997, 38, 6235–6242 CrossRef CAS.
- W. N. E. van Dijk Wolthuis, M. J. vanSteenbergen, W. J. M. Underberg and W. E. Hennink, J. Pharm. Sci., 1997, 86, 413–417 CrossRef CAS.
- J. A. Cadee, C. J. de Groot, W. Jiskoot, W. den Otter and W. E. Hennink, J. Controlled Release, 2002, 78, 1–13 CrossRef CAS.
- C. J. De Groot, J. A. Cadee, J. W. Koten, W. E. Hennink and W. Den Otter, Int. J. Cancer, 2002, 98, 134–140 CrossRef CAS.
- O. Franssen, L. Vandervennet, P. Roders and W. E. Hennink, J. Controlled Release, 1999, 60, 211–221 CrossRef CAS.
- W. E. Hennink, O. Franssen, W. N. E. vanDijkWolthuis and H. Talsma, J. Controlled Release, 1997, 48, 107–114 CrossRef CAS.
- W. E. Hennink, H. Talsma, J. C. H. Borchert, S. C. DeSmedt and J. Demeester, J. Controlled Release, 1996, 39, 47–55 CrossRef.
- R. J. H. Stenekes, O. Franssen, E. M. G. van Bommel, D. J. A. Crommelin and W. E. Hennink, Int. J. Pharm., 1999, 183, 29–32 CrossRef CAS.
- O. Franssen and W. E. Hennink, Int. J. Pharm., 1998, 168, 1–7 CrossRef CAS.
- R. J. H. Stenekes, O. Franssen, E. M. G. van Bommel, D. J. A. Crommelin and W. E. Hennink, Pharm. Res., 1998, 15, 557–561 CrossRef CAS.
- T. Thorsen, R. W. Roberts, F. H. Arnold and S. R. Quake, Phys. Rev. Lett., 2001, 86, 4163–4166 CrossRef CAS.
- S. Xu, Z. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin and G. M. Whitesides, Angew. Chem., Int. Ed., 2005, 44, 3799–3799 CrossRef CAS.
- B. G. De Geest, J. P. Urbanski, T. Thorsen, J. Demeester and S. C. De Smedt, Langmuir, 2005, 21, 10275–10279 CrossRef.
- M. T. Gokmen, B. G. De Geest, W. E. Hennink and F. E. Du Prez, ACS Appl. Mater. Interfaces, 2009, 1, 1196–1202 Search PubMed.
- K. Olbrich, W. Rawicz, D. Needham and E. Evans, Biophys. J., 2000, 79, 321–327 CrossRef CAS.
- W. Rawicz, K. C. Olbrich, T. McIntosh, D. Needham and E. Evans, Biophys. J., 2000, 79, 328–339 CrossRef CAS.
- S. R. Van Tomme, C. F. Van Nostrum, S. C. De Smedt and W. E. Hennink, Biomaterials, 2006, 27, 4141–4148 CrossRef CAS.
- S. R. Van Tomme, M. J. van Steenbergen, S. C. De Smedt, C. F. van Nostrum and W. E. Hennink, Biomaterials, 2005, 26, 2129–2135 CrossRef.
- P. F. Kiser, G. Wilson and D. Needham, Nature, 1998, 394, 459–462 CrossRef CAS.
- P. F. Kiser, G. Wilson and D. Needham, J. Controlled Release, 2000, 68, 9–22 CrossRef CAS.
- S. Buck, P. S. Pennefather, H. Y. Xue, J. Grant, Y. L. Cheng and C. J. Allen, Biomacromolecules, 2004, 5, 2230–2237 CrossRef CAS.
- C. C. Ng, Y. L. Cheng and P. S. Pennefather, Macromolecules, 2001, 34, 5759–5765 CrossRef CAS.
- T. Jin, P. Pennefather and P. I. Lee, FEBS Lett., 1996, 397, 70–74 CrossRef CAS.
- S. Mornet, O. Lambert, E. Duguet and A. Brisson, Nano Lett., 2005, 5, 281–285 CrossRef CAS.
- A. L. Troutier and C. Ladaviere, Adv. Colloid Interface Sci., 2007, 133, 1–21 CrossRef CAS.
- A. L. Troutier, L. Veron, T. Delair, C. Pichot and C. Ladaviere, Langmuir, 2005, 21, 9901–9910 CrossRef CAS.
- A. L. Troutier, T. Delair, C. Pichot and C. Ladaviere, Langmuir, 2005, 21, 1305–1313 CrossRef CAS.
- Z. Y. Tang, Y. Wang, P. Podsiadlo and N. A. Kotov, Adv. Mater., 2007, 19, 906–906 CrossRef.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- G. Decher and J. D. Hong, Makromol. Chem., Macromol. Symp., 1991, 46, 321–327 Search PubMed.
- G. Decher and J. D. Hong, Ber. Bunsen-Ges. Phys. Chem., 1991, 95, 1430–1434 CAS.
- G. Decher, J. D. Hong and J. Schmitt, Thin Solid Films, 1992, 210–211, 831–835 CrossRef.
- G. Decher and J. Schlenoff, Multilayer Thin Films, Wiley-VCH, Weinheim, 2002 Search PubMed.
- G. B. Sukhorukov, E. Donath, H. Lichtenfeld, E. Knippel, M. Knippel, A. Budde and H. Mohwald, Colloids Surf., A, 1998, 137, 253–266 CrossRef.
- S. R. Van Tomme, C. F. van Nostrum, S. C. de Smedt and W. E. Hennink, Biomaterials, 2006, 27, 4141–4148 CrossRef CAS.
- B. G. De Geest, C. Dejugnat, G. B. Sukhorukov, K. Braeckmans, S. C. De Smedt and J. Demeester, Adv. Mater., 2005, 17, 2357–2361 CrossRef CAS.
- B. G. De Geest, C. Dejugnat, E. Verhoeven, G. B. Sukhorukov, A. M. Jonas, J. Plain, J. Demeester and S. C. De Smedt, J. Controlled Release, 2006, 116, 159–169 CrossRef CAS.
- R. A. McAloney, V. Dudnik and M. C. Goh, Langmuir, 2003, 19, 3947–3952 CrossRef CAS.
- R. A. McAloney, M. Sinyor, V. Dudnik and M. C. Goh, Langmuir, 2001, 17, 6655–6663 CrossRef CAS.
- A. A. Antipov and G. B. Sukhorukov, Adv. Colloid Interface Sci., 2004, 111, 49–61 CrossRef CAS.
- A. A. Antipov, G. B. Sukhorukov, S. Leporatti, I. L. Radtchenko, E. Donath and H. Mohwald, Colloids Surf., A, 2002, 198–200, 535–541 CrossRef CAS.
- B. G. De Geest, C. Dejugnat, M. Prevot, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Adv. Funct. Mater., 2007, 17, 531–537 CrossRef CAS.
- A. Birkett, K. Lyons, A. Schmidt, D. Boyd, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle and E. Nardin, Infect. Immun., 2002, 70, 6860–6870 CrossRef CAS.
- B. G. De Geest, R. E. Vandenbroucke, A. M. Guenther, G. B. Sukhorukov, W. E. Hennink, N. N. Sanders, J. Demeester and S. C. De Smedt, Adv. Mater., 2006, 18, 1005 CrossRef.
- S. De Koker, B. G. De Geest, C. Cuvelier, L. Ferdinande, W. Deckers, W. E. Hennink, S. C. De Smedt and N. Mertens, Adv. Funct. Mater., 2007, 17, 3754–3763 CrossRef CAS.
- J. A. Cadee, M. J. A. van Luyn, L. A. Brouwer, J. A. Plantinga, P. B. van Wachem, C. J. de Groot, W. den Otter and W. E. Hennink, J. Biomed. Mater. Res., 2000, 50, 397–404 CrossRef CAS.
- S. De Koker, B. G. De Geest, C. Cuvelier, L. Ferdinande, W. Deckers, W. E. Hennink, S. De Smedt and N. Mertens, Adv. Funct. Mater., 2007, 17, 3754–3763 CrossRef CAS.
- I. Pastoriza-Santos, B. Scholer and F. Caruso, Adv. Funct. Mater., 2001, 11, 122–128 CrossRef CAS.
- H. G. Zhu and M. J. McShane, Langmuir, 2005, 21, 424–430 CrossRef CAS.
- B. G. De Geest, M. J. McShane, J. Demeester, S. C. De Smedt and W. E. Hennink, J. Am. Chem. Soc., 2008, 130, 14480 CrossRef.
- M. Bedard, B. G. De Geest, H. Mohwald, G. B. Sukhorukov and A. Skirtach, Soft Matter, 2009, 5, 3927 RSC.
- B. G. De Geest, S. De Koker, K. Immesoete, J. Demeester, S. C. De Smedt and W. E. Hennink, Adv. Mater., 2008, 20, 3687 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111–1114 CrossRef CAS.
- E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Mohwald, Angew. Chem., Int. Ed., 1998, 37, 2202–2205 CrossRef.
- G. B. Sukhorukov, E. Donath, S. Davis, H. Lichtenfeld, F. Caruso, V. I. Popov and H. Mohwald, Polym. Adv. Technol., 1998, 9, 759–767 CrossRef CAS.
- B. G. De Geest, N. N. Sanders, G. B. Sukhorukov, J. Demeester and S. C. De Smedt, Chem. Soc. Rev., 2007, 36, 636–649 RSC.
- A. I. Petrov, D. V. Volodkin and G. B. Sukhorukov, Biotechnol. Prog., 2005, 21, 918–925 CrossRef CAS.
- D. V. Volodkin, N. I. Larionova and G. B. Sukhorukov, Biomacromolecules, 2004, 5, 1962–1972 CrossRef CAS.
- D. V. Volodkin, A. I. Petrov, M. Prevot and G. B. Sukhorukov, Langmuir, 2004, 20, 3398–3406 CrossRef CAS.
- R. De Rose, A. N. Zelikin, A. P. R. Johnston, A. Sexton, S. F. Chong, C. Cortez, W. Mulholland, F. Caruso and S. J. Kent, Adv. Mater., 2008, 20, 4698 CrossRef CAS.
- O. E. Selina, S. Y. Belov, N. N. Vlasova, V. I. Balysheva, A. I. Churin, A. Bartkoviak, G. B. Sukhorukov and E. A. Markvicheva, Russ. J. Bioorg. Chem., 2009, 35, 103–110 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
