Polycarbazoles for plastic electronics
Pierre-Luc T.
Boudreault
,
Serge
Beaupré
and
Mario
Leclerc
*
Canada Research Chair on Electroactive and Photoactive Polymers, Department of Chemistry, Université Laval, Quebec City, Quebec, Canada G1V 0A6. E-mail: mario.leclerc@chm.ulaval.ca
First published on 25th November 2009
Abstract
This Review covers recent progress made in the synthesis of carbazole-based monomers and polymers and their applications in plastic electronics. That includes the first photoactive carbazole-containing polymer, namely PVK, conjugated poly(3,6-carbazole)s, and finally the latest developments in poly(2,7-carbazole) derivatives. We will mainly discuss photovoltaic cell applications because of the impressive performances that have been obtained in the past couple of years. Semi-ladder poly(indolo[3,2-b]carbazole)s that have recently shown interesting features will also be presented.
 Pierre-Luc T. Boudreault | Pierre-Luc Boudreault received his BSc in chemistry from Université Laval in 2005. He then joined the group of Professor Mario Leclerc to pursue a PhD in chemistry where he is a fourth-year graduate student. Meanwhile, he received two important scholarships: the FQRNT graduate scholarship (Doctorate B2) in 2007 and since then the NSERC (BESC S) scholarship. During his graduate studies, he spent 6 months in the laboratories of Professor Zhenan Bao as a visiting scholar at Stanford University. His research interests include the study of new indolo[3,2-b]carbazole and carbazole-based oligomers and polymers for applications in organic field-effect transistors and photovoltaic cells. |
 Serge Beaupré | Serge Beaupré received his BSc in chemistry from Université de Montréal in 1997. He then joined the group of Professor Mario Leclerc and worked on new polyfluorene derivatives for applications in polymeric light-emitting diodes. He received a Masters degree in chemistry in 1999 and a PhD degree in chemistry in 2004 both from Université Laval. He then joined the Laboratory of electroactive and photoactive polymers as research associate and project leader. His research interests include the synthesis and characterization of new poly(2,7-fluorene) and poly(2,7-carbazole) derivatives for applications in electro-optical devices such as polymeric light-emitting diodes, electrochromic devices, and photovoltaic cells. |
 Mario Leclerc | Mario Leclerc was awarded a PhD in chemistry from Université Laval, in 1987, under the guidance of Prof. R.E. Prud’homme. After a short post-doctoral stay at INRS-Energie et Matériaux near Montréal with professor L.H. Dao, he joined the Max-Planck-Institute for Polymer Research, Germany, as a post-doctoral fellow in the research group of Prof. Dr G. Wegner. In 1989, he accepted a position of professor at the department of chemistry of Université de Montréal. He returned to Université Laval in 1998 where he has held the Canada Research Chair on Electroactive and Photoactive Polymers since 2001. He has co-authored 200 papers published in leading scientific journals which have been cited more than 7500 times. According to Science Citation Index, he has a H-index of 50. His current research activities include the synthesis and characterization of new conjugated oligomers and polymers for applications in micro- and nano-electronics, electro-optics, genomics, and proteomics. |
1 Introduction
Since the discovery of high electrical conductivity in doped polyacetylene,1 the search for new conjugated polymers (CPs) has never stopped.2,3 They exhibit never-seen-before properties that could lead to many applications. For instance, sensors,4 light-emitting diodes (LEDs),5 field-effect transistors (FETs),6 and photovoltaic cells (PCs)7,8 have all been carefully studied by many academic and industrial researchers. Because of the insolubility and relative instability of polyacetylene,3 many other classes of CPs rapidly emerged. Polyaniline (PAni)9 and polypyrrole (PPy)10 were among the first ones to attract interest. These nitrogen-containing conjugated polymers became very popular because of the possibility to polymerize them from different electrochemical and chemical methods. Because of its great versatility, chemical oxidation seems to be the most efficient method to produce these polymers in a multi-gram scale.11 Other developments have led to new processable polymers such as poly(2,5-thiophene)s,12poly(1,4-phenylene)s,13 and poly(2,7-fluorene)s.14,15 These polymeric materials are particularly promising for the development of the so-called plastic electronics (organic light-emitting diodes, solar cells, transistors, etc.)Interestingly, an organic field-effect transistor is very analogous to its inorganic counterpart: in a three-electrode device, a voltage is applied to the gate controlling the current floating between the source and drain electrodes.16 More precisely, when a voltage is applied at the gate electrode, there is an accumulation of charges at the dielectric/organic interface. Since there is a voltage applied between the source and drain, the measured current will depend on how effectively the charges circulate in the organic semiconductor.
On the other hand, polymeric light-emitting diodes are made from a positive hole-injecting electrode (usually transparent) with a high work function such as indium tin oxide (ITO) or a conducting polymer, a negative electron-injecting electrode with a low work function such as Al, In, Mg, or Ca, and the light-emitting polymer film sandwiched between these two electrodes. In this layered structure, the injected holes and electrons migrate across the polymer layer, combine to form excitons, which then decay with photon emission. Depending on the bandgap of the polymer, different colors can be obtained.17
Finally, organic photovoltaic cells are often fabricated via the bulk heterojunction (BHJ) architecture.18 The fundamental BHJ concept involves self-assembly of nano-scale heterojunction by spontaneous phase separation of the polymeric electron donor and fullereneelectron acceptor (usually [6,6]-phenyl C61 butyric methyl ester (PCBM)). There are several challenges in obtaining efficient photovoltaic cells with high power conversion efficiencies (PCEs). For instance, it is important to develop polymers with a LUMO level of about −3.8 to −4.0 eV while keeping the bandgap between 1.2 and 1.9 eV. These parameters are expected to maximize the open circuit voltage while keeping a good overlap with the emission spectrum of the sun, a good electron transfer to PCBM, and a good air-stability. Mobilities of about 10−3 cm2 V−1 s−1 are also required.19
Up to now, the most studied material is certainly regioregular poly(3-hexylthiophene) (rr-P3HT) because of its remarkable performances as the active layer in FET and PC applications. For instance, in FET applications, rr-P3HT has been the most promising material for a long period of time, with hole-mobility of 0.1 cm2 V−1 s−1,20 before being recently surpassed by some new polythiophene derivatives.21 In polymeric PCs, a PCE of 5% has been achieved with rr-P3HT.8 Even though a great number of industrial and academic research laboratories have worked on the optimization of these performances, the intrinsic limits of this particular material have probably been reached. Indeed, the relatively high bandgap (∼1.9 eV) limits the fraction of the solar spectrum that can be harvested and the small energy difference between the HOMO of P3HT and LUMO of the PCBM results in a relatively low open-circuit voltage (VOC ≈ 0.6 V).18 To allow PCEs higher than 6%, these fundamental parameters (polymer bandgap and HOMO energy level) must be decreased.
Along these lines, a new class of polymers has been developed to try to combine the promising properties of PAni, PPy and that of polyfluorenes, namely polycarbazoles. In spite of some structural similarities with PAni and PPy, optical and electrical properties of polycarbazoles are completely different from these two polymers. This is one of the reasons why carbazole has been greatly studied over the past decade and still is today. Also, 9H-carbazole is a very cheap starting material and its fully aromatic character provides a good environmental stability. Moreover, carbazole can be substituted or polymerized either at the 3- and 6- positions or 2- and 7- positions and a wide variety of alkyl and aryl chains can be added on the nitrogen atom without altering the planar conformation of the resulting polymers.22 For instance, in the case of PPy, the addition of chains on the nitrogen will create torsion of the main chain which hinders the packing of the polymer chain in three dimensions. The same rule applies for substituted polyanilines.23
Throughout this Review, we will describe the synthesis of different homopolymers and mostly copolymers based on the carbazole unit.24 The polymerization techniques will be described thoroughly to give an understanding of why certain strategies are suitable and others are not. We will mainly focus on low bandgap polymers for PC applications that have been synthesized recently. However, we will also briefly discuss several other polymers that have been synthesized for PLED and FET applications since a high mobility is an important parameter for all plastic electronic applications. As an extension of carbazole-based materials, indolo[3,2-b]carbazoles have also been developed and we will also describe these new electroactive and photoactive carbazole derivatives. In addition, we will discuss the synthesis of the monomers since it is necessary to produce them at a very low cost, efficiently, and with a high purity level to enable the commercialization of the corresponding polymers.25
2 Polycarbazoles
Polycarbazoles have now been studied for more than 30 years.22 First studies were devoted to the remarkable photoconducting properties of poly(N-vinylcarbazole) (PVK).26 Since then, it has been used as a photoconductive polymer in photocopiers.27 Moreover, recent progress has shown that PVK could be very useful as the host material in white organic light-emitting diodes, with a combination of other layers, because of its high energy blue-emissive singlet excited state and the absence of low energy triplet state.28 Moreover, PVK has also been used in dye-sensitized solar cells blended with TiO2 as the electron donor and hole transporting material.29 Because PVK is not a conjugated polymer, charge transport occurs viaradical cation hopping among the discrete carbazole units.302.1 Poly(3,6-carbazole)s
After the synthesis and commercialization of PVK,31 new carbazole-based conjugated materials have been synthesized. In this regard, 3,6-disubstituted carbazoles were particularly investigated. These monomers are easily obtained from 9H-carbazole; indeed, because of very high reactivity at the 3- and 6- positions, the carbazole unit can be directly brominated with N-bromosuccinimide (NBS).22 A wide variety of side chains can also be added on the nitrogen atoms (9H- position) to provide a much better solubility in common solvents and to protect the reactive secondary amine at the 9-position. The carbazole framework can be polymerized by electrochemical or chemical oxidation.32Several other pathways have been used over the years to synthesize poly(3,6-carbazole)s like reductive polymerization of 3,6-dihalocarbazole from Grignard,33 electrochemical,34 and chemical palladium- or nickel-catalyzed35 coupling reactions. The solubility and the processability of the polymeric materials clearly depend upon the nature and length of the side chains on the nitrogen atom. Long and bulky side chains combined with the well-known Ni(0)-catalyzed Yamamoto reductive homocoupling36 led to higher molecular weights (MW of 105). Lower molecular weights (MW around 104) were obtained by using shorter side chains.
Unfortunately, the performances of 3,6-carbazole-based polymers did not meet the requirements for several opto-electronic applications. Some problems with these materials seemed recurrent such as relatively low molecular weights and poor conjugation of the electrons within the backbone. As shown in Scheme 1, it was demonstrated that the oxidation of the material leads to the delocalization of the π electrons only on two monomeric units.37 This is one of the main drawbacks of these materials for FET devices and other applications that require high conductivity and hole (or electron) mobility. However, 3,6-carbazole-based polymers showed interesting performances in LEDs because of a relatively good hole transport. The first device using a poly(3,6-carbazole) was fabricated in 1996.38Homopolymers from 3,6-carbazoles mostly led to blue light emission because of a short conjugation length due to the high dihedral angle between two adjacent carbazole monomer.39 Most of the studies have focused on the blue light-emission but few studies investigated lower bandgap polymers that emit yellow, green, or red light.40
Studies involving 3,6-carbazole-based materials for solar cell applications are also relatively rare and do not show high PCEs. Early results in 2000 by Jenekhe et al. have shown that using a Schottky barrier type PC, ITO/copolymer (thickness of 50–100 nm)/Al allowed PCE of 0.1–0.2% which are very similar with many single-layer conjugated polymer solar cells.41 Other low bandgap polymers (1.8 to 2.0 eV) have been synthesized lately by Li and co-workers.42 Solar cells should be fabricated in further studies to see the true potential of such 3,6-carbazole-based copolymers.
2.2 Poly(2,7-carbazole)s
Poly(2,7-carbazole)s were reported later on because their preparation is not as straightforward as the synthesis of poly(3,6-carbazole)s. Indeed, functional groups cannot be directly added at the 2,7-positions of the carbazole unit. In order to synthesize the desired monomers, several synthetic strategies have been developed over the years. All these pathways will be described in the following sections, numbering their pros and cons.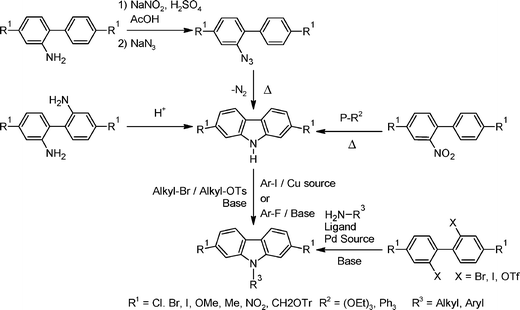 | ||
| Scheme 2 Synthesis pathways leading to 2,7-functionalized carbazoles. | ||
The first synthesis of 2,7-functionalized carbazoles was performed in 1951 by Smith and Brown.43 The functional groups on the aminobiphenyl derivatives are necessary to obtain the targeted compound. The two main steps of this pathway are the formation of the azide compound from the amine group which is converted into a diazonium salt . The remaining step is the ring-closure reaction with the azide as the starting compound. In 2001, Morin and Leclerc utilized this azide strategy (Scheme 3);24 starting from a dinitroaminobiphenyl derivative (4); they have been able to prepare a soluble 2,7-dihalogenocarbazole derivative (9) in five steps, a very useful derivative for the synthesis of conjugated polymers by palladium-catalyzed cross-coupling reaction.
 | ||
| Scheme 3 Synthesis of 2,7-diiodocarbazole through the azide strategy. | ||
Leditschke reported in 1953 that 2,7-disubstituted carbazoles can be directly obtained from 2,2′-diaminobiphenyl by treating it with phosphoric acid at 200 °C with yields of around 80% depending on the substituents on the backbone.44 This synthesis was optimized by using other proton sources, such as a Nafion-H membrane, which helped to improve the yields up to 87–93%.45 In 1965, a very useful ring-closure reaction was developed by Cadogan.46 The fact that this reaction is directly performed on the 2-nitrobiphenyl derivatives is a great advantage. The starting compound just has to be treated in triethylphosphite (P(OEt)3) and heated at high temperatures (140–150 °C) for 2 to 4 h. The relatively low yields (around 50–60%) are mainly due to the formation of N-ethylcarbazole during the reaction. The optimization of this reaction has attracted much attention and interesting avenues were found by using microwave synthesis47 or by treating the starting compound with triphenylphosphine (PPh3) instead of P(OEt)3 which led to high yields (70–90%).48 Since its discovery, the Cadogan ring-closure has been widely used because it allows 2,7-carbazole derivatives in only 2 or 3 easy steps from commercially available starting compounds. One particular nice example has been developed by Müllen et al. and involves two simple steps from a commercially available starting material, i.e.4,4′-dibromobiphenyl (Scheme 4).24,49,50
 | ||
| Scheme 4 Simple and rapid synthesis of 2,7-dibromocarbazole. | ||
 | ||
| Scheme 5 Blue-light-emitting poly(2,7-carbazole) derivatives. | ||
To obtain a highly efficient blue PLED, the polymer needs to have a large bandgap, good air stability, good and balanced charge injection and charge transport. The advantage of carbazole over fluorene or other heterofluorene derivatives is that the nitrogen atom provides a lone electron pair that gives a fully aromatic structure and therefore, a better stability.14,57 Preliminary data were obtained from homopolymers with different side chain on the nitrogen atom. They exhibit blue fluorescence (415–450 nm) with relatively high quantum yields.45,58Polymer13-A has a low molecular weight and poor solubility and was therefore difficult to process. To solve this problem, several alkyl chains were developed and added to the nitrogen atom of the carbazole (13-B to E).58–61 Bulky side chains (13-C, E) and aryl side chains (13-F to K)60,62,63 improved considerably the solubility of the materials and make them promising for PLED applications. Using this strategy, the solid state quantum yield was increased up to 0.40 with strong blue emission.63 In parallel, copolymers have also been developed for blue light-emission (14).64 In order to avoid cross-linking at the 3- and 6-positions,55,65 they were protected with methyl or nitrile group.66 Interesting performances have been obtained with these polymers but especially for polymers13-H, 13-K and 15 with blue electroluminescence up to 1500 cd m−2 at 10 V.67
| Polymer | Eg/eV b | JSC/mA cm−2c | VOC/V d | FF e | η e (%)f | Reference |
|---|---|---|---|---|---|---|
| a Blended with PDI as the acceptor. b Solid state optical band gap. c Short-circuit current. d Open-circuit voltage. e Fill factor. f Power conversion efficiency. | ||||||
| 13-E a | 3.00 | 0.23 | 0.71 | 0.37 | 0.6 | 61 |
| 16 | 2.30 | 0.89 | 0.85 | 0.46 | 0.4 | 69 |
| 17 | 2.00 | 0.76 | 0.50 | 0.43 | 0.2 | 69 |
| 18 | 2.20 | 1.1 | 0.70 | 0.45 | 0.4 | 69 |
| 19 | 2.10 | 0.86 | 0.55 | 0.48 | 0.3 | 69 |
| 20 | 1.70 | 1.6 | 0.80 | 0.55 | 0.8 | 69 |
| 21 | 2.02 | 3.0 | 0.95 | 0.56 | 1.8 | 70 |
| 22 | 1.89 | 2.6 | 0.90 | 0.44 | 1.1 | 70 |
| 23 -A | 1.88 | 6.9 | 0.90 | 0.53 | 3.6 | 71 |
| 23 -B | 1.88 | 10.7 | 0.91 | 0.59 | 5.7 | 72 |
| 23 -C | 1.88 | 10.6 | 0.88 | 0.66 | 6.1 | 18 |
| 24 | 1.75 | 2.9 | 0.71 | 0.32 | 0.7 | 70 |
| 25 | 1.87 | 3.7 | 0.96 | 0.60 | 2.4 | 70 |
| 26 | 1.67 | 1.4 | 0.85 | 0.60 | 0.8 | 70 |
| 27 | 1.10 | 5.2 | 0.41 | 0.29 | 0.6 | 73 |
| 28 -A | 1.57 | 5.2 | 0.85 | 0.37 | 1.6 | 74 |
| 28 -B | 1.63 | 5.4 | 0.76 | 0.56 | 2.3 | 75 |
In the meantime, 2,7-carbazole-based copolymers with lower bandgaps were synthesized by Leclerc and co-workers.69 As shown in Scheme 6, copolymers16–19 were synthesized via a Horner-Emmons reaction76 (copolymer20 was obtained by Stille coupling). Interestingly, this polymerization reaction is performed without any metal-containing catalysts. The purification of the resulting polymers is then much easier. Even though the bandgaps of the polymers were in a good range (1.7 to 2.3 eV), the device performances were very poor (up to 0.8%). That can be explained by the low molecular weight as well as the poor solubility of these copolymers.
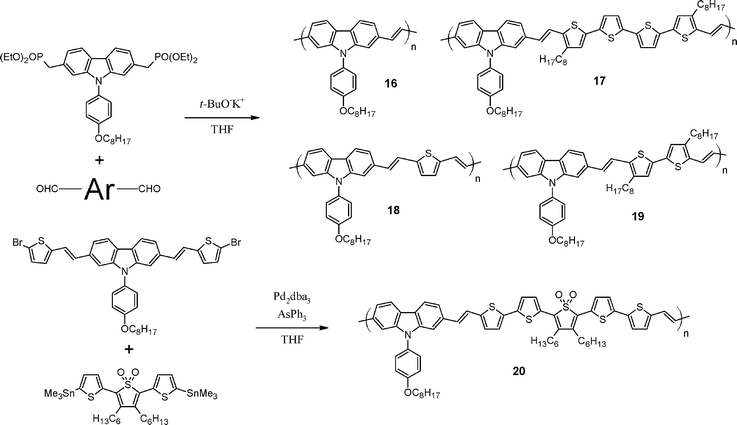 | ||
| Scheme 6 Synthesis pathways for poly(2,7-carbazolenevinylene) derivatives.69 | ||
Keeping in mind these first disappointing results (Table 1), new low bandgap polymers were developed. First, these new polymers had to be more soluble in common solvents. A new double alkyl side chain (see homopolymer13-C) has been used for the synthesis of these new polymers. The combination of an electron donor (such as carbazole) and an electron acceptor such as units 21 to 26 (Scheme 7) gave low bandgap polymers with better processability.
 | ||
| Scheme 7 Structure and synthesis of some 2,7-carbazole-based low bandgap polymers. | ||
Through optimized Suzuki cross-coupling polymerization, high molecular weights were obtained with polymers21, 23, and 25. However, polymers22, 24, and 26 showed only low molecular weights.70 Metal analyses indicated that the pyrazine-containing copolymers trap palladium particles which could explain their lower molecular weights. The complete study of these polymers has demonstrated that PCDTBT (23) has a lot more potential than the other materials. With a hole mobility of 3 × 10−3 cm2 V−1 s−1 and an optical bandgap of 1.9 eV, the initial power conversion efficiency was 3.6%.71 Numerous parameters were studied such as the effect of the molecular weight, the solution concentration during the processing of the film, the donor: acceptor ratio, the thickness of the active layer as well as the nature of the acceptor (PCBMC60 or C70). These studies allowed an improvement of the power conversion efficiency up to 5.7%.72,77 Further optimizations by Heeger and co-workers led to a PCE of 6.1% which is one of the best results reported for organic PCs so far.18 Moreover, this particular material also demonstrated a remarkable stability and good field-effect mobility. A hole mobility of 0.02 cm2 V−1 s−1) was obtained and the performances were stable up to 150 °C under ambient conditions and up to 350 °C in a nitrogen atmosphere.78
Recently, other new copolymers have also shown some promises for PC applications. First, Iraqi and co-workers73 have reported copolymers based on 6,7-diphenyl-4,9-bis-(thiophen-2-yl)-[1,2,5]thiadiazolo[3,4-g]quinoxaline79 and 2,7-carbazole (27) (Scheme 8). These materials have a low bandgap (1.1 to 1.3 eV) but show poor PCE (0.61%).73 These results may be due to the small difference between the HOMO of the copolymers (4.8 eV) and the LUMO of the PCBM (3.8 to 4.3 eV) which is one of the reason why the VOC of the device is so low (≈ 0.4 V).
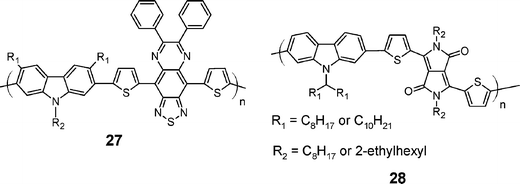 | ||
| Scheme 8 Chemical structures of newly synthesized low bandgap 2,7-carbazole-based copolymers. | ||
Later on, another class of comonomers has been used, namely diketopyrrolopyrrole (DPP),80 and also resulted in low bandgap copolymers (see polymers28).74,81 These copolymers exhibit good mechanical properties mainly because of their relatively high molecular weights (Mn of 30 kDa). Field-effect transistors were fabricated from these polymers because of previous interesting results with this type of structure.82 The field-effect mobility obtained was one of the best obtained with carbazole-based polymers (μh = 0.02 cm2 V−1 s−1) together with an on/off ratio of 1.4 × 106 and a threshold voltage of −16 V.74 Used in PCs, initial results for 28 showed a PCE of 1.6%, but the performances rapidly reached 2.26% by optimizing the alkyl chains on both the carbazole unit (n–C10H21) and the DPP core (2-ethylhexyl).75
3 Polyindolo[3,2-b]carbazoles
Other carbazole derivatives with an extended conjugated backbone have also been explored. For example, diindolocarbazoles54,83 and bisindenocarbazoles84 have been synthesized to reproduce the structure of pentacene but with a better stability and processability. Unfortunately, performances of these molecules and related polymers did not meet the expectations.85 Many problems with these materials are difficult to overcome such as the long and tedious synthesis of the monomers. However, interesting transport properties were obtained with indolo[3,2-b]carbazoles (ICs). Materials based on this structure usually demonstrate high crystallinity.3.1 Synthesis
There are several pathways for synthesizing ICs that usually require multi-step procedures. Among them, two different approaches are shown in Scheme 9. The first one was developed on the basis of a Cadogan ring-closure (which was discussed above). The first strategy that was developed involved a nine-step synthetic pathway to obtain compound 29 with the final step being a single Cadogan ring-closure.86Methylprotecting groups were crucial in order to obtain the desired regioisomer. A more rapid synthesis was developed starting from compound 30 and involved a double Cadogan ring-closure. The main problem with this reaction is that it is not regioselective which means that several regioisomers can be obtained. Therefore, the yields for this reaction is very low (6–8%)85 but still provides the IC framework very rapidly compared to the pathway mentioned before.86 More recently, Kawaguchi et al. also synthesized different IC derivatives using a palladium-catalyzed double N-arylation (starting from compound 31) as depicted in Scheme 9.87 Even though this method allows the synthesis of many different ICs and carbazoles (as described above), it is somewhat limited regarding the functional groups that can be tolerated during the ring-closure reaction. | ||
| Scheme 9 Synthesis of ICs viaCadogan ring-closure and double N-arylation. | ||
The most common way to synthesize efficiently ICs is via double Fischer indolisation of cyclohexane-1,4-dione bis(phenylhydrazone) in strong acidic conditions (H2SO4 in AcOH), which was developed by Robinson in 1963.88 A wide variety of IC-based molecules have been obtained through the indolisation reaction with various functional groups.89 For example, 2,8-dimethoxy-5,11-diBOC-indolo[3,2-b]carbazole was obtained in a 20% yield and 2,8-difluoro-5,11-dihydroindolo[3,2-b]carbazole was synthesized with yields from 26 to 48%. Despite the low yields, the two-step Fischer indolisation reaction (Scheme 10) is still the most common method to synthesize indolo[3,2-b]carbazoles.
 | ||
| Scheme 10 Synthesis of ICs via the Fischer indolisation reaction. | ||
3.2 Polyindolo[3,2-b]carbazole derivatives for PCs
Because of their chemical structure, it was thought that the most suitable application for IC-based materials would be FETs. In this regard, good performances were indeed obtained.90 With a hole mobility of 0.14 cm2 V−1 s−1 for 2,8-dichloro-5,11-didodecylindolo[3,2-b]carbazole, there was great deal of interest that came out for this class of materials. Subsequently, several research groups investigated other type of IC derivatives and the best performances were obtained in 2007 and 2009 by Leclerc and co-workers with two materials (Scheme 11) with very similar structures but completely different thin film organizations.91 Hole mobilities of up to 0.22 cm2V−1 s−1 were achieved with compound 35.![3,9-Disubstituted indolo[3,2-b]carbazole for FET applications.](/image/article/2010/PY/b9py00236g/b9py00236g-s11.gif) | ||
| Scheme 11 3,9-Disubstituted indolo[3,2-b]carbazole for FET applications. | ||
At the same time, IC-based polymers have also attracted a lot of interest.85,92 Even if there has been some progress during the past few years, it is still very difficult to obtain good performances with this type of material for numerous reasons (low molecular weights, low solubility, and poor processability). Because of the necessity to add bulky side chains on the nitrogen atoms to obtain high molecular weights, IC-based polymers will probably not reach good performances in FETs. Nevertheless, the hole mobility of these type of polymers is high enough to think that new derivatives could be developed for PC applications. A few groups have investigated this avenue and were able to generate some interesting and promising results. In 2008, Lu et al. were able to polymerize, viaSuzuki cross-coupling, the 3,9-diboronate IC unit with 4,7-bis(3,4′-dioctyl-2,2′-bithiophen-5-yl)-2,1,3-benzothiadiazole (DTBT) to afford polymer36 (Scheme 12) with a number-average molecular weight (Mn) of 19.5 kDa. As mentioned above, the use of long bulky side chains was necessary to obtain a good molecular weight. Solar cells were fabricated via a BHJ architecture blended with PCBM (C60) with a PCE as high as 3.6%, a VOC = 0.69 V, JSC = 9.17 mA cm−2, and a FF = 0.57.93 Moreover, Tsai et al. synthesized several new 2,8- and 3,9-IC-based copolymers using a similar strategy.94 Unfortunately, the devices suffer from the low molecular weights of the polymers. The best performances were obtained with polymer37 (2,8-disubstituted IC) (Scheme 12) which exhibited a weight-average molecular weight (Mw) of 10.6 kDa. The best PCE value is 1.4% when blended with PCBM C70. The relatively low performances could be due to the low JSC (4.5 mA cm−2) which can, in part, be linked to the relatively low hole mobility of the polymer (1 × 10−4 cm2 V−1s −1).
![Indolo[3,2-b]carbazole-based low bandgap polymers.](/image/article/2010/PY/b9py00236g/b9py00236g-s12.gif) | ||
| Scheme 12 Indolo[3,2-b]carbazole-based low bandgap polymers. | ||
Finally, it is worth noting recent developments in the synthesis of ladder and semi-ladder carbazole-based conjugated polymers.95 These polymers are processable and air-stable and seem to exhibit the required electronic properties to make interesting photovoltaic devices. Consequently, new developments should come through from this promising class of conjugated polymers.
4 Concluding remarks
All results presented above witness of the strong research activities performed by both academic and industrial laboratories on polycarbazoles. Because of the latest discoveries with poly(2,7-carbazole) derivatives, it is clear that the interest in this field will be growing in the coming years and hopefully, the performances will go in the same direction. Interestingly, during the revision of this manuscript, Inganäs and co-workers reported a new poly(2,7-carbazole) derivative that gives a power conversion efficiency of 5.4% when blended with PCBM C70.96 These authors believe that further improvements in the solar cell configurations should lead to a PCE higher than 6%. Moreover, completely new carbazole-based polymers such as poly(1,8-carbazole)s97 and 3,9- or 2,9-linked polycarbazoles98 should soon be tested in different electronic devices and might pave the way for new exciting developments for polycarbazoles. Ladder-like structures also generated a lot of attention and are suitable candidates for extensive structure-property studies.91 Keeping all this in mind, we foresee great developments for polycarbazoles. This could be especially true for the development of the solar cells of tomorrow.Acknowledgements
The authors wish to gratefully acknowledge Professor Jean-François Morin from Université Laval for fruitful discussions throughout the writing of this Review.References
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1977, 578 RSC.
- T. A. Skotheim and J. R. Reynolds, Handbook of Conducting Polymers, Taylor & Francis Group, Boca Raton, 2007 Search PubMed; H. Klauk, Organic Electronics, Wiley-VCH, Weinheim, 2006 Search PubMed.
- G. Hadziioannou and G. G. Malliaras, Semiconducting Polymers, Wiley-VCH, Weinheim, 2007 Search PubMed.
- M. Leclerc and K. Faid, Adv. Mater., 1997, 9, 1087–1094 CrossRef; D.T. McQuade, A.E. Pullen and T.M. Swager, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS.
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS.
- C. D. Dimitrakopoulos and P. R. L. Malenfant, Adv. Mater., 2002, 14, 99 CrossRef CAS; H. E. Katz, Chem. Mater., 2004, 16, 4748 CrossRef CAS.
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS; K. M. Coakley and M. D. McGehee, Chem. Mater., 2004, 16, 4533–4542 CrossRef CAS.
- H. Hoppe and N. S. Sariciftci, J. Mater. Res., 2004, 19, 1924 CAS.
- E. T. Kang, K. G. Neoh and K. L. Tan, Prog. Polym. Sci., 1998, 23, 277–324 CrossRef CAS; A. G. Green and A. E. Woodhead, J. Chem. Soc. Trans., 1910, 97, 2388 RSC; J. C. Chiang and A. G. MacDiarmid, Synth. Met., 1986, 13, 193 CrossRef CAS; A. G. MacDiarmid, J. C. Chiang, A. F. Richter and A. J. Epstein, Synth. Met., 1987, 18, 285 CrossRef CAS; C. R. Martin, Acc. Chem. Res., 1995, 28, 61 CrossRef CAS; J. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851 CrossRef CAS.
- J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS.
- J. Roncali, R. Garreau, A. Yassar, P. Marque, F. Garnier and M. Lemaire, J. Phys. Chem., 1987, 91, 6706 CrossRef CAS; M. Angelopoulos, G. E. Asturias, S. P. Ermer, A. Ray, E. M. Scherr, A. G. Macdiarmid, M. Akhtar, Z. Kiss and A. J. Epstein, Mol. Cryst. Liq. Cryst., 1988, 160, 151–163; J. Y. Lee, D. Y. Kim and C. Y. Kim, Synth. Met., 1995, 74, 103 CrossRef.
- J. Roncali, Chem. Rev., 1992, 92, 711–738 CrossRef CAS; R. D. McCullough and R. D. Lowe, J. Chem. Soc., Chem. Commun., 1992, 70 RSC; R. S. Loewe, S. M. Khersonsky and R. D. McCullough, Adv. Mater., 1999, 11, 250 CrossRef CAS.
- D. Y. Kim, H. N. Cho and C. Y. Kim, Prog. Polym. Sci., 2000, 25, 1089–1139 CrossRef CAS.
- U. Scherf and E. J. W. List, Adv. Mater., 2002, 14, 477–487 CrossRef CAS.
- M. Leclerc, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2867–2873 CrossRef CAS.
- C. Reese, M. Roberts, M.-M. Ling and Z. Bao, Mater. Today, 2004, 7, 20–27 CrossRef CAS.
- A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 402–428 CrossRef.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297–302 Search PubMed.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- H. Sirringhaus, N. Tessler and R. H. Friend, Science, 1998, 280, 1741 CrossRef CAS.
- I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. Macdonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, S. W. Zhang, M. L. Chabinyc, R. J. Kline, M. D. McGehee and M. F. Toney, Nat. Mater., 2006, 5, 328 CrossRef CAS; H. Pan, Y. Li, Y. Wu, P. Liu, B. S. Ong, S. Zhu and G. Xu, J. Am. Chem. Soc., 2007, 129, 4112–4113 CrossRef CAS.
- J.-F. Morin, M. Leclerc, D. Adès and A. Siove, Macromol. Rapid Commun., 2005, 26, 761–778 CrossRef CAS.
- L. H. Dao, M. Leclerc, J. Guay and J. W. Chevalier, Synth. Met., 1989, 29, 377–382 CrossRef.
- J. F. Morin and M. Leclerc, Macromolecules, 2001, 34, 4680–4682 CrossRef CAS.
- J. Huang, S. Virji, B. H. Weiller and B. H. Kaner, J. Am. Chem. Soc., 2003, 125, 314 CrossRef CAS.
- J. V. Grazulevicius, P. Strohriegl, J. Pielichowski and K. Pielichowski, Prog. Polym. Sci., 2003, 28, 1297 CrossRef CAS; J. H. Pearson and M. Stolka, Polymer Monographs, Gordon and Breach, New York, 1981 Search PubMed; H. Narmann and P. Strohriegl, Handbook of Polymer Synthesis, M. Dekker, New York, 1992 Search PubMed; P. M. Borsenberger and D. Weiss, Organic Photoreceptors for Xerography, M. Dekker, New York 1998 Search PubMed; W. E. Moerner and S. M. Silence, Chem. Rev., 1994, 94, 127–155 Search PubMed.
- G. Burton, J. Holman, J. Lazonby, G. Pilling and D. Waddington, in Chemical Storylines, 2000, pp. 121–122 Search PubMed.
- X. Tang, J. Yu, L. Li, W. Wen and Y. Jiang, Chin. J. Chem. Phys., 2008, 21, 510–514 CrossRef CAS; X. Tang, J. Yu, L. Li, L. Zhang and Y. Jiang, Displays, 2009, 30, 123–127 CrossRef CAS; H. Xia, M. Li, D. Lu, C. B. Zhang, W. J. Xie, X. D. Liu, B. Yang and Y. G. Ma, Adv. Funct. Mater., 2007, 17, 1757–1764 CrossRef CAS.
- J. Wagner, J. Pielichowski, A. Hinsch, K. Pielichowski, D. Bogdal, M. Pajda, S. S. Kurek and A. Burczyk, Synth. Met., 2004, 146, 159–165 CrossRef CAS; N. Ikeda and T. Miyasaka, Chem. Commun., 2005, 1886–1888 RSC; T. X. Lav, F. Tran-Van, F. Vidal, S. Péralta, C. Chevrot, D. Teyssié, J. V. Grazulevicius, V. Getautis, H. Derbal and J. M. Nunzi, Thin Solid Films, 2008, 516, 7223–7229 CrossRef CAS; C. Dridi, V. Barlier, H. Chaabane, J. Davenas and H. B. Ouada, Nanotechnology, 2008, 19, 375201 CrossRef; V. Barlier, V. Bounor-Legaré, G. Boiteux, J. Davenas, A. Slazak, A. Rybak and J. Jung, Synth. Met., 2009, 159, 508–512 CrossRef CAS.
- P. M. Borsenberger and D. S. Weiss, Organic Photoreceptors for Imaging Systems, Marcel Dekker, New York, 1993 Search PubMed.
- J. M. Pearson and M. Stolka, Poly (N-vinylcarbazole), Gordon and Breach, New York, 1981 Search PubMed.
- J. F. Ambrose and R. F. Nelson, J. Electrochem. Soc., 1968, 115, 1159 CAS; J. F. Ambrose, L. L. Carpenter and R. F. Nelson, J. Electrochem. Soc., 1975, 122, 876; P. Beresford, D. H. Iles, L. J. Kricka and A. Ledwith, J. Chem. Soc., Perkin Trans. 1, 1974, 276 RSC; A. Siove and D. Adès, Polymer, 2004, 45, 4045 CrossRef CAS.
- S. T. Wellinghoff, Z. Deng, J. F. Reed and J. Racchini, Polym. Prepr., 1984, 25, 238 CAS.
- A. Siove, D. Adès, C. Chevrot and G. Froyer, Makromol. Chem., 1989, 190, 1361 CrossRef CAS; A. Siove, D. Adès, E. Ngbilo and C. Chevrot, Synth. Met., 1990, 38, 331 CrossRef CAS; A. Siove, A. Aboulkassim, K. Faïd and D. Adès, Polym. Int., 1995, 37, 171 CrossRef CAS.
- U. Geissler, M. L. Hallensleben, A. Rienecker and N. Rohde, Polym. Adv. Technol., 1997, 8, 87 CrossRef CAS; A. Iraqi and I. Wataru, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 6041 CrossRef CAS.
- Z. B. Zhang, M. Fujiki, H.-Z. Tang, M. Motonaga and K. Torimistu, Macromolecules, 2002, 35, 1988 CrossRef CAS.
- X. Jiang, R. A. Register, K. A. Killeen, M. E. Thompson, F. Pschenitzka, T. R. Hebner and J. C. Sturm, J. Appl. Phys., 2002, 91, 6717–6724 CrossRef CAS.
- D. B. Romero, M. Schaer, M. Leclerc, D. Adès, A. Siove and L. Zuppiroli, Synth. Met., 1996, 80, 271 CrossRef CAS.
- K. Lmimouni, C. Legrand and A. Chapoton, Synth. Met., 1998, 97, 151–155 CrossRef CAS; Y. Yang and Q. Pei, Appl. Phys. Lett., 1997, 70, 1926–1928 CrossRef CAS.
- H.-J. Cho, D.-H. Hwang, J.-D. Lee, N.-S. Cho, S.-K. Lee, J. Lee, Y. K. Jung and H.-K. Shim, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 979–988 CrossRef CAS; H. K. Kim, M.-K. Ryu, K.-D. Kim, S.-M. Lee, S.-W. Cho and J.-W. Park, Macromolecules, 1998, 31, 1114–1123 CrossRef CAS; L. Ying, J. Zou, A. Zhang, B. Chen, W. Yang and Y. Cao, J. Organomet. Chem., 2009, 694, 2727–2734 CrossRef CAS.
- S. A. Jenekhe and S. Yi, Appl. Phys. Lett., 2000, 77, 2635–2637 CrossRef CAS.
- H. Chen, X. Cai, Z. Xu, T. Zhang, B. Song, Y. Li, Q. Jiang and M. Xie, Polym. Bull., 2008, 60, 581–590 CrossRef CAS.
- P. A. S. Smith and B. B. Brown, J. Am. Chem. Soc., 1951, 73, 2435–2437 CrossRef CAS; P. A. S. Smith and B. B. Brown, J. Am. Chem. Soc., 1951, 73, 2438–2441 CrossRef CAS.
- H. Leditschke, Chem. Ber., 1953, 86, 522–524 CrossRef CAS.
- T. Yamato, C. Hideshima, K. Suehiro, M. Tashiro, G. K. S. Prakash and G. A. Olah, J. Org. Chem., 1991, 56, 6248–6250 CrossRef CAS.
- J. I. G. Cadogan, M. Cameron-Wood, R. K. Mackie and R. J. G. Searle, J. Chem. Soc., 1965, 4831–4837 RSC; J. I. G. Cadogan, Synthesis, 1969, 11–17 CrossRef CAS.
- P. Appukkuttan, E. Van der Eycken and W. Dehaen, Synlett, 2005, 2005, 127–133.
- A. W. Freeman, M. Urvoy and M. E. Criswell, J. Org. Chem., 2005, 70, 5014–5019 CrossRef CAS.
- J.-F. Morin, N. Drolet, Y. Tao and M. Leclerc, Chem. Mater., 2004, 16, 4619 CrossRef CAS.
- F. Dierschke, A. C. Grimsdale and K. Müllen, Synthesis, 2003, 2470–2472 CAS.
- T. Yamamoto, A. Morita, Y. Miyazaki, T. Maruyama, H. Wakayama, Z. H. Zhou, Y. Nakamura, T. Kanbara, S. Sasaki and K. Kubota, Macromolecules, 1992, 25, 1214–1223 CrossRef CAS; T. Yamamoto, Synlett, 2003, 425 CrossRef CAS.
- A. D. Schlüter, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1533–1556 CrossRef CAS.
- Z. Bao, W. K. Chan and L. Yu, J. Am. Chem. Soc., 1995, 117, 12426–12435 CrossRef CAS; F. Babudri, S. R. Cicco, G. M. Farinola, F. Naso, A. Bolognesi and W. Porzio, Macromol. Rapid Commun., 1996, 17, 905–911 CrossRef CAS.
- J. Bouchard, S. Wakim and M. Leclerc, J. Org. Chem., 2004, 69, 5705–5711 CrossRef CAS.
- G. Zotti, G. Schiavon, S. Zecchin, J.-F. Morin and M. Leclerc, Macromolecules, 2002, 35, 2122–2128 CrossRef CAS.
- F. Dierschke, A. C. Grimsdale and K. Müllen, Macromol. Chem. Phys., 2004, 205, 1147–1154 CrossRef CAS.
- E. J. W. List, R. Guentner, P. Scanducci de Freitas and U. Scherf, Adv. Mater., 2002, 14, 374–378 CrossRef CAS.
- A. Iraqi and I. Wataru, Chem. Mater., 2004, 16, 442–448 CrossRef CAS.
- J.-F. Morin and M. Leclerc, Macromolecules, 2002, 35, 8413–8417 CrossRef CAS.
- S. Wakim, N. Blouin, E. Gingras, Y. Tao and M. Leclerc, Macromol. Rapid Commun., 2007, 28, 1798–1803 CrossRef CAS.
- J. Li, F. Dierschke, J. Wu, A. C. Grimsdale and K. Mullen, J. Mater. Chem., 2006, 16, 96–100 RSC.
- A. Iraqi, T. G. Simmance, H. Yi, M. Stevenson and D. G. Lidzey, Chem. Mater., 2006, 18, 5789–5797 CrossRef CAS; H. Yi, A. Iraqi, M. Stevenson, C. J. Elliott and D. G. Lidzey, Macromol. Rapid Commun., 2007, 28, 1155–1160 CrossRef CAS.
- N. Kobayashi, R. Koguchi and M. Kijima, Macromolecules, 2006, 39, 9102–9111 CrossRef CAS.
- J.-F. Morin, P.-L. Boudreault and M. Leclerc, Macromol. Rapid Commun., 2002, 23, 1032–1036 CrossRef CAS; Y. Fu and Z. Bo, Macromol. Rapid Commun., 2005, 26, 1704–1710 CrossRef CAS.
- J.-F. Morin, S. Beaupré, M. Leclerc, I. Lévesque and M. D'Iorio, Appl. Phys. Lett., 2002, 80, 341–343 CrossRef CAS; N. Drolet, S. Beaupre, J.-F. Morin, Y. Tao and M. Leclerc, J. Opt. A: Pure Appl. Opt., 2002, 4, S252–S257 CrossRef CAS.
- A. Iraqi, D. F. Pickup and H. Yi, Chem. Mater., 2006, 18, 1007–1015 CrossRef; A. Iraqi, R. C. Pegington and T. G. Simmance, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3336–3342 CrossRef CAS.
- N. Kobayashi and M. Kijima, Appl. Phys. Lett., 2007, 91, 081113 CrossRef; N. Drolet, Ph. D. Thesis, Université Laval, Québec City, 2006.
- P.-L. T. Boudreault, N. Blouin and M. Leclerc, Adv. Polym. Sci., 2008, 212, 99–124 CAS; N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110–1119 CrossRef CAS.
- N. Leclerc, A. Michaud, K. Sirois, J. F. Morin and M. Leclerc, Adv. Funct. Mater., 2006, 16, 1694–1704 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletete, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732–742 CrossRef CAS.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295–2300 CrossRef CAS.
- T.-Y. Chu, S. Alem, P. G. Verly, S. Wakim, J. Lu, Y. Tao, S. Beaupre, M. Leclerc, F. Belanger, D. Desilets, S. Rodman, D. Waller and R. Gaudiana, Appl. Phys. Lett., 2009, 95, 063304 CrossRef.
- H. Yi, R. G. Johnson, A. Iraqi, D. Mohamad, R. Royce and D. G. Lidzey, Macromol. Rapid Commun., 2008, 29, 1804–1809 CrossRef CAS.
- Y. Zou, D. Gendron, R. Badrou-Aïch, A. Najari, Y. Tao and M. Leclerc, Macromolecules, 2009, 42, 2891–2894 CrossRef CAS.
- E. Zhou, S. Yamakawa, K. Tajima, C. Yang and K. Hashimoto, Chem. Mater., 2009, 21, 4055–4061 CrossRef CAS.
- W. S. Wadsworth and W. D. Emmons, J. Am. Chem. Soc., 1961, 83, 1733 CrossRef CAS.
- S. Wakim, S. Beaupre, N. Blouin, B.-R. Aich, S. Rodman, R. Gaudiana, Y. Tao and M. Leclerc, J. Mater. Chem., 2009, 19, 5351–5358 RSC.
- S. Cho, J. H. Seo, S. H. Park, D.-Y. Kim, S. Beaupré, M. Leclerc, K. Lee and A. J. Heeger, Adv. Mater. DOI:10.1021/ja9057986.
- X. Wang, E. Perzon, J. L. Delgado, P. d. l. Cruz, F. Zhang, F. Langa, M. Andersson and O. Inganas, Appl. Phys. Lett., 2004, 85, 5081–5083 CrossRef CAS; E. Perzon, X. Wang, S. Admassie, O. Inganäs and M. R. Andersson, Polymer, 2006, 47, 4261–4268 CrossRef CAS.
- L. Bürgi, M. Turbiez, R. Pfeiffer, F. Bienewald, H.-J. Kirner and C. Winnewisser, Adv. Mater., 2008, 20, 2217–2224 CrossRef CAS.
- Y. Zou, D. Gendron, R. Neagu-Plesu and M. Leclerc, Macromolecules, 2009, 42, 6361–6365 CrossRef CAS.
- M. M. Wienk, M. Turbiez, J. Gilot and R. A. J. Janssen, Adv. Mater., 2008, 20, 2556–2560 CrossRef CAS.
- S. Wakim, J. Bouchard, N. Blouin, A. Michaud and M. Leclerc, Org. Lett., 2004, 6, 3413–3416 CrossRef CAS.
- A. K. Mishra, M. Graf, F. Grasse, J. Jacob, E. J. W. List and K. Mullen, Chem. Mater., 2006, 18, 2879–2885 CrossRef CAS.
- N. Blouin, A. Michaud, S. Wakim, P.-L. T. Boudreault, M. Leclerc, B. Vercelli, S. Zecchin and G. Zotti, Macromol. Chem. Phys., 2006, 207, 166 CrossRef CAS.
- S. Wakim, J. Bouchard, M. Simard, N. Drolet, Y. Tao and M. Leclerc, Chem. Mater., 2004, 16, 4386–4388 CrossRef CAS.
- K. Kawaguchi, K. Nakano and K. Nozaki, J. Org. Chem., 2007, 72, 5119–5128 CrossRef CAS.
- B. Robinson, J. Chem. Soc., 1963, 3097 RSC.
- L. N. Yudina and J. Bergman, Tetrahedron, 2003, 59, 1265–1275 CrossRef CAS; J. Bergman, Tetrahedron, 1970, 26, 3353–3355 CrossRef CAS; J. Tholander and J. Bergman, Tetrahedron, 1999, 55, 6243–6260 CrossRef CAS.
- Y. Li, Y. Wu, S. Gardner and B. S. Ong, Adv. Mater., 2005, 17, 849 CrossRef CAS.
- P.-L. T. Boudreault, S. Wakim, N. Blouin, M. Simard, C. Tessier, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2007, 129, 9125 CrossRef CAS; P.-L. T. Boudreault, S. Wakim, M. L. Tang, Y. Tao, Z. Bao and M. Leclerc, J. Mater. Chem., 2009, 19, 2921–2928 RSC.
- N. Blouin, M. Leclerc, B. Vercelli, S. Zecchin and G. Zotti, Macromol. Chem. Phys., 2006, 207, 175 CrossRef CAS; Y. Li, Y. Wu and B. S. Ong, Macromolecules, 2006, 39, 6521–6527 CrossRef CAS.
- J. Lu, F. Liang, N. Drolet, J. Ding, Y. Tao and R. Movileanu, Chem. Commun., 2008, 5315–5317 RSC.
- J.-H. Tsai, C.-C. Chueh, M.-H. Lai, C.-F. Wang, W.-C. Chen, B.-T. Ko and C. Ting, Macromolecules, 2009, 42, 1897–1905 CrossRef CAS.
- A. C. Grimsdale and K. Müllen, Macromol. Rapid Commun., 2007, 28, 1676–1702 CrossRef CAS.
- R. Qin, W. Li, C. Li, C. Du, C. Veit, H.-F. Schleiermacher, M. Andersson, Z. Bo, Z. Liu, O. Inganäs, U. Wuerfel and F. Zhang, J. Am. Chem. Soc., 2009, 131, 14612 CrossRef CAS.
- T. Michinobu, H. Osako and K. Shigehara, Macromol. Rapid Commun., 2008, 29, 111–116 CrossRef CAS.
- K. Tamura, M. Shiotsuki, N. Kobayashi, T. Masuda and F. Sanda, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3506–3517 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |

