Micelle formation of poly(ethylene oxide-b-sodium 2-(acrylamido)-2-methyl-1-propane sulfonate-b-styrene) and its interaction with dodecyl trimethyl ammonium chloride and dibucaine†
Bishnu Prasad
Bastakoti
a,
Sudhina
Guragain
a,
Airi
Yoneda
a,
Yuuichi
Yokoyama
b,
Shin-ichi
Yusa
b and
Kenichi
Nakashima
*a
aDepartment of Chemistry, Faculty of Science and Engineering, Saga University, 1 Honjo-machi, Saga, 840-8502, Japan. E-mail: nakashik@cc.saga-u.ac.jp
bDepartment of Materials Science and Chemistry, University of Hyogo, 2167 Shosha, Himeji, 671-2280, Japan
First published on 24th December 2009
Abstract
Micelles of a new ABC triblock copolymer, poly(ethylene oxide-b-sodium 2-(acrylamido)-2-methyl-1-propanesulfonate-b-styrene) (PEO-b-PAMPS-b-PS), were prepared in aqueous solutions. The physicochemical properties of the micelle were investigated by combining different techniques: dynamic light scattering, fluorescence spectroscopy, scanning electron microscopy, and transmission electron microscopy (TEM). The micelles have a spherical morphology with a hydrophobic PS core, a hydrophilic and anionic PAMPS shell, and a hydrophilic and nonionic PEO corona as revealed by TEM measurements. The interactions of dibucaine (DC) and dodecyl trimethyl ammonium chloride (DTAC) with PEO-b-PAMPS-b-PS were also studied. The binding of cationic DC and DTAC to the anionic PAMPS shell was confirmed by zeta-potential measurements. It is elucidated that hydrophobic interactions contribute to the binding of DTAC and DC to the polymer, although electrostatic interactions play a primarily important role.
Introduction
Amphiphilic block copolymers, constituting an important class of materials, have attracted major scientific interest in recent years due to their intriguing self-assembly behavior in aqueous media and their potential applications as nanocarriers, nanoreactors, and nanotemplates.1–3 Consequently a significant amount of effort has been expended in attempts to better understand the factors that govern the physicochemical behavior of macromolecules in aqueous media. Furthermore complexation of ionic copolymers with oppositely charged additives has been investigated intensively. Interaction of polymeric micelles with low molecular weight surfactants has been extensively studied with the aim of producing new aggregates,4 which may be useful in cosmetics and detergent formulations.5Among the amphiphilic block copolymers, ABC asymmetric triblock terpolymers have attracted great interest (in comparison to AB diblock and ABA symmetric triblock copolymers) due to a number of different morphologies that have not been observed for the micelles of AB and ABA block copolymers.6 The main motivation for studying ABC triblock terpolymer micelles is related to the presence of a third nano-sized compartment in the micellar structure. Moreover, the introduction of a third block may introduce interesting new functionalities and offer additional parameters to control the copolymer properties, thereby influencing deeply the self-assembly process.7 Gohy et al. reported the formation of micelles from the poly(ethylene oxide-b-2-vinylpyridine-b-styrene) (PEO-b-P2VP-b-PS) triblock copolymer, which has a PS core, a P2VP shell, and a PEO corona, in aqueous solutions.8 They also observed the change in micellar morphology from sphere to rod shapes of the same polymeric micelles in the presence of benzene as co-solvent.9 Nakashima and coworkers investigated the effect of various low molecular-weight counter ions on the morphology of triblock copolymer micelles.10–12 They also tried to incorporate dextran sulfate (DS) into micelles of PEO-b-P2VP-b-PS13 because DS is known as one of the polymeric medicines which have anticoagulant activity.14 As another interesting example, characterization of aqueous solutions of poly(ethylene oxide-b-N-isopropylacrylamide-b-isoprene) copolymer shows that small spherical micelles are formed at low temperatures and large vesicles are favored at higher temperatures.15 The micellization of triblock copolymers in aqueous solutions has been reported by different authors.16–20
In the present study we have synthesized a new ABC triblock copolymer, poly(ethylene oxide-b-sodium 2-(acrylamido)-2-methyl-1-propanesulfonate-b-styrene) (PEO-b-PAMPS-b-PS). The structure of the polymer is shown in Scheme 1. This polymer is expected to form a hydrophobic PS core, an ionizable PAMPS shell and a hydrophilic PEO corona in aqueous solutions. We address the physicochemical properties of the PEO-b-PAMPS-b-PS micelles. One of the features of the present polymer, compared to those in the previous studies,11,12,21 is that the PS chain is short enough to have a lower glass transition temperature (Tg).
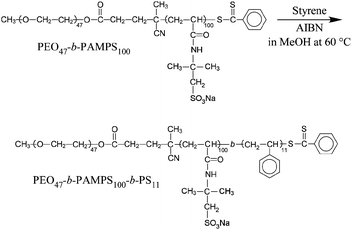 | ||
| Scheme 1 Synthesis route to PEO-b-PAMPS-b-PS. | ||
Furthermore, we have extended our study to the interaction of amphiphilic additives with the PEO-b-PAMPS-b-PS micelles. We chose dodecyl trimethyl ammonium chloride (DTAC) as a surfactant and dibucaine (DC) as a drug to bind to the triblock copolymer (see Scheme 2 for the structures of DTAC and DC). DTAC and DC are cationic counter ions, and thus may preferentially bind to anionic PAMPS block of PEO-b-PAMPS-b-PS. As expected, we obtained nanoaggregates formed from PEO-b-PAMPS-b-PS and the additives. The binding was confirmed by zeta-potential measurements, while the aggregate formation was confirmed by dynamic light scattering (DLS), fluorescence spectroscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
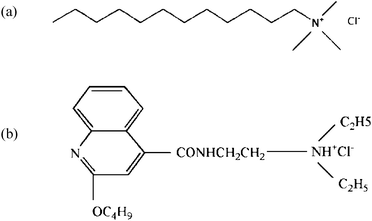 | ||
| Scheme 2 Structures of (a) dodecyl trimethyl ammonium chloride (DTAC) and (b) dibucaine·HCl (DC). | ||
Experimental
Materials
Poly(ethylene oxide-b-sodium 2-(acrylamido)-2-methyl propanesulfonate) (PEO-b-PAMPS) based chain transfer agent was prepared as reported previously.22 The number-averaged molecular weight (Mn) and molecular weight distribution (Mw/Mn) values determined by gel-permeation chromatography (GPC) were 1.92 × 104 and 1.28, respectively. The values of number-averaged degree of polymerization (DP) for the PEO and PAMPS blocks were 47 and 100, respectively, estimated by 1H NMR. 2,2′-Azobis(isobutyronitrile) (AIBN, 98%) from Wako Pure Chemical Industries Ltd. was recrystallized from methanol. Styrene (99%) from Wako Pure Chemical Industries Ltd. was washed with an aqueous alkaline solution and distilled from calcium hydride under reduced pressure. Methanol was dried over 4 Å molecular sieves and distilled. DTAC (Tokyo Chemical Industry) and DC (Aldrich) were used without further purification.Preparation of PEO-b-PAMPS-b-PS
Synthesis route to PEO-b-PAMPS-b-PS is shown in Scheme 1. Styrene (526 mg, 5.05 mmol), AIBN (3.26 mg, 0.02 mmol), and PEO47-b-PAMPS100 (1.26 g, 0.05 mmol) were dissolved in methanol (83.2 mL). The solution was degassed by purging with Ar gas for 60 min. The polymerization was carried out at 60 °C for 24 h. The polymerization mixture was poured into a large excess of tetrahydrofuran (THF) to precipitate the resulting polymer. The polymer was purified by reprecipitating from methanol into a large excess of THF two times.The precipitate was dissolved in water and the obtained polymer (PEO-b-PAMPS-b-PS) was recovered by a freeze-drying technique (1.25 g). DP of the PS block is 11 as estimated by 1H NMR in DMSO-d6 at 100 °C (Fig. 1). The Mn (NMR) value of PEO-b-PAMPS-b-PS is 2.63 × 104.
 | ||
| Fig. 1 1H NMR of PEO-b-PAMPS-b-PS in DMSO-d6 at 100 °C. | ||
Preparation of polymeric micelles
A known amount of PEO-b-PAMPS-b-PS was dissolved in water and gently agitated with a magnetic stirrer at room temperature till a clear solution was obtained. The solution was transferred to a volumetric flask to obtain a stock solution with a concentration of 1 g L−1. A known amount of this stock solution was transferred to a volumetric flask and diluted with water to make a working solution.Titration of PEO-b-PAMPS-b-PS micelle solution with DTAC or DC
A known amount of PEO-b-PAMPS-b-PS solution was titrated with DTAC or DC. The amount of added DTAC or DC is expressed in terms of apparent degree of neutralization (DN), which is defined as | (1) |
During titration the samples were agitated with a magnetic stirrer for 1 min to accelerate the interaction between the micelle and the counter ion. All the samples were kept at room temperature for one day before the measurements.
It should be noted that the calculation of DN was based on the assumption that the nitrogen atom in the aromatic ring is not protonated. This assumption is justified because the pH of the sample solution is 6 while the pKa value of the conjugate acid of quinoline (mother compound of dibucaine) is 4.94.23
Gel-permeation chromatography (GPC)
GPC measurements for PEO-b-PAMPS were performed using a refractive index detector equipped with a Shodex GF-7M HQ column working at 40 °C under a flow rate of 0.6 mL min−1. A phosphate buffer (pH 8) containing 10 vol% acetonitrile was used as eluent. Mn and Mw/Mn were calibrated with standard sodium poly(styrenesulfonate) samples.Nuclear magnetic resonance (NMR)
1H NMR spectra were obtained with a Bruker DRX-500 spectrometer operating at 500 MHz.DLS measurements
DLS measurements were carried out with an Otsuka ELS-8000 electrophoretic light scattering instrument. All the measurements were carried out at 25 °C. All the solutions were passed through a 0.2 μm cellulose filter before the measurements. The scattered light of a vertically polarized He–Ne laser (632.8 nm) was measured at an angle of 90° and was collected on an autocorrelator. Correlation functions were analyzed by the histogram method and used to determine the diffusion coefficient (D) of the samples. Hydrodynamic diameter (Dh) was calculated from D using the Stokes–Einstein equation.| Dh = kBT/(3πηD) | (2) |
Zeta-potential measurements
The electrophoresis mobility (EPM) was measured using an Otsuka ELS-8000 apparatus. The zeta-potential (ζ) was calculated from the EPM using the Smoluchowski equation.| μE = ζε/η | (3) |
Fluorescence spectroscopy
The fluorescence spectra were recorded on a JASCO F-6500 fluorescence spectrophotometer (right angle geometry, 1 cm × 1 cm quartz cell). Pyrene was employed as a fluorescent probe. The sample was excited at 339 nm in the measurement of fluorescence spectra, and the band widths were 3 and 1 nm on the excitation and emission sides, respectively. In the measurement of excitation spectra, the emission wavelength is 390 nm, and the band widths were 1 and 5 nm on the excitation and emission sides, respectively.SEM measurements
SEM measurements were carried out on a Hitachi SU-1500 electron microscope at an accelerating voltage of 15 kV. The samples were prepared by dropping the micelle solution onto a carbon seal (Nisshin Em Co.) attached to the glass plate, followed by drying in air for 1 day.TEM measurements
TEM measurements were carried out using a JEOL JEM-1210 transmission electron microscope at an accelerating voltage of 80 kV. The samples were prepared by casting onto a copper grid and the excess solution was evaporated by drying at room temperature.Results and discussion
Characteristics of neat PEO-b-PAMPS-b-PS micelles
The micelle formation of PEO-b-PAMPS-b-PS was confirmed by SEM measurements. Fig. 2 represents the SEM image of the sample. We clearly see spherical micelle particles with homogeneous size distribution. The average diameter of the particles is about 70 nm. The picture provides evidence for the micelle formation of the triblock copolymer in aqueous solution. | ||
| Fig. 2 SEM image of PEO-b-PAMPS-b-PS micelles. The sample for the SEM measurement was prepared from a solution in which the concentration of polymer is 1 g L−1. | ||
It is expected that the micelles have a PS core, a PAMPS shell, and a PEO corona as in the case of poly(ethylene oxide-b-[3-(methacryloylamino)propyl]trimethylammonium chloride-b-styrene) (PEO-b-MAPTAC-b-PS).12 We carried out TEM measurements (ESI† Fig. S1). We can see several spherical dark particles. The dark portion of the particles corresponds to the PAMPS block of the micelles stained with Fe3+ ions. The central part of the spherical particles is not as dark as the outer part showing the PS core of PEO-b-PAMPS-b-PS micelles. As the PS block is very short (DP for PS is 11), we could not observe a clear PS core which should appear as a bright spot.12
We carried out DLS measurements of the polymer solution at different concentrations. Fig. 3 shows a plot of Dh of the PEO-b-PAMPS-b-PS nanoaggregates against the polymer concentration. It was found that Dh is almost constant (approximately 70 nm) at polymer concentrations greater than 0.2 g L−1.
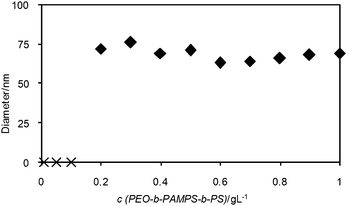 | ||
| Fig. 3 Dependence of Dh of micelles of PEO-b-PAMPS-b-PS on the polymer concentration. When the polymer concentration is less than 0.2 g L−1, the scattering intensity was too low to give reliable Dh. Therefore, we plot such data on the horizontal axis using different symbols (×). | ||
Although it is shown by DLS measurements that the micelles are formed at the polymer concentration of 0.2 g L−1, this concentration cannot be regarded as the CMC, because the sensitivity of DLS is not high enough to determine the CMC. Therefore, we employed fluorescence measurements by using pyrene as a probe. One of the useful fluorescence parameters of pyrene is the intensity ratio between the 0–0 band at 373 nm and the b1g vibronic band at 384 nm (the so-called I1/I3 ratio).24 When pyrene is added to micelle solutions, it is incorporated into the hydrophobic domain of the micelles. The incorporation of pyrene into the hydrophobic domain of the micelles produces a lower I1/I3 value than that in the aqueous bulk phase. Thus, we can observe a significant change in the I1/I3 ratio if we plot this parameter versus the polymer concentration. Fig. 4 shows the dependence of the I1/I3 ratio on the concentration of polymer.
 | ||
| Fig. 4 Dependence of I1/I3 ratios of pyrene fluorescence on the concentration of PEO-b-PAMPS-b-PS in aqueous solutions. The concentration of pyrene is 0.6 μM. The samples were excited at 339 nm and the band widths were 3 and 1 nm on the excitation and emission sides, respectively. | ||
At lower concentrations, the I1/I3 ratio is almost constant. However, this ratio starts to decrease when the concentration of polymer increases. The initial break is observed at 0.05 g L−1, in the I1/I3 value. In contrast to the case of low molecular-weight surfactants, however, this value cannot be regarded as the CMC of the polymeric micelles, but corresponds to the upper bound of the CMC.25 According to Winnik et al., an accurate CMC of polymeric micelles can be obtained from the excitation spectra of pyrene.25 Therefore, we measured the excitation spectra, but we could not determine the CMC because we did not observe any significant red shift in the excitation spectra upon increasing the polymer concentration.
It should be noted here that the PS block in the present polymer is small as stated in introduction section (i.e. DP = 11). We estimated the Tg value of the PS block from its molecular weight,26 and obtained a value of 13 °C. This value indicates that the PS block is in the liquid state at room temperature. We expect the micelles to be in equilibrium with their free unimers in solution. However, this assumption may not be entirely satisfied in the experiments, depending on the copolymer characteristics and/or on the micelle formation mode.27,28 Different ways of preparing sample solutions lead to micelles with different Dh (data not shown), indicating the frozen character of the PEO-b-PAMPS-b-PS self-assembly.
Interaction of PEO-b-PAMPS-b-PS micelles with DTAC
It is expected in the PEO-b-PAMPS-b-PS micelles that the anionic PAMPS block strongly interacts with organic and/or inorganic cations. We tried to bind DTAC, a typical example of a cationic surfactant, to the PAMPS block to obtain hybrid micelles. Fig. 5a shows a plot of Dh of the DTAC/PEO-b-PAMPS-b-PS nanoaggregates as a function of DN. The concentration of the polymer solution is fixed at 0.05 g L−1, where the polymer cannot form micelles without additives (see Fig. 3). At lower values of DN, the formation of nanoaggregates cannot be detected. However, the nanoaggregate formation seems to begin at about 60%. This can be attributed to the insolubilization of the PAMPS block by the charge neutralization with DTAC. The Dh value of DTAC/PEO-b-PAMPS-b-PS nanoaggregates decreases as the DN value increases which is indicative of non-equilibrium micelles. The decrease in Dh suggests that the charge neutralization of the PAMPS block by added DTAC leads to a more compact form of the nanoaggregates. However, Dh begins to increase after 120% as seen in Fig. 5a. The reason for this is currently unclear. One of the possibilities is that DTAC is bound to the nanoaggregates of DTAC/PEO-b-PAMPS-b-PS by a hydrophobic interaction and DTAC forms bridges between the nanoaggregates to produce secondary aggregates. Kabanov et al. reported that a complex between sodium polyacrylate and alkyl trimethyl ammonium bromide could form a highly ordered supramolecular structure.29 Khokhlov et al. showed that the complexes between a cationic polyelectrolyte gel, poly(diallyldimethylammonium chloride), and an anionic surfactant, sodium dodecyl sulfate, exhibited a highly ordered hexagonal supramolecular structure.30 If we take these observations by other groups into account, it is probable that DTAC causes the formation of secondary aggregates in our case. | ||
| Fig. 5 Dependence of (a) Dh and (b) zeta-potential of DTAC/PEO-b-PAMPS-b-PS on DN. The concentration of PEO-b-PAMPS-b-PS is fixed at 0.05 g L−1. When the DN % is less than 60, the scattering intensity was too low to give reliable Dh in (a). Therefore, we plot such data on the horizontal axis using different symbols (×). | ||
In order to confirm the binding of DTAC to the anionic block of PEO-b-PAMPS-b-PS, we carried out zeta-potential measurements, because it is expected that the negative zeta-potential of the PEO-b-PAMPS-b-PS nanoaggregates will be increased by the binding of positively-charged DTAC. Fig. 5b shows the zeta-potential of DTAC/PEO-b-PAMPS-b-PS nanoaggregates as a function of DN. The zeta-potential increases as the DN is increased up to 100%. This confirms the binding of DTAC to the PAMPS shell. However, at DN 100% the zeta-potential is not 0 mV, indicating non-stoichiometric binding of DTAC to the PAMPS block.
Fig. 6 shows the dependence of Dh of DTAC/PEO-b-PAMPS-b-PS nanoaggregates on the polymer concentration at the constant DN of 100%. A sharp rise in the diameter at a polymer concentration of 0.01 g L−1 was found which indicates the nanoaggregate formation. We cannot regard this concentration as a critical aggregate concentration (CAC) of DTAC/PEO-b-PAMPS-b-PS at 100% DN, because the DLS measurement does not have sufficient sensitivity for CAC determination. However, the micelle formation of neat PEO-b-PAMPS-b-PS was detected at the concentration of 0.2 g L−1 by DLS (see Fig. 3), while the aggregate formation of the DTAC/PEO-b-PAMPS-b-PS system was detected at the polymer concentration of 0.01 g L−1, about twenty times lower concentration. This fact indicates that the aggregate formation of PEO-b-PAMPS-b-PS is greatly enhanced by the existence of DTAC. The diameter remains almost constant (62 nm) at higher concentrations of polymer. It should be noted that the hydrodynamic diameter at 0.01 g L−1 is considerably large compared to that at higher concentrations. The reason for this is currently unclear.
 | ||
| Fig. 6 Dependence of Dh of DTAC/PEO-b-PAMPS-b-PS nanoaggregates on polymer concentration at constant DN of 100%. | ||
The morphology of the DTAC/PEO-b-PAMPS-b-PS nanoaggregates with different degrees of neutralization and different concentrations was investigated by SEM. Fig. 7 shows the SEM picture for the sample having a concentration of 0.1 g L−1 with 100% DN. We can see the spherical shape of the particles. The morphologies of the particles at different DN and different polymer concentrations are the same as this case.
Incorporation of DC into the PEO-b-PAMPS-b-PS micelles
It is interesting to incorporate DC, a local anesthetic, into the PEO-b-PAMPS-b-PS micelles, because the micelles can be regarded as a model system of drug carriers. Fig. 8a shows the dependence of the Dh value of the DC/PEO-b-PAMPS-b-PS aggregates on the DN value. The Dh of the nanoaggregates rises at DN = 20%, and decreases with increasing DN. The decrease in the diameter after 20% DN is attributed to the neutralization of the anionic sulfonate ion of the PAMPS block by positively charged DC. When DN is small (20–60%), the electrostatic repulsion between negative PAMPS chains is significant, leading to an extended conformation of this block. Due to the extended conformation of the PAMPS block, the nanoaggregates show larger Dh. However, as the negative charge is neutralized by the bound DC, the repulsion between the PAMPS chains is weakened, and this block undergoes a conformational change from the extended to the shrunken form. Thus, the diameter of the nanoaggregates decreases. After 60% DN, the hydrodynamic diameter of the aggregates is almost constant, around 29 nm. This implies that the conformational change in the PAMPS block is completed at 60% DN. It should be noted that Dh does not increase after 100% DN in contrast to the case of the DTAC/PEO-b-PAMPS-b-PS system. This implies that the formation of secondary aggregates does not occur after 100% DN. The difference between the two cases seems to arise from the fact that DTAC has a long alkyl chain to be used for bridging the primary aggregate particles whereas DC does not.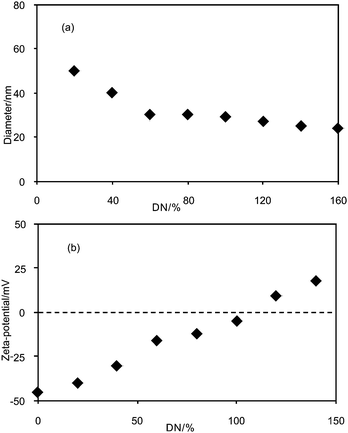 | ||
| Fig. 8 Dependence of (a) Dh and (b) zeta-potential of DC/PEO-b-PAMPS-b-PS nanoaggregates on DN. The concentration of polymer is fixed at 0.1 g L−1. | ||
Fig. 8b shows the zeta-potential of the aggregates of DC/PEO-b-PAMPS-b-PS as a function of DN. The increase in DN results in an almost linear increase in the zeta-potential. At 100% DN, the zeta-potential is almost zero, indicating that the binding of DC to the PEO-b-PAMPS-b-PS is stoichiometric and thus the charges of PAMPS are completely neutralized by DC at 100% DN. When DN exceeds 100%, the zeta-potential becomes positive. This is not surprising because a hydrophobic interaction is also possible between PEO-b-PAMPS-b-PS and DC beside the electrostatic interaction. A hydrophobic interaction between the alkyl and aryl tails of DC and hydrophobic moieties of the polymer seems to contribute to the binding of DC after 100% DN.
Fig. 9 shows the change in the hydrodynamic diameter of the DC/PEO-b-PAMPS-b-PS aggregates as a function of the polymer concentration at DN = 100%. Since the diameter sharply rises at the polymer concentration of 0.05 g L−1, the CAC seems to fall in the range below 0.05 g L−1. The diameter becomes almost constant at higher concentrations of polymer.
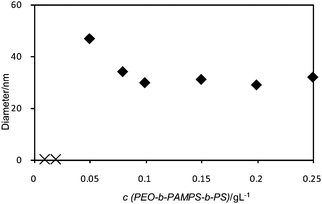 | ||
| Fig. 9 Dependence of Dh of DC/PEO-b-PAMPS-b-PS nanoaggregates on concentration of polymer at constant DN of 100%. When the polymer concentration is less than 0.05 g L−1, the scattering intensity was too low to give reliable Dh. Therefore, we plot such data on the horizontal axis using different symbols (×). | ||
The morphology of the DC/PEO-b-PAMPS-b-PS nanoaggregates with different degrees of neutralization and different concentrations was investigated by SEM. Fig. 10 shows the SEM picture for the sample having a concentration of 0.1 g L−1 at a DN of 100%. We can see a spherical shape of the particles. The morphologies of the particles at different DN and different polymer concentrations are the same as this case.
Conclusions
We have synthesized a new ABC triblock copolymer, PEO-b-PAMPS-b-PS. The degree of polymerization of the polymer is 47, 100 and 11 for PEO, PAMPS and PS blocks, respectively. The obtained triblock copolymer was investigated by focusing on the nature of its micelles in aqueous solutions. The micelles consist of a PS core, a PAMPS shell and a PEO corona. SEM and TEM measurements provide evidence for the formation of spherical micelles. DLS measurements give the hydrodynamic diameter of approximately 70 nm. Fluorescence spectroscopy gives the upper limit of the CMC (0.05 g L−1).We also investigated the binding of DTAC and DC to the PAMPS block of the PEO-b-PAMPS-b-PS nanoaggregates. The binding of the respective organic cations to the PAMPS shell was confirmed by zeta-potential measurements. The zeta-potential increased with increasing DN, showing the electrostatic binding of cationic DTAC or DC to the anionic PAMPS block of PEO-b-PAMPS-b-PS. However, there was such a difference between DTAC and DC binding that DC showed stoichiometric binding, while DTAC did not. Furthermore, both DTAC and DC continued to bind to the micelles by hydrophobic interactions after the DN value exceeded 100%. These facts suggest that hydrophobic interactions contribute to the binding of DTAC and DC to the polymer, although electrostatic interactions are primarily important. The binding of DTAC and DC did not induce a change in the shape (spherical) of the particles but induced a decrease in the size. The decrease in the particle size is attributed to the change in the PAMPS block from an extended to a shrunken conformation.
Acknowledgements
The authors thank Mr Toshini Tabata for the measurement of TEM. The present study was supported by a Grant-in-Aid for Scientific Research (20310054) from the Japan Society for the Promotion of Science (JSPS).References
- K. Nakashima and P. Bahadur, Adv. Colloid Interface Sci., 2006, 123–126, 75–96 CrossRef CAS.
- L. B. Luo and A. Eisenberg, Angew. Chem., Int. Ed., 2002, 41, 1001–1004 CrossRef CAS.
- H. Lee and K. Char, ACS Appl. Mater. Interfaces, 2009, 1, 913–920 Search PubMed.
- S. E. Burke and A. Eisenberg, Langmuir, 2001, 17, 8341–8347 CrossRef CAS.
- S. Pispas and N. Hadjichristidis, Langmuir, 2003, 19, 48–54 CrossRef CAS.
- H. Mori and A. H. E. Muller, Prog. Polym. Sci., 2003, 28, 1403–1439 CrossRef CAS.
- C. A. Fustin, V. Abetz and J. F. Gohy, Eur. Phys. J. E, 2005, 16, 291–302 CrossRef CAS.
- J. F. Gohy, N. Willet, S. Varshney, J. X. Zhang and R. Jerome, Angew. Chem., Int. Ed., 2001, 40, 3214–3216 CrossRef CAS.
- L. Lei, J. F. Gohy, N. Willet, S. Varshney, J. X. Zhang and R. Jerome, Macromolecules, 2004, 37, 1089–1094 CrossRef CAS.
- A. Khanal, Y. Li, N. Takisawa, N. Kawasaki, Y. Oishi and K. Nakashima, Langmuir, 2004, 20, 4809–4812 CrossRef CAS.
- A. Khanal, S. Yusa and K. Nakashima, Langmuir, 2007, 23, 10511–10517 CrossRef CAS.
- L. Jingjing, L. Dian, Y. Yuuichi, S. Yusa and K. Nakashima, Langmuir, 2009, 25, 739–743 CrossRef.
- Y. Li, A. Khanal, N. Kawasaki, Y. Oishi and K. Nakashima, Bull. Chem. Soc. Jpn., 2005, 78, 529–533 CrossRef CAS.
- Y. Tajima, Biomed. Res. Trace Elem., 2002, 13, 46 CAS.
- A. Sundararaman, T. Stephan and R. B. Grubbs, J. Am. Chem. Soc., 2008, 130, 12264–12265 CrossRef CAS.
- O. Lambert, S. Reutenauer, G. Hurtrez and P. Dumas, Macromol. Symp., 2000, 161, 97 CrossRef CAS.
- Z. Zhu, S. P. Armes and S. Liu, Macromolecules, 2005, 38, 9803–9812 CrossRef CAS.
- Y. Liu, S. H. Chen and J. S. Huang, Macromolecules, 1998, 31, 2226–2233.
- X. Li, K. Y. Mya, X. Ni, C. He, K. W. Leong and J. Li, J. Phys. Chem. B, 2006, 110, 5920–5926 CrossRef CAS.
- M. Even, D. M. Haddleton and D. Kukulj, Eur. Polym. J., 2003, 39, 633–639 CrossRef CAS.
- R. Xu, M. A. Winnick, G. Riess, B. Chu and M. D. Croucher, Macromolecules, 1992, 25, 644–652 CrossRef CAS.
- S. Yusa, Y. Yokoyama and Y. Morishima, Macromolecules, 2009, 42, 376–383 CrossRef CAS.
- R. E. Lokhov, Chem. Heterocycl. Compd., 1981, 17, 72–76 CrossRef.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039–2044 CrossRef CAS.
- M. Wilhelm, C. L. Zhao, Y. Wang, R. Xu, M. A. Winnik, J. L. Mura, G. Riess and M. D. Croucher, Macromolecules, 1991, 24, 1033–1040 CrossRef CAS.
- A. Jada, G. Hurtrez, B. Siffert and G. Riess, Macromol. Chem. Phys., 1996, 197, 3697–3710 CrossRef CAS.
- O. Colombani, M. Ruppel, M. Burkhardt, M. Drechsler, M. Schumacher, M. Gradzielski, R. Schweins and A. H. E. Muller, Macromolecules, 2007, 40, 4351–4362 CrossRef CAS.
- M. Jacquin, P. Muller, R. Talingting-Pabalan, H. Cottet, J. F. Berret, T. Futterer and O. Théodoly, J. Colloid Interface Sci., 2007, 316, 897–911 CrossRef CAS.
- Y. V. Khandurina, A. T. Dembo, V. B. Rogacheva, A. B. Zezin and V. A. Kabanov, Polym. Sci., 1994, 36, 189–194 Search PubMed.
- B. Chu, F. Yeh, E. L. Sokolov, S. G. Starodubtzev and A. R. Khokhlov, Macromolecules, 1995, 28, 8447–8449 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image. See DOI: 10.1039/b9py00231f |
| This journal is © The Royal Society of Chemistry 2010 |


