Non-resonant two-photon photochemistry of a Barton ester, N-phenylacetyloxy-2-pyridinethione
Norman P. Schepp*, Christopher J. M. Green and Frances L. Cozens*
Department of Chemistry, Dalhousie University, Halifax, Nova Scotia, Canada. E-mail: nschepp@dal.ca; fcozens@dal.ca
First published on 9th December 2009
Abstract
N-Acetyloxy-2-pyridinethiones, otherwise known as Barton esters, are a class of molecules that can be easily photolysed via single-photon excitation to facilitate the controlled release of carbon or oxygen-centred radicals. In the present work, we investigate the two-photon chemistry of a simple Barton ester, and show that this material can be photolysed via two-photon excitation, with a two-photon bleaching cross section value of 0.13 ± 0.01 GM.
1. Introduction
N-Oxy-2-pyridinethione derivatives are efficient and versatile precursors for the photogeneration of oxygen-, nitrogen- and carbon-centered radicals. This class of compounds, especially N-acetyloxy-2-pyridinethiones commonly known as Barton or PTOC esters,1 have been used extensively as precursors for radicals in synthetic chemistry,2 as well as radicals in mechanistic studies.3–5 A variety of N-oxy-2-pyridinethione derivatives have also been used for the generation of radicals in biological studies. For example, N-hydroxy-, N-alkoxy- and N-acetyloxy-2-pyridinethione derivatives have all been used as precursors to generate radicals photochemically in the presence of DNA to examine the ability of radicals to induce DNA strand cleavage.6–10In more recent studies, 2-pyridinethione derivatives have been used as precursors for the light-induced production of intracellular radicals to examine, for example, the effect of radicals on cell growth and viability.11–14 Such studies are often complicated by the fact that 2-pyridinethione derivatives, like most small organic chromophores, require excitation wavelengths in the UV region. This can be problematic because UV light cannot penetrate biological material as well as longer wavelengths can, and may cause peripheral damage within the target organism.
One solution to the problems associated with using UV light is non-resonant two-photon excitation,15 initiated using high-intensity femtosecond laser pulses. This method involves the virtually simultaneous absorption of two lower-energy photons, typically in the red or near-infrared region, to cause the formation of an excited state that would otherwise require a single photon of higher energy. Two-photon excitation therefore provides the possibility of conducting studies with small organic chromophores using wavelengths of light that are compatible with biological systems.
Recently, it was shown that tightly focused 750 nm femtosecond irradiation of N-hydroxy-2-pyridinethione in the presence of tryptophan leads to the formation of 5-hydroxytryptophan, presumably by initial formation of the hydroxyl radical by multi-photon excitation of N-hydroxy-2-pyridinethione.16 While this report shows that N-hydroxy-2-pyridinethione is susceptible to multi-photon excitation, information concerning the efficiency of this process continues to be lacking. Furthermore, there has not yet been a study conducted to probe the multi-photon photochemistry of more complicated N-oxy-2-pyridinethione derivatives, such as Barton esters. In the present work, we describe results that demonstrate that N-phenylacetyloxy-2-pyridinethione 1 (Scheme 1), a prototypical Barton ester, is sensitive to two-photon induced photodecomposition, and quantify that sensitivity by meauring the two-photon action cross section of 1 using 775 nm femtosecond laser irradiation.
 | ||
| Scheme 1 Photolytic cleavage of ester 1. | ||
2. Experimental
2.1 General
UV-Vis spectra were obtained on a Varian Cary 100 Bio UV-Vis spectrophotometer. NMR spectra were obtained using a Bruker AVANCE 500 MHz spectrometer. Spectroscopy-grade acetonitrile was used in all photochemical experiments.2.2 Laser irradiation
Solutions of 1 were prepared at a concentration of about 0.1 mM in acetonitrile. 1.8 mL samples were irradiated in a 1 cm × 1 cm quartz cuvette with either 355 nm or 775 nm laser pulses. The 355 nm pulses (37 mJ per pulse, 6 ns FWHM) were from a Spectra-Physiks Nd:YAG laser. The 775 nm pulses (0.710 mJ per pulse, 210 fs FWHM) were delivered using a Clark-MXR CPA 2001 femtosecond laser. To avoid photodecomposition of 1 from room light, all photolysis and analytical experiments were carried out in a dark room.For the 355 nm laser dose experiments, six pulses were delivered 3 s apart, with mixing of the samples by inversion between each pulse. The intensity of the laser pulses was reduced by varying amounts using calibrated (at 355 nm) absorptive glass neutral density filters. Before passing through the neutral density filters, each laser pulse had a total energy of 37 mJ.
For the 775 nm laser dose experiments, samples were irradiated for two hours at a pulse rate of 1000 Hz with continuous stirring using a magnetic stir bar. There was no appreciable change in sample temperature over the course of an irradiation. The intensity of the laser pulses was reduced using calibrated (at 775 nm) absorptive glass neutral density filters. Laser power after the neutral density filters was measured with power meter.
Data for the cross-section calculation was obtained by measuring UV-Vis absorption of a sample of 1 irradiated with 775 nm femtosecond laser light at increasing irradiation time. Because only a fraction of the sample cell was irradiated by the laser, the irradiation time required to achieve photolysis of 1 was scaled down according to the percentage of the sample cell that was irradiated. The cross sectional area of the laser beam was measured to be 0.229 cm2, so the volume of sample irradiated was therefore 0.229 cm3, which is 12.7% of the total volume 1.8 mL. The time of each measurement in Fig. 3 is therefore scaled down by a factor of 0.127.17
2.3 Cross section calculation
The rate of two-photon induced decomposition of a chromophore is described by eqn (1), which is easily derived from Beer's Law for two-photon absorption.17 | (1) |
To determine the value of the integral of I2, the time dependence of laser intensity was assumed to be perfectly Gaussian for each laser pulse. Based on this assumption, an explicit expression for the laser intensity, I, over the course of a pulse was derived using the general equation for a Gaussian curve centered at t = 0, eqn (2).
 | (2) |
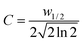 | (3) |
2.4 Preparation of N-phenylacetyloxy-2-pyridinethione, 1
The ester 1 was prepared in 40% yield using a literature procedure 18 and recrystallized from a mixture of hexanes and dichloromethane.Melting point: 86–89 °C (dec.) (lit.:18 93–95 °C (dec.)); λmax(CH3CN)/nm 290 (ε = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 M−1 cm−1), 364 (ε = 4848 M−1 cm−1); 1H NMR (CDCl3): δ 7.74 (ddd, 1H, J = 0.52, 1.80, and 8.84), 7.56 (ddd, 1H, J = 0.52, 1.56, and 6.94), 7.46–7.35 (m, 5H), 7.24 (ddd, 1H, J = 1.56, 6.82, and 8.84), 6.64 (ddd, 1H, J = 1.80, 6.82 and 6.94), 4.09 (s, 2H); 13C DEPTQ-135 (CDCl3): δ 175.8(−), 167.4(−), 137.5(+), 137.4(+), 133.5(+), 131.4(−), 129.6(+), 128.9(+), 127.9(+), 112.6(+), 38.2(−); HRMS: calculated 245.053 g mol−1; found 245.051 ± 0.003 g mol−1.
050 M−1 cm−1), 364 (ε = 4848 M−1 cm−1); 1H NMR (CDCl3): δ 7.74 (ddd, 1H, J = 0.52, 1.80, and 8.84), 7.56 (ddd, 1H, J = 0.52, 1.56, and 6.94), 7.46–7.35 (m, 5H), 7.24 (ddd, 1H, J = 1.56, 6.82, and 8.84), 6.64 (ddd, 1H, J = 1.80, 6.82 and 6.94), 4.09 (s, 2H); 13C DEPTQ-135 (CDCl3): δ 175.8(−), 167.4(−), 137.5(+), 137.4(+), 133.5(+), 131.4(−), 129.6(+), 128.9(+), 127.9(+), 112.6(+), 38.2(−); HRMS: calculated 245.053 g mol−1; found 245.051 ± 0.003 g mol−1.
3. Results and discussion
Photodecomposition of N-acetyloxy-2-pyridinethiones can be easily monitored via UV-Vis spectroscopy by observing the disappearance of strong absorption bands at 364 and 290 nm as a function of light dosage. Fig. 1a shows the progressive decomposition of 1 in acetonitrile as a function of the number of 355 nm pulses from a Nd:YAG laser. The decrease in absorption at 364 and 290 nm is accompanied by an increase in absorption at 240 nm, which is consistent with the formation of 2,2′-dipyridyl disulfide,19 shown from previous studies to be a major product upon irradiation of 1.20 | ||
| Fig. 1 Absorption spectra of 1 (a) before (●) and after one (○) and two (■) 355 nm nanosecond laser (Nd:YAG) and (b) before (●) and after 2.4 × 106 (○) and 3.6 × 106 (■) 775 nm femtosecond laser (Ti:Sapphire) pulses in acetonitrile. The insets show the difference spectra obtained by Absno irradiation− Absafter irradiation. | ||
N-Phenylacetyloxy-2-pyridinethione 1 has no absorption at wavelengths above 430 nm; thus, irradiation with 775 nm light would not be expected to induce photodecomposition if the only mode of excitation involved absorption of a single 775 nm photon. However, upon irradiation with 775 nm femtosecond laser pulses, 1 appeared to undergo substantial photodecomposition, with the degree of photoreaction increasing with increasing irradiation time as illustrated in Fig. 1b. In a manner identical to that observed upon single-photon excitation using 355 nm light, irradiation using high intensities of 775 nm light resulted in a decrease in absorption at 364 and 290 nm, accompanied by an increase in absorption at 240 nm. Difference spectra shown as insets in Fig. 1 highlight the similarity between the 355 nm and 775 nm irradiations, which provides good evidence that identical photoreactions are occurring at the two different laser wavelengths.
Evidence that the observed photoreaction occurring at 775 nm is indeed due to two-photon excitation was obtained by taking advantage of the quadratic dependence that two-photon excitation has on light intensity. Fig. 2a shows the percentage decomposition of 1 as a function of 355 nm laser intensity using a Nd:YAG laser. As expected, at the low laser doses, the relationship is linear, indicating that photodecomposition with 355 nm light is a single-photon phenomenon. The downward curvature observed at higher laser intensities is presumably caused by saturation effects, where the number of photons in the laser pulse exceeds the number of excitable substrates. When 1 was irradiated with varying intensities of 775 nm femtosecond laser pulses, the percentage decomposition had a dramatically different dependence on light intensity (Fig. 2b). Unlike the linear relationship obtained using the 355 nm light, the data in Fig. 2b show a distinct upward curvature in a manner that is consistent with a multiphoton initiated photoreaction. A logarithmic plot of the percentage decomposition versus laser intensity has a slope of 1.9 ± 0.1, suggesting that the photochemical decomposition of 1 with the 775 nm light has a quadratic dependence on light intensity, and is therefore a two-photon process.
 | ||
| Fig. 2 (a) Decomposition of 1 as a function of 355 nm laser intensity in acetonitrile. (b) Decomposition of 1 as a function of 775 nm femtosecond laser intensity in acetonitrile. The inset shows log–log plot with a slope of 1.9 ± 0.1. | ||
A key parameter needed to establish the efficiency of two-photon photochemistry is the observed two-photon cross section, σobs. To determine σobs, the disappearance of 1 was monitored as a function of irradiation time, Fig. 3. These data were fit using a non-linear least squares method to eqn (1), and a value of σobs = 0.13 ± 0.01 GM was obtained. This observed two-photon cross section represents the photosensitivity of Barton ester 1 to two-photon induced decomposition and corresponds to the photodecomposition action cross-section at 775 nm. Two-photon action cross sections of 0.10 GM to 10 GM have been suggested as lower limits for biological applications of two-photon chromophores.21–23 The value of σobs for 1 lies within this range, which suggests that Barton esters should be useful precursors for the generation of radicals in biological environments. Furthermore, the photochemical events that lead to N–O bond cleavage and the subsequent thermal decarboxylation step are rapid, taking place with rate constants greater than 1 × 107 s−1.5,24 Thus, in those biological applications where photolysis takes within the focus of a pulsed laser, radical generation would be sufficiently rapid in comparison to diffusion to ensure greater accumulation of photogenerated radicals within the focal volume of the laser beam.22,23
 | ||
| Fig. 3 Decomposition of Barton ester 1 as a function of irradiation time. The data were fit using eqn (1), with linear least squares analysis giving σ = 0.13 ± 0.01 GM. | ||
Under ideal circumstances, σobs can be converted to the true two-photon absorption cross section, σ, using the relationship σ = σobs/ϕ2PE, where ϕ2PE is the two-photon quantum yield for photoinduced decomposition of 1. Values for ϕ2PE are not typically measured explicitly, but are instead assumed, due to Kasha's rule, to be identical to the quantum yield for the same photoreaction under single-photon conditions, ϕ2PE = ϕ1PE.17 The quantum yield for single-photon N–O bond cleavage of 1 in acetonitrile has been measured to be near 0.5;20 however, this is not the quantum yield for decomposition, since photochemical decomposition of N-acetyloxy-2-pyridinethione derivatives can involve radical chain reactions, where the 2-pyridinethiyl radical generated by photolytic cleavage of the N–O bond in an excited state N-acetyloxy-2-pyridinethione can attack a ground state substrate to induce a subsequent N–O bond cleavage.25 At low substrate concentrations, such as those used in the present work, the quantum yield of photodecomposition of 1 has been found to be near ϕ1PE = 1,20 which would yield a two-photon absorption cross-section for ester 1 of σ≈σobs = 0.13 GM.
4. Conclusion
We have observed that a prototypical Barton ester, N-phenylacetyloxy-2-pyridinethione, is sensitive to efficient two-photon photochemistry. This finding provides reason to consider Barton esters and other similar 2-pyridinethione derivatives as viable precursors for radical generation by two-photon excitation, and furthermore, provides an impetus for development of modified Barton esters with even higher two-photon cross sections.Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council of Canada for generous funding of this research.Notes and references
- M. F. Saraiva, M. R. C. Couri, M. Le Hyaric and M. V. de Almeida, The Barton ester free-radical reaction: a brief review of applications, Tetrahedron, 2009, 65, 3563 CrossRef CAS.
- D. H. R. Barton, The invention of chemical-reactions – the last 5 years, Tetrahedron, 1992, 48, 2529 CrossRef CAS.
- A. L. J. Beckwith and B. P. Hay, Generation of alkoxy radicals from N-alkoxypyridinethiones, J. Am. Chem. Soc., 1988, 110, 4415 CrossRef CAS.
- C. Ha, J. H. Horner, M. Newcomb, T. R. Varick, B. R. Arnold and J. Lusztyk, Kinetic-studies of the cyclization of the 6,6-diphenyl-5-hexenyl radical – a test of the accuracy of rate constants for reactions of hydrogen transfer agents, J. Org. Chem., 1993, 58, 1194 CrossRef CAS.
- C. Bohne, R. Boch and J. C. Scaiano, Exploratory studies of the photochemistry of N-hydroxypyridine-2-thione esters – Generation of excited radicals by laser flash-photolysis and in a conventional fluorescence spectrometer, J. Org. Chem., 1990, 55, 5414 CrossRef CAS.
- W. Adam, D. Ballmaier, B. Epe, G. N. Grimm and C. R. Sahamoller, N-Hydroxypyridinethiones as photochemical hydroxyl radical sources for oxidative DNA-damage, Angew. Chem., Int. Ed. Engl., 1995, 34, 2156 CrossRef CAS.
- W. Adam, G. N. Grimm, S. Marquardt and C. R. Saha-Moller, Are pyridinethiones reliable photochemical oxyl-radical sources for photobiological studies? The importance of secondary photolysis products in the guanine oxidation of 2′-deoxyguanosine and cell-free DNA, J. Am. Chem. Soc., 1999, 121, 1179 CrossRef CAS.
- E. A. Theodorakis and K. M. Wilcoxen, N-aroyloxy-2-thiopyridones as efficient oxygen-radical generators: Novel time-controlled DNA photocleaving reagents, Chem. Commun., 1996, 1927 RSC.
- E. A. Theodorakis, X. Xiang and P. Blom, Photochemical cleavage of duplex DNA by N-benzoyloxy-2-thiopyridone linked to 9-aminoacridine, Chem. Commun., 1997, 1463 RSC.
- P. Blom, A. X. Xiang, D. Kao and E. A. Theodorakis, Design, synthesis, and evaluation of N-aroyloxy-2-thiopyridones as DNA photocleaving reagents, Bioorg. Med. Chem., 1999, 7, 727 CrossRef CAS.
- B. M. Aveline and R. W. Redmond, Exclusive free radical mechanisms of cellular photosensitization, Photochem. Photobiol., 1998, 68, 266 CrossRef.
- M. Moller, W. Adam, C. R. Saha-Moller and H. Stopper, Studies on cytotoxic and genotoxic effects of N-hydroxypyridine-2-thione (Omadine) in L5178Y mouse lymphoma cells, Toxicol. Lett., 2002, 136, 77 CrossRef CAS.
- M. Moller, W. Adam, S. Marquardt, C. R. Saha-Moller and H. Stopper, Cytotoxicity and genotoxicity induced by the photochemical alkoxyl radical source N-tert-butoxypyridine-2-thione in L5178Y mouse lymphoma cells under UVA irradiation, Free Radical Biol. Med., 2005, 39, 473 CrossRef.
- G. Nocentini, E. Castagnino, A. Salvatori, S. Corsano and M. C. Fioretti, In vitro evaluation of the potential antitumor-activity of an N-acridyl-pentanoyloxypyridine-2-thione derivative, Arzneimittelforschung, 1995, 45-2, 1127 CAS.
- M. Goppert-Mayer, Ann. Phys. (Leipzig), 1931, 9, 273 Search PubMed.
- S. W. Botchway, A. G. Crisostomo, A. W. Parker and R. H. Bisby, Near infrared multiphoton-induced generation and detection of hydroxyl radicals in a biochemical system, Arch. Biochem. Biophys., 2007, 464, 314 CrossRef CAS.
- N. K. Urdabayev, A. Poloukhtine and V. V. Popik, Two-photon induced photodecarbonylation reaction of cyclopropenones, Chem. Commun., 2006, 454 RSC.
- D. H. R. Barton and J. A. Ferreria, N-hydroxypyridine-2(1H)-thione derivatives of carboxylic acids as activated esters. 1. The synthesis of carboxamides., Tetrahedron, 1996, 52, 9347 CrossRef CAS.
- S. Stoyanov, I. Petkov, L. Antonov, T. Stoyanova, P. Karagiannidis and P. Aslanidis, Thione-thiol tautomerism and stability of 2-mercaptopyridines and 4-mercaptopyridines and 2-mercaptopyrimidines, Can. J. Chem., 1990, 68, 1482 CAS.
- B. M. Aveline, I. E. Kochevar and R. W. Redmond, Photochemistry of N-hydroxypyridine-2 thione derivatives - Involvement of the 2-pyridylthiyl radical in the radical-chain reaction-mechanism, J. Am. Chem. Soc., 1995, 117, 9699 CrossRef CAS.
- T. Furuta, S. S. H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Callaway, W. Denk and R. Y. Tsien, Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1193 CrossRef CAS.
- N. I. Kiskin and D. Ogden, Two-photon excitation and photolysis by pulsed laser illumination modelled by spatially non-uniform reactions with simultaneous diffusion, Eur. Biophys. J. Biophys. Lett., 2002, 30, 571 Search PubMed.
- N. I. Kiskin, R. Chillingworth, J. A. McCray, D. Piston and D. Ogden, The efficiency of two-photon photolysis of a “caged” fluorophore, o-1-(2-nitrophenyl)ethylpyranine, in relation to photodamage of synaptic terminals, Eur. Biophys. J. Biophys. Lett., 2002, 30, 588 Search PubMed.
- D. P. Decosta and J. A. Pincock, Photochemistry of Substituted 1-Naphthylmethyl Esters of Phenylacetic and 3-Phenylpropanoic Acid – Radical Pairs, Ion-Pairs, and Marcus Electron-Transfer, J. Am. Chem. Soc., 1993, 115, 2180 CrossRef.
- D. H. R. Barton, P. Blundell and J. C. Jaszberenyi, Quantum yields in the photochemically induced radical chemistry of acyl derivatives of thiohydroxamic acids, J. Am. Chem. Soc., 1991, 113, 6937 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2010 |
