In vivo quantification of kleptoplastic chlorophyll a content in the “solar-powered” sea slug Elysia viridis using optical methods: spectral reflectance analysis and PAM fluorometry
João
Serôdio
,
Sílvia
Pereira
,
Joana
Furtado
,
Raquel
Silva
,
Helena
Coelho
and
Ricardo
Calado
Departamento de Biologia and CESAM–Centro de Estudos do Ambiente e do Mar, Universidade de Aveiro, Campus de Santiago, 3810-193, Aveiro, Portugal. E-mail: jserodio@ua.pt; Fax: +351 23432587; Tel: +351 234370787
First published on 30th November 2009
Abstract
Kleptoplasty is a particularly remarkable type of symbiosis, consisting of the presence of functional chloroplasts in the tissues of a host of another species. One of the most well-studied types of kleptoplasty is the association between sacoglossan molluscs (sea slugs) and algal chloroplasts. After ingestion, the chloroplasts remain photosynthetically functional and provide photosynthates to the host, therefore named as “solar-powered” sea slugs. This study evaluated the use of two optical methods, spectral reflectance analysis and in vivo Chl fluorescence, as measured by pulse amplitude modulated (PAM) fluorometry, for the in vivo quantification of kleptoplastic chlorophyll (Chl) a content in the sacoglossan Elysia viridis (Montagu, 1804) bearing chloroplasts of the macroalgae Codium tomentosum var. mucronatum (G. Hamel) Ardré. The Chl a content of E. viridis specimens was compared to a number of reflectance-based indices and to the dark-level fluorescence, Fo. Most reflectance-based indices varied linearly with the symbiosis Chl a content over the whole range of pigment content variation. Most significant correlations (P < 0.001) were found between indices using as reference the reflectance at 750 nm, with the proportion of pigment content explained by the indices varying between 63.5% and 85.9%. Fo varied linearly with the Chl a content only for low pigment levels (below 4–6 μg Chl a per individual), above which it followed a saturation-like pattern. The use of optical methods was illustrated by monitoring the changes in Chl a content of specimens during periods of starvation and subsequent recovery. The results of this study suggest that, if basic requirements of signal detection and reproducible measuring geometry are verified, these optical methods may be readily applied to other photosynthetic symbioses.
Introduction
Photosynthetic symbioses between animal hosts and algal endosymbionts occur in several groups of marine invertebrates. Hosts are predominantly of the phyla Porifera (sponges) and Cnidaria (e.g. sea anemones or corals), while the endosymbiont is usually a green alga (Chlorophyta, e.g. genus Chlorella) or a dinoflagellate (Dinophyta, typically of the genus Symbiodinum).1 The algal endosymbiont (zooxanthellae) remains photosynthetically active within the animal's tissues, so that the photosynthates are made available to the host cells, constituting a major source of organic matter.A particularly remarkable kind of photosynthetic symbiosis is the association formed between sacoglossan molluscs (sea slugs), animals with a much more complex internal organization than the more common zooxanthellae-bearing hosts, and the chloroplasts of macroalgae. This very particular type of biological association, termed kleptoplasty, is an extreme case of photosynthetic symbiosis, in which only the algal organelles responsible for photosynthesis—the chloroplasts, in this context named kleptoplasts—are maintained structurally intact within the host's tissues.2–5 Kleptoplasty occurs when sacoglossa feed on macroalgae and, whilst most of the ingested algal material is readily digested, the chloroplasts remain functional for variable periods of time, ranging from a single day to several months.4,6,7 The most unique aspect of this symbiosis is that kleptoplasts remain photosynthetically active in the absence of algal nucleus control, a feature recently shown to result from the horizontal gene transfer between the macroalgae and the animal host.8,9 Kleptoplasty is also ecologically relevant as the photosynthates produced by the kleptoplasts represent an important source of organic matter, enabling the animal to withstand periods of food shortage.2,10–12 This remarkable adaptation has granted the label “solar-powered”† to these sea slugs.6
As with other types of invertebrate/algal symbiosis, a key parameter for functionally characterising kleptoplastic associations is the host content in chlorophyll (Chl) a. Chl a is a photosynthetic pigment present in all photosynthetic eukaryotes and cyanobacteria, its content being the most commonly used index for photosynthetic microalgae biomass (e.g. phytoplankton abundance). The determination of the Chl a content of kleptoplastic sacoglossans is thus a common form of assessing the presence and abundance of kleptoplasts.2,4,13–15 The monitoring of kleptoplastic Chl a content may be used to detect periods of algal food shortage or changes in food preference,3 assess the nutritional dependency on photosynthesis,11 or characterise the stability and turn over rates of kleptoplasts.12,16,17 Chl a content is further required for the detection of changes in sacoglossan development phases (post-larval initiation of algal ingestion18) and the normalization of photosynthetic rates (calculation of Chl a-specific rates19).
The Chl a content of kleptoplast-bearing sacoglossans has been commonly assessed through pigment extraction and quantification, a destructive method requiring the death of the animals.3,4,13,14,20,21 The introduction of pulse amplitude modulated (PAM) fluorometry22 to this field,23 introduced the possibility of performing non-destructive measurements of kleptoplasts photosynthetic activity.7,24 Besides the regularly-used photophysiological Chl fluorescence-based indices, fluorescence parameters measurable through PAM fluorometry (such as the dark-level Fo; see below) have also been used to estimate the Chl a content in microalgal-containing systems (e.g. sedimentary biofilms25). Although this technique has already been employed to study sacoglossans,7,23,24 the relationship between fluorescence parameters and Chl a content has not been experimentally confirmed.24
This study evaluates the use of optical methods for the in vivo, non-destructive quantification of kleptoplastic Chl a content, using as a biological model the association between the sacoglossan Elysia viridis (Montagu, 1804) and the chloroplasts of the green macroalgae Codium tomentosum var. mucronatum (G. Hamel) Ardré. Two techniques commonly used for the remote sensing of photosynthetic pigment content in a variety of organisms were tested: spectral reflectance, through which Chl a is quantified based on its distinctive visible light absorption features, and in vivo Chl fluorescence, as measured by PAM fluorometry, based on the active excitation of the light-harvesting complexes (LHC) pigments and detection of fluorescence emitted by photosystem II (PSII).
Materials and methods
Specimen collection and stocking
Specimens of E. viridis were collected by hand in stands of C. tomentosum var. mucronatum growing on intertidal rocky shores at two locations on the west coast of Portugal (north-eastern Atlantic), Aguda (41° 02′ N, 8° 39′ W) and Cabo Raso (38° 42′ N, 9° 29′ W) in June 2008. A total of 62 animals were used in the study, 43 from Aguda and 19 from Cabo Raso. The animals were maintained in 20 × 40 cm plastic trays (ca. 20 individuals per tray) with filtered natural seawater and recently-collected Codium until used for experiments. The trays were kept in a growth chamber (Growth Cabinet, Sanyo, Osaka, Japan) at 20 °C in a 12 h light–12 h dark cycle, illuminated by fluorescent lamps (FL40SS w/37, Sanyo) delivering a PAR irradiance of 50 μmol m−2 s−1 at the surface of the tray.Measuring setup
Spectral reflectance and in vivo Chl fluorescence were measured by carefully placing each animal in the centre of the well of a concavity microscope slide (15 mm diameter well, 0.5 mm deep), filled with seawater and covered with a coverslip, as described by Vieira et al.24 Measurements were made on the animals' dorsal surface with opened parapodia. Because of the continuous movements of the animals, even when placed between the microscope slide and the coverslip, measurements were made immediately after placing the animals in the desired position. Both types of measurements were carried out maintaining a fixed distance between the microscope slide and the tip of the fiberoptics of the spectroradiometer or the fluorometer (see below).Spectral reflectance
Reflectance spectra were measured over the 350–1000 nm bandwidth with a spectral resolution of 0.33 nm, using a USB2000 spectrometer (USB2000-VIS-NIR, grating #3, Ocean Optics, Duiven, The Netherlands) connected to a 400 μm diameter fiber optic (QP400-2-VIS/NIR-BX, Ocean Optics). The light spectrum reflected from each sample was normalized to the spectrum reflected from a reference white panel (WS-1-SL Spectralon Reference Standard, Ocean Optics), placed at the same distance from the fiberoptics as the sample (see below), and beneath the coverslip and concavity microscope slide filled only with seawater. Sample and reference spectra were measured under a constant irradiance of 70 μmol m−2 s−1 of cold white light, provided by an halogen lamp (Quartzline DDL 150 W, General Electric, Fairfield, USA) in a fiberoptics illuminator (Intralux 5000-1, Volpi, Schlieren, Switzerland). A reflectance spectrum measured in the dark was subtracted from both spectra to account for the dark current noise of the spectrometer. The fiberoptics was maintained at a fixed distance of 30 mm from the microscope slide, set so that the view field covered the total animal surface. A minimum of five replicated spectra were measured on each individual and occasion, and the mean spectrum was smoothed using a 10 point moving average filter before it was used for subsequent calculations. Replication was found necessary due to the continuous movements of the animals and possible changes in the surface exposed to light.A number of indices were calculated from reflectance spectra and compared to the Chl a content of E. viridis individuals. The different reflectance indices use various wavelengths of the visible spectrum, and most use the reflectance at 750 nm (R750) as a reference, as it is expected not to be affected by light absorption of photosynthetic pigments. Wavelengths and formulae used in the calculation of the indices tested in the present study are listed in Table 1. These indices were derived under a range of different contexts, such as the mapping of terrestrial vegetation or the quantification of microalgal biomass on aquatic sediments, and more detailed descriptions can be found elsewhere.26
| λ ref | λ abs | Index | Formula | Reference | Aguda | Cabo Raso | All |
|---|---|---|---|---|---|---|---|
| IR | Red | NDVI | (R750−R675)/(R750 + R675) | Rouse et al.27 | 0.799*** | 0.719*** | 0.752*** |
| RVI | R 750/R672 | Jordan28 | 0.766*** | 0.771*** | 0.759*** | ||
| NDVImin | (R750−Rmin(650–700))/(R750 + Rmin(650–700)) | This study | 0.753*** | 0.717*** | 0.733*** | ||
| Green | Green NDVI | (R750−R550)/(R750 + R550) | Gitelson et al.29 | 0.774*** | 0.832*** | 0.803*** | |
| R 750/R550 | Lichtenthaller et al.30 | 0.724*** | 0.859*** | 0.794*** | |||
| NDVI575 | (R750−R575)/(R750 + R575) | This study | 0.773*** | 0.841*** | 0.806*** | ||
| Blue | IR-B | (R750−R435)/(R750 + R435) | Kromkamp et al.31 | 0.769*** | 0.635*** | 0.684*** | |
| Other | Red | R 700/R675 | Gitelson et al.32 | 0.461** | 0.217* | 0.310*** | |
| R 562/R647 | Murphy et al.26 | 0.598** | 0.316* | 0.408*** | |||
| Blue | NPCI | (R680−R430)/(R680 + R430) | Peñuelas et al.33 | 0.530** | 0.187ns | 0.320*** | |
| BG | (R590−R435)/(R590 + R435) | Kromkamp et al.31 | 0.616** | 0.125ns | 0.299** |
In vivo Chl fluorescence
The Chl a content and the physiological status of the kleptoplasts of individual animals were measured non-destructively using PAM fluorometry, as described by Vieira et al.24 Variable Chl fluorescence was measured on single individuals using a fluorometer comprising a computer-operated PAM-Control Unit (Walz, Effeltrich, Germany) and a WATER-EDF-Universal emitter-detector unit (Gademann Instruments, Würzburg, Germany). Measuring, actinic and saturating light were provided by a blue LED lamp (peaking at 450 nm, half-bandwidth of 20 nm), and were delivered to the studied specimens by a 6 mm diameter Fluid Light Guide fiberoptics bundle. The fluorometer fiberoptics was positioned perpendicularly to the microscopy slide containing the animal, and all measurements were made at a fixed distance of 1 mm, controlled by a micromanipulator (MM33, Märtzhäuser, Germany). The fluorometer was zeroed using the concavity microscopy slide and coverslip filled only with seawater.Measurements were always carried out at the same time of the day, at least 2 h after the start of the daylight period, to ensure the full activation of the photosynthetic apparatus. At each occasion, immediately following the measurement of the reflectance spectra, the animals were dark-adapted for 15 min, after which one saturation pulse (0.8 s) was applied to determine the minimum- or dark-level fluorescence, Fo, a parameter expected to correlate with the Chl a content,24,25 and the maximum fluorescence, Fm. Fo and Fm were used to determine the maximum quantum yield of PSII, Fv/Fm (= (Fm−Fo)/Fm).22
Pigment quantification
Animals were freeze-dried overnight, dry-weighted, and their photosynthetic pigments were extracted in 1.5 ml of 90% acetone at 4° C in the dark for 24 h. The extracts were centrifuged (1300 rpm, 10 min) and the Chl a and Chl b content of individual animals were determined spectrophotometrically (Genesys 6, Thermo Spectronic, Whalthman, USA) using the equations of Jeffrey & Humphrey34 for Chl b-containing organisms. The equations were adapted to yield pigment content in units of μg per individual.Relationship between optical indices and Chl a content
To test for the existence of functional relationships between optical indices and kleptoplastic Chl a content of living animals, reflectance spectra and Chl fluorescence were measured on specimens with varying Chl a content. To obtain a wide range of kleptoplastic pigment content, a total of recently-collected and well-fed 32 individuals (13 from Aguda, 19 from Cabo Raso) were submitted to a period of starvation, known to induce the gradual loss of Chl a.7,24 Every two days, 2–3 animals were withdrawn from stocking trays and used for reflectance spectra and Chl fluorescence measurements. Animal length was measured under a stereo-microscope (SZX10, Olympus) by averaging at least three independent determinations of maximum length. Each specimen was then frozen (−80° C) until pigment extraction and quantification.Monitoring of changes in Chl a content on individual sacoglossans
To illustrate the use of the optical methods to monitor the variation in individual kleptoplastic Chl a content, a second set of specimens were subjected to starvation, to induce the loss of Chl a, after which they were re-fed with fresh Codium, to follow the recovery in kleptoplastic pigment content. During a period of 27 days (13 days of starvation and 14 days of recovery), reflectance spectra were measured on individual specimens to quantify Chl a, and Fv/Fm was determined to assess the photophysiological status of ingested kleptoplasts. To test the possible effects of animal size on the loss and recovery of kleptoplastic pigments, this experiment was carried out in parallel on two groups of specimens, corresponding to two size classes, below and above 8 mm in length (range of body sizes: 5.4–7.5 mm and 8.4–13.0 mm). All animals used in this experiment were collected at Aguda.Statistical methods
The existence of functional relationships between Chl a content and reflectance spectra indices or Chl fluorescence parameters was tested by linear regression analysis. Regression equations (slope and intercept) were compared by applying Analysis of Covariance (ANCOVA). Statistical analyses were carried out following Sokal and Rohlf.35Results
Spectral reflectance indices vs. Chl a
Fig. 1 shows typical reflectance spectra as measured on E. viridis individuals and its variation over time following the decrease in kleptoplastic Chl a content. On well-nourished individuals (at the beginning of the experiment, t0), the reflectance spectrum was characterised by low reflectance values throughout the 400–700 nm wavelength range, denoting the high absorption of visible light by photosynthetic pigments, causing the typical dark green appearance of the animals (Fig. 1). A steep increase in reflectance was observed in the 700–750 nm region, and much higher (ca. three-fold) reflectance levels were recorded for the near-infrared region (>750 nm), compatible with the low absorption by photosynthetic pigments on this spectral region. In the visible wavelength range, reflectance spectra showed a well-defined and wide inverted peak in the red 650–700 nm region, with a minimum at 672–675 nm, corresponding to the in vivo absorption peak of Chl a. Also clearly visible is a second local reflectance minimum at ca. 650 nm, corresponding to the in vivo absorption maximum of Chl b. Local reflectance maxima in the yellow-green region (525–650 nm) separate the red region reflectance minima from a second spectral region of low reflectance, on the blue region of the spectrum (<525 nm), caused by the high absorption by Chls a and b and carotenoids. Considerably noisier spectra were usually recorded for wavelengths below 475 nm due to lower incident irradiance levels provided by the used light source in this spectral region. | ||
| Fig. 1 (a) Changes in reflectance spectra during loss of kleptoplastic Chl a in one Elysia viridis specimen 0, 13 and 27 days after the beginning of starvation. Vertical grey bars represent one standard error. (b) Average values normalized to the reflectance at 750 nm (R750), by dividing the reflectance at each wavelength by R750. Arrows indicate main wavelengths used for the calculation of reflectance indices tested for estimation of Chl a content. | ||
Major changes in reflectance spectra with decreasing Chl a content consisted mainly of the decrease in reflectance levels in the near infrared region (>700 nm), with values in the visible spectral range presenting a relatively much smaller variation, and retaining the features present in the beginning of the experiment (Fig. 1A). The accentuated decrease in the reflectance above 750 nm was caused by the reduction in non-photosynthetic absorption due to the reduction of animal's size, as indicated by the correlation found between R750 and the animals’ length (r2 = 0.503; P < 0.001; Fig. 2) or dry weight (r2 = 0.353; P < 0.001), and between Chl a content and these two optical biomass proxies (r2 = 0.702, P < 0.001; r2 = 0.575, P < 0.001). This caused the decrease in the total area of light interception (light absorption cross section) and thus of the amount of reflected infrared light. However, the overall absorption in the visible range decreased markedly in relation to that in the infrared region, as highlighted when comparing the reflectance spectra normalized to R750 (Fig. 1B). This denotes the loss of photosynthetic pigment content, and of the change in overall coloration from dark green to pale yellow.
 | ||
| Fig. 2 Linear relationship between the reflectance at 750 nm (R750) and length of Elysia viridis collected at two locations, Aguda and Cabo Raso. Line represents the linear regression equation fitted to the data of both sampling locations pooled together. Each data point represents one individual. Significant correlations were found also for the data from each sampling site separately (Aguda: r2 = 0.398, P < 0.05; Cabo Raso: r2 = 0.683, P < 0.001). | ||
As a consequence, reflectance indices using R750 as a reference, like the case of the majority of the indices used to estimate microalgal biomass, are expected to decrease following the loss in Chl a content. In fact, all tested reflectance indices based on R750 were found to vary linearly with the total Chl a content of E. viridis individuals (NDVI, RVI, NDVImin, Green NDVI, R750/R550, NDVI575 and IR-B; Table 1, Fig. 3). For all these indices, the correlation between the index value and the individual Chl a content was highly significant (P < 0.001), both when considering data from each sampling site separately or when pooling the data from the two sites together, with the proportion of pigment content explained by the index varying between 63.5% and 85.9%. For all these indices, no significant differences were found between the regression lines (slope and intercept) fitted on data from the two sampling sites (ANCOVA, P > 0.05 in all cases), allowing to fit a single regression line to the whole set of data points.
 | ||
| Fig. 3 Linear relationship between various reflectance indices and the Chl a content of Elysia viridis collected at two locations, Aguda and Cabo Raso. Lines represent linear regression equations fitted to the data of both sampling locations pooled together. (a) NDVI. (b) R750/R550. (c) Green NDVI575. (d) RVI. Each data point represents one individual. | ||
Amongst the reflectance indices using infrared wavelengths as a reference, those based on reflectance minima on the green region were the ones that yielded the strongest correlations with Chl a, especially when considering the pooled dataset (Green NDVI, R750/R550 and NDVI575; Table 1). The index NDVI575, a modified version of the Green NDVI based on the reflectance at 575 nm (instead of R675), provided the highest correlation for the pooled data, explaining 80.6% of the variability in Chl a content (Table 1, Fig. 3A). The other two green–infrared indices yielded only slightly lower correlations, still explaining a very high percentage of Chl a variability, always above 72% (Table 1, Fig. 3B). Correlations with the indices based on the infrared and red regions (NDVI, RVI; Table 1; Fig. 3C,D) were in most cases lower, although the proportion of the Chl a variability explained was decreased on average by only 4.6% (pooled data). In the case of NDVI, the most often used vegetation and microalgal biomass index, the proportion of the Chl a variability explained varied between 71.9% (Cabo Raso) and 79.9% (Aguda), being the index that yielded the highest predictive ability for the Aguda population. NDVImin, a modified version of NDVI designed to use to the reflectance minimum in the 650–700 wavelength interval, did not result in an improvement in predictive ability relatively to the green–infrared or the other tested red–infrared indices. The blue–infrared index IR-B did not yield better results than the remaining infrared-based indices (Table 1).
The reflectance indices not using infrared wavelengths as a reference were in all cases clearly the worst predictors of the Chl a content. The index R562/R647, recently proposed for estimating the biomass of sediment-inhabiting microalgae, provided the best correlation with Chl a, but explained only 40.8% of Chl a variability (pooled data, Table 1). The worst results were obtained for the indices based on the reflectance minimum in the blue region (NPCI and BG), which for the Cabo Raso dataset did not correlate significantly with Chl a (Table 1). As a very strong correlation was found between the content in Chl a and in Chl b (r2 = 0.960, P < 0.001), very similar relationships were obtained between each reflectance indices and the content of the two pigments (data not shown).
Chl fluorescence vs. Chl a
Unlike reflectance indices, the Chl fluorescence parameter Fo did not increase linearly for the whole range of Chl a content. A linear relationship between Fo and Chl a content was found for low Chl a values (<6 μg Chl a per individual; r2 = 0.594, P < 0.001), both for the data from each sampling site separately as for the pooled data. No significant differences were found between slope (ANCOVA, F[1,26] = 0.018, P = 0.893) and intercept (ANCOVA, F[1,27] = 1.615, P = 0.215) of data from the two sampling sites. Above 4–6 μg Chl a per individual, Fo did not increase with Chl a content and a clear saturation-like pattern became apparent (Fig. 4). A positive intercept was found for the linear part of the Fovs. Chl a relationship, denoting the emission of a measurable fluorescence signal from animal tissues without Chl a. | ||
| Fig. 4 Relationship between dark-level Chl fluorescence, Fo, and the Chl a content of Elysia viridis collected at two locations, Aguda and Cabo Raso. The line represents the linear regression equation fitted to the data of both sampling locations pooled together, for Chl a content <6 μg per individual. Each data point represents one individual. | ||
Effects of starvation and refeeding on Chl a content
Fig. 5 illustrates the inter-individual variability in the response to starvation of E. viridis and subsequent refeeding with Codium. On a minority of individuals (2 out of 30), starvation during 13 days resulted in the loss of their recovery capacity after refeeding and subsequent death. In these cases, Chl a content decreased following a negative exponential pattern until reaching very low values at the end of the starvation period, and did not recover after the start of the refeeding (Fig. 5A). Fv/Fm started to decrease after 9 days of starvation, reaching ca. 50% of initial values at the end of the starvation period, and continued to decrease almost linearly until attaining null values (Fig. 5A).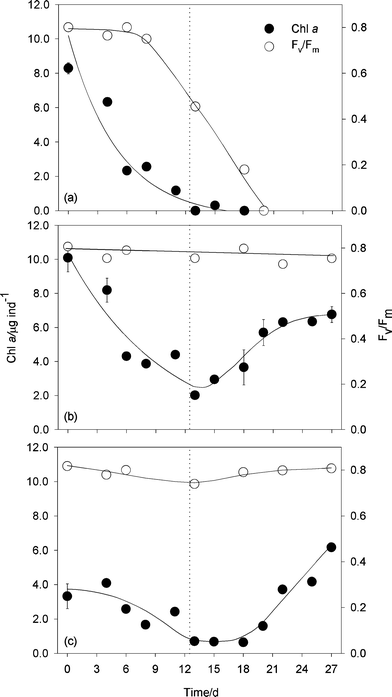 | ||
| Fig. 5 Examples of daily variation in Chl a content and photosynthetic activity of three Elysia viridis specimens during starvation and subsequent refeeding, as estimated non-destructively from the reflectance index NDVI and maximum PSII quantum efficiency, Fv/Fm, respectively. The vertical dotted line represents the end of the starvation period and start of refeeding with Codium. Each panel represents one individual. Panels (a) and (b) represent two individuals of the size class >8 mm (initial lengths of 9.8 and 10.1 mm, respectively) and panel (c) represents an individual of the size class <8 mm (initial length of 7.0 mm). Bars represent one standard error. | ||
Nonetheless, most specimens were able to successfully recover from starvation and increase their kleptoplastic Chl a content upon refeeding, although the pattern of response varied with the initial size of each animal (Fig. 5B,C). On large animals (>8 mm), the Chl a content decreased also in an exponential-like manner until the end of the starvation period, starting to increase immediately after the animal being refed with Codium (Fig. 5B). Recovery was completed after 10 days, with Chl a content usually stabilizing on values similar or slightly lower than the initial levels. Despite this large variation in Chl a content, no significant changes occurred regarding Fv/Fm throughout the whole duration of the experiment (Fig. 5B).
On smaller individuals (<8 mm), the decrease from initially lower Chl a levels resulted in the reaching of values close to zero at the end of the starvation period (Fig. 5C). However, unlike large animals, recovery in Chl a content became noticeable only after a lag of 3–5 days after start of refeeding. Chl a content continued to increase reaching levels clearly above those measured at the beginning of the experiment, an indication that these individuals had a larger growth potential. Again, Fv/Fm remained virtually constant during the whole experiment, only showing a slight decline near the end of the starvation period (Fig. 5C).
Despite the large inter-individual variability in the initial levels of Chl a content, the different response pattern of the two size classes were still detectable when considering the whole dataset (Fig. 6). The significantly different initial Chl a content of large and small animals decreased to similar levels after 13 days. Upon refeeding, both classes of individuals recovered at an identical rate so that, after 14 days, they no longer significantly differed in their Chl a content. Larger animals returned to Chl a contents similar to initial levels, while smaller specimens attained Chl a levels significantly higher than those initially recorded.
 | ||
| Fig. 6 Variation in Chl a content of Elysia viridis of two size classes (<8 mm and >8 mm) during starvation (days 0–13) and refeeding with Codium (days 14–27), as estimated from the reflectance index NDVI. Average of measurements made on 5 individuals. Bars represent one standard error, and thick and thin horizontal lines inside the boxes represent the mean and the median, respectively. Identical letters indicate non-significant differences. | ||
Discussion
Optical estimation of Chl a: spectral reflectance vs. Chl fluorescence
Both optical methods tested in this study proved capable of non-destructively estimating the kleptoplastic Chl a content of the E. viridis/Codium sp. symbiotic association. In comparison to Chl fluorescence, the use of spectral reflectance indices has the main advantages of allowing measurements to be taken under ambient light and, by using Chl a-specific absorption bands, to be largely insensitive to short-term changes in algal physiological status. The results of this study showed that various reflectance-based indices varied linearly with the symbiosis Chl a content over the whole range of pigment content variation. The small differences found between the predictive ability of these reflectance indices in the present study do not support the recommendation of any of these indices in particular. Nevertheless, the use of this technique to estimate the Chl a content of E. viridis individuals requires the previous calibration of these reflectance-based indices by means of the determination of the regression equation that allows converting index values into Chl a contents. The strong correlations consistently found between these indices and the Chl a content ensures that, even without determining a specific linear relationship between each index and the pigment content, changes in the index value may be taken as representing a proportional variation in the Chl a content.By providing detailed information on the absorption features over the whole visible spectrum, spectral reflectance analysis may further provide a rapid and non-invasive form of quantifying photosynthetic pigments other than Chl a. In particular, this technique may potentially be used to assess the operation of the xanthophyll cycle, a central photoprotective processes in intact algal and plant cells36 but to our knowledge never studied in kleptoplasts, by quantifying the changes in the relative amount of pigments involved in this process.37–39 Another possible application is, through the characterization of the animal's coloration, to quantify the relative content of different algal material (e.g. green versus red algal material12) and thus infer its recent feeding habits.
The present results also support the use of spectral reflectance analysis as a tool to estimate the animal's dry weight, based on the strong linear relationship found with R750. Dry weight is often used as a direct measure of the animal's biomass, but also used to normalise Chl a contents19 or photosynthetic rates,21 which has up to now required the sacrifice of the specimens under study.
Comparative to spectral reflectance analysis, the use of in vivo Chl fluorescence presents a number of a priori advantages. First, by being based on the response to an active excitation of LHC pigments, fluorescence emission presents a higher specificity and sensitivity for Chl a than reflectance-bases indices, since these rely solely on the passive absorption of light by all of the materials constituting the sample, not necessarily only Chl a. Secondly, given that Chl a content can be estimated by a single fluorescence parameter (Fo), determined almost instantaneously, this method does not require the time-consuming and complex mathematical treatment required for the analysis of reflectance spectra. Finally, the use of PAM fluorometry, along with the measurement of Fo, enables researchers to gather detailed information on the photophysiology of the sample, e.g. by determining Fv/Fm or a number of other fluorescence indices informative on various aspects of the functioning photosynthetic apparatus.40 In fact, the estimation of Chl a content may represent a minor use of PAM fluorometry, as this technique's main application is the quantification of PSII electron transport rates, achieved through the unique saturation pulse method, leading to the transient closure of PSII reaction centers and the detection of the corresponding fluorescence maxima.22
However, the positive aspects mentioned above are matched by several comparative disadvantages. One derives from the fact that, unlike reflectance indices, the fluorescence signal is strongly influenced by the distance between the fiberoptics and the sample surface. This makes it crucial to control the measuring geometry and, because a high signal-to-noise ratio may require a short measuring distance (few mm), this may result in a sampling area similar to the fiberoptics diameter, thus smaller than the total animal's surface. In the case of a heterogeneous Chl a distribution in the animal's tissues, this may introduce an additional source of error in the estimation of total Chl a content. Another shortcoming of the use of Fo is the need to dark-adapt the sample during a period of several minutes, a time-consuming operation that may potentially introduce errors in the Chl a determination.24 Also, although Fo is known to be the fluorescence parameter less influenced by chloroplast physiology, changes in physiological status may still affect the Fo emission per Chl a unit.25
The results of the present study further showed that the use of Fo to estimate kleptoplastic Chl a content may be less favourable than the use of spectral reflectance-based indices, mainly due to the non-linearity of the Fovs. Chl a relationship observed for high pigment contents. This saturation of Fo values for high Chl a levels may be due to self-shading of kleptoplasts accumulated in high numbers within the sea slug tissues. This aspect may be expected to cause a significant attenuation of (unidirectional) downwelling exciting light as well as a strong re-absorption of upwelling emitted fluorescence, and cause the overall, depth-integrated fluorescence emission not to follow proportionally the increase in Chl a content. As with reflectance-based indices, the use of Fo to quantify the Chl a content would require the establishment of a calibration curve in order to convert arbitrary fluorescence values (largely determined by instrument settings and measuring geometry) into meaningful Chl a content levels.
A potential application of these techniques is the development of optical-based indices for the estimation of photosynthetic production by the algal endosymbiont. Since the discovery of the close relationship between the Chl fluorescence index ΔF/Fm′ and the effective quantum yield of PSII in higher plants,41 fluorescence-based indices have been successfully developed for microalgal-containing systems, such as phytoplankton42 and microphytobenthos.43 However, an essential parameter for the estimation of absolute rates of photosynthesis is the light absorption efficiency of the sample, i.e. the fraction of incident light that is absorbed for photosynthesis, a quantity closely related to the Chl a content. Thus, reflectance indices may provide a way to non-destructively assess the symbiosis photosynthetic light absorption and thus, coupled with the estimation of the photosynthetic quantum yield provided by Chl fluorescence, be used to construct an optically-based index for the kleptoplastic photosynthate production.
Monitoring of changes in the Chl a content of individual sacoglossans
The results of the starvation/recovery experiments showed that spectral reflectance can be successfully used to trace and characterise in detail the pattern of change in the Chl a content of individual specimens of E. viridis. The method proved to be valuable to assess inter-individual variability response to shortage in algal food, as it is impossible to obtain these data using destructive methods. The results further illustrate the importance of measuring Chl a content to assess and predict the evolution of the overall physiological status of the symbiosis. The total amount of photosynthates provided to the animal host is determined by both the functionality of kleptoplasts (assessed by Fv/Fm), and by their total number, for which Chl a content is a direct proxy. Because Fv/Fm and other fluorescence parameters used to assess photophysiological status are independent of the absolute number of kleptoplasts or Chl a content, they cannot be used to predict processes dependent on the total amount of photosynthates produced. This is exemplified by the fact that Fv/Fm can remain virtually constant regardless of the accentuated decrease in Chl a content. As already shown by Vieira et al.,24 when the Chl a content decreases below a certain critical level, the Fv/Fm of the symbiosis drops abruptly, reflecting the irreversible degradation of general physiological condition and results in the animal's rapid death.The lack of differences regarding the survival and recovery of animals of the two size classes suggests that the capacity to resist periods of algal food shortage may not depend on the absolute body size but on the relative content in functional kleptoplasts. A possible explanation lies in the fact that the decrease in the number of kleptoplasts is followed by a decrease in body size, causing the decrease in photosynthates production to be matched by a proportional reduction in overall nutritional demands. The parallel decrease in amount of kleptoplasts and body size has been consistently observed for E. viridis.10,44
One difference recorded between the response of the animals of the two tested size classes is the time lag observed in smaller animals between the beginning of the refeeding period and the start of recovery in kleptoplastic Chl a content. This may be an indication that chloroplasts ingested in the beginning of the refeeding period are completely digested to fulfil nutritional needs. On the other hand, larger animals, which may possibly have larger nutritional reserves, could immediately start to accumulate photosynthetically active kleptoplasts upon the start of refeeding with fresh algal material.
Application to other invertebrate/algal symbiotic associations
This study establishes the possibility of using optical methods to quantify the Chl a content in the association of E. viridis and C. tomentosum var. mucronatum. However, considering the basic general requirements of signal detection and reproducible measuring geometry, these techniques may be readily applied to other photosynthetic symbioses. However, the assumption made in this study that all the Chl a present in each E. viridis can be optically detected may not hold in the case of larger animals. First, because the total surface of the animal may be larger than the area that can be monitored. In this context, spectral reflectance analysis should be preferred as the dimension of measuring area can be adjusted simply by increasing the distance between the fiberoptics and the sample. In the case of Chl fluorescence, this is not feasible as measuring distances above a few millimetres lower the fluorescence signal below the detection threshold. Second, because larger animals may be optically thicker, causing only the Chl a (and other pigments) present in the most superficial layers to be actually detected. Spectral reflectance analysis can nevertheless be used to quantitatively characterise the colouration of the surface tissue layers of kleptoplast- or zooxanthellae-bearing symbioses, as already done for large reef-building corals.45,46Yet another source of problems may be the movement of the animals during the measurements, changing their relative position to the optical probe and thus possibly affecting the fraction of the total pigment that is quantified. While this issue is not exclusive to larger animals, it was possible to overcome it in the case of Elysia viridis by making the measurements rapidly after positioning the animal in the microscope slide. This, however, may not efficient in the case of other species. Due to all of these factors, the application of optical indices to estimate Chl a content of animals containing kleptoplasts or zooxanthellae requires the previous verification of a linear relationship, and the determination of the corresponding linear regression equation, between the two variables. On the other hand, both techniques can be used to map the distribution of kleptoplasts or zooxanthellae within animal tissues,15 with PAM fluorometry having the additional advantage of allowing mapping of the distribution of photosynthetic activity over the host body.
Abbreviations
| BG | Blue-green index |
| Chl | Chlorophyll |
| F o and Fm | Minimum and maximum fluorescence of a dark-adapted sample |
| F v/Fm | Maximum quantum yield of PSII |
| IR | Infrared |
| IR-B | Blue-infrared index |
| LHC | Light-harvesting complexes |
| NDVI | Normalised difference vegetation index |
| NPCI | Normalized pigment Chl a index |
| PAM | Pulse amplitude modulation |
| PAR | Photosynthetically active radiation |
| PSII | Photosystem II |
| R λ | Reflectance at wavelength λ |
| RVI | Ratio vegetation index |
| t | Time after the start of the starvation period |
Acknowledgements
The authors thank Ana Ré for technical support in sea slug collection and stocking. H. Coelho is a recipient of a FCT–Fundação para a Ciência e a Tecnologia doctoral grant (SFRH/BD/23720/205). J. Furtado is a recipient of a FCT Integration into Research Grant (BII/C2008/CESAM-UA/Biologia). We thank two anonymous reviewers for critical comments on the manuscript.References
- A. A. Venn, J. E. Loram and A. E. Douglas, Photosynthetic symbiosis in animals, J. Exp. Bot., 2008, 59, 1069–1080 CrossRef CAS.
- R. W. Greene, Symbiosis in sacoglossan opisthobranchs: functional capacity of symbiotic chloroplasts, Mar. Biol., 1970, 7, 138–142 CrossRef CAS.
- G. R. Waugh and K. B. Clark, Seasonal and geographic variation in chlorophyll level of Elysia tuca (Ascoglossa: Opisthobranchia), Mar. Biol., 1986, 92, 483–487 CrossRef.
- K. B. Clark, K. R. Jensen and H. M. Stirts, Survey for functional kleptoplasty among West Atlantic Ascoglossa (=Sacoglossa) (Mollusca: Opisthobranchia), Veliger, 1990, 33, 339–345 Search PubMed.
- B. J. Green, W.-Y. Li, J. R. Manhart, T. C. Fox, E. J. Summer, R. A. Kennedy, S. K. Pierce and M. E. Rumpho, Mollusc-algal chloroplast endosymbiosis. Photosynthesis, thylakoid protein maintenance, and chloroplast gene expression continue for many months in the absence of the algal nucleus, Plant Physiol., 2000, 124, 331–342 CrossRef CAS.
- M. E. Rumpho, E. J. Summer and J. R. Manhart, Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis, Plant Physiol., 2000, 123, 29–38 CrossRef CAS.
- J. Evertsen, I. Burghardt, G. Johnsen and H. Wägele, Retention of functional chloroplasts in some sacoglossans from the Indo-Pacific and Mediterranean, Mar. Biol., 2007, 151, 2159–2166 CrossRef.
- S. K. Pierce, N. E. Curtis, J. J. Hanten, S. L. Boerner and J. A. Schwartz, Transfer, integration and expression of functional nuclear genes between multicellular species, Symbiosis, 2007, 43, 57–64 Search PubMed.
- M. E. Rumpho, J. M. Worfula, J. Leeb, K. Kannana, M. S. Tylerc, D. Bhattacharyad, A. Moustafad and J. R. Manharte, Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17867–17871 CrossRef CAS.
- R. Hinde and D. C. Smith, The role of photosynthesis in the nutrition of the mollusc Elysia viridis, Biol. J. Linn. Soc., 1975, 7, 161–171 Search PubMed.
- F. Giménez-Casalduero and C. Muniain, The role of kleptoplasts in the survival rates of Elysia timida (Risso, 1818): (Sacoglossa, Opisthobranchia) during periods of food shortage, J. Exp. Mar. Biol. Ecol., 2008, 357, 181–187 CrossRef.
- B. Teugels, S. Bouillon, B. Veuger, J. J. Middelburg and N. Koedam, Kleptoplasts mediate nitrogen acquisition in the sea slug Elysia viridis, Aquat. Biol., 2008, 4, 15–21 Search PubMed.
- R. K. Trench, J. E. Boyle and D. C. Smith, The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. II. Chloroplast ultrastrucutre and photosynthetic carbon fixation in E. viridis, Proc. R. Soc. London, Ser. B, 1973, 184, 63–81 CrossRef CAS.
- K. B. Clark and M. Bussaca, Feeding specificity and chloroplast retention in four tropical ascoglossa, with a discussion of the extent of chloroplast symbiosis and evolution of the order, J. Molluscan Stud., 1978, 44, 272–282.
- A. Gallop, J. Bartrop and D. C. Smith, The biology of chloroplast acquisition by Elysia viridis, Proc. R. Soc. London, Ser. B, 1980, 207, 335–349 CrossRef CAS.
- R. Hinde and D. C. Smith, Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa), Nat. New Biol., 1972, 239, 30–31 Search PubMed.
- R. K. Trench and S. Olhorst, The stability of chloroplasts from siphonaceous algae in symbiosis with sacoglossan mollusca, New Phytol., 1976, 76, 99–109 CrossRef CAS.
- C. D. Trowbridge, The missing links: larval and post-larval development of the ascoglossan opisthobranch Elysia viridis, J. Mar. Biol. Assoc. U. K., 2000, 80, 1087–1094 Search PubMed.
- K. B. Clark, K. R. Jensen, H. M. Stirts and C. Fermin, Chloroplast symbiosis in a non-elysiid mollusc, Costasiella liljanae Marcus (Hermaeidae: Ascoglossa (=Sacoglossa): Effects of temperature, light intensity, and starvation on carbon fixation rate, Biol. Bull., 1981, 160, 43–54 CrossRef.
- R. K. Trench and D. C. Smith, Synthesis of pigment in symbiotic chloroplasts, Nature, 1970, 227, 196–197 CAS.
- F. Giménez-Casalduero and C. Muniain, Photosynthetic activity of the solar-powered lagoon mollusc Elysia timida (Risso, 1818) (Opisthobranchia: Sacoglossa), Symbiosis, 2006, 41, 151–158 Search PubMed.
- U. Schreiber, U. Schliwa and W. Bilger, Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer, Photosynth. Res., 1986, 10, 51–62 CrossRef CAS.
- H. Wägele and G. Johnsen, Observations on the histology and photosynthetic performance of “solar-powered” opisthobranchs (Mollusca, Gastropoda, Opisthobranchia) containing symbiotic chloroplasts or zooxanthellae, Org. Diversity Evol., 2001, 1, 193–210 Search PubMed.
- S. Vieira, R. Calado, H. Coelho and J. Serôdio, Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis, Mar. Biol., 2009, 156, 1007–1020 CrossRef CAS.
- J. Serôdio, J. Marques da Silva and F. Catarino, Use of in vivo chlorophyll a fluorescence to quantify short-term variations in the productive biomass of intertidal microphytobenthos, Mar. Ecol.: Prog. Ser., 2001, 218, 45–61 CrossRef CAS.
- R. J. Murphy, T. J. Tolhurst, M. G. Chapman and A. J. Underwood, Estimation of surface chlorophyll-a on an emersed mudflat using field spectrometry: accuracy of ratios and derived-based approaches, Int. J. Remote Sens., 2005, 26, 1835–1859 CrossRef.
- J. W. Rouse, R. H. Haas Jr, J. A. Schell and D. W. Deering, Monitoring vegetation systems in the Great Plains with ERTS, Proc. ERTS-1 Symp., 3rd, Greenbelt, MD, 10–15 Dec. 1973, NASA, Washington, DC, SP-351, vol. 1, pp. 309–317 Search PubMed.
- J. T. O. Jordan, Determination of leaf area index from quality of light on the forest floor, Ecology, 1969, 50, 663–666 CrossRef.
- A. A. Gitelson, Y. J. Kaufman and M. N. Merzlyak, Use of a green channel in remote sensing of global vegetation from EOS-MODIS, Remote Sens. Environ., 1996, 58, 289–298 CrossRef.
- H. K. Lichtenthaler, A. Gitelson and M. Lang, Non-destructive determination of chlorophyll content of leaves of a green and an aurea mutant of tobacco by reflectance measurements, J. Plant Physiol., 1996, 148, 483–493 CAS.
- J. C. Kromkamp, E. P. Morris, R. M. Forster, C. Honeywill, S. Hagerthey and D. M. Paterson, Relationship of intertidal surface sediment chlorophyll concentration to hyperspectral reflectance and chlorophyll fluorescence, Estuaries Coasts, 2006, 29, 183–196 Search PubMed.
- A. Gitelson, G. Garbuzov, F. Szilagyi, K.-H. Mittenzwey, A. Karnielli and A. Kaiser, Quantitative remote sensing methods for real-time monitoring of inland waters quality, Int. J. Remote Sens., 1993, 14, 1269–1295 CrossRef.
- J. Peñuelas, J. A. Gamon, K. L. Griffin and C. B. Field, Assessing community type, plant biomass, pigment composition, and photosynthetic efficiency of aquatic vegetation from spectral reflectance, Remote Sens. Environ., 1993, 46, 110–118 CrossRef.
- S. W. Jeffrey and G. F. Humphrey, New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae, and natural phytoplankton, Biochem. Physiol. Pflanz., 1975, 167, 191–194 CAS.
- R. R. Sokal and F. J. Rohlf, Biometry, W. H. Freeman, New York, 2nd edn, 1981 Search PubMed.
- P. Müller, X.-P. Li and K. Niyogi, Non-photochemical quenching. A response to excess light energy, Plant Physiol., 2001, 125, 1558–1566 CrossRef CAS.
- J. Peñuelas, I. Filella, J. A. Gamon and C. Field, Assessing photosynthetic radiation-use efficiency of emergent aquatic vegetation from spectral reflectance, Aquat. Bot., 1997, 58, 307–315 CrossRef.
- J. A. Gamon and J. S. Surfus, Assessing leaf pigment content and activity with a reflectometer, New Phytol., 1999, 143, 105–117 Search PubMed.
- B. Jesus, J.-L. Mouget and R. G. Perkins, Detection of diatom xanthophyll cycle using spectral reflectance, J. Phycol., 2008, 44, 1349–1359 CrossRef CAS.
- K. Roháček, Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships, Photosynthetica, 2002, 40, 13–29 CrossRef CAS.
- B. Genty, J.-M. Briantais and N. R. Baker, The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence, Biochim. Biophys. Acta, Gen. Subj., 1989, 990, 87–92 Search PubMed.
- Z. Kolber and P. G. Falkowski, Use of active fluorescence to estimate phytoplankton photosynthesis in situ, Limnol. Oceanogr., 1993, 38, 1646–1665 CAS.
- J. Serôdio, S. Vieira and F. Barroso, Relationship of variable chlorophyll fluorescence indices to photosynthetic rates in microphytobenthos, Aquat. Microb. Ecol., 2007, 49, 71–85 Search PubMed.
- J. Evertsen and G. Johnsen, In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis, Mar. Biol., 2009, 156, 847–859 CrossRef CAS.
- E. J. Hochberg, M. J. Atkinson, A. Apprill and S. Andréfouët, Spectral reflectance of coral, Coral Reefs, 2004, 23, 84–95 CrossRef.
- N. Stambler and N. Shashar, Variation in spectral reflectance of the hermatypic corals, Stylophora pistillata and Pocillopora damicornis, J. Exp. Mar. Biol. Ecol., 2007, 351, 143–149 CrossRef.
Footnote |
| † Term adapted from Dr Bill Rudman, Australian Museum, and the Sea Slug Forum (http://www.seaslugforum.net/factsheet.cfm?base=solarpow). |
| This journal is © The Royal Society of Chemistry and Owner Societies 2010 |
