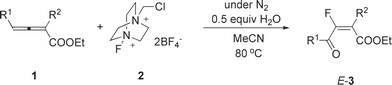
Study on the selectivity in the electrophilic monofluorination of 2,3-allenoates with Selectfluor™: an efficient synthesis of 4-fluoro-2(5H)-furanones and 3-fluoro-4-oxo-2(E)-alkenoates†
Bo Lü, Chunling Fu and Shengming Ma*
Laboratory of Molecular Recognition and Synthesis, Department of Chemistry, Zhejiang University, Hangzhou, 310027, Zhejiang, P. R. China. E-mail: masm@mail.sioc.ac.cn; Fax: (+86) 21-62609305
First published on 19th November 2009
Abstract
Different from the reaction of 2,3-allenoic acids with Selectfluor™, 4-fluoro-2(5H)-furanones and (E)-3-fluoro-4-oxo-2-alkenoates were highly selectively generated from 2,4-disubstituted 2,3-allenoates with Selectfluor™ under different conditions in moderate yields. The reaction of 2,4,4-trisubstituted 2,3-allenoates afforded the corresponding 4-fluoro-2(5H)-furanones highly selectively with up to 95% yield under different conditions. The scope of the substrates has been carefully explored. Due to the more readily availability of 2,3-allenoates as compared to 2,3-allenoic acids, new 4-fluoro-2(5H)-furanones were prepared. Based on the isolation and characterization of the minor fluorohydroxylation product E-5m, a mechanism has been proposed.
Introduction
Many fluorinated organic compounds have been identified as biologically active molecules,1 probably due to the fact that the fluorine most closely resembles hydrogen in size among the atoms but is much more electronegative than oxygen.2 Monofluorination has been usually conducted using the common but very dangerous and environmentally unfriendly fluorine gas. Considering this problem, chemists have developed a series of highly efficient and easily controlled electrophilic fluorination reagents such as DAST,3 CF3OF,4 XeF2,5N-fluoropyridinium triflate,6 NFSI,7 Selectfluor™,8,9etc. Among these reagents, Selectfluor™ has been proven to be a highly efficient fluorination reagent, and thus, has been widely used in organic synthesis.In 2008, we reported that 4-fluoro-2(5H)-furanones could be synthesized conveniently by the cyclization of 2,3-allenoic acid in the presence of Selectfluor™.8a Recently, this type of fluorocyclization reaction using 4,5-allenoic acid and Selectfluor™ has also been reported by Zhao, Zhu, and their co-workers.9 Since the acids are usually prepared from the hydrolysis of esters,10 it is desirable to prepare lactones directly from 2,3-allenoates.11a,b In this paper, we wish to report our recent study on the reaction of 2,3-allenoates with Selectfluor™, which affords 4- fluoro-2(5H)-furanones and 3-fluoro-4-oxo-2(E)-alkenoates under different reaction conditions, respectively.12
Results and discussion
The initial experiment was carried out by using ethyl 2-methyl-4-phenyl-2,3-butadienoate 1a and 1.2 equiv of Selectfluor™2 in MeCN at 80 °C (Table 1, entry 1). Interestingly, 4-fluoro-3-methyl-5-phenyl-2(5H)-furanone 4a was formed together with an unknown product. However, from 1H NMR and 13C NMR, IR, MS and HRMS analysis, we identified this new product as ethyl 3-fluoro-2-methyl-4-oxo-4-phenyl-2(E)-butenoate 3a.13,14
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | 2a | Solvent | Additive (equiv) | Time/h | NMR Yield (%)b | Ratio (3a/4a)b | 1a (%)c | |
| E-3a | 4a | |||||||
| a Equivalents of 2.b Determined by 1H NMR analysis of the crude reaction mixture using 1,3,5-trimethylbenzene as the internal standard.c 1a recovered after the reaction.d Determined by 19F NMR analysis.e The reaction was carried out under N2; MeCN was refluxed in the presence of calcium hydride for 10 h and distilled immediately before use. | ||||||||
| 1 | 1.2 | MeCN | — | 19.5 | 19 | 35 | 35/65 | 7 |
| 2 | 1.2 | MeCN | Li2CO3 (2.0) | 11.5 | 12.5 | 4.5 | 74/26 | 8 |
| 3 | 2.0 | MeCN | Li2CO3 (2.0) | 14 | 32 | 13 | 71/29 | — |
| 4 | 2.0 | MeCN | Na2CO3 (2.0) | 19.3 | 9 | 3 | 75/25 | 3.5 |
| 5 | 2.0 | MeCN | K2CO3 (2.0) | 19.3 | 10 | — | 87/13d | 28 |
| 6 | 2.0 | MeCN | Cs2CO3 (2.0) | 19 | Trace | Trace | — | 33 |
| 7 | 3.0 | MeCNe | — | 9 | 26 | — | 98/2d | — |
| 8 | 3.0 | MeCNe | H2O(0.5) | 10.5 | 60 | 1.6 | 97/3 | — |
| 9 | 3.0 | MeCNe | H2O (1.0) | 10 | 26 | 1.3 | 95/5 | — |
| 10 | 1.5 | MeCN–H2O = 5/1 | 12 | — | 54 | 1/99 d | 3 | |
| 11 | 1.5 | MeCN–H2O = 3/1 | 11.5 | — | 50 | 1/99d | 2 | |
| 12 | 1.5 | MeCN–H2O = 2/1 | 11.5 | — | 57 | 1/99d | 4 | |
| 13 | 1.5 | MeCN–H2O = 1/1 | 10.5 | — | 44 | 1/99d | — | |
| 14 | 1.7 | MeCN/H2O = 2/1 | 14.7 | — | 56 | 1/99d | — | |
With the addition of Li2CO3,13 the selectivity for the formation of 3a was improved; however, the yield was still very low, indicating the difference of the reaction of 2,3-allenoates with PhSeCl and Selectfluor™ (Table 1, entry 2). Increasing the amount of Selectfluor™ could also improve the yield (Table 1, entry 3). Other bases such as Na2CO3, K2CO3, and Cs2CO3 are also unsuitable in this reaction (Table 1, entries 4–6). The carbonyl functionality formed in compound 3a should have something to do with the trace amount of water in the commercially available MeCN or air, thus, the reaction was carried out under N2 in anhydrous MeCN, which had been refluxed in the presence of calcium hydride for 10 h and distilled immediately before use, to afford E-3a in 26% yield with a selectivity of 3a/4a as high as 98/2 (Table 1, entry 7). Encouraged by these results, a series of experiments were carried out by adding a fixed amount of water (Table 1, entries 8–9). The best results were given when 0.5 equiv of water was used to afford E-3a in 60% yield with the 3a/4a ratio being 97/3 (Table 1, entry 8, Conditions A). In addition, it is interesting to observe that further increasing the amount of water led to almost exclusive formation of 4a (Table 1, entries 10–13). When running the reaction with 1.7 equiv of Selectfluor™ in MeCN–H2O = 2/1 at 80 °C, lactone 4a was formed as the only product in 56% yield (Table 1, entry 14, Conditions B).
The configuration of the carbon–carbon double bond in E-3a was determined by 1H–19F HOESY analysis (heteronuclear Overhauser effect spectroscopy, see Fig. 1 and ESI†).
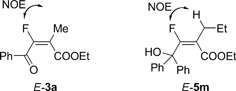 | ||
| Fig. 1 1H–19F HOESY analysis of E-3a and E-5m. | ||
The scope of selective fluorination of 2,3-allenoates to form (E)-3-fluoro-4-oxo-2-butenoates 3 was then explored under Conditions A. Some typical results are summarized in Table 2. The R1 group could be substituted phenyl group; R2 could be alkyl or benzyl group.
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | 1 | R1 | R2 | Time/h | Product | Yield (%)b | 3/4c |
| a Conditions A: A solution of 1 (0.2–0.3 mmol), 2 (3 equiv), and water (0.5 equiv) was stirred in 2–3 mL of anhydrous MeCN under N2 at 80 °C.b Isolated yield.c The ratio of 3/4 was determined by 19F NMR spectra of crude products. | |||||||
| 1 | 1a | Ph | Me | 10.5 | E-3a | 55 | 97/3 |
| 2 | 1b | Ph | Et | 10 | E-3b | 56 | 98/2 |
| 3 | 1c | Ph | n-Pr | 11 | E-3c | 53 | 98/2 |
| 4 | 1d | Ph | Bn | 11.5 | E-3d | 45 | 98/2 |
| 5 | 1e | p-FC6H4 | Me | 10 | E-3e | 45 | 99/1 |
| 6 | 1f | p-ClC6H4 | Me | 10.5 | E-3f | 46 | 98/2 |
| 7 | 1g | p-BrC6H4 | Me | 12 | E-3g | 55 | 98/2 |
| 8 | 1h | p-BrC6H4 | n-Pr | 10.5 | E-3h | 46 | 98/2 |
| 9 | 1i | p-MeC6H4 | Me | 10 | E-3i | 43 | 98/2 |
| 10 | 1j | p-MeC6H4 | Et | 9.5 | E-3j | 39 | 98/2 |
However, the reaction could not be extended to 4-alkyl-substituted 2,3-allenoate 1k (Scheme 1). The reaction of ethyl 4-(2-chlorophenyl)-2-methyl-2,3-butadienoate (1l) afforded ketone E-3l together with lactone 4l in a ratio of 87/13 (3l/4l) (Scheme 1).
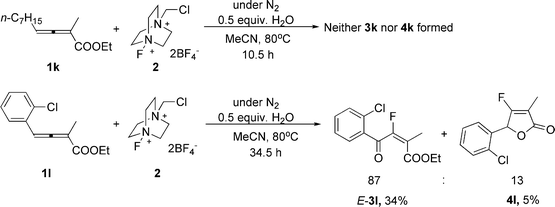 | ||
| Scheme 1 | ||
The fluorolactonization of 2,3-allenoates was also demonstrated under Conditions B (Table 3): When R2 is ethyl or propyl, 2.5 equiv of Selectfluor™ were required to form the corresponding lactones (Table 3, entries 2 and 3). The reaction of fully substituted 1m afforded 4m under Conditions B in 86% yield together with the fluorohydroxylation product, i.e., ethyl 3-fluoro-4-hydroxyl-4,4-diphenyl-2-propyl-2(E)-butenoate (5m) in 7% yield, indicating the existence of a 5m-type intermediate for this type of transformation (Scheme 2). Treatment of 1m with Selectfluor™ under Conditions A still led to the formation of lactone 4m in 93% isolated yield together with E-5m in 5% isolated yield (Scheme 2). The configuration of the carbon–carbon double bond in E-5m was determined by 1H-19F HOESY analysis (see Fig. 1 and ESI†). Further screening led us to observe that 4m could be afforded when 1m was treated with just 1.2 equiv of Selectfluor™ in MeCN at 80 °C (Conditions C) in 90% isolated yield with a selectivity of 4m/5m being 98/2 as determined by 19F NMR analysis of the crude reaction mixture (Scheme 2).
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | 1 | Conditionsa | t/h | Yield (%)b | 4/3b | 4/5b | ||
| R1 | R3 | R2 | ||||||
| a Conditions B: A solution of 1 (0.2–0.4 mmol) and 2 (1.7 equiv) was stirred in 2–4 mL of MeCN–H2O (2/1 (v/v)) at 80 °C; Conditions C: A solution of 1 (0.2 mmol) and 2 (1.2 equiv) was stirred in 2 mL of MeCN at 80 °C.b The ratios of 4/3 and 4/5 were determined by the 19F NMR analysis of the crude products.c A quantity of 2.5 equiv of 2 was used.d MeNO2 was used instead of MeCN. | ||||||||
| 1 | Ph | H | Me (1a) | B | 14.7 | 51 (4a) | >99/1 | — |
| 2 | Ph | H | Et (1b) | Bc | 23.5 | 50 (4b) | >99/1 | — |
| 3 | Ph | H | n-Pr (1c) | Bc | 23.5 | 47 (4c) | >99/1 | — |
| 4 | p-FC6H4 | H | Me (1e) | B | 26 | 60 (4e) | >99/1 | — |
| 5 | p-BrC6H4 | H | Me (1g) | B | 21.5 | 42 (4g) | >99/1 | — |
| 6 | p-MeC6H4 | H | Me (1i) | B | 21 | 64 (4i) | >99/1 | — |
| 7 | Ph | Me | Me (1n) | C | 5.3 | 80 (4n) | — | 99/1 |
| 8 | Ph | Ph | Me (1o) | C | 5.5 | 93 (4o) | — | 99/1 |
| 9 | Ph | Et | Me (1p) | C | 5.5 | 95 (4p) | — | 98/2 |
| 10 | Ph | Et | n-Pr (1q) | C | 5 | 91 (4q) | — | 99/1 |
| 11 | Ph | Et | H (1r) | C | 5 | 76 (4r) | — | 98/2 |
| 12 | -(CH2)5- | Me (1s) | C | 11 | 30 (4s) | — | 98/2 | |
| 13 | -(CH2)5- | Me (1s) | Cd | 10.8 | 42 (4s) | — | 98/2 | |
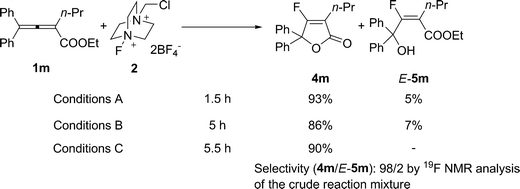 | ||
| Scheme 2 | ||
A series of 4,4-disubstituted 4-aryl 2,3-allenoates were then treated with Selectfluor™ under Conditions C. The corresponding 4-fluoro-2(5H)-furanones could also be afforded in excellent yields (Table 3, entries 7–11, Conditions C). MeNO2 is more effective than MeCN leading the lactonization reaction of 2,4,4-trialkyl substituted ethyl 3-cyclohexylidene-2-methylacrylate (1s) to afford 4s in relatively higher yields (Table 3, entries 12 and 13).
However, complicated products were observed when 2,4-disubstituted 2,3-allenoate 1k was treated with Selectfluor™ under either Conditions B or Conditions C (Scheme 3). For 4,4-dialkyl substituted allenoate 1t, either in MeCN or in MeNO2, the corresponding lactone could not be afforded (Scheme 3).
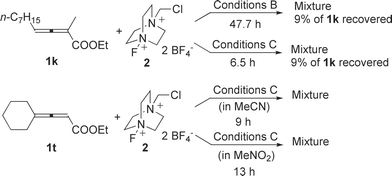 | ||
| Scheme 3 | ||
A plausible mechanism was proposed for this reaction (Scheme 4): The relatively electron-rich carbon–carbon double bond of 2,3-allenoate firstly interacts with F+ in Selectfluor™ to form cyclic intermediate 6, which explains the E-stereoselectivity observed here. Hydroxylation at the 5-position would lead to the formation of fluorohydroxylation product 5, which was confirmed by the isolation and characterization of E-5m (Scheme 2). If R3 = H, subsequent oxidation by Selectfluor™15 would form ketone 3 (Path I, Scheme 4). Corresponding dealkylation of intermediate 6 may lead to lactonization to form 4 (Path II, Scheme 4).
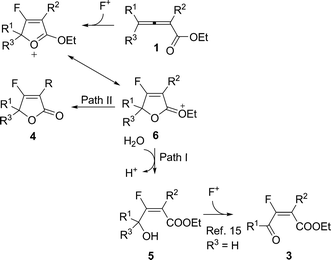 | ||
| Scheme 4 | ||
This reaction may easily be carried out with 5 mmol of ethyl 4-phenyl-2,3-butadienoate 1a to afford 3a and 4a, respectively (Scheme 5).
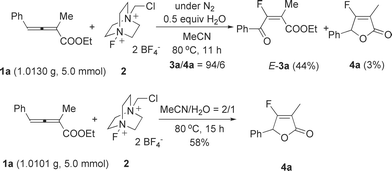 | ||
| Scheme 5 | ||
Conclusions
In conclusion, we have developed an efficient way to synthesize 3-fluoro-4-oxo-2(E)-alkenoates and 4-fluoro-2(5H)-furanones from the same starting materials 4-aryl-2-alkyl or benzyl-2,3-allenoates and Selectfluor™ in acetonitrile. The reaction of fully substituted 2,3-allenoates with Selectfluor™ afforded 4-fluoro-2(5H)-furanones highly selectively in excellent yields under Conditions A, B, and C. A mechanism has been proposed based on the isolation and characterization of the minor fluorohydroxylation product E-5m. Due to the readily availability of 2,3-allenoates and the formation of different types of monofluorinated products with functionalities for further elaboration under different conditions, this method shows more potential than that of 2,3-allenoic acids. Further studies in this area are being conducted in our laboratory.Experimental section
1. Preparation of 3-fluoro-4-oxo-2(E)-butenoates (3a–j)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica gel afforded 3a (25.8 mg, 55%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.96–7.89 (m, 2H), 7.66–7.58 (m, 1H), 7.53–7.45 (m, 2H), 3.99 (q, J = 7.2 Hz, 2H), 2.05 (d, J = 4.2 Hz, 3H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 187.2 (d, J = 28.1 Hz), 166.1 (d, J = 17.4 Hz), 158.9 (d, J = 276.2), 134.6, 134.1, 129.2, 128.8, 114.9 (d, J = 16.9 Hz), 61.4, 13.4, 10.7 (d, J = 5.4 Hz); 19F NMR (282 MHz, CDCl3) δ−100.5; IR (neat) ν/cm−1 3064, 2984, 2936, 1725, 1682, 1598, 1583, 1451, 1369, 1302, 1202, 1176, 1113, 1082, 1019; MS (70 eV, EI) m/z (%): 236 (M+, 7.13), 105 (100); HRMS calcd for C13H13O3F (M+): 236.0849. Found: 236.0842.
1) on silica gel afforded 3a (25.8 mg, 55%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.96–7.89 (m, 2H), 7.66–7.58 (m, 1H), 7.53–7.45 (m, 2H), 3.99 (q, J = 7.2 Hz, 2H), 2.05 (d, J = 4.2 Hz, 3H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 187.2 (d, J = 28.1 Hz), 166.1 (d, J = 17.4 Hz), 158.9 (d, J = 276.2), 134.6, 134.1, 129.2, 128.8, 114.9 (d, J = 16.9 Hz), 61.4, 13.4, 10.7 (d, J = 5.4 Hz); 19F NMR (282 MHz, CDCl3) δ−100.5; IR (neat) ν/cm−1 3064, 2984, 2936, 1725, 1682, 1598, 1583, 1451, 1369, 1302, 1202, 1176, 1113, 1082, 1019; MS (70 eV, EI) m/z (%): 236 (M+, 7.13), 105 (100); HRMS calcd for C13H13O3F (M+): 236.0849. Found: 236.0842.The following compounds were prepared according to this typical procedure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a liquid; 1H NMR (300 MHz, CDCl3) δ 7.81–7.73 (m, 2H), 7.67–7.60 (m, 2H), 4.05 (q, J = 7.2 Hz, 2H), 2.54–2.42 (m, 2H), 1.67–1.55 (m, 2H), 1.06 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 186.0 (d, J = 30.0 Hz), 165.9 (d, J = 15.4 Hz), 157.8 (d, J = 275.0 Hz), 133.5, 132.2, 130.6 (d, J = 2.0 Hz), 129.4, 120.8 (d, J = 16.0 Hz), 61.5, 27.6 (d, J = 3.5 Hz), 21.3 (d, J = 2.2 Hz), 13.8, 13.5; 19F NMR (282 MHz, CDCl3) δ 105.3; IR (neat) ν/cm−1 2965, 2935, 2874, 1728, 1687, 1586, 1484, 1464, 1399, 1369, 1311, 1272, 1228, 1199, 1173, 1124, 1070, 1011; MS (70 eV, EI) m/z (%): 344 (M+(81Br), 20.01), 342 (M+(79Br), 20.23), 185 (98.06), 183 (100); HRMS calcd for C15H16O379BrF (M+): 342.0267. Found: 342.0263.
1) as a liquid; 1H NMR (300 MHz, CDCl3) δ 7.81–7.73 (m, 2H), 7.67–7.60 (m, 2H), 4.05 (q, J = 7.2 Hz, 2H), 2.54–2.42 (m, 2H), 1.67–1.55 (m, 2H), 1.06 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 186.0 (d, J = 30.0 Hz), 165.9 (d, J = 15.4 Hz), 157.8 (d, J = 275.0 Hz), 133.5, 132.2, 130.6 (d, J = 2.0 Hz), 129.4, 120.8 (d, J = 16.0 Hz), 61.5, 27.6 (d, J = 3.5 Hz), 21.3 (d, J = 2.2 Hz), 13.8, 13.5; 19F NMR (282 MHz, CDCl3) δ 105.3; IR (neat) ν/cm−1 2965, 2935, 2874, 1728, 1687, 1586, 1484, 1464, 1399, 1369, 1311, 1272, 1228, 1199, 1173, 1124, 1070, 1011; MS (70 eV, EI) m/z (%): 344 (M+(81Br), 20.01), 342 (M+(79Br), 20.23), 185 (98.06), 183 (100); HRMS calcd for C15H16O379BrF (M+): 342.0267. Found: 342.0263.2. Preparation of 4-fluoro-2(5H)-furanone (4a–i)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica gel afforded 4a8a (19.9 mg, 51%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.45–7.38 (m, 3H), 7.36–7.30 (m, 2H), 5.79–5.75 (m, 1H), 1.88 (t, J = 1.8 Hz, 3H); 19F NMR (282 MHz, CDCl3) δ−110.0.
1) on silica gel afforded 4a8a (19.9 mg, 51%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.45–7.38 (m, 3H), 7.36–7.30 (m, 2H), 5.79–5.75 (m, 1H), 1.88 (t, J = 1.8 Hz, 3H); 19F NMR (282 MHz, CDCl3) δ−110.0.The following compounds were prepared according to this typical procedure.
3. Preparation of 4-fluoro-2(5H)-furanone (4m–s)
The formation of 4m and E-5m under Conditions A:
The reaction of 1m (0.6162 g, 2.01 mmol) and Selectfluor™ (95%, 2.2435 g, 6.02 mmol) in 20 mL of H2O–MeCN (premixed, 0.9 μL mL−1) at 80 °C under nitrogen afforded 4m (0.5558 g, 93%) and E-5m (0.0375 g, 5%) (petroleum ether–ethyl acetate = 30 : 1 to 5 : 1) as a liquid.
4m: 1H NMR (300 MHz, CDCl3) δ 7.43–7.30 (m, 10H), 2.35–2.27 (m, 2H), 1.73-1.57 (m, 2H), 0.95 (t, J = 7.4 Hz, 3H).
E-5m: liquid; 1H NMR (300 MHz, CDCl3) δ 7.45–7.30 (m, 10H), 4.25 (q, J = 7.1 Hz, 2H), 2.92 (d, J = 2.1 Hz, 1H), 1.98–1.89 (m, 2H), 1.34–1.15 (m, 5H), 0.65 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) 167.6 (d, J = 5.0 Hz), 159.5 (d, J = 261.8 Hz), 142.7, 128.3, 127.5, 117.8 (d, J = 13.3 Hz), 80.7 (d, J = 30.4 Hz), 61.1, 28.6 (d, J = 3.2 Hz), 21.8 (d, J = 2.6 Hz), 14.2, 13.7; 19F NMR (282 MHz, CDCl3) δ−96.5; IR (neat) ν/cm−1 3479, 3061, 3028, 2962, 2932, 2873, 1713, 1600, 1493, 1464, 1449, 1368, 1295, 1232, 1181, 1110, 1031; MS (ESI) m/z: 397 (M+ + Na + MeOH), 365 (M+ + Na), 360 (M+ + NH4), 325 (M+− OH); HRMS calcd for C21H23FO3Na (M++Na): 365.1523. Found: 365.1536.
The formation of 4m under Conditions B:
The reaction of 1m (61.8 mg, 0.20 mmol) and Selectfluor™ (95%, 126.3 mg, 0.34 mmol) in a mixture of 1.3 mL of MeCN and 0.65 mL of H2O at 80 °C afforded 4m (51.6 mg, 86%) and E-5m (4.9 mg, 7%) (petroleum ether–ethyl acetate = 20 : 1 to 5 : 1) as a liquid.
4m: 1H NMR (300 MHz, CDCl3) δ 7.42–7.33 (m, 10H), 2.36–2.27 (m, 2H), 1.73–1.58 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H).
E-5m: 1H NMR (300 MHz, CDCl3) δ 7.45–7.30 (m, 10H), 4.25 (q, J = 7.1 Hz, 2H), 2.91 (d, J = 2.1 Hz, 1H), 1.98–1.88 (m, 2H), 1.35–1.14 (m, 5H), 0.65 (t, J = 7.4 Hz, 3H).
The following compounds were prepared according to the Conditions C.
The reaction of (1s) (37.8 mg, 0.19 mmol) and Selectfluor™ (95%, 88.5 mg, 0.24 mmol) in 2 mL of MeNO2 at 80 °C afforded 4s8a (14.9 mg, 42%) as a liquid.1H NMR (300 MHz, CDCl3) δ 1.85–1.58 (m, 12H), 1.35–1.18 (m, 1H); 19F NMR (282 MHz, CDCl3) δ−112.8.
4. Large scale reactions
Acknowledgements
Financial support from the National Basic Research Program of China (NO. 2009CB825300) and National Natural Sciences Foundation of China (20972135) is greatly appreciated. Shengming Ma is a Qiu Shi Adjunct Professor at Zhejiang University. We thank Mr Jing Li for reproducing the results presented in Table 2, entries 4 and 9 and Table 3 entries 5, 14, and 15.References
- (a) For reviews on the bioactivities of fluorinated compounds, see: B. E. Smart, J. Fluorine Chem., 2001, 109, 3 Search PubMed; (b) P. Kirsch, Modern Fluoroorganic Chemistry, 1st edn, Wiley-VCH, Weinhein, 2004 Search PubMed; (c) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS.
- N. Shibata, T. Ishimaru, S. Nakamura and T. Toru, J. Fluorine Chem., 2007, 128, 469 CrossRef CAS.
- W. J. Middleton, J. Org. Chem., 1975, 40, 574 CrossRef CAS.
- D. H. R. Barton, L. S. Godinho, R. H. Hesse and M. M. Pechet, Chem. Commun., 1968, 804 RSC.
- M. A. Tius, Tetrahedron, 1995, 51, 6605 CrossRef CAS.
- (a) T. Umemoto, K. Kawada and K. Tomita, Tetrahedron Lett., 1986, 27, 4465 CrossRef CAS; (b) T. Umemoto, S. Fukami, G. Tomizawa, K. Harasawa, K. Kawada and K. Tomita, J. Am. Chem. Soc., 1990, 112, 8563 CrossRef CAS.
- (a) T. D. Beeson and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 8826 CrossRef CAS; (b) M. Marigo, D. Fielenbach, A. Braunton, A. Kjœrsgaard and K. A. Jørgensen, Angew. Chem., Int. Ed., 2005, 44, 3703 CrossRef CAS; (c) Y. Hamashima, K. Yagi, H. Takano, L. Tamás and M. Sodeoka, J. Am. Chem. Soc., 2002, 124, 14530 CrossRef CAS; (d) Y. Hamashima, H. Takano, D. Hotta and M. Sodeoka, Org. Lett., 2003, 5, 3225 CrossRef CAS.
- (a) C. Zhou, Z. Ma, Z. Gu, C. Fu and S. Ma, J. Org. Chem., 2008, 73, 772 CrossRef CAS; (b) C. Zhou, J. Li, B. Lü, C. Fu and S. Ma, Org. Lett., 2008, 10, 581 CrossRef CAS; (c) R. G. Syvret, K. M. Butt, T. P. Nguyen, V. L. Bulleck and R. D. Rieth, J. Org. Chem., 2002, 67, 4487 CrossRef CAS; (d) For reviews on fluorination reactions, see: P. T. Nyffeler, S. G. Durón, M. D. Burkart, S. P. Vincent and C.-H. Wong, Angew. Chem., Int. Ed., 2005, 44, 192 Search PubMed; (e) J.-A. Ma and D. Cahard, Chem. Rev., 2004, 104, 6119 CrossRef CAS; J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS; (f) R. P. Singh and J. M. Shreeve, Acc. Chem. Res., 2004, 37, 31 CrossRef CAS; (g) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305 Search PubMed; (h) G. S. Lal, G. P. Pez and R. G. Syvret, Chem. Rev., 1996, 96, 1737 CrossRef CAS; (i) M. Shimizu and T. Hiyama, Angew. Chem., Int. Ed., 2005, 44, 214 CrossRef CAS.
- H. Cui, Z. Chai, G. Zhao and S. Zhu, Chin. J. Chem., 2009, 27, 189 CrossRef CAS.
- (a) S. Ma and S. Wu, J. Org. Chem., 1999, 64, 9314 CrossRef CAS; (b) H. D. Venkruijsse, L. Brandsma, Synthesis of Acetylenes, Allenes and Cumulenes.A Laboratory Manual, Elsevier, Amsterdam, The Netherlands, 1981, p. 33 Search PubMed.
- (a) G. Chen, C. Fu and S. Ma, Tetrahedron, 2006, 62, 4444 CrossRef CAS; (b) C. Fu and S. Ma, Eur. J. Org. Chem., 2005, 3942 CrossRef CAS.
- For a review on ionic addition of allenes, see: S. Ma, Chapter 10 in Modern Allene Chemistry, Wiley-VCH, Weinheim, 2004. Search PubMedFor an account on electrophilic addition and cyclization reactions of allenes, see: S. Ma, Acc, Chem. Res., 2009, 42, 1679 Search PubMed.
- G. Chen, C. Fu and S. Ma, J. Org. Chem., 2006, 71, 9877 CrossRef CAS.
- Y. Wang and D. J. Burton, Org. Lett., 2006, 8, 1109 CrossRef CAS.
- (a) J. Anaya, M. C. Grande, M. Grande, A.-I. Patino and P. Torres, Synlett, 1999, 1429 CrossRef CAS; (b) G. R. Krow, D. Gandla, W. Guo, R. A. Centafont, G. Lin, C. DeBrosse, P. E. Sonnet, C. W. Ross, III, H. G. Ramjit and K. C. Cannon, J. Org. Chem., 2008, 73, 2122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis of new 2,3-allenoates 1j, 1l, and 1q and 1H, and 13C spectra for all compounds. See DOI: 10.1039/b917793k |
| This journal is © The Royal Society of Chemistry 2010 |