Mechanistic insights into seeded growth processes of gold nanoparticles†
Jörg
Polte
a,
Martin
Herder
a,
Robert
Erler
a,
Simone
Rolf
a,
Anna
Fischer
b,
Christian
Würth
a,
Andreas F.
Thünemann
a,
Ralph
Kraehnert
b and
Franziska
Emmerling
*a
aBAM Federal Institute for Materials Research and Testing, Richard Willstätter-Straße 11, 12489, Berlin, Germany. E-mail: franziska.emmerling@bam.de; Fax: +00 (0)49 30 8104 1137; Tel: +00 (0)49 30 8104 1133
bTechnische Universität Berlin, Technische Chemie, Straße des 17. Juni 124, 10623, Berlin, Germany
First published on 29th September 2010
Abstract
A facile approach for the synthesis of monodisperse gold nanoparticles with radii in the range of 7 to 20 nm is presented. Starting from monodisperse seeds with radii of 7 nm, produced in the first step, the addition of a defined amount of additional precursor material permits distinct size regulation and the realization of predicted nanoparticle sizes. These information were derived from ex- and in situ investigations by comprehensive small angle X-ray scattering (SAXS), X-ray absorption near edge structure (XANES) and UV-Vis data to obtain information on the physicochemical mechanisms. The obtained mechanisms can be transferred to other seeded growth processes. Compared to similar approaches, the presented synthesis route circumvents the use of different reducing or stabilizing agents. The size of resulting nanoparticles can be varied over a large size range presented for the first time without a measurable change in the shape, polydispersity or surface chemistry. Thus, the resulting nanoparticles are ideal candidates for size dependence investigations.
1. Introduction
Nanoparticles are intensively investigated due to their wide range of potential applications in fields such as medicine,1 biotechnology,2 and catalysis.3 Among these, gold nanoparticles (GNP) are the most prominent nanoscale materials. GNPs can be prepared via various synthesis routes including chemical, sonochemical or photochemical paths.4 The most common route is the reduction of a dissolved gold precursor, e.g. (tetrachloroauric acid, HAuCl4), by a reducing agent such as sodium citrate, ascorbic acid, sodium boron hydride or block-copolymers. A further stabilizing agent is typically required to prevent agglomeration or further growth of the particles. In many applications the properties of metallic nanoparticles are determined by parameters such as size, shape, composition or crystalline structure. In principle, it is possible to adjust the behaviour of nanoparticles in such applications in the desired manner by controlling one of the listed properties.Control over the size of GNPs can be achieved by varying the synthesis parameters, e.g. the concentration of the precursor and reducing agent or the temperature (see ref 4 and references therein). A precise size control is often difficult to achieve, since small changes in the above-mentioned parameters often lead to pronounced differences in the size distribution. In most cases it is also difficult to keep the polydispersity low when changing the reaction parameters in order to obtain larger particles. A prominent example for challenges faced in controlling particle-size distribution is the frequently employed synthesis of gold nanoparticles by reduction of HAuCl4 with sodium citrate.5 For this GNP synthesis, Frens et al.5 demonstrated control over particle size by varying the citrate concentration; hence the synthesis is referred to as “Frens method”.6–8 Based on this method, several approaches of changing the initial reaction parameters were developed, but none of them could assure a specific particle size, spherical shape and the same low polydispersity concurrently.5,6,9,10 Unfortunately, these characteristics are required for applications of GNPs in which the size effect is utilized, e.g. for well-defined surface plasmon resonances in chemical and biomedical detection and analysis.11
An alternative approach to overcome such difficulties is “seeded growth”, the use of previously prepared small particles, which act as seeds for a further particle growth. For precise control of the particle size it is necessary to suppress further nucleation events during seed-mediated growth and to promote heterogeneous growth. However, seed-mediated growth is predominantly used for controlled growth of particles of distinct elongated shapes.12–16 To the best of our knowledge, an additional stabilizing and/or reducing agent has been required in the previously reported studies to control particle size and shape during seeded growth.4,6–8,10,17–21
In the following, a procedure for a size-controlled, seed-mediated growth of spherical GNPs is presented. It solely relies on the reduction of HAuCl4 with sodium citrate, without additional stabilizing or reducing agents, thus in the following this approach is referred to as ‘self-seeded’ growth. Hence, side reactions inducing further nucleation can be prevented. By applying this novel procedure, the size of the GNPs can be adjusted while the spherical shape, as well as the low polydispersity and the surface chemistry is retained. The precision of size control depends solely on the analytic determination of the particle sizes, as is demonstrated by using the two complementary methods SAXS and SEM. The data obtained from in situ SAXS, XANES and UV-Vis analysis throughout the synthesis provides information about the underlying growth mechanism. The potential of the concept is demonstrated for the synthesis of monodisperse GNPs with adjustable radii ranging from 7 to 20 nm.
2. Experimental
2.1 Nanoparticle synthesis
Gold nanoparticles were synthesized according to the procedure described by Turkevich et al.,22i.e. chemical reduction of the gold precursor HAuCl4 by dissolved trisodium citrate at 75 °C from aqueous solutions containing 0.25 mmol/L and 2.5 mmol/L of gold precursor and citrate, respectively. Prior to each experiment 35 mL aqueous (Millipore) solution of gold precursor (7 mg HAuCl4 × 3H2O, Aldrich) and 35 mL aqueous solution of trisodium citrate (51.45 mg Na3C6H5O7 × 2 H2O, Aldrich) were prepared and pre-heated to the reaction temperature (75 °C). The synthesis was carried out under stirring in a flask immersed in a temperature-controlled water bath, adding the citrate solution to the gold solution. Self-seeded growth of GNPs at 65 °C was initiated by adding volumes of 1, 2 or 5 ml of aqueous solutions of HAuCl4 (1 mmol/L) to 10 mL of the seed solution under stirring, after adding the respective 10-fold amount of citrate to the seeds. The amount of gold salt nadd needed to obtain a specific gold nanoparticle radius can be simply determined from stoichiometry as nadd = (r3final − r3initial)/r3initial × ninitial, where ninitial is the molar amount of gold in the nanoparticle stock solution, and rinitial and rfinal are the initial and final radii. Liquid samples were extracted by pipetting for SAXS/XANES, UV analysis or pH measurement (5.8 to 6.2). For SEM imaging, ca. 0.1 mL of the final colloid was dried on a Si wafer.2.2 Nanoparticle characterization
At different reaction times, 10 μl of the liquid samples were extracted from the batch of reaction solution and approx. 5 μl were placed as droplets in an acoustic levitator (Tec5, Oberursel, Germany) used as sample holder for X-ray analysis. The acoustic levitator was positioned in the μSpot beamline at BESSY II synchrotron (Berlin, Germany) as described in ref 23 to measure time-resolved combined SAXS and XANES (for details see Supporting Information, S1). The in-house scattering experiments were carried out using the Kratky camera type system SAXSess (Anton Paar, Graz, Austria).Scanning electron microscopy (SEM) imaging of desiccated nanoparticles was performed on a JEOL JSM-7401F instrument, operated with an acceleration voltage of 4 kV, at a working distance of 2.8 mm, covering the Si wafer that carried the nanoparticles with a gold mesh to reduce surface charging. The size determination was performed using Image J software (http://rsbweb.nih.gov/ij/). For each sample three SEM images from non overlapping areas were taken. From these images about 200 particles were measured in size to provide statistical significance. UV absorption (UV-Vis) spectra were recorded on an Avantes AvaSpec-2048TEC-2 (Deuterium halogen light source), connected to a 10 mm optical path length cuvette holder via fibre optical cables.
3. Results and discussion
The concept of size-controlled GNP synthesis extends our previous work,24 which focussed on understanding the nucleation and growth process of monodisperse gold nanoparticles synthesized according to the Turkevich method.22 Briefly, it was shown that the growth mechanism consists of four steps. Starting with a rapid reduction of the gold precursor and nucleation, the process is followed by coalescence (resulting in a GNP radius of about 4 nm) and a diffusional growth step (rGNP = 5 nm). The last growth step is a rapid and complete consumption of the remaining gold precursor retaining the number of particles as well as constant polydispersity, i.e. without any further nucleation. An autocatalytic surface reduction was proposed as the underlying process.These previous results suggest that the final size of the GNPs obtained by applying the synthesis procedure described above is determined by two factors, the number and size of particles formed initially due to nucleation and coalescence, as well as the remaining quantity of precursor material available in the final step of particle growth. Thus, adding additional amounts of gold precursor to the colloidal solution during or after the GNP formations final step should increase the particle size, whereas the particle number and polydispersity should remain constant. According to the proposed mechanism the autocatalytic surface growth will continue until the added gold precursor is completely consumed and deposited onto the existing particles.
To prove this hypothesis, a coupled SAXS and XANES in situ experiment was performed using the same setup and data evaluation procedure previously reported.24 In this context SAXS delivers information about the size, shape, and number of particles. The potential of this method for growth studies is demonstrated in several recent contributions.12,24–31 XANES provides information on the oxidation state of the gold atoms in the solution and thus on the progress of the reduction process. The proof of principle of such self-seeded growth was tested for the citrate reduction at 85 °C, a temperature close to the conditions for the typical Turkevich synthesis.22 The standard synthesis for GNPs was carried out until completion. Thereafter, an additional amount of gold precursor solution (HAuCl4) was added to induce self-seeded particle growth.
The time-dependent results of the evaluated respective SAXS and XANES data are provided in Fig. 1. The radii and number of particles (a) as well as polydispersities (b) were calculated from fitted SAXS curves. The inset in (b) exemplarily shows the size distributions of the GNPs at different reaction times, including seed formation and particle growth processes. Oxidation states and volume fractions derived from XANES are plotted in (c). Both SAXS and XANES show the expected phases of formation and growth of the seeds being completed within ca. 40 min.
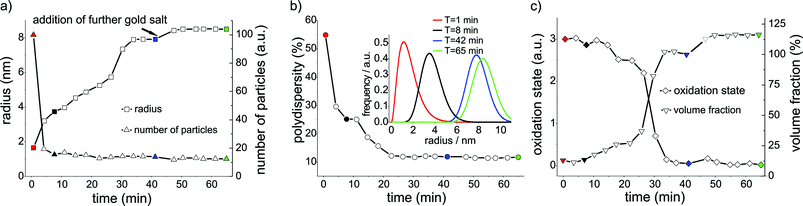 | ||
| Fig. 1 Results of the evaluated SAXS and XANES data as a function of time, recorded for the synthesis of gold seeds (at 85 °C) via citrate method (0 to 45 min) and the addition of further gold precursor solution to the seed solution (45 min). Significant points of the experiments are indicated by different colours: start (red), first phase (black), before (blue) and after the (green) addition of further precursor material. a) The evaluation of the mean radius and the normalized number of particles as a function of time. b) Polydispersity of particles as a function of time and the corresponding particle size distribution of the Schulz-Zimm distribution exemplarily shown for four reaction times (inset). c) Average formal oxidation state of gold derived from normalized XANES spectra plotted vs. reaction time; volume fraction of particles calculated from SAXS data. | ||
By adding an additional amount of 25% of the gold precursor (with respect to the starting solution) to the reaction solution, a rapid increase in the particle radius from 7.9 nm to 8.5 nm was observed (Fig. 1a) whereas the polydispersity remained constant (Fig. 1b). Correspondingly, the total volume of the gold particles increases by approx. 25%, suggesting complete conversion of the added precursor. Whereas the total number of GNP remained constant, the number of particles per unit volume decreased due to the dilution resulting from the addition of more solvent (Fig. 1a). For the same reason the volume fraction of gold particles increases only by about 13% and not by 25% as would be expected for a constant total volume of solvent (Fig. 1c). The XANES results confirm the rapid reduction as well as the full stoichiometric conversion of the gold salt (see Fig. 1c). The results indicate that further nucleation and formation of GNPs can be suppressed by applying the described synthesis procedure. Consequently, the final size of the particles can be regulated by the controlled addition of further precursor material.
To provide further evidence for GNP-accelerated reduction of the added gold precursor, the same experiment (0.06125 mmol/L HAuCl4, 2.5 mmol/L sodium citrate) was carried out at 65 °C with and without nanoparticles being present in the solution (for corresponding UV-vis results see ESI (S2)†). Whereas complete reduction of gold precursor requires less than 15 min when GNP are present, more than 200 min are required in the absence of already formed GNP. Hence, the consumption of gold precursor is significantly accelerated in presence of colloidal GNP.
Further experiments using SAXS as the analytical technique were performed to test whether it is possible to increase the radius of the GNPs step by step by adding additional amounts of gold-precursor solution. The seeds were synthesized at 75 °C employing a slight modification of the standard procedure described by Turkevich et al.22 to obtain particles with a radius of approximately 7 nm. The subsequent self-seeded growth was carried out at 65 °C. At this temperature the reduction rate is slowed down and therefore the probability of nucleation events is minimized. Since sodium citrate acts both as reducing and stabilizing agent, it is important to keep the molar concentration of sodium citrate constant at 2.5 mmol throughout the growth process to avoid the aggregation of the gold particles.
Thus, proceeding in a stepwise manner, first 1 mL, then 2 mL and finally 5 mL of HAuCl4 solution (1 mmol) were added slowly to 10 mL of the seed solution. For each addition of HAuCl4 solution the same volume of citrate solution (5 mmol/L) was added. This alternating addition was repeated until the particles reached the desired size. Between two additions, a time span of approximately 5 min was found to be sufficient to ensure the complete conversion of the gold salt.
In Fig. 2a the evolution of the scattering curves during the growth period is displayed (SAXS measured at the synchrotron setup). The integration time for each scattering curve was 30 s and every 3 min a fresh sample was taken for SAXS analysis. Further gold salt was added after the particles reached the calculated radius. An interval of at least 5 min was allowed between two additions of aliquots of gold precursor. As evident from Fig. 2a the shape of the scattering curves persists while the curves are shifted to smaller q-values with addition of further reactants. This indicates without mathematical modelling that the particles grow while retaining their spherical shape and low polydispersity. Fig. 2b illustrates the good agreement of five selected scattering curves (black) and their respective mathematical fits (red). The fit assumes spherical particles having a Schulz size distribution,32 which was found to be a good approximation in the previous analysis of the growth process of citrate stabilized GNPs.24
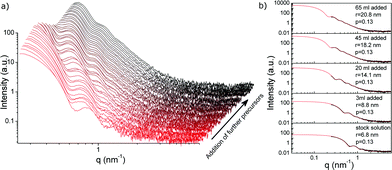 | ||
| Fig. 2 SAXS data and data evaluation for the stepwise growth of GNP at 65 °C via addition of further reactants (Na3Ct, HAuCl4): a) SAXS data as a function of the precursor addition obtained during the reduction of HAuCl4 with sodium citrate. The SAXS data indicate the formation of spherical nanoparticles with a narrow size distribution. b) Data evaluation showing examples for measured vs. fitted scattering curves for different particle radii obtained after addition of corresponding quantities of precursor ranging from GNP seeds (bottom, 6.8 nm radius) to a radius of 20.8 nm (top). | ||
For the stepwise self-seeded growth of GNPs from 6.8 to 20.8 nm radius, the results of fitted scattering curves are displayed in Fig. 3. Each vertical blue line corresponds to the addition of 1, 2 or 5 mL of 1 mmol L−1 gold precursor solution to the seed colloid. In Fig. 3a the “theoretical radius” calculated from stoichiometry and the measured radius (“experimental radius”) for each step of the addition is given. Fig. 3b shows the corresponding number of particles, with the dilution due to the addition of solvent being corrected for and the volume fraction.
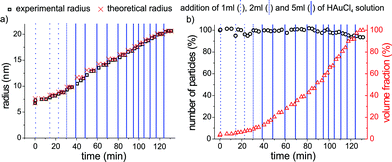 | ||
| Fig. 3 Evaluation of radius, particle number and volume fraction as a function of time for the self-seeded growth of GNPs at 65 °C, with repeated injection of additional reactant solution (Na3Ct, HAuCl4). Each vertical blue line indicates the addition of the respective volume of precursor material. Dashed lines correspond to the addition of a volume of 1 mL, dotted lines the addition of 2 mL and solid lines the addition of 5 mL of gold precursor solution. a) Particle radius vs. time: the values for the radii derived from SAXS (squares) are in excellent agreement with the radii predicted by the stoichiometry of precursor addition, assuming complete conversion (crosses). b) The number of particles derived from SAXS (circles) remains nearly constant. The volume fraction of GNP normalized to the initial reaction volume (triangles) increases continuously with further addition of precursor solution. | ||
Upon adding HAuCl4 solution to the gold seeds, the particle radius increases exactly as predicted by the reaction's stoichiometry (Fig. 3a), whereas the number of particles (Fig. 3b) and their polydispersity remained constant at a value of 13% throughout the experiment. A small decrease in the number of particles of about 5% is observed as the particle radius exceeds 18 nm. This might be due to precipitation of some particles onto the stirrer or the glass wall (not directly observed), and related to gradually failing stabilisation of the GNPs via citrate. As clearly seen in Fig. 3a the calculated theoretical radius agrees very well with the particle radius derived experimentally from SAXS. Hence, the repeatedly added gold precursor is completely reduced and grown within 3 to 5 min onto the existing particles.
The SAXS data obtained on the synchrotron for all final samples (Fig. 2, Fig. 3) were confirmed experimentally using a lab-scale SAXS instrument (SAXSess, Anton Paar GmbH, Graz, Austria), which allows measurements at lower q-values and thus a more precise size determination of particles with radii >15 nm (see Fig. 5).
GNP size and polydispersity deduced from SAXS evaluation were also confirmed by scanning electron microscopy (SEM). Fig. 4 shows SEM images of three different steps of the self-seeded growth process, as well as histograms indicating the particle size distribution derived from SEM images. The SEM images confirm that while the particle radius increases from 6.2 to 20.4 nm, the predominantly spherical shape of the particles is preserved. The formed GNPs retain a low polydispersity of less than 13%. TEM images of particles synthesized with the method mentioned above are in accordance with the SEM and SAXS results (for details see ESI (S4)†).
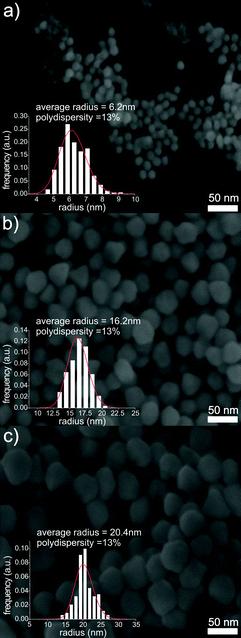 | ||
| Fig. 4 SEM images from different stages of the self-seeded growth of GNP at 65 °C: GNP seeds (a), after the addition of 30 mL of precursor (b), and after the addition of 65 mL of precursor (c). Scale bars: 50 nm. The insets show the size distribution of the particles (Schulz size distribution) as used for the analysis of the SAXS data. | ||
The GNP radius and polydispersity derived from SAXS and SEM results reveals that the particle sizes determined with the two methods agree to within 4 to 5%. The direct comparison of particle radii obtained via SAXS at the synchrotron and in the lab as well as from SEM is displayed in Fig. 5, plotting experimental values vs. theoretically calculated radii. The observation that the data lies almost perfectly on the angle bisector confirms the high accuracy of the SAXS analysis. The results illustrate the capabilities of SAXS as a reliable tool for size determination of nanoparticles. Furthermore, SAXS allows online monitoring of nanoparticle growth, especially when in situ experiments are needed or further sample preparation is undesirable. However it should be noted that with increasing heterogeneity in the shape of the particles the information obtained from SAXS is limited and thus electron microscopy is in general the preferred choice. The seed-mediated growth process presented here meets the criteria for a SAXS analysis in particular, since the formed particles exhibit a spherical shape.
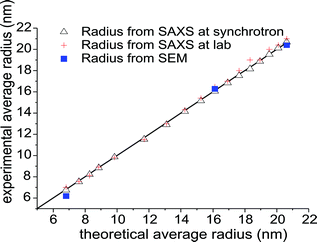 | ||
| Fig. 5 Comparison of the experimentally determined average GNP radii obtained from SAXS (synchrotron-based and SAXSess) as well as SEM, plotted as a function of the calculated theoretical radius. The agreement between experimental and theoretical radii is indicated by the straight parity line. | ||
The results derived from SAXS, XANES, and SEM are in agreement with the previously suggested interpretation of the growth step as autocatalytic reduction. However, further nucleation may occur but these small gold clusters/particles can undergo coalescent processes, which would be in accordance with recent findings in the NaBH4 system, where the growth of GNP occurs exclusively due to coalescence in the absence of a stabilizing agent.25 At first glance it is surprising that SAXS does not indicate a temporary increase in the particle number, but in fact this is not contradictory. When one considers that signal intensity in SAXS analysis scales proportional to the sixths power of the particle radius,33 it is practically impossible to detect gold clusters with radii smaller that a 1 nm in the presence of GNPs with a radius larger than 7 nm, since the intensity ratio between new formed cluster and GNP seeds would be in the order of 1 to 105.
However, a further nucleation process should be apparent from the optical properties of the GNPs (see Fig. 6), since the UV-Vis spectra of particles with radii of about 1 nm differ clearly from the seeds.34 After addition of HAuCl4 to the seeds, the solution colour turned from wine red to violet within seconds. Subsequently, it returns to wine red within 5 to 10 min (see Fig. 6a), which is in agreement with the time span of a self-seeded growth step. Thus, the seed-mediated GNP growth was monitored using UV-Vis spectroscopy with a time resolution of 10 s. The resulting spectra, the absorbance values and the wavelength shift of the GNPs plasmon resonance are displayed in Fig. 6b and c for GNPs growing 3 monolayers with a thickness of 0.3 nm (the thickness of a monolayer is based on the dimension of the unit cell) onto the seed particles thus from a starting radius of 7.2 nm to 8.1 nm determined with SAXS. It can be seen that the maximum in the absorption spectrum shifts from 518 nm to 530 nm within 15 s after adding an aliquot of gold precursor (black and red curve in Fig. 6b), i.e. the colour changes from wine red to violet. Within the next 5 to 15 min the location of the plasmon resonance band returns to the initial position of 518 nm (blue curve in Fig. 6b). Fig. 6c displays in a time-resolved manner the corresponding shift of the band as well as the absorbance at the peak maximum position (inset in Fig. 6c). Hence, the UV-Vis data confirm the results from SAXS analysis that the seeded growth process requires about 5–10 min for completion.
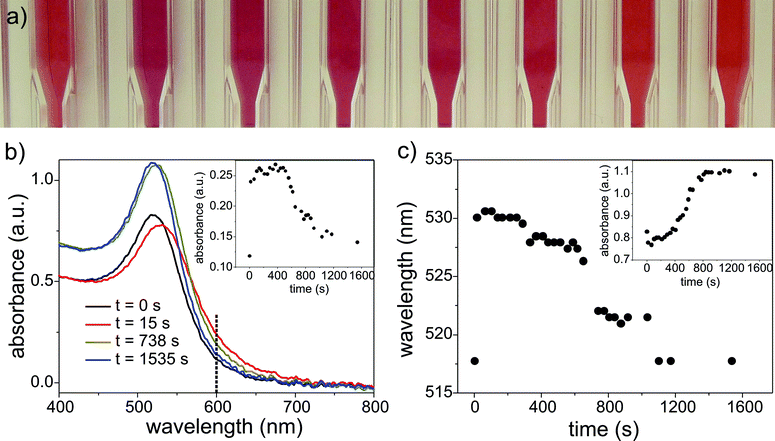 | ||
| Fig. 6 (a) Color change of the GNP solution due to the addition of further precursor and the subsequent self-seeded growth. (b) UV-Vis spectra obtained during GNP growth from seeds with radii of 7.2 nm recorded at different time intervals after adding an aliquot of gold salt. The inset shows the absorbance at 600 nm as a function of the reaction time. (c) The wavelength shift at the absorbance maximum with respect to the time of the reaction process. The inset depicts the corresponding absorbance at the maximum. | ||
Unfortunately, obtaining quantitative information from UV-Vis is only possible for GNPs larger than 5 nm in diameter.34 Thus the spectra of small gold clusters can only provide qualitative information. The initially observed shift towards higher wavelength in the absorption spectrum can hardly be explained by the existence of small gold cluster/particles in the vicinity of the larger GNP seeds. This change in the absorption matches neither the UV-Vis spectra measured in the beginning of the standard citrate synthesis when predominantly very small particles are present (radius ≈ 1 nm) nor other GNP synthesises in which the final particle size is small.25
Apart from the presence of additional small GNPs, a plausible explanation for the observed reversible change of the plasmon resonance band towards higher wavelengths might be the temporary decrease in the pH of the reactant solution upon adding the acidic precursor solution. It has been reported that decreasing the pH of GNP colloids leads to a shift of the plasmon band towards higher wavelengths.35,36 In fact, upon adding the acidic precursor solution (pH = 4), the solutions pH should decrease. After the complete consumption of the gold precursor, the pH would return to its initial value. However, it has been also reported that sodium citrate acts as buffer, so that only small changes of the pH are observed throughout the reduction/growth process. A sufficiently accurate experimental determination of the present pH value did not succeed. That colour changes result from temporary aggregation of large GNPs, as possibly suggested by the shift of the plasmon resonance towards higher wavelengths, can be excluded on the basis of the SAXS data: no aggregates were observed, although even small numbers of aggregates would produce a strong increase of the SAXS intensity at low q-values. To support the interpretation of the temporary colour change being pH related, but not due to aggregation, the addition of HAuCl4 solution to a GNP colloid was repeated at room temperature complemented by simultaneously UV-vis, SAXS and pH analysis using indicator paper. The described colour change and the shift in the UV-vis spectrum towards higher wavelengths is reproducibly observed, whereas SAXS curves did not show any changes upon adding HAuCl4, solution (see ESI (S3)†). Hence, the particle size distribution did not change upon adding gold precursor. Thus, the temporary shift of the GNP plasmon resonance cannot be explained by aggregation of the present GNP.
The presented results on self-seeded GNP growth are in agreement with previous publications on seeded growth in which the addition rate of further metal precursor was discussed as crucial factor.8,17,37 Jana et al.8 showed in detail for a common method for seeded growth of gold nanoparticles (GNP seeds prepared via citrate method, whereas for further growth ascorbic acid is used as a reducing agent) that a heterogeneous growth and thus a precise size control is only possible when adding small amounts of gold precursor step by step to the seed solution, thus avoiding further nucleation and preserving the spherical shape and low polydispersity.
A seed mediated growth in which the addition rate had no influence for a heterogeneous seeded growth was described by Niu et al.38 They described a ‘one pot’ GNP seeded growth synthesis with citrate-reduced and -stabilized seeds, where the success of the heterogeneous growth shows that it is independent of the addition rate. The further growth of the seeds was carried out using 2-mercaptosuccinic acid (MSA) which acts as reducing and stabilizing agent. The authors presume a surface enhanced reduction of HAuCl4, based on the gold affinity of the mercapto groups that was derived because the MSA doesn't reduce the HAuCl4 in the absence of the seeds. Their findings are in accordance with our investigations since the surface reduction in their case is much stronger than the reduction in solution. The authors state that the UV-Vis spectra for the growth processes do not show any aggregation.
As a consequence of this study, and the comparison to the recent literature, it appears that successful seeded growth procedures (heterogeneous growth) are primarily governed by the addition rate, which is depending or influenced by mainly two factors. The first factor is the relation of the surface reduction rate and the reduction rate in solution separate from any other particle. The second influencing factor is the need for the preservation of the nanoparticle stabilization in each step of addition.
4. Conclusions
The present study shows that from a comprehensive understanding of the mechanism of particle growth based on time-resolved in situ studies, it is possible to derive a synthesis strategy for size-controlled self-seeded growth of gold nanoparticles. Analysis by SAXS, SEM and UV-Vis confirm that the radius of GNP can be precisely adjusted between 7 and 20 nm in GNP synthesis from HAuCl4 and citrate, by adding incremental amounts of reactants in the absence of additional stabilizing or reducing agents. These GNP are ideal candidates for size dependence investigations. Moreover, the mechanistic results imply that rapid reduction of added precursor in presence of seed particles is a crucial factor for a heterogeneous seeded growth. Comprehensive investigation of related nanoparticle-synthesis procedures with the employed analytical tools will show whether the presented self-seeded growth procedure and synthesis strategy also applies to the formation of other noble-metal nanoparticles.Acknowledgements
R.K. acknowledges generous funding from the BMBF within the frame of the NanoFutur program (FKZ 03X5517A).Notes and references
- J. A. Copland, M. Eghtedari, V. L. Popov, N. Kotov, N. Mamedova, M. Motamedi and A. A. Oraevsky, Mol. Imaging Biol., 2004, 6, 341–349 Search PubMed.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959–1964 CrossRef CAS.
- G. C. Bond, C. Louis and D. T. Thompson, Catalysis by Gold, Imperial College Press 2006 Search PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- G. Frens, Nature-Physical Science, 1973, 241, 20–22 Search PubMed.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042 CrossRef CAS.
- K. R. Brown, D. G. Walter and M. J. Natan, Chem. Mater., 2000, 12, 306–313 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Mater., 2001, 13, 2313–2322 CrossRef CAS.
- G. Frens, Phys. Lett. A, 1973, 44, 208–210 CrossRef.
- X. H. Ji, X. N. Song, J. Li, Y. B. Bai, W. S. Yang and X. G. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS.
- A. M. Schwartzberg and J. Z. Zhang, J. Phys. Chem. C, 2008, 112, 10323–10337 CrossRef CAS.
- A. Henkel, O. Schubert, A. Plech and C. Sonnichsen, J. Phys. Chem. C, 2009, 113, 10390–10394 CrossRef CAS.
- H. A. Keul, M. Moller and M. R. Bockstaller, Langmuir, 2007, 23, 10307–10315 CrossRef CAS.
- P. H. Qiu and C. B. Mao, J. Nanopart. Res., 2009, 11, 885–894 CrossRef CAS.
- T. K. Sau and C. J. Murphy, Langmuir, 2004, 20, 6414–6420 CrossRef CAS.
- Y. Xia, Y. J. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1393 CrossRef CAS.
- J. L. Niu, T. Zhu and Z. F. Liu, Nanotechnology, 2007, 18 Search PubMed.
- J. Rodriguez-Fernandez, J. Perez-Juste, F. J. G. de Abajo and L. M. Liz-Marzan, Langmuir, 2006, 22, 7007–7010 CrossRef CAS.
- T. Zhu, K. Vasilev, M. Kreiter, S. Mittler and W. Knoll, Langmuir, 2003, 19, 9518–9525 CrossRef CAS.
- B. V. Enustun and J. Turkevich, J. Am. Chem. Soc., 1963, 85, 3317 CrossRef CAS.
- O. Paris, C. H. Li, S. Siegel, G. Weseloh, F. Emmerling, H. Riesemeier, A. Erko and P. Fratzl, J. Appl. Crystallogr., 2006, 40, s466–S470 CrossRef.
- J. Polte, T. T. Ahner, F. Delissen, S. Sokolov, F. Emmerling, A. F. Thunemann and R. Kraehnert, J. Am. Chem. Soc., 2010, 132, 1296–1301 CrossRef CAS.
- J. Polte, R. Erler, A. F. Thunemann, S. Sokolov, T. T. Ahner, K. Rademann, F. Emmerling and R. Kraehnert, ACS Nano, 2010, 4, 1076–1082 CrossRef CAS.
- M. Eichelbaum, K. Rademann, A. Hoell, D. M. Tatchev, W. Weigel, R. Stosser and G. Pacchioni, Nanotechnology, 2008, 19, 135701 CrossRef.
- B. Abecassis, F. Testard, O. Spalla and P. Barboux, Nano Lett., 2007, 7, 1723–1727 CrossRef CAS.
- J. Polte, F. Emmerling, M. Radtke, U. Reinholz, H. Riesemeier and A. F. Thunemann, Langmuir, 2010 Search PubMed.
- T. Morita, E. Tanaka, Y. Inagaki, H. Hotta, R. Shingai, Y. Hatakeyama, K. Nishikawa, H. Murai, H. Nakano and K. Hino, J. Phys. Chem. C, 2010, 114, 3804–3810 CrossRef CAS.
- G. Sankar and W. Bras, Catal. Today, 2009, 145, 195–203 CrossRef CAS.
- B. Abecassis, F. Testard and O. Spalla, Phys. Rev. Lett., 2008, 100, 115504 CrossRef.
- M. Kotlarchyk, R. B. Stephens and J. S. Huang, J. Phys. Chem., 1988, 92, 1533–1538 CrossRef CAS.
- O. Glatter and O. Kratky, Small Angle X-ray Scattering, Academic Press, London, 1982 Search PubMed.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79, 4215–4221 CrossRef CAS.
- Y. Shiraishi, D. Arakawa and N. Toshima, Eur. Phys. J. E, 2002, 8, 377–383 CAS.
- A. J. Viudez, R. Madueno, T. Pineda and M. Blazquez, J. Phys. Chem. B, 2006, 110, 17840–17847 CrossRef CAS.
- S. M. Humphrey, M. E. Grass, S. E. Habas, K. Niesz, G. A. Somorjai and T. D. Tilley, Nano Lett., 2007, 7, 785–790 CrossRef CAS.
- J. L. Niu, T. Zhu and Z. F. Liu, Nanotechnology, 2007, 18, 7.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data. See DOI: 10.1039/c0nr00541j |
| This journal is © The Royal Society of Chemistry 2010 |
