Hydrothermal transformation from Au core–sulfide shell to Au nanoparticle-decorated sulfide hybrid nanostructures†
Zhihong
Bao
ab,
Zhenhua
Sun
a,
Manda
Xiao
a,
Linwei
Tian
*b and
Jianfang
Wang
*a
aDepartment of Physics, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China. E-mail: jfwang@phy.cuhk.edu.hk; Fax: +852 2603 5204; Tel: +852 3163 4167
bSchool of Public Health and Primary Care, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China. E-mail: linweit@cuhk.edu.hk; Fax: +852 2606 3500; Tel: +852 2252 8879
First published on 7th June 2010
Abstract
Core–shell Au nanorod–AgAuS nanostructures were hydrothermally prepared from Au nanorods and metal thiobenzoates and then transformed into Au nanoparticle-decorated sulfide nanostructures.
Using preformed nanocrystals as reactants for chemical transformation into derivative nanostructures has proven a powerful method for the preparation of complex nanocrystalline solids. Successful examples include the synthesis of hollow metal nanocrystals through galvanic replacement,1,2 fabrication of chalcogenide nanocrystals3 and nanorod superlattices4via ion exchange, preparation of metal oxide5 and chalcogenide6,7 nanocrystals through diffusion.
Gold nanocrystals are chemically stable and exhibit rich plasmonic properties.8,9 They have been synthetically integrated together with other components, such as magnetic nanoparticles10,11 and semiconductor nanocrystals,12–14 to achieve multiple functionalities and synergistic properties. Noble metal chalcogenides are semiconductors with narrow band gaps.15 They exhibit interesting optical limiting16 and superionic conduction properties.17 They have been used in photovoltaic cells15 and coupled with titania18 to enhance solar light adsorption for energy conversion and photodegradation of toxic molecules, respectively. To date, there have been a few experiments on the synthesis of core–shell and heterogeneous Au–Ag2S nanostructures.19–21 Herein we report on the hydrothermal preparation of core–shell Au nanorod–AgAuS nanostructures and the subsequent transformation of the core–shell nanostructures into Au nanoparticle-decorated sulfide nanostructures.
The procedure for the production of the two types of nanostructures is shown schematically in Fig. 1, where Au nanorods and thiobenzoates are utilized as the starting reactants. The Au nanorods were grown using a seed-mediated method.22 They were washed by centrifugation and dispersed in 0.1 M cetyltrimethylammonium bromide (CTAB) solutions. Their diameter and length, measured from their transmission electron microscopy (TEM, Fig. 2a) images, are 16 ± 2 and 69 ± 8 nm, respectively.
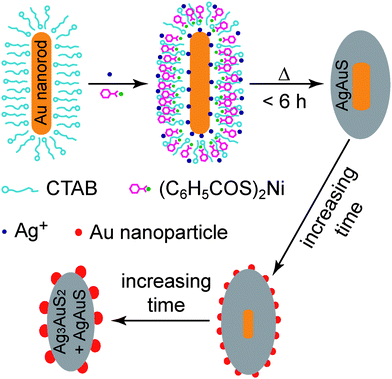 | ||
| Fig. 1 Schematic illustrating the formation of the core–shell and Au nanoparticle-decorated sulfide nanostructures. | ||
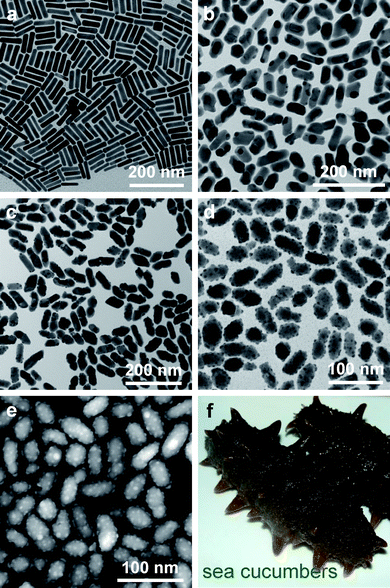 | ||
| Fig. 2 (a) TEM (FEI CM120) image of the starting nanorods. (b)–(d) TEM images of the hybrid gold–gold silver sulfide nanostructures obtained from the hydrothermal process for 3, 9, and 24 h, respectively. (e) HAADF-STEM (FEI Tecnai F20) image of the same sample as shown in (d). (f) Digital photo of two sea cucumbers. | ||
We have recently described a general strategy for the synthesis of core–shell Au nanocrystal–metal sulfide nanostructures.23 The method was employed in this experiment to prepare the core–shell Au nanorod–AgAuS nanostructures by mixing together the Au nanorod solution (10 mL), nickel thiobenzoate (0.002 g), and AgNO3 (0.01 M, 0.25 mL) and then transferring the mixture solution to a 15-mL Teflon-lined stainless steel autoclave. The autoclave was kept at 140 °C for varying periods of time and then taken out for natural cooling in air. In our experiments, the Au nanorod concentration was controlled by adjusting the extinction value at the longitudinal plasmon peak to be 1.8 at an optical path length of 0.5 cm. Nickel thiobenzoate acted as a source of sulfur. The amount of sulfur was in excess, with the molar ratio between sulfur and the total amount of the added Ag+ ions plus the Au atoms from the nanorods was estimated to be ∼2. The dissolution of the nickel complex in the aqueous reaction solution was enhanced by CTAB. Because the Au nanorods were encapsulated with CTAB molecules, the hydrothermal decomposition of the nickel complex embedded in the CTAB layer led to the formation of the AgAuS shell on the nanorod. The Ag and Au ingredients in the shell arose from the Ag+ ions in the solution and the Au nanorod, respectively.
The morphological evolution of the hybrid nanostructures versus the hydrothermal time is revealed by TEM and scanning electron microscopy (SEM) imaging (Fig. 2b–d and Figs. S1 and S2 of the ESI†). When the reaction time is shorter than ∼6 h, the products are mainly composed of the core–shell Au nanorod–AgAuS nanostructures. The Au nanorods are shortened gradually and become nearly spherical, with an accompanying increase in the size distribution. The overall shape of the nanostructures turns spheroidal, while the starting Au nanorods are cylindrical. When the reaction time is extended to 9 h, small Au nanoparticles start to appear on the outer surface of the sulfide shell. Within 9–18 h of the reaction time, the spherical Au cores get smaller and more Au nanoparticles are produced on the shell surface. When the reaction time is within 18–36 h, the Au cores are completely etched, with an accompanying increase in the number and size of the Au nanoparticles on the outer surface. The resultant nanostructures are similar in morphology to sea cucumbers (Fig. 2f). When the reaction time is longer than 36 h, the Au nanoparticles start to detach from the nanostructures and form much larger, separate nanoparticles (Fig. S1e and f, Fig. S2h and I of the ESI†), presumably through Ostwald ripening.
The Au nanoparticle-decorated sulfide nanostructures were further characterized with high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM, Fig. 2e), which clearly reveals that the Au core is absent from most of the nanostructures and that the Au nanoparticles are attached on the outer surface and protrude out. Therefore, in comparison to the morphology of sea cucumbers, the gold silver sulfide core and decorating Au nanoparticles are analogous to the body and feet of sea cucumbers, respectively.
The crystalline structure of both the core–shell and Au nanoparticle-decorated sulfide products was characterized with high-resolution TEM (HRTEM). Fig. 3a and b show the HRTEM images acquired from two different regions on one core–shell nanostructure. Lattice fringes are clearly observed in the shell. They are ascribed to crystalline AgAuS. Fig. 3c and d show the HRTEM images recorded on one sulfide nanostructure decorated with Au nanoparticles. The clearly-resolved lattice fringes are ascribed to crystalline Ag3AuS2 and Au. The HRTEM results are consistent with the powder X-ray diffraction (XRD) measurements (Fig. S3 of the ESI†). Most of the XRD peaks of the core–shell nanostructures can be indexed as a combination of the monoclinic Petrovskaite form of AgAuS (JCPDS 38-0396) and the face-centered-cubic form of Au (JCPDS 65-2870). Most of the XRD peaks of the Au nanoparticle-decorated sulfide nanostructures can be indexed as a combination of the Petrovskaite form of AgAuS, the tetragonal Liujinyinite form of Ag3AuS2 (JCPDS 44-1466), and the face-centered-cubic form of Au.
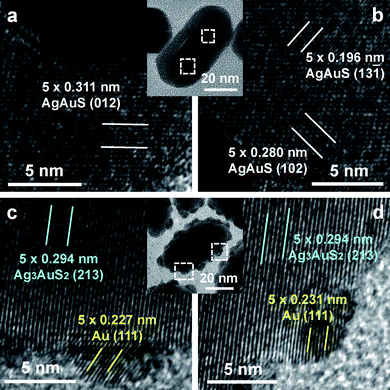 | ||
| Fig. 3 (a, b) HRTEM (FEI Tecnai F20) images of one core–shell Au nanorod–AgAuS nanostructure (inset) obtained from the hydrothermal process for 3 h. (c, d) HRTEM images of one sulfide nanostructure decorated with Au nanoparticles (inset). The sample was obtained from the hydrothermal process for 24 h. | ||
The lengths and middle diameters of the hybrid nanostructures were measured from their TEM images. Compared to the starting Au nanorods, the core–shell Au nanorod–AgAuS nanostructures produced after a short hydrothermal time show a decrease in length and an increase in diameter (Fig. 4a). As the hydrothermal time is increased, the average length first increases and then decreases steadily, while the diameter stays unchanged. The Au nanoparticles decorated on the sulfide nanostructures range from 3–9 nm in size and show an increased size broadening as the reaction time is prolonged (Fig. S4 of ESI†).
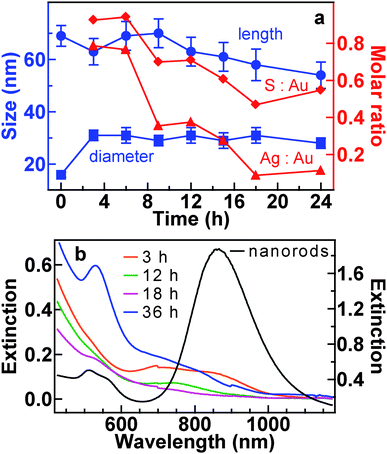 | ||
| Fig. 4 (a) Variations in the length, middle diameter, and elemental molar ratios of the hybrid nanostructures versus the hydrothermal time. (b) Extinction (Hitachi U-3501 UV–visible/NIR spectrophotometer) spectra of the starting Au nanorods (right axis) and the nanostructures (left axis) obtained after varying periods of the hydrothermal time. For the extinction spectral measurements, 1 mL of each sample solution was transferred to a quartz cuvette with an optical path length of 0.5 cm. | ||
No nickel is detected in the nanostructures by energy-dispersive X-ray (EDX) analysis (Fig. S5 of the ESI†), which confirms that the role of the nickel complex is to supply sulfur for the formation of the sulfides. Moreover, the S-to-Au and Ag-to-Au molar ratios are observed to decrease as a function of the hydrothermal reaction time (Fig. 4a).
Combining all of the observations together, we believe that in the early stage of the reaction, the Ag+ ions, sulfur species, and Au nanorods react together to form the core–shell Au nanorod–AgAuS nanostructures. As the reaction time is prolonged, the Au cores are gradually etched and at the same time, small Au nanoparticles start to form on the outer surface of the shell. The etching is believed to start with the electron transfer from the Au core to the sulfide shell.24 The Au ions generated from the electron transfer substitute the cations in the sulfide lattice. They then diffuse through consecutive substitutions to the outer surface of the sulfide shell, where they are reduced by obtaining electrons from the sulfide or the solution to produce Au nanoparticles. As the reaction proceeds further, more Au nanoparticles are produced, yielding the Au nanoparticle-decorated sulfide nanostructures. The net result of the entire reaction is that the Au atoms diffuse from the inner core to the outside surface.
The starting Au nanorods have a transverse and longitudinal plasmon wavelength of 512 and 860 nm, respectively (Fig. 4b). After the formation of the AgAuS shell, the longitudinal plasmon peak is weakened and broadened over a range of 700–850 nm, due to the etching of the nanorods. The extinction spectra of the Au nanoparticle-decorated sulfide nanostructures exhibit a weak peak at ∼520 nm, which is ascribed to the plasmon resonance of the small Au nanoparticles. As a result, the solutions of the hybrid nanostructures show a color change from pale yellow to red as the hydrothermal time is increased (Fig. S6 of the ESI†).
In sumamry, a hydrothermal method has been employed for both the synthesis of the core–shell Au nanorod–AgAuS nanostructures and their subsequent transformation into the Au nanoparticle-decorated sulfide nanostructures. As the reaction time is increased, the Au cores are observed to be gradually etched, while small Au nanoparticles are formed on the outer surface. We believe that this preparation method could potentially be used to synthesize other metal–semiconductor nanostructures with desirable optical and photocatalytic properties.
This work was supported by the NSFC and RGC Joint Research Scheme (Ref. No.: N_CUHK454/08, Project Code: 2900331; Ref. No.: N_CUHK465/09, Project Code: 2900339).
References
- Y. J. Xiong, B. Wiley, J. Y. Chen, Z.-Y. Li, Y. D. Yin and Y. N. Xia, Angew. Chem., Int. Ed., 2005, 44, 7913 CrossRef CAS.
- J. Y. Chen, F. Saeki, B. J. Wiley, H. Cang, M. J. Cobb, Z.-Y. Li, L. Au, H. Zhang, M. B. Kimmey, X. D. Li and Y. N. Xia, Nano Lett., 2005, 5, 473 CrossRef CAS.
- D. H. Son, S. M. Hughes, Y. D. Yin and A. P. Alivisatos, Science, 2004, 306, 1009 CrossRef CAS.
- R. D. Robinson, B. Sadtler, D. O. Demchenko, C. K. Erdonmez, L.-W. Wang and A. P. Alivisatos, Science, 2007, 317, 355 CrossRef CAS.
- M. Yin, C.-K. Wu, Y. B. Lou, C. Burda, J. T. Koberstein, Y. M. Zhu and S. O'Brien, J. Am. Chem. Soc., 2005, 127, 9506 CrossRef CAS.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS.
- K. Jang, S. Y. Kim, K. H. Park, E. Jang, S. Jun and S. U. Son, Chem. Commun., 2007, 4474 RSC.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. X. Gao, L. F. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857 CrossRef CAS.
- L. M. Liz-Marzán, Langmuir, 2006, 22, 32 CrossRef CAS.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. H. Sun, Nano Lett., 2005, 5, 379 CrossRef CAS.
- E. V. Shevchenko, M. I. Bodnarchuk, M. V. Kovalenko, D. V. Talapin, R. K. Smith, S. Aloni, W. Heiss and A. P. Alivisatos, Adv. Mater., 2008, 20, 4323 CrossRef CAS.
- T. Mokari, E. Rothenberg, I. Popov, R. Costi and U. Banin, Science, 2004, 304, 1787 CrossRef CAS.
- T. Mokari, C. G. Sztrum, A. Salant, E. Rabani and U. Banin, Nat. Mater., 2005, 4, 855 CrossRef.
- V. Subramanian, E. E. Wolf and P. V. Kamat, J. Am. Chem. Soc., 2004, 126, 4943 CrossRef CAS.
- T. B. Nasrallah, H. Dlala, M. Amlouk, S. Belgacem and J. C. Bernède, Synth. Met., 2005, 151, 225 CrossRef.
- W. L. Jia, E. P. Douglas, F. G. Guo and W. F. Sun, Appl. Phys. Lett., 2004, 85, 6326 CrossRef CAS.
- E. Bychkov, Solid State Ionics, 2009, 180, 510 CrossRef CAS.
- M. C. Neves, J. M. F. Nogueira, T. Trindade, M. H. Mendonça, M. I. Pereira and O. C. Monteiro, J. Photochem. Photobiol., A, 2009, 204, 168 CrossRef CAS.
- M. Z. Liu and P. Guyot-Sionnest, J. Mater. Chem., 2006, 16, 3942 RSC.
- J. Yang and J. Y. Ying, Chem. Commun., 2009, 3187 RSC.
- J. M. Zhu, Y. H. Shen, A. J. Xie and L. Zhu, J. Mater. Chem., 2009, 19, 8871 RSC.
- W. H. Ni, X. S. Kou, Z. Yang and J. F. Wang, ACS Nano, 2008, 2, 677 CrossRef CAS.
- Z. H. Sun, Z. Yang, J. H. Zhou, M. H. Yeung, W. H. Ni, H. K. Wu and J. F. Wang, Angew. Chem., Int. Ed., 2009, 48, 2881 CrossRef CAS.
- J. Yang and J. Y. Ying, J. Am. Chem. Soc., 2010, 132, 2114 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The TEM and SEM images, digital photos, XRD patterns, and EDX spectra of the hybrid nanostructures, and the size distributions of the Au nanoparticles on the nanostructures. See DOI: 10.1039/c0nr00302f |
| This journal is © The Royal Society of Chemistry 2010 |
