Fabrication of self-supporting porous silicon membranes and tuning transport properties by surface functionalization
Leonora
Velleman
a,
Cameron James
Shearer
a,
Amanda Vera
Ellis
a,
Dusan
Losic
b,
Nicolas Hans
Voelcker
a and
Joseph George
Shapter
*a
aCentre for NanoScale Science and Technology, Flinders University, School of Chemical and Physical Sciences, 5040, Australia. E-mail: Joe.shapter@flinders.edu.au
bIan Wark Research Institute, University of South Australia, Mawson Lakes Campus, 5095, Australia
First published on 7th July 2010
Abstract
This study presents a simple approach to perform selective mass transport through freestanding porous silicon (pSi) membranes. pSi membranes were fabricated by the electrochemical etching of silicon to produce membranes with controlled structure and pore sizes close to molecular dimensions (∼12 nm in diameter). While these membranes are capable of size-exclusion based separations, chemically specific filtration remains a great challenge especially in the biomedical field. Herein, we investigate the transport properties of chemically functionalized pSi membranes. The membranes were functionalized using silanes (heptadecafluoro-1,1,2,2-tetrahydrodecyl)dimethylchlorosilane (PFDS) and N-(triethoxysilylpropyl)-o-polyethylene oxide urethane (PEGS) to give membranes hydrophobic (PFDS) and hydrophilic (PEGS) properties. The transport of probe dyes tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate (Rubpy) and Rose Bengal (RB) through these functionalized membranes was examined to determine the effect surface functionalization has on the selectivity and separation ability of pSi membranes. This study provides the basis for further investigation into more sophisticated surface functionalization and coupled with the biocompatibility of pSi will lead to new advances in membrane based bio-separations.
Introduction
In recent years, there has been a great deal of interest in the development and characterization of well-organized, stable and reproducible membranes capable of molecular separation. Potentially porous silicon (pSi) membranes have the capability of performing low cost, high throughput and high selectivity separations. pSi membranes can be fabricated with highly controlled morphologies, pore size and surface area and due to their tuneable pore size, size selective separations could potentially be performed. In addition to these advantageous structural characteristics, pSi is also a biocompatible material.1,2 Consequently pSi is of great importance in the development of nano-bio applications such as molecular-sensing, bio-sensing, drug delivery and tissue engineering.3–6 Additionally, pSi's high porosity and large internal surface area (up to 800 m2 g−1) can accommodate large amounts of functional molecules.2Emerging applications for pSi membranes are based on the ability to functionalize the surfaces of the membrane with organic monolayers to provide rich surface chemistries and a platform capable of incorporating other functional groups and materials, thus providing a membrane where the surface chemistry can be tailored and optimized for a specific purpose. Surface functionalization of pSi has given access to a broad range of novel organic–inorganic hybrid materials relevant to a variety of applications.1,3,4,7 The modification of inorganic materials by the adsorption of organic molecules is a common technique for tailoring interface properties.8,9 The attachment of a variety of functional molecules onto pSi membranes is possible via attachment to the silanol (Si–OH) groups present on the inner and outer surfaces after oxidation. Silanes can attach to the silanol groups to form highly ordered self-assembled monolayers (SAMs). In addition to the ease of preparation of these SAMs, they possess advantageous characteristics such as high structural stability due to covalent attachment between the silane molecules and silicon surface.10–12
There is much interest in increasing the efficacy of surface chemistry driven molecular transport and separation.8 However, the detailed mechanism behind transport properties through functionalized membranes is not sufficiently understood. This work is a preliminary investigation into the effect on the transport properties of pSi membranes coated with various SAMs. The pores in pSi can be made controllably with molecular dimensions and hence provide an opportunity to develop readily tunable, molecularly selective membranes.
The silanes (heptadecafluoro-1,1,2,2-tetrahydrodecyl)dimethylchlorosilane (PFDS) and N-(triethoxysilylpropyl)-o-polyethylene oxide urethane (PEGS) were chosen to functionalize pSi membranes due to their hydrophobic and hydrophilic properties respectively.13 Through examining the transport of probe dyes through these functionalized membranes the transport mechanism of molecules through SAM modified pores can be probed. Perfluorinated SAMs are highly hydrophobic and provide optimal properties such as high resistance to thermal decomposition and stability towards various chemicals.14 Furthermore, PFDS and PEGS are used due to their ability to form low free energy surfaces14–17 and their non-fouling properties.18–21
To our knowledge, there has not been any previous research performed regarding the transport of molecules through pSi membranes primarily due to the fragility of the membranes. In this work, we describe a simple procedure to provide support to the pSi membranes in order to directly observe the transport properties of these membranes. Furthermore, we present the ability for these freestanding pSi membranes to perform molecular separations and investigate the selectivity of silane-functionalized membranes towards the transport of organic dyes with different hydrophilicity.
Experimental
Materials
Silicon wafers (100 orientation, p-type, Boron doped, 0.00055–0.001 Ω cm) were purchased from Virginia Semiconductor (USA). The silanes, (heptadecafluoro-1,1,2,2-tetrahydrodecyl)dimethylchlorosilane (PFDS) was purchased from Gelest, Inc. (USA) and N-(triethoxysilylpropyl)-o-polyethylene oxide urethane (PEGS) was purchased from Fluorochem (UK). The dyes tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate (Rubpy) and Rose Bengal (RB) were supplied from Aldrich (Australia). Hydrofluoric acid (48% (w/w) aqueous, HF) was purchased from Merck. The ethanol used for pSi etching was 100% undenatured and purchased from Chem-Supply (Australia). All other reagents, dichloromethane (DCM), methanol, acetone and toluene were purchased from Aldrich (Australia) and used as received. Milli-Q water (18 MΩ) was used for rinsing and preparation of solutions.Fabrication of pSi membranes
A custom made Teflon cell with an etching area of 1.767 cm2 was used. Prior to etching, the silicon wafer was washed with methanol, acetone and DCM and dried under a stream of nitrogen. The first etching step was carried out at a constant current of 100 mA (using a Keithley 2425 source meter) for 30 minutes in a solution of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) 48% aqueous HF/ethanol. The HF solution was removed and replaced with a solution of 1
1 (v/v) 48% aqueous HF/ethanol. The HF solution was removed and replaced with a solution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) HF/ethanol for the second etching step. A current of 450 mA was applied for 60 s. The HF solution was then removed and the pSi substrate was washed repeatedly with ethanol, removed from the etching cell and allowed to dry.
1 (v/v) HF/ethanol for the second etching step. A current of 450 mA was applied for 60 s. The HF solution was then removed and the pSi substrate was washed repeatedly with ethanol, removed from the etching cell and allowed to dry.
Functionalization of pSi membranes
After etching, the freestanding pSi membranes were carefully placed in tube furnace (Labec, Australia) and thermally annealed for 1 h at 400 °C. The membranes were removed from the furnace and allowed to cool and then either investigated for molecule transport properties or further functionalized with a silane. The pSi membranes were treated with the hydrophobic silane PFDS via a neat silanization method by placing the membrane within a glass Petri dish, depositing 20 µL of the silane on-top of the membrane, covering the Petri dish and then placing in an oven at 80 °C for 15 min.22 The membrane was then washed repeatedly with ethanol and allowed to dry. The pSi membranes were treated with the hydrophilic PEGS via a solution phase deposition method by immersing the pSi in a solution of 1% (v/v) of the PEGS in dry toluene for 10 min. The now hydrophilic membrane was then washed in toluene and then repeatedly washed in ethanol and allowed to dry.Characterization of pSi membranes
Scanning electron microscopy (SEM) (Helios NanoLab DualBeam, FEI, Adelaide Microscopy) was used to characterize the structure of the fabricated pSi membranes. The static contact angle of water droplets on the surfaces of pristine pSi and silanized pSi was determined with a custom-built contact angle goniometer. Samples were affixed to a glass slide and a droplet of 1 µL of Milli-Q water was applied to the surface. Images of the drops were recorded using a horizontal digital microscope. Contact angle measurements were determined from drop images using drop shape analysis software (Image J, NIH, USA). The mean value of the contact angles was calculated from at least 3 individual measurements taken at different locations on the examined substrates.Investigation of the transport properties of pSi membranes
The fragile freestanding pSi membranes were supported by sealing the membrane between two pieces of mica with epoxy. Each piece of mica contained a hole with an area of 0.05 cm2. The membrane was sandwiched between the pieces of mica and the holes in the mica were aligned thus forming an effective open area of pores of 0.05 cm2. The support of the mica provided the ability to clamp the pSi membrane into the transport cell without breakage. Transport experiments were performed using a U-tube permeation cell in which the membrane separates two half-cells: the feed cell and the permeate cell. The transport properties of the pSi membranes were determined with two probe dyes: Rubpy, with more hydrophobic properties and the RB, with more hydrophilic properties. Separate permeation experiments were performed with Rubpy and RB in which a 1 mM solution of the dye in ethanol was added to the feed cell and pure solvent was added to the permeate cell. The diffusion of the dye from the feed cell to the permeate cell was continuously monitored with a UV-Vis fiber optic spectrophotometer (Ocean Optics, USA) at 286 nm and 454 nm for Rubpy or 549 nm for RB. The ratio of the flux for Rubpy to the flux for RB (flux ratio) was determined. Additional transport experiments were performed with feed cell concentrations of 500 µM and 1.5 mM in order to plot the flux of the dye molecule against the concentration difference across the membrane to obtain the permeability.Results and discussion
Membrane characterization
Permeable pSi substrates were prepared by a two-step electrochemical etching of silicon in a solution of ethanoic hydrofluoric acid. The two-step process involves an initial etching step to create the pores and a final electropolishing step to liberate the porous layer from the silicon wafer. The created pSi membrane was quite brittle but was strong enough to be held with tweezers. SEM was used to calculate the pore size and depth of pSi membranes created. Fig.1(a) is an example of a typical image with the mean pore size calculated to be 12.8 nm with a range of 2 to 30 nm (Fig. 1(c)). This pore size region was chosen because it was considered to be large enough for silane penetration into the pore while small enough that the effect of the silanes on transport will be maximized. It should be noted that the pores investigated in this study are considerably smaller than those of commonly used materials in these transport experiments (e.g., Anodisc porous alumina, pore size 20–200 nm). The total thickness of the pSi membrane was found to be 70 µm and a small portion of the cross-section showing the pore structure is shown in Fig. 1(b).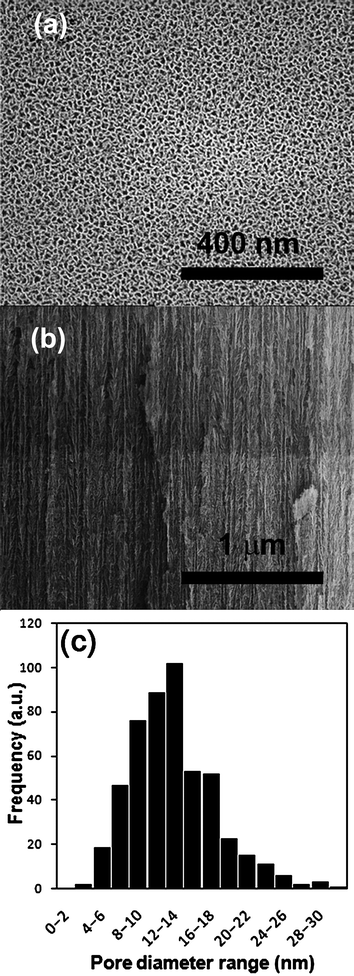 | ||
| Fig. 1 SEM images of (a) top down and (b) side view of the pSi membranes and (c) a pore size distribution graph (as determined by SEM analysis). | ||
To improve the level of molecular separation of the pSi membranes, surface functionalization was completed to make the surface either hydrophobic or hydrophilic. This was achieved by first oxidising the surface thermally and then depositing silane layers utilising simple silane surface chemistry (Fig. 2). Surface functionalization of the pSi membranes was confirmed by contact angle measurements. The thermally oxidized pSi membrane showed a hydrophilic contact angle of approximately 20° (Table 1). This value is due to the surface chemistry consisting of silicon to oxygen bonds (e.g. –Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Si–OH and –Si–O–Si–). When functionalized with the hydrophobic PFDS silane the contact angle was found to be 135°. The high value is attributed to two factors: the highly hydrophobic nature of the PFDS surface coating and the roughness of the porous structure which has been shown to increase contact angle values.23,24Table 1 also shows a contact angle of approximately 13° for the hydrophilic PEGS modified pSi surface. Values of between 10 and 15° have previously been reported for a similar PEGylated silane on a PDMS surface.25 These contact angle values indicate that the surface functionalization was successful and that both hydrophobic and hydrophilic pSi surfaces have been created.
O, Si–OH and –Si–O–Si–). When functionalized with the hydrophobic PFDS silane the contact angle was found to be 135°. The high value is attributed to two factors: the highly hydrophobic nature of the PFDS surface coating and the roughness of the porous structure which has been shown to increase contact angle values.23,24Table 1 also shows a contact angle of approximately 13° for the hydrophilic PEGS modified pSi surface. Values of between 10 and 15° have previously been reported for a similar PEGylated silane on a PDMS surface.25 These contact angle values indicate that the surface functionalization was successful and that both hydrophobic and hydrophilic pSi surfaces have been created.
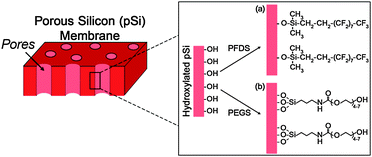 | ||
| Fig. 2 Schematic of the chemical modification of pSi membranes with (a) a hydrophobic fluorinated silane (PFDS) and (b) a hydrophilic silane (PEGS). | ||
| Surface modification | Contact angle/° |
|---|---|
| Unfunctionalized | 19.5 ± 4.5 |
| PFDS | 135 ± 13 |
| PEGS | 12.9 ± 1.8 |
Dye transport
To explore the transport and selectivity properties of hydrophobic (PFDS) and hydrophilic (PEGS) modified pSi membranes, the flux of the dyes, Rubpy (hydrophobic) and RB (hydrophilic), was measured. Fig. 3 presents the transport of both probe dyes through unfunctionalized pSi membranes and pSi membranes functionalized with PFDS and PEGS. Straight-line plots are obtained indicating steady state (Fick's first law26) diffusion across the membrane.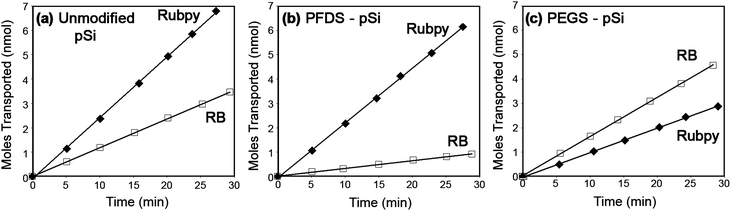 | ||
| Fig. 3 Transport of Rubpy (hydrophobic properties) and RB (hydrophilic properties) through pSi membranes where (a) is an unfunctionalized membrane, (b) is a hydrophobic (PFDS) functionalized membrane and (c) is a hydrophilic (PEGS) functionalized membrane. | ||
A summary of the permeation data of these dyes through functionalized and unfunctionalized pSi membranes is presented in Table 2. The pSi membrane permeability coefficients were determined from Fick's first law of diffusion (eqn (1)) for each of the dyes by documenting the flux of the dyes through the various surface functionalized pSi membranes at several feed concentrations: 500 µm, 1 mM and 1.5 mM (data for 500 µm and 1.5 mM not shown).
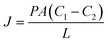 | (1) |
| Surface modification | Flux of permeate molecule/mol cm−2 h−1 | Flux ratio of Rubpy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RB RB |
Membrane permeability, P/cm2 h−1 | ||
|---|---|---|---|---|---|
| Rubpy | RB | Rubpy | RB | ||
| Unfunctionalized | 3.08 ± 0.17 × 10−7 | 1.46 ± 0.10 × 10−7 | 2.11 | 2.72 ± 0.32 × 10−9 | 1.35 ± 0.22 × 10−9 |
| PFDS | 2.76 ± 0.12 × 10−7 | 3.87 ± 0.16 × 10−8 | 7.13 | 2.88 ± 0.07 × 10−9 | 4.73 ± 0.74 × 10−10 |
| PEGS | 1.24 ± 0.01 × 10−7 | 1.92 ± 0.03 × 10−7 | 0.65 | 1.12 ± 0.06 × 10−9 | 1.39 ± 0.21 × 10−9 |
The permeability can be expressed as,
| P = DK, | (2) |
| c1 = KC1 | (3) |
For an unfunctionalized pSi membrane we find that the flux of RB is 2.11 times smaller than Rubpy (Table 2 and Fig. 3(a)). The observed difference in transport is due to the variation in the diffusion coefficients of the dyes which are attributed to factors such as the size, charge, shape and solubilities of the molecules in the solvent. The transport data obtained from the functionalized membranes are compared to the unfunctionalized membrane transport data in order to determine the impact of functionalization on the transport properties of the membrane.
Fig. 3(b) displays the transport of Rubpy and RB through the hydrophobic PFDS modified membrane in which the transport of Rubpy through the PFDS modified membrane remains relatively unchanged in comparison to the transport through the unfunctionalized membrane (Fig. 3(a)), resulting in similar permeability coefficients (Table 2). Therefore the diffusion of the hydrophobic dye through the hydrophobic functionalized membrane is not hindered with the addition of the adsorbed monolayer and is allowed to pass freely through the membrane. Modification with silanes would be expected to slightly decrease the membrane pore size and consequently decrease the transport. However, it is also important to consider the partitioning of organic solutes into the organic SAM formed within the pores. The ability for molecules to partition into molecular layers such as SAMs and lipid bilayers and the impact this has on transport properties have been explored and reported in the recent literature.28–32 In our case, it is likely that the hydrophobic dye can partition into the hydrophobic silane layer and thus diffusion across the membrane can occur through both the silane layer and the solvent in the centre of the pore resulting in similar Rubpy transport rates through PFDS-modified and unfunctionalized membranes. From Fig. 3(b) it can be seen that the transport of RB through the PFDS modified membrane is significantly reduced in comparison to the transport through the unfunctionalized membrane (Fig. 3(a)). Table 2 shows that the permeability coefficient of RB into the PFDS–pSi membrane is approximately 2.8 times smaller than the permeability coefficient of the dye into the unfunctionalized membrane. Therefore the partition coefficient for RB transport in the PFDS–pSi membrane has decreased (eqn (2)). The PFDS–pSi membrane exhibits a hydrophobic environment in which the hydrophilic dye would not enter easily. Furthermore, it is unlikely that the RB will partition into the hydrophobic organic monolayer and hence the reduction of the transport rate can also be attributed to a reduction in the pore diameter from the adsorbed monolayer. These contributing factors lead to an overall decrease in the transport of RB through the PFDS–pSi membrane. Thus the PFDS–pSi membrane facilitates the transport of hydrophobic species through the membrane while hindering the transport of hydrophilic species.
The transport rates of Rubpy and RB through the hydrophilic PEGS modified membrane is presented in Fig. 3(c). In this case the transport of RB through the membrane is faster than Rubpy resulting in a flux ratio of 0.65 (Table 2). The permeability coefficients (Table 2) of RB through the PEG–pSi membrane and the unfunctionalized membrane are similar therefore the ability for RB to partition into the PEG-functionalized membrane is unaffected. Furthermore, the permeability of Rubpy through the membrane has decreased considerably which is due to a reduction in the partition coefficient explained by the poor solubility of Rubpy into the hydrophilic environment formed within the pores of the membrane due to adsorption of the hydrophilic PEGS. Therefore, complementary to the transport results obtained from the PFDS–pSi membranes, the transport of hydrophilic species through the PEGS modified membrane is facilitated while the transport of the hydrophobic species is hindered due to the hydrophilic environment exhibited by the PEGS modified membrane.
From Fig. 3 it is apparent that the PFDS-functionalized membrane favours the transport of the hydrophobic dye over the hydrophilic dye (Fig. 3(b)) and the PEGS-functionalized membrane favours the transport of the hydrophilic dye over the hydrophobic dye (Fig. 3(c)). After PFDS modification it is seen that the flux ratio of Rubpy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RB has increased by a factor of 3.38 (from 2.11 to 7.13) while after PEGS modification the flux ratio of Rubpy
RB has increased by a factor of 3.38 (from 2.11 to 7.13) while after PEGS modification the flux ratio of Rubpy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RB has decreased by a factor of 3.25 (from 2.11 to 0.65) (Table 2). Silane modification has therefore improved the selectivity properties of pSi membranes considerably. Thus chemical sensitivity has been imparted to the membrane through the adsorption of silanes onto the inner and outer surfaces of the membrane resulting in an enhancement in the degree of separation between hydrophilic and hydrophobic molecules. These results confirm that surface modifications can be tailored to favor the transport of molecules with a specific chemical nature.
RB has decreased by a factor of 3.25 (from 2.11 to 0.65) (Table 2). Silane modification has therefore improved the selectivity properties of pSi membranes considerably. Thus chemical sensitivity has been imparted to the membrane through the adsorption of silanes onto the inner and outer surfaces of the membrane resulting in an enhancement in the degree of separation between hydrophilic and hydrophobic molecules. These results confirm that surface modifications can be tailored to favor the transport of molecules with a specific chemical nature.
Conclusions
The transport properties of porous silicon membranes can be varied by modifying the inner and outer surfaces with silanes. The transport properties vary in a chemically rational way with the chemical nature of the silane. Porous silicon membranes were modified with the highly hydrophobic silane, PFDS, and the hydrophilic silane, PEGS. Contact angle measurements confirmed the hydrophobic and hydrophilic nature imparted to the membrane after PFDS and PEGS functionalization, respectively. The transport data were found to support these results where the PFDS–pSi membrane facilitated the transport of hydrophobic molecules while hindering the diffusion of hydrophilic molecules. Transport of hydrophilic species through the PEGS modified membrane was facilitated while the transport of the hydrophobic species was hindered. This preliminary investigation provides the first study into mass flow through a pSi layer and is a basis for further investigation into more sophisticated surface functionalization and the tailoring of surface chemistry to improve the control of membrane selectivity and separation towards specific molecules. The impact of surface modification coupled with the biocompatibility of pSi membranes will provide new advances in membrane based bio-applications such as drug delivery, biomolecule sensing and artificial organs. In particular, the employment of freestanding pSi with open ended pore structures in biomolecule separation applications such as hemodialysis will be greatly beneficial.Notes and references
- L. T. Canham, Adv. Mater., 1995, 7, 1033–1041 CAS.
- M. P. Stewart and J. M. Buriak, Adv. Mater., 2000, 12, 859–869 CrossRef CAS.
- E. J. Anglin, L. Y. Cheng, W. R. Freeman and M. J. Sailor, Adv. Drug Delivery Rev., 2008, 60, 1266–1277 CrossRef CAS.
- A. Jane, R. Dronov, A. Hodges and N. H. Voelcker, Trends Biotechnol., 2009, 27, 230–239 CrossRef CAS.
- W. Sun, J. E. Puzas, T. J. Sheu, X. Liu and P. M. Fauchet, Adv. Mater., 2007, 19, 921–926 CrossRef CAS.
- T. A. Desai, D. J. Hansford, L. Leoni, M. Essenpreis and M. Ferrari, Biosens. Bioelectron., 2000, 15, 453–462 CrossRef CAS.
- S. P. Low, K. A. Williams, L. T. Canham and N. H. Voelcker, Biomaterials, 2006, 27, 4538–4546 CrossRef CAS.
- B. J. Ravoo, D. N. Reinhoudt and S. Onclin, Angew. Chem., Int. Ed., 2005, 44, 6282–6304 CrossRef CAS.
- L. de Smet, H. Zuilhof, E. J. R. Sudholter, G. Wittstock, M. S. Duerdin, L. H. Lie, A. Houlton and B. R. Horrocks, Electrochim. Acta, 2002, 47, 2653–2663 CrossRef CAS.
- S. R. Cohen, R. Naaman and J. Sagiv, J. Phys. Chem., 1986, 90, 3054–3056 CrossRef CAS.
- R. Maoz and J. Sagiv, Langmuir, 1987, 3, 1034–1044 CrossRef CAS.
- A. Seeboth and W. Hettrich, J. Adhes. Sci. Technol., 1997, 11, 495–505 CrossRef CAS.
- A. M. M. Jani, E. J. Anglin, S. J. P. McInnes, D. Losic, J. G. Shapter and N. H. Voelcker, Chem. Commun., 2009, 3062–3064 RSC.
- R. Banga, J. Yarwood, A. M. Morgan, B. Evans and J. Kells, Langmuir, 1995, 11, 4393–4399 CrossRef CAS.
- M. K. Chaudhury and G. M. Whitesides, Science, 1992, 255, 1230–1232 CAS.
- E. Lindner and E. Arias, Langmuir, 1992, 8, 1195–1198 CrossRef CAS.
- C. P. Tripp, R. P. N. Veregin and M. L. Hair, Langmuir, 1993, 9, 3518–3522 CrossRef CAS.
- Y. H. M. Chan, R. Schweiss, C. Werner and M. Grunze, Langmuir, 2003, 19, 7380–7385 CrossRef CAS.
- Z. H. Yang, J. A. Galloway and H. U. Yu, Langmuir, 1999, 15, 8405–8411 CrossRef CAS.
- A. Papra, N. Gadegaard and N. B. Larsen, Langmuir, 2001, 17, 1457–1460 CrossRef CAS.
- S. Jo and K. Park, Biomaterials, 2000, 21, 605–616 CrossRef CAS.
- S. Fiorilli, P. Rivolo, E. Descrovi, C. Ricciardi, L. Pasquardini, L. Lunelli, L. Vanzetti, C. Pederzolli, B. Onida and E. Garrone, J. Colloid Interface Sci., 2008, 321, 235–241 CrossRef CAS.
- Y. H. Liu, X. K. Wang, J. B. Luo and X. C. Lu, Appl. Surf. Sci., 2009, 255, 9430–9438 CrossRef CAS.
- A. Ressine, D. Finnskog, G. Marko-Varga and T. Laurell, NanoBiotechnology, 2008, 4, 18–27 CrossRef CAS.
- V. Sharma, M. Dhayal, Govind, S. M. Shivaprasad and S. C. Jain, Vacuum, 2007, 81, 1094–1100 CrossRef CAS.
- A. Fick, Ann. Phys. (Weinheim, Ger.), 1855, 94, 59–86 Search PubMed.
- E. L. Cussler, Diffusion: Mass Transfer in Fluid Systems, Cambridge University Press, 3rd ed, 2009 Search PubMed.
- D. Bemporad, J. W. Essex and C. Luttmann, J. Phys. Chem. B, 2004, 108, 4875–4884 CrossRef CAS.
- S. Paula, A. G. Volkov, A. N. VanHoek, T. H. Haines and D. W. Deamer, Biophys. J., 1996, 70, 339–348 CrossRef CAS.
- A. Janshoff and C. Steinem, Anal. Bioanal. Chem., 2006, 385, 433–451 CrossRef CAS.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- D. J. Odom, L. A. Baker and C. R. Martin, J. Phys. Chem. B, 2005, 109, 20887–20894 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
