Synthesis and surface activity of single-crystalline Co3O4 (111) holey nanosheets†
Lifang
Chen
a,
Juncheng
Hu
*b,
Ryan
Richards
*c,
Sergey
Prikhodko
d and
Suneel
Kodambaka
d
aState Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai, 20023, P.R. China
bKey Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission & Ministry of Education South-Central University for Nationalities, Wuhan, 430074, P.R. China. E-mail: junchenghuhu@hotmail.com
cDepartment of Chemistry and Geochemistry, Colorado School of Mines, Golden, CO 80401, USA. E-mail: rrichard@mines.edu; Fax: +1 303 2733629
dDepartment of Materials Science and Engineering, University of California Los Angeles, Los Angeles, CA 90095, USA
First published on 18th June 2010
Abstract
Single crystalline, thermally stable, Co3O4 (111) holey nano-sheets were prepared by an efficient, template-free, wet chemical synthetic approach. The high energy (111) surfaces formed can be used as highly active heterogeneous catalysts for methanol decomposition.
The development of novel nanostructured transition metal oxides with controlled shapes and morphologies has stimulated considerable research interest for their novel properties and potential applications in materials science, physics and chemistry.1–3 Various two dimensional nanostructures such as nanosheets have been successfully synthesized.4,5 However, holey (or porous) nanosheets have not been widely studied owing to less existing knowledge for their preparation. Relatively few papers exist regarding the synthesis of holey sheets and they pertain to ZnO nanoplates obtained by a hydrothermal method6 and we previously reported on the synthesis of NiO nanosheets with hexagonal holes.7 The fabrication of holey architectures on the nanometre scale enables greater control of local chemical environment, with potential applications in gas sensors, sorbents, catalysts, and other fields.8–10 It is widely accepted that synthesis of metal oxide nanocrystals with exposed high-energy surfaces is important because these facets can endow nanocrystals with a high activity, thus facilitating their potential applications such as highly efficient catalysts.10,11 Typically, 111-teminated transition-metal oxides with rock salt structure have high surface reactivities, consisting of alternating polar monolayers of anions and cations.7,12 Thus, it still remains a major challenge to synthesize porous metal oxides nanosheets with high energy surfaces.
In particular, nanostructured cobalt oxides have been widely used in many applications, such as toxic gas sensors,9 catalysts,10,13 magnetic semiconductors14 and rechargeable battery materials.15 Several different cobalt oxide nanostructures such as wires, cubes, and platelets have been successfully synthesized via calcination, hydrothermal processes as well as inorganic templates.13,16 In this communication, we report an efficient template-free wet chemical method to synthesize porous 〈111〉-terminated Co3O4 nanosheets with high yield (>95%). These Co3O4 nano-sheets are catalytically active for methanol decomposition at low temperature, which shows their potential application in alternative energy technologies.
Wet chemistry is a widely used method to synthesize inorganic nanomaterials in the laboratory. The pores are formed when organic moieties are removed by calcination. The diagram of holey Co3O4 nanosheet preparation is depicted in Scheme 1. The first step comprises formation of an as-synthesized Co3O4 sheet during a methanol thermal process. The as-synthesized Co3O4 sheet containing Co3+, H2O and methanol results in the formation of an intermediate Co–OH and Co–O–CH3 species. Subsequently, the as-synthesized Co3O4 maintains its sheet-like structure without distortion and shrinkage through the supercritical fluid drying treatment (methanol critical point: 79.9 bar, 240 °C). Lastly, porous Co3O4 nanosheets were generated after calcination of the sheet-like precursor at 350 °C for 3 h and 500 °C for 2 h (ESI†).
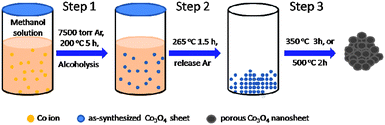 | ||
| Scheme 1 Multistage synthesis of porous Co3O4 nanosheets by a wet-chemical method. | ||
Fig. 1a and b show the as-synthesized Co3O4 powders with different magnifications, composed of very thin sheet-like nanostructures about 10–15 µm in diameter. The sizes and shapes of the flower-like powders do not appear to change significantly due to calcination. However, nanometre-sized holey architectures were formed in the nanosheets, as shown in Fig. 1d. The powder X-ray diffraction (XRD) analysis of the sheet-like precursor is shown in Fig. 2a. The presence of diffraction peaks at 2θ = 11.5° and 33.5° is indicative of crystalline product. After calcination at 500 °C, additional peaks with higher intensities appear at 2θ = 31.3°, 36.8°, 38.5°, 44.7°, 55.7°, 59.4° and 65.2° (see Fig. 2b). According to standard Co3O4 XRD pattern (JCPDS card no. 43-1003), these peaks are assigned to (111), (220), (311), (222), (400), (422), (511) and (440) diffraction lines of cubic spinel Co3O4 phase (space group: Fd3m), corresponding to d spacings of 4.6670, 2.8580, 2.4374, 2.3337, 2.0210, 1.6501, 1.5558 and 1.4292 Å, respectively. No other secondary or amorphous phase was observed. Diffuse reflectance infrared Fourier transform spectra (DRIFTS) result affirm the presence of organic species in the as-synthesized material prior to annealing (Fig. S1a†). The bands at 1074, 1161, 1274, 1387, 1469, 2800, 2870 and 2924 cm−1 are indicative of the presence of methoxyl or dioxymethyl groups, while the bands at 1640 cm−1 correspond to the bending vibrations of OH. The bands at 2110, 2176 and 2576 cm−1 are stretching vibrations of OH groups in the presence of surface carboxylic acids that may result from the decomposition of methanol at high temperature during supercritical fluid drying.17 After calcination at 500 °C (Fig. S1b†), these methoxyl and hydroxyl groups are completely removed and the pores are formed. However, the sheet-like morphology is still maintained.
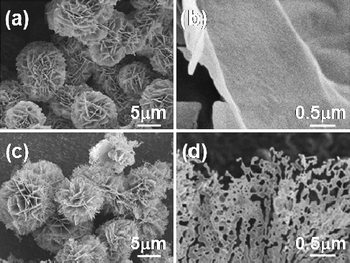 | ||
| Fig. 1 Scanning electron microscopy (SEM) images of Co3O4 powders at different magnifications. (a) and (b) As-synthesized products. (c) and (d) After annealing at 500 °C for 2 h. | ||
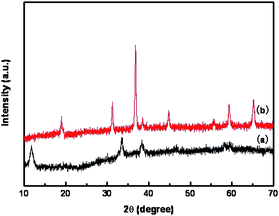 | ||
| Fig. 2 XRD patterns of (a) as-synthesized product and (b) after annealing at 500 °C for 2 h. | ||
Fig. 3a is a bright-field TEM image of a single sheet and Fig. 3b is the corresponding SAED pattern prior to calcination. The sheet shown in Fig. 3a is found to be lying parallel to the TEM grid which enabled determination of its surface crystal orientation. Fig. 3c is the indexed SAED pattern from which we identify the crystal structure as cubic Co3O4 # 43-1003 (JCPDS), Fd3m (227) (a = 8.084 Å), matching well with our XRD data. From the SAED pattern, we determine that the entire sheet is a single crystal with its surface perpendicular to 〈111〉 direction. In Fig. 3c, the larger spots correspond to fundamental reflections, from the families of the planes {224} and {220}. The smaller spots are super-reflections that appear as a result of the ordered Fd3m structure of Co3O4. We measured interplanar distances (dm) of four non-symmetrical spots labeled 1, 2, 3 and 4 in Fig. 3b. Table S1† shows dm values along with JCPDS values (dt) for Fd3m structure and the corresponding planes. Spots 2 and 4 are the data for super reflections. We find that the measured values are, within experimental errors of ∼5%, in agreement with the JCPDS data.
![(a) Transmission electron microscopy (TEM) image, (b) selected area electron diffraction (SAED) pattern of the as-synthesized Co3O4 powder sample prior to calcination, and (c) indexed diffraction pattern in b (zone axis on the sketch is [1̄11] direction).](/image/article/2010/NR/c0nr00205d/c0nr00205d-f3.gif) | ||
Fig. 3 (a) Transmission electron microscopy (TEM) image, (b) selected area electron diffraction (SAED) pattern of the as-synthesized Co3O4 powder sample prior to calcination, and (c) indexed diffraction pattern in b (zone axis on the sketch is [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11] direction). 11] direction). | ||
Typically, crystallization of amorphous materials via heat treatment leads to undesired morphology.18,19 In this case, it is interesting to note that after calcination, the Co3O4 maintained the morphology: sheet-like structure aggregated into “flowers” with a nominal diameter up to ∼10 µm and a sheet thickness of several nm. Moreover, a large number of holes form in the nano-sheets (Fig. 4b and c); the surface areas of the powders are 70 m2 g−1 and 24 m2 g−1 after annealing at 350 °C for 3 h and 500 °C for 2 h, respectively. TEM images in Fig. 4 show that the sheets become holey after calcination, in agreement with the SEM observations. Moreover, the hole size and their number density increase with increasing calcination temperatures. For example, calcination at 350 °C for 3 h results in pores with an average radius of 5.7 nm and an areal fraction of 12.4%. Calcination at 500 °C for 2 h yields pores with an average radius of 15.7 nm and an areal fraction of 18.4%. From the SAED patterns (shown as insets in Fig. 4a–c), we confirm that the crystal structure is unaffected during calcination. The arrows in the SAED patterns highlight the strongest intensity line corresponding to {311}. Moreover, the sharpness and the intensity of the diffraction reflections increase upon calcination, indicative of improved crystallinity of the powders. From the fast Fourier transform (Fig. S2b†), the spots labeled 1, 2, and 3 yield interplanar spacings of 2.9, 1.7 and 1.4 Å respectively, corresponding to {220}, {422} and {440} planes of Fd3m structure, and orientation of entire sheet is close to the (111) axis. After calcination at 500 °C (Fig. S2f†), we identify the orientation of the tilted sheet to be close to the 〈110〉 zone axis. Spots 1, 2, 3, 4 and 5 are identified as {200}, {311}, {220}, {422} and {440}, respectively. This also shows that the sheet surface is (111)-oriented and the one in Fig. S2d† is tilted along the [011] direction. From the image of the tilted sheet in Fig. S2d,† we can estimate the sheet thickness h as h = a/sin(θ), where a is the measured edge thickness in the tilted image and θ is the tilt angle. Here, θ = 35°16′ the angle between (111) and [011], and a = 5.88 nm yielding h = 10.2 nm. Table S2† shows the measured (dm) interplanar spacings for the powders calcined at 350 °C for 3 h and 500 °C for 2 h along with the JCPDS values (dt) for Co3O4. The measured data are, within the experimental uncertainties of ∼3%, in good agreement with the JCPDS values.
 | ||
| Fig. 4 TEM images and SAED patterns of Co3O4 powders (a) as-synthesized, (b) after calcination at 350 °C for 3 h, and (c) 500 °C for 2 h. The scale bars in the figures are 200 nm. Arrows highlight the diffraction ring corresponding to {311}. | ||
Methanol is a “smart” molecular probe that can provide fundamental information about the number and the nature of active surface sites.20,21 Methanol decomposition has been found to be structure sensitive, and the reaction processes depend on the arrangement of the surface atoms.22 Methanol can be used as a combustible or in methanol fuel cells.19 In the present work, we investigated methanol adsorption and reaction on the surface of Co3O4 nanostructures by DRIFT spectroscopic techniques at low temperatures. 10 mg of sample were pretreated at 500 °C for 2 h under nitrogen to remove adsorbed water and impurities from its surface. A spectrum of clean sample surface was collected at the adsorption temperature (room temperature or 70 °C) under 1 Torr and was used as the reference. Methanol was introduced into the reaction chamber at 0.05 Torr while the sample was maintained at the adsorption temperature. DRIFT spectra of Co3O4 nano-sheets and conventionally prepared CP-Co3O4 (CP-Co3O4 from cobalt acetate decomposition at 500 °C for 5 h with a specific surface area of 8.6 m2 g−1 and SEM image in Fig. S3†) exposed to methanol vapor pressures of 0.05 Torr at room temperature were collected at different times (Fig. 5a). Typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bands at 1740 cm−1, associated with a series of bands at 1453, 1347, 1215 cm−1 are observed (Fig. 5a, traces 1 and 2), and assigned to the OCO asymmetric and symmetric stretching modes of an intermediate formate species adsorbed on the Co3O4 nano-sheet surface.7,23–25 This result indicates that methanol can be dissociated and oxidized on the surface of Co3O4 nano-sheets at room temperature. However, no dissociated adsorption or oxidation occurs on the surface of CP-Co3O4 (Fig. 5a, trace 3 and 4). When Co3O4 nano-sheets were exposed to methanol at 70 °C for 5 minutes (Fig. 5b, trace 1), a small amount of CO2 (peaks at 2361 and 2342 cm−1) and CO (peaks at 2075 and 2053 cm−1) formed. The amount of CO2 increased with time (from Fig. 5b trace 1 to trace 2), while the adsorbed intermediate species (CO) peaks are similar on both traces. In contrast, only a small amount of CO was observed and no CO2 formed when CP-Co3O4 was exposed to methanol (Fig. 5b, traces 3 and 4). This suggests that Co3O4 nano-sheets are very active for methanol decomposition in comparison with CP-Co3O4. The DRIFT spectra for methanol adsorption and decomposition on the two different forms of Co3O4 and the interaction of methanol with Co3O4 are elaborated in Fig. S4 and S5† in addition to Fig. 5. After exposing the sample to methanol vapor at room temperature or 70 °C, the DRIFT spectra show C–O (between 950 and 1080 cm−1), O–H (between 3600 and 3800 cm−1), and C–H (between 2800 and 3200 cm−1) stretching bands, characteristic of methanol adsorption.22 These results indicate that most of the methanol is adsorbed on the surface of the sample. For methanol oxidation and decomposition, the activity of Co3O4 (111) nanosheets is higher than that of CP-Co3O4.
O stretching bands at 1740 cm−1, associated with a series of bands at 1453, 1347, 1215 cm−1 are observed (Fig. 5a, traces 1 and 2), and assigned to the OCO asymmetric and symmetric stretching modes of an intermediate formate species adsorbed on the Co3O4 nano-sheet surface.7,23–25 This result indicates that methanol can be dissociated and oxidized on the surface of Co3O4 nano-sheets at room temperature. However, no dissociated adsorption or oxidation occurs on the surface of CP-Co3O4 (Fig. 5a, trace 3 and 4). When Co3O4 nano-sheets were exposed to methanol at 70 °C for 5 minutes (Fig. 5b, trace 1), a small amount of CO2 (peaks at 2361 and 2342 cm−1) and CO (peaks at 2075 and 2053 cm−1) formed. The amount of CO2 increased with time (from Fig. 5b trace 1 to trace 2), while the adsorbed intermediate species (CO) peaks are similar on both traces. In contrast, only a small amount of CO was observed and no CO2 formed when CP-Co3O4 was exposed to methanol (Fig. 5b, traces 3 and 4). This suggests that Co3O4 nano-sheets are very active for methanol decomposition in comparison with CP-Co3O4. The DRIFT spectra for methanol adsorption and decomposition on the two different forms of Co3O4 and the interaction of methanol with Co3O4 are elaborated in Fig. S4 and S5† in addition to Fig. 5. After exposing the sample to methanol vapor at room temperature or 70 °C, the DRIFT spectra show C–O (between 950 and 1080 cm−1), O–H (between 3600 and 3800 cm−1), and C–H (between 2800 and 3200 cm−1) stretching bands, characteristic of methanol adsorption.22 These results indicate that most of the methanol is adsorbed on the surface of the sample. For methanol oxidation and decomposition, the activity of Co3O4 (111) nanosheets is higher than that of CP-Co3O4.
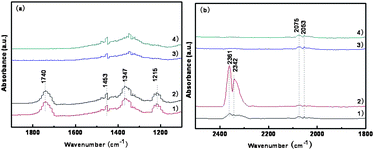 | ||
| Fig. 5 (a) DRIFTS in the range of 1100–1900 cm−1 of methanol vapor of 0.05 Torr at room temperature (1) 5 min, (2) 20 min in equilibrium with porous Co3O4 (111) nanosheets (calcination at 500 °C for 2 h), and (3) 5 min and (4) 20 min in equilibrium with CP-Co3O4. (b) DRIFTS in the range of 1800–2500 cm−1 of methanol adsorption and reaction at 70 °C on Co3O4 nanosheets at (1) 5 min, (2) 20 min, and on CP-Co3O4 at (3) 5 min and (4) 20 min. | ||
Catalytic performance of nanoscale-materials can be finely tuned by their composition or shape. Composition mediates electronic structures and shape determines surface arrangement and coordination.11 Most catalytic reactions reported to date for rock salt type metal oxides are related to the well-known (100) surface.26,27 The (111) facet is more interesting as it is composed of alternating monolayers of anions and cations.28 Recently, Shen et al. reported that the presence of active Co3+ species at the surface has a high reaction rate for CO oxidation.10 We have also found that the polar (111) surface oriented MgO nano-sheets showed ultrahigh activity for the Claisen–Schmidt condensation and methanol decomposition due to the high concentration of surface O2− Lewis basic sites.12,22 The polar (111) surface NiO nanosheets exhibited efficient activity for methanol decomposition and adsorbents for dye pollutants removal.7,29,30 In comparison with polar NiO (111) nanosheets, there are two additional peaks at 1215 cm−1 and 1740 cm−1 observed (see Fig. 5a, traces 1 and 2), contributing to stretching OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands, respectively. Finally, the preparation is simple and has a potential to prepare metal oxides with highly ionic (111) as the major surface of other oxides.
O bands, respectively. Finally, the preparation is simple and has a potential to prepare metal oxides with highly ionic (111) as the major surface of other oxides.
In summary, we have experimentally demonstrated the direct synthesis of single crystalline Co3O4 holey nano-sheets by an efficient wet chemical approach, where the (111) facets form the main surfaces. We have used SEM and TEM to characterize the morphology and structure of Co3O4 powders, and find these powders are composed of ∼10 nm thick single-crystalline sheets aggregated in the form of flower-like architectures. From the SAED and XRD patterns, we determine the crystal structure to be cubic Fd3m and that the individual sheets are oriented along high energy (111) surface. Upon calcination, the sheets exhibit a high density of nanoscale, strongly facetted, holes while the macroscopic shape, size, and structure remain the same. These materials with higher energy (111) surfaces can be used as highly active heterogeneous catalysts (providing a prototype for the study of surface structure and surface reactions of polar oxide surfaces) and have potential applications as nanoscale devices, tools and in alternative-energy technologies.
Acknowledgements
This work is supported by East China University of Science and Technology, the Fundamental Research Funds for Central Universities, Science and Technology Commission of Shanghai Municipality (10ZR1407200), Colorado School of Mines, South-Central University for Nationalities, National Natural Science Foundation of China (20803096), and the American Chemical Society Petroleum Research Fund.Notes and references
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- J. Polleux, A. Gurlo, N. Barsan, U. Weimar, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2005, 45, 261.
- N. Pinna and M. Niederberger, Angew. Chem., Int. Ed., 2008, 47, 5292 CrossRef CAS.
- L. Hu, Q. Peng and Y. Li, J. Am. Chem. Soc., 2008, 130, 16136 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc, 2004, 126, 8124 CrossRef CAS.
- Z. Jing and J. Zhan, Adv. Mater., 2008, 20, 4547 CrossRef CAS.
- J. Hu, K. Zhu, L. Chen, H. Yang, Z. Li, A. Suchopar and R. Richards, Adv. Mater., 2008, 20, 267 CrossRef CAS.
- L. Chen, Z. Song, X. Wang, S. V. Prikhodko, J. Hu and R. Richards, ACS Appl. Mater. Interfaces, 2009, 1, 1391 Search PubMed.
- R. Wu, J. Wu, T. Tsai and C. Yeh, Sens. Actuators, B, 2006, 120, 104 CrossRef.
- X. Xie, Y. Li, Z.-Q. Liu, M. Haruta and W. Shen, Nature, 2009, 758, 746 CrossRef.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- K. Zhu, J. Hu, C. Kuebel and R. Richards, Angew. Chem., Int. Ed., 2006, 45, 7277 CrossRef CAS.
- X. Xie and W. Shen, Nanoscale, 2009, 1, 50 RSC.
- E. L. Salabas, A. Rumplecker, F. Kleiz, F. Radu and F. Schueth, Nano Lett., 2006, 6, 2977 CrossRef CAS.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885 CrossRef CAS.
- H. Tűysűz, Y. Liu, C. Weidenthaler and F. Schűth, J. Am. Chem. Soc., 2008, 130, 14108 CrossRef.
- Y. V. Rashkes, J. Appl. Spectrosc., 1967, 6, 330 CrossRef.
- M. Niederberger, G. Garnweitner, N. Pinna and M. Antonietti, J. Am. Chem. Soc., 2004, 126, 9120 CrossRef CAS.
- K. T. Ranjit and K. J. Klabunde, Chem. Mater., 2005, 17, 65 CrossRef CAS.
- G. A. Olah, Angew. Chem., Int. Ed., 2005, 44, 2636 CrossRef CAS.
- W. Cheng, Acc. Chem. Res., 1999, 32, 685 CrossRef CAS.
- J. Hu, K. Zhu, L. Chen, C. Kuebel and R. Richards, J. Phys. Chem. C, 2007, 111, 12038 CrossRef CAS.
- R. C. Millikan and K. S. Pitzer, J. Am. Chem. Soc., 1958, 80, 3515 CrossRef CAS.
- T. C. Schilke, I. A. Fisher and A. T. Bell, J. Catal., 1999, 184, 144 CrossRef CAS.
- K. D. Jung and A. T. Bell, J. Catal., 2000, 193, 207 CrossRef CAS.
- M. B. Jensen, L. Pettersson, O. Swang and U. Olsbye, J. Phys. Chem. B, 2005, 109, 16774 CrossRef CAS.
- J. Rudberg and M. Foster, J. Phys. Chem. B, 2004, 108, 18311 CrossRef CAS.
- P. W. Tasker, J. Phys. C: Solid. State Phys., 1979, 12, 4977 CrossRef CAS.
- Z. Song, L. Chen, J. Hu and R. Richards, Nanotechnology, 2009, 20, 275707 CrossRef (9p).
- Y. Liu, L. Chen, J. Hu, J. Li and R. Richards, J. Phys. Chem. C, 2010, 114, 1641 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional DRIFTS, interplanar spacings, TEM and fast Fourier transform. See DOI: 10.1039/c0nr00205d |
| This journal is © The Royal Society of Chemistry 2010 |
