Access to small size distributions of nanoparticles by microwave-assisted synthesis. Formation of Ag nanoparticles in aqueous carboxymethylcellulose solutions in batch and continuous-flow reactors
Satoshi
Horikoshi
*a,
Hideki
Abe
b,
Kanjiro
Torigoe
b,
Masahiko
Abe
ab and
Nick
Serpone
*c
aResearch Institute for Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan. E-mail: horikosi@rs.noda.tus.ac.jp
bDepartment of Pure and Applied Chemistry, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan
cGruppo Fotochimico, Dipartimento di Chimica Organica, Universita di Pavia, Via Taramelli 10, Pavia, 27100, Italy. E-mail: nick.serpone@unipv.it
First published on 26th May 2010
Abstract
This article examines the effect(s) of the 2.45-GHz microwave (MW) radiation in the synthesis of silver nanoparticles in aqueous media by reduction of the diaminesilver(I) complex, [Ag(NH3)2]+, with carboxymethylcellulose (CMC) in both batch-type and continuous-flow reactor systems with a particular emphasis on the characteristics of the microwaves in this process and the size distributions. This microwave thermally-assisted synthesis is compared to a conventional heating (CH) method, both requiring a reaction temperature of 100 °C to produce the nanoparticles, in both cases leading to the formation of silver colloids with different size distributions. Reduction of the diaminesilver(I) precursor complex, [Ag(NH3)2]+, by CMC depended on the solution temperature. Cooling the reactor during the heating process driven with 390-Watt microwaves (MW-390W/Cool protocol) yielded silver nanoparticles with sizes spanning the range 1–2 nm. By contrast, the size distribution of Ag nanoparticles with 170-Watt microwaves (no cooling; MW-170W protocol) was in the range 1.4–3.6 nm (average size ∼3 nm). The overall results suggest the potential for a scale-up process in the microwave-assisted synthesis of nanoparticles. Based on the present data, a flow-through microwave reactor system is herein proposed for the continuous production of silver nanoparticles. The novel flow reactor system (flow rate, 600 mL min−1) coupled to 1200-Watt microwave radiation generated silver nanoparticles with a size distribution 0.7–2.8 nm (average size ca. 1.5 nm).
1. Introduction
Recent years have witnessed active research in the synthesis of nanoparticles using the effective heating provided by microwave radiation. Parameters that affect the size and size distribution of the dispersed silver nanoparticles that are formed have been described.1,2 They include the nature and concentration of the stabilizer (e.g. poly(vinylpyrrolidone), β-cyclodextrin, and micelles, among others), the nature of the complexed silver, the nature, concentration and strength of the reducing agent (alcohol, formaldehyde, and others), and the pH of the initial solution. Using formaldehyde as reducing agent and citrate as complexing agent for the silver(I) cation yielded small-sized Ag nanoparticles.3 Alcohol reduction of silver salts of fatty acids led to formation of nanoparticles in the range 4.9–7.4 nm on irradiation with 2.45-GHz microwaves for 1 to 5 min at 140–157 °C depending on the alkyl chain lengths of the fatty acids.4 The microwave-assisted synthesis of nanoparticles yielded smaller and a more narrow distribution of silver particles than did conventional heating owing to the faster rate of reduction in the former case.1 The temperature attained by microwave dielectric heating also affected the shape of the nanoparticles generated.5 Microwaves have also been used to produce core–shell bimetallic nanoparticles (e.g. Au–Pt.6 Ag–Cu,7 Au–Ag8) with a stepwise process using alcohols as reducing agents.In all earlier studies the role of the microwave radiation, other than as a heat source, was only of minor interest in organic syntheses9,10 and in the syntheses of nanoparticles.11–15 Early stages in the microwave-assisted formation of Ag nanoparticles in solution were monitored by an in situ surface-enhanced Raman scattering technique.16 Experiments confirmed the formation of uniform seed nanoparticles in solution from a uniform increase of temperature through a homogeneous increase in microwave heating. Heating effects were a key factor in the synthesis of nanoparticles with uniform size.16 It is clear, therefore, that some special thermal effect(s) of the microwaves must be involved in the growth of these metallic (and bimetallic) nanoparticles.
Our overall strategy in this study was to clarify some of the characteristics of the microwave methodology in nanoparticle synthesis. Accordingly, the principal objective was to use the synthesis of silver nanoparticles in the presence of CMC, which acts as both the reducing agent and a stabilizer of the colloids, as the model reaction with which to examine the thermal features of the microwaves in such a process. In particular, the thermal features of the 2.45-GHz microwave radiation (MW) used in the synthesis of silver nanoparticles are compared to conventional heating (CH), with a special emphasis on the temperature effects and the characteristics (if any) of different microwave synthesizers.
2. Experimental setup
High pure grade AgNO3 (Kanto chemical Co. Inc.; 3.14 g), aqueous NH3 (Kanto Chemical Co. Inc.; 28%; 3 mL), and ultrapure H2O (10 mL) were used to prepare the diaminesilver(I) complex, following which a 40-mL solution (60 mM) of [Ag(NH3)2]+ was added to aqueous carboxymethylcellulose (CMC; 0.05% w/v; volume of CMC solution, 40 mL). The resulting solution was then introduced into the 150-mL Pyrex glass batch-type cylindrical reactor {Taiatsu Techno Co.; size, 160 mm (H) × 37 mm (i.d.)} illustrated in Fig. 1. Continuous microwave irradiation (power, 64 Watts) was provided by a Hitachi Kyowa Engineering Co. Ltd. 2.45-GHz microwave generator (maximal power, 800 W), coupled to an isolator (air cooling device), a power monitor and a three-stub tuner; henceforth this method is denoted as the MW method. The reactor was sealed with two Byton O-rings and a stainless steel cap containing an Allihn condenser (Fig. 1).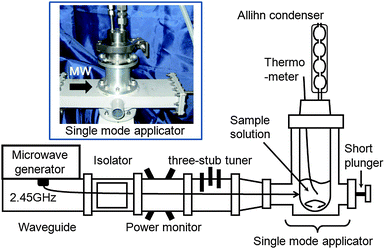 | ||
| Fig. 1 Experimental setup of the microwave apparatus with a single mode applicator used in the synthesis of silver nanoparticles. | ||
Unless noted otherwise, the reaction mixture was continually stirred magnetically under near-reflux conditions. The rate of increase of temperature of the aqueous media under microwave irradiation was not influenced by the presence of the magnetic stirring bar. The continuous microwaves emitted by the magnetron were monitored using the power monitor. After heating the sample solution either with microwaves or conventionally with an oil bath, the reactor contents were rapidly cooled in an ice/water bath to arrest the synthesis of the Ag nanoparticles, which would have otherwise continued as a result of residual heat from the microwave and oil-bath heating. Temperatures from heating and cooling were strictly monitored and measured with a K-type thermocouple. The latter thermometric device was chosen because a fiber optic thermometer would have caused generation of a hot spot upon microwave heating as a result of adsorption of nanoparticles onto the fiber optic polymer sensor surface.
Exploratory experiments ascertained that fluctuations in temperatures measured by both the fiber optic thermometer (Anritsu Meter Co., Ltd. FL-2000) and the thermocouple were less than 1 °C. Moreover, the reactor was positioned such that irradiation was achieved by the microwaves' electric field at its maximal intensity by adjustments of the short plunger and the three-stub tuner. The conventional heating source (CH method) was a silicone oil bath.
The size distributions and morphologies of the silver nanoparticles were characterized by transmission electron microscopy (TEM) using a Hitachi High-Technologies Co. H-7650 electron microscope. Particle size distributions were also determined by light scattering techniques with the Particle Sizing Systems NICOMP 380ZSL. Further confirmation of the formation of the Ag nanoparticles was provided by an analysis of the localized surface plasmon resonance (LSPR)17,18 absorption recorded on an Agilent 8453 UV-visible spectrophotometer.
3. Results and discussion
3.1 Microwave heating effects in nanoparticle synthesis
The microwave-assisted synthesis of nanoparticles is characterized by rapid and homogeneous heating in contrast to a conventional heat-assisted synthesis, even though the thermal effects are similar to those of other heating methods.19 In addition, the heating performances of microwaves can differ from one microwave apparatus to another. Accordingly, the rate of temperature increase of an initial solution with the microwave apparatus of Fig. 1 was measured and compared to the rate from the oil-bath heating method. Continuous 64-Watt (applied power) microwave irradiation showed a rate of temperature rise of 0.51 °C s−1 for the aqueous CMC/diaminesilver(I) solution, whereas for the oil-bath heating method the rate was 0.09 °C s−1 for a power consumption of 400 Watts. The 16% lower power of the microwaves led to a 5.7-fold enhancement of the heating rate relative to oil-bath heating.The rapid heating by microwaves observed herein was controlled and closely monitored so as to extract the characteristic thermal features of the microwaves. Temperature conditions of both heating methods (MW and CH) were closely matched in the nanoparticle synthesis. This was achieved by soaking the cylindrical reactor in the oil bath pre-heated to 190 °C, followed by determination of the temperature rise of the solution (dashed line, Fig. 2). The rise in temperature of the solution exposed to microwave radiation was subsequently matched to the temperature rise in the oil-bath heating using microwave power levels in the range 60–70 Watts at 1-Watt increments controlled by the proportional–integral–derivative (PID) device available in the microwave apparatus. The solid line in Fig. 2 represents the temperature rise in the solution exposed to the 64-Watt microwaves. Following the temperature match between microwave heating and oil-bath heating, formation of silver nanoparticles was monitored at heating times of 0.5, 1, 2, 3, 4 and 5 min by the LSPR band around 420 nm in the UV-visible absorption spectra. Results showed that silver nanoparticles formed after a 3-min heating period by both MW and CH methods. In both cases, the synthesis of Ag nanoparticles necessitated a temperature of 100 °C reached only after this time period. The UV-visible spectra of Fig. 3a and 3b, recorded after a 4-min and 5-min heating time, revealed a LSPR band at 417 nm and 424 nm (MW method) and 413 nm and 421 nm (CH method), respectively, in accord with absorption spectra of as-prepared silver nanoparticles reported earlier by Zhang and coworkers.20 Note that the 5-min UV-visible spectra of the samples were obtained after a 4-fold dilution of the respective colloidal sols. Optical properties of silver nanosized specimens are highly dependent on size and shape.21–23
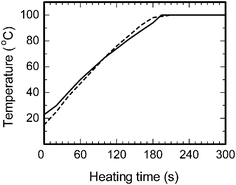 | ||
| Fig. 2 Temperature-time profiles of the aqueous CMC/diaminesilver(I) solution by microwave heating (applied power, 64 Watts; solid line) and oil bath heating (consumed power, 400 Watts; dashed line). | ||
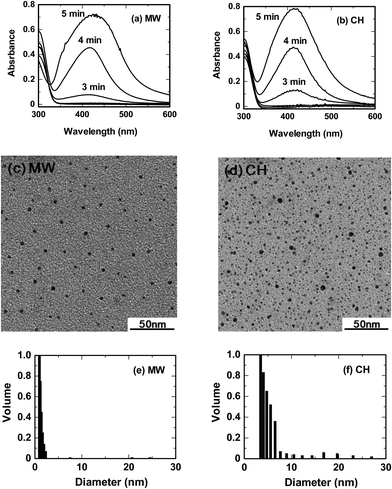 | ||
| Fig. 3 UV-visible absorption spectra, TEM images and particle size distribution with light scattering of the silver nanoparticles produced by microwave and oil bath heating methods (MW heating, a, c and e; oil bath heating, b, d and f). Note that the 5-min UV-visible absorption bands of the samples were obtained after a 4-fold dilution of the respective colloidal sols. Concentrations: CMC, 0.05% w/v and diaminesilver(I) aqueous solution, 60 mM. | ||
Fig. 3c and 3d illustrate the corresponding TEM images of the resulting silver nanoparticles after the 5-min heating time. A fairly monodispersed particle size distribution was observed in TEM images of the colloids obtained from microwave heating (1.8 to 3.6 nm; average size ∼3 nm) under temperature conditions otherwise identical to those of conventional heating, which produced a broader size distribution (1–5 nm). By contrast, light scattering measurements indicate a size distribution by MW heating in the range 1–2.3 nm (Fig. 3e), whereas for the CH heating method they revealed polydipsersed nanoparticles mostly in the 3–5.7 nm range (Fig. 3f), with some up to 30 nm. Despite the above size discrepancies between TEM and light scattering, it is clear that even though the reaction temperature conditions for the microwave and oil-bath methods were identical, there was an otherwise noticeable difference in the rate of formation of the silver nanoparticles and their size distributions.
For a better understanding of the microwave heating mechanism we next examined the dielectric characteristics of the CMC aqueous solution (0.05% w/v), the diaminesilver(I) aqueous solution (60 mM), water, and the CMC/diaminesilver(I) solution after 0 and 5 min irradiation with an Agilent Technologies HP-85070B Network Analyzer. Results are summarized in Table 1.
| Parameters | CMC/H2O | Ag(NH3)2+/H2O | H2O | Sample solution (0 min of irradiation) | Sample solution (5 min of irradiation) |
|---|---|---|---|---|---|
| Dielectric loss factor (ε′′) | 11.3 | 15.2 | 10.7 | 15.3 | 15.2 |
| Dielectric Constant (ε′) | 78.5 | 78.1 | 78.3 | 77.9 | 77.9 |
| Dielectric loss tangent (tan δ = ε′′/ε′) | 0.144 | 0.195 | 0.137 | 0.196 | 0.195 |
The dielectric loss factor, which reflects the degree to which the electromagnetic energy of the microwaves is transformed into thermal energy (heat), of the diaminesilver(I) solution was 1.4-fold greater than that of pure water and greater than the loss factor of the aqueous CMC system indicating that preferential microwave heating occurs at the diaminesilver(I) complex. By contrast, the dielectric constants remained rather constant relative to water, whereas the dielectric loss tangent of the initial solution and of the diaminesilver(I)/water system were otherwise identical to the silver colloidal sol after the 5-min MW heating period but somewhat greater than those of pure water and the CMC/water solution.
The major source of heating from the microwaves originates with the electric field of the microwaves, the magnitude of which can be estimated from eqn (1): i.e. the thermal energy P produced per unit volume originating from the microwaves' electric field is given by,
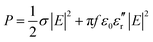 | (1) |
The penetration depth of the 2.45-GHz microwaves in water increases 2.7-fold from ca. 1.8 cm to 4.8 cm when the temperature increases from near-ambient (22 °C) to 99 °C.25 Note that the internal diameter of the Pyrex reactor was 3.7 cm. Thus, to the extent that the 2.45-GHz microwaves penetrate the aqueous media, the increase in temperature by MW heating infers that microwave energy was supplied directly and preferentially to the diamine–silver(I) complex (see above). On the other hand, with oil-bath heating the aqueous solution was heated non-selectively by thermal conduction and convection through the reactor walls, first to the solvent and then to the substrate(s). Thus, mechanistic variations in MW heating versus CH heating are expected to cause differences in heat interchange at the microscopic level which leads ultimately to variations in size distributions of thermally generated Ag nanoparticles.
3.2 Some features of microwave heating
Variations in the two heating methods are clearly evident in the results illustrated in Fig. 4. Five minutes into the microwave irradiation of the aqueous CMC/diaminesilver(I) solution led to formation of a slightly yellow-colored sol in the reactor (Fig. 4a), whose concentration did not change even after 1 h of irradiation. Silver nanoparticles that adsorbed on the reactor walls were easily removed by simple washing with water. By contrast, the silver film (mirror) coating the inner reactor walls formed after 4 min by CH heating (Fig. 4b), at otherwise identical temperature conditions as the MW method, resisted such washings.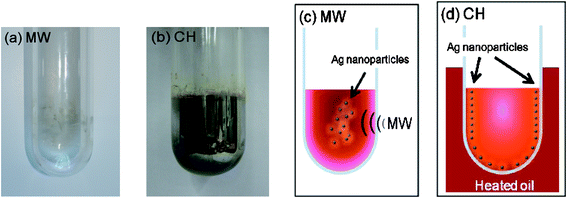 | ||
| Fig. 4 Photograph of the reactor after the sample discharge (a) after 4 min of microwave irradiation, and (b) after 5 min of oil bath heating. Cartoon representing the temperature distribution in the reactor (c) after the 4 min of microwave heating, and (d) after the 4 min of oil bath heating. Note that the photographs in (a) and (b) were taken immediately after the 4-min heating time. Experiments carried out under non-stirring conditions. Concentrations: CMC, 0.05% w/v and diaminesilver(I) aqueous solution, 60 mM. | ||
The initial temperature distributions and formation of silver nanoparticles produced under non-stirring conditions by both MW and CH heating methods are illustrated, respectively, in the cartoons of Fig. 4c and 4d. In the former case, microwave radiation penetrated the CMC/diaminesilver(I) solution causing the temperature to rise by dielectric loss and to some extent by conduction loss heating, followed by subsequent loss of heat to the surroundings through the reactor walls. As such, the temperature near the inner walls of the reactor tended to be lower than at the center of the reactor. That is, the synthesis of Ag nanoparticles by the microwave-assisted process progresses outward from the center of the reactor to the inner reactor walls owing to a temperature gradient that gave rise to a concentration gradient (thermophoretic migration, i.e. the Ludwig–Soret effect26). On the other hand, heat from the oil bath was most prominent at the reactor walls and was subsequently transmitted to the solution by thermal conduction and convection mechanisms. This led ultimately to formation of a silver film at the reactor inner walls as the concentration of the nanoparticles was greatest at this position. Germane to the present discussion, Schanche27 and Kappe28 have described temperature distributions produced from MW and CH heating methods in various organic syntheses. In a later study, the Kappe group noted that the temperature distribution in the MW method may not always be uniform.29 That is, the location where heat is generated may be different from the position where the endothermic process occurs. This calls attention to the fact that even completely homogeneous solutions must be stirred/agitated when using single-mode microwave reactors so as to avoid temperature gradients from developing, a consequence of the inherent field inhomogeneities that exist inside the single-mode microwave cavity.29
Also related to the present study, selective microwave heating in the syntheses of CdSe and CdTe nanoparticles in microwave non-absorbing alkane solvents showed that the heating rate dependencies support precursor activation by selective heating that triggers nucleation.30 Such precursor activation appears to be a peculiar heating phenomenon of the microwaves. Moreover, the mechanism of crystal growth suggested that the growth behavior could be controlled microscopically providing control of precursor activation, nucleation and growth, and that variances in heating rate, cooling rate and thermal gradients in the reaction lead to batch-to-batch variations in the size and shape of the nanoparticles.30,31
3.3 Nanoparticle synthesis with microwaves under cooling conditions
Recent studies have examined external cooling of reactions subjected to microwave dielectric heating as a special case of microwave-assisted organic syntheses.32,33 For instance, cooling microwave-assisted reactions of radical species caused removal of excess heat and maintained reaction temperature at near ambient. This led to relatively high product yields and significant minimization of generated side-products (impurities), two important and attractive outcomes of microwave heating when coupled to a cooling methodology. In addition, cooling allows a higher level of microwave power to be administered directly to the reaction mixture, thus potentially enhancing nonthermal microwave effects that rely on the electric field strength, while at the same time preventing overheating by the continuous removal of heat.29The characteristics of cooling in the microwave-assisted nanoparticle synthesis was therefore examined using an integrated Pyrex flask reactor equipped with a cooling system consisting of a cooling coil and a silicone oil bath fabricated with microwave non-absorbing materials.34 Microwave radiation (frequency, 2.45 GHz) was generated from a Tokyo Rikakikai Co., Ltd. MWO-1000S system consisting of a microwave generator and a multimode applicator. Reaction temperature was controlled with a Tokyo Rikakikai Co., Ltd. MWO-1000 chemical reaction system for microwave heating and cooling using a circulation system. Different protocols were thus examined in the synthesis of silver nanoparticles: (i) irradiation of the sample solution with 390-Watt microwaves under cooling conditions (MW-390W/Cool protocol); (ii) microwave irradiation alone with 170-Watt microwaves (MW-170W protocol); and (iii) irradiation with 170-Watt microwaves under cooling conditions (MW-170W/Cool protocol). Rates of temperature rise with the MW-390W/Cool and MW-170W protocols were 0.50 °C min−1 and 0.51 °C min−1, respectively, reaching a temperature of 100 °C in ca 3.5 min. By contrast, the rate of temperature rise for the MW-170W/Cool protocol was 0.27 °C min−1 and the temperature reached after ca. 4 min was 67 °C (Fig. 5a). Thus the coolant inhibited the temperature rise by 46% in the MW-170W/Cool protocol.
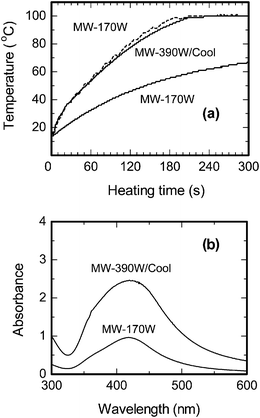 | ||
| Fig. 5 (a) Temperature-time profiles of the aqueous CMC/diaminesilver(I) solution using the MW heating method with 170-Watt microwaves and by the MW/Cooling hybrid methods with 390-Watt and 170-Watt microwaves. (b) Plasmon resonance absorption of Ag nanoparticles using the MW heating method with 170-Watt microwaves and by the MW/Cooling hybrid method with 390-Watt microwaves for 5 min. Note that the sample solution of the MW/Cooling method was diluted 8-fold with water, whereas for the MW-170 method the solution was diluted 4-fold. Concentrations: CMC, 0.05% w/v and diaminesilver(I) aqueous solution, 60 mM. | ||
Maximal absorption of the LSPR band of the silver colloids produced by the MW-390W/Cool and MW-170W protocols occurred at 420 nm, with the band intensity being 5.5-fold greater for the colloids formed with the former protocol (Fig. 5b). Note that the samples were diluted 8-fold and 4-fold, respectively, prior to recording the spectra. The rate of formation of silver nanoparticles was enhanced with the 390-Watt microwaves under cooling conditions (MW-390W/Cool protocol; temperature, 100 °C), relative to the usage of 170-Watt microwaves even though the temperature was also 100 °C.
Transmission electron microscope images of Ag nanoparticles produced by the MW-390W/Cool and MW-170W protocols are displayed in Fig. 6. In the former case, the size distribution of the nanoparticles spanned the 1–2 nm range, whereas for the latter protocol the sizes ranged from 1.4 to 3.6 nm. Clearly, smaller silver nanoparticles are formed at a higher concentration under cooling conditions and at the higher microwave radiation power. The weaker 170-Watt microwaves (MW-170W protocol) led to formation of somewhat larger size nanoparticles (average ∼3 nm; compare the TEMs of Fig. 6a and 6b).
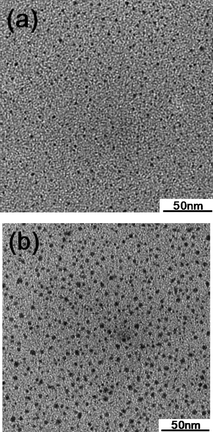 | ||
| Fig. 6 TEM images of Ag nanoparticles generated from (a) the 390-Watt MW/Cool hybrid protocol and from (b) the 170-Watt microwave heating protocol for a 5 min reaction period. | ||
No silver nanoparticles were produced with the MW-170W/Cool protocol as evidenced by both UV-visible absorption spectroscopy and by TEM microscopy, as the maximum temperature reached after 5 min was only 67 °C (Fig. 5a). Recall that a reaction temperature of ca. 100 °C is necessary to nucleate the growth of the silver nanoparticles. Accordingly, a certain threshold of the thermal energy is a necessary condition in the synthesis of silver nanoparticles.
3.4 Microwave-assisted nanoparticle synthesis in a continuous flow reactor
Thus far, silver nanoparticles were herein synthesized in a batch-type reactor (Fig. 1). As part of our long-standing interests in (photo)reactor designs, a continuous-flow reactor system is now proposed for the synthesis of silver nanoparticles taking full advantage of microwave dielectric heating. In this regard, a flow-type reactor system that incorporated a microwave discharge electrodeless lamp device (MDEL) was reported for the first time by Horikoshi and coworkers for the treatment of wastewaters.35,36 Continuous-flow synthesizers are now being examined actively in the field of organic syntheses.37,38An appropriate continuous-flow microwave reactor design must take into account the penetration depth (Dp) of the microwaves into the reacting solution (eqn (2)).
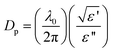 | (2) |
The proposed continuous-flow reactor system consists of a Pyrex pipe (length = 135 mm; internal diameter, 8 mm) placed horizontally in the microwave waveguide through which the aqueous CMC/diaminesilver(I) solution was circulated by means of a peristaltic pump. Irradiation of the reactor contents was performed with 1200-Watt microwaves obtained from a microwave generator (maximal power, 3000 Watts). In the photograph of Fig. 7a, the microwaves emanate from the back toward the reactor with the maximum of the microwaves' electric field positioned at the center of the reactor. A metal mesh closed the waveguide to prevent microwave leakages and to observe events occurring in the reactor. For maximal heating efficiency the flow rate of the peristaltic pump was fixed at 600 mL min−1; the residence time of the precursor solution in the MW reactor was ca. 0.7 s. The solution temperature increased rapidly to 100 °C under microwave irradiation to yield a colloidal sol of Ag nanoparticles which were then collected in the receiver flask and rapidly cooled with an ice-water bath to arrest any further reaction.
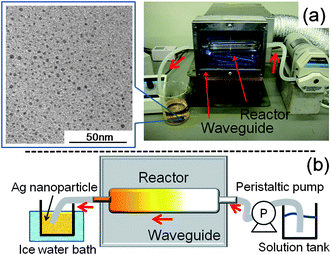 | ||
| Fig. 7 (a) Photograph of the continuous flow reactor system for the microwave-assisted synthesis of silver nanoparticles; the TEM image of the resulting silver colloids is also displayed. (b) Schematic image of the overall experimental setup. Concentrations: CMC, 0.05% w/v and diaminesilver(I) aqueous solution, 60 mM. | ||
The TEM image of the nanoparticles displayed in Fig. 7a confirmed the generation of a fairly narrow size distribution of silver nanoparticles (range, 0.7–2.8 nm; average size ∼1.5 nm) with this reactor setup. The cartoon in Fig. 7b summarizes the reactor setup and our observations. Interestingly, the internal reactor walls showed no visible evidence of a yellow stain (seen above in the batch reactor) by this continuous-flow synthesis of silver nanoparticles even after 7 min of microwave irradiation. With microwave power levels greater than 1200 Watts, we had some difficulty in controlling the temperature, which could (in principle) be corrected by cooling the reactor externally with a cooling device and by a faster solution flow rate to enhance synthesis efficiency. However, addition of any cooling device (e.g. a cooling jacket, cold wind) would unnecessarily complicate the reactor setup. In the present instance, with the 1200-Watt microwaves there was no necessity for cooling and as such this microwave power level was maintained throughout the synthesis. Understandably, a similar continuous-flow rapid synthesis of silver nanoparticles of similar size distribution (i.e., 0.7–2.8 nm) would be somewhat difficult to achieve in a reactor system that relied on the conventional heating method.
4. Concluding remarks
The dielectric characteristics of the aqueous media and the silver colloidal sol in the reactor revealed clear differences in the heating mechanisms between conventional heating and microwave heating. The uniform shape and rapid synthesis of the silver nanoparticles originated from the microscopic heating by microwaves. The basic energy required for the reaction under our conditions was indeed thermal energy. An external cooling system was effective from the viewpoint of size control and the rate of formation of the nanoparticles. A batch-type reactor and a continuous flow-through reactor system have been described for the microwave-assisted synthesis of nanoparticles.Acknowledgements
Financial support to S. H. from the Japan Society for the Promotion of Science (JSPS) through a Grant-in-aid for young scientists (No. B-21750210) and the National Institute for Fusion Science Foundation is gratefully appreciated. One of us (N. S.) thanks Prof. Albini and his group at the Universita di Pavia, Italy, for their continued kind hospitality during the many semesters spent in their laboratory since 2002. We are also grateful to the personnel of the Shinkou Kagaku Co. Ltd, the Hitachi Kyowa Engineering Co. Ltd., and the Tokyo Rikakikai Co., Ltd. for technical assistance.Notes and references
- B. He, J. J. Tan, K. Y. Liew and H. Liu, J. Mol. Catal. A: Chem., 2004, 221, 121–126 CrossRef CAS.
- M. Maillard, S. Giorgio and M. P. Pileni, Adv. Mater., 2002, 14, 1084–1086 CrossRef CAS.
- H. Yin, T. Yamamoto, Y. Wada and S. Yanagida, Mater. Chem. Phys., 2004, 83, 66–70 CrossRef CAS.
- T. Yamamoto, Y. Wada, T. Sakata, H. Mori, M. Goto, S. Hibino and S. Yanagida, Chem. Lett., 2004, 33, 158–159 CrossRef CAS.
- M. Tsuji, N. Miyamae, S. Lim, K. Kimura, X. Zhang, S. Hikino and M. Nishio, Cryst. Growth Des., 2006, 6, 1801–1807 CrossRef CAS.
- R. Harpeness and A. Gedanken, Langmuir, 2004, 20, 3431–3434 CrossRef CAS.
- T. Nakamura, Y. Tsukahara, T. Yamauchi, T. Sakata, H. Mori and Y. Wada, Chem. Lett., 2007, 36, 154–155 CrossRef CAS.
- M. Tsuji, M. Nishio, P. Jiang, N. Miyamae, S. Lim, K. Matsumoto, D. Ueyama and X.-L. Tang, Colloids Surf., A, 2008, 317, 247–255 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546–1557 RSC.
- V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS.
- S. Komarneni, R. Pidugu, Q. H. Li and R. Roy, J. Mater. Res., 1995, 10, 1687–1692 CAS.
- S. Komarneni, D. Li, B. Newalkar, H. Katsuki and A. S. Bhalla, Langmuir, 2002, 18, 5959–5962 CrossRef CAS.
- F. Gao, Q. Lu and S. Komarneni, Chem. Mater., 2005, 17, 856–860 CrossRef CAS.
- B. Hu, S.-B. Wang, K. Wang, M. Zhang and S.-H. Yu, J. Phys. Chem. C, 2008, 112, 11169–11174 CrossRef CAS.
- B. Baruwati, V. Polshettiwar and R. S. Varma, Green Chem., 2009, 11, 926–930 RSC.
- Y. Tsukahara, T. Nakamura, T. Kobayashi and Y. Wada, Chem. Lett., 2006, 35, 1396–1397 CrossRef CAS.
- E. Hutter and J. H. Fendler, Adv. Mater., 2004, 16, 1685–1706 CrossRef CAS.
- S. Link and M. A. El-Sayed, Annu. Rev. Phys. Chem., 2003, 54, 331–366 CrossRef CAS.
- W. Tu and H. J. Liu, J. Mater. Chem., 2000, 10, 2207–2211 RSC.
- J. Zhang, X. Li, X. Sun and Y. Li, J. Phys. Chem. B, 2005, 109, 12544–12548 CrossRef CAS.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS.
- T. Wriedt, J. Quant. Spectrosc. Radiat. Transfer, 2008, 109, 1543–1548 CrossRef CAS.
- C. F. Bohren, D. R. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, New York, 1983 Search PubMed.
- S. Horikoshi and N. Serpone, J. Photochem. Photobiol., C, 2009, 10, 96–110 CrossRef CAS.
- S. Horikoshi, F. Sakai, M. Kajitani, M. Abe and N. Serpone, Chem. Phys. Lett., 2009, 470, 304–307 CrossRef CAS.
- (a) S. Wiegand, J. Phys.: Condens. Matter, 2004, 16, R357–R379 CrossRef CAS; (b) J. Kreft and Y.-L. Chen, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2007, 76, 021912 CrossRef.
- J.-S. Schanche, Mol. Diversity, 2003, 7, 293–300 Search PubMed.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS.
- M. A. Herrero, J. M. Kremsner and C. O. Kappe, J. Org. Chem., 2008, 73, 36–47 CrossRef CAS.
- A. L. Washington II and G. F. Strouse, J. Am. Chem. Soc., 2008, 130, 8916–8922 CrossRef CAS.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS.
- S. Horikoshi, N. Ohmori, M. Kajitani and N. Serpone, J. Photochem. Photobiol., A, 2007, 189, 374–379 CrossRef CAS.
- S. Horikoshi, J. Tsuzuki, M. Kajitani, M. Abe and N. Serpone, New J. Chem., 2008, 32, 2257–2262 RSC.
- See Figure 5 in: S. Horikoshi, A. Matsubara, S. Takayama, M. Sato, F. Sakai, M. Kajitani, M. Abe and N. Serpone, Appl. Catal., B, 2009, 91, 362–367 Search PubMed.
- S. Horikoshi, H. Hidaka and N. Serpone, Environ. Sci. Technol., 2002, 36, 5229–5237 CrossRef CAS.
- S. Horikoshi, M. Abe and N. Serpone, Photochem. Photobiol. Sci., 2009, 8, 1087–1104 RSC.
- O. Benali, M. Deal, E. Farrant, D. Tapolczay and R. Wheeler, Org. Process Res. Dev., 2008, 12, 1007–1011 Search PubMed.
- M. Damm, T. N. Glasnov and C. O. Kappe, Org. Process Res. Dev., 2010, 14, 215–224 Search PubMed.
- D. Bogdal, Tetrahedron Org. Chem. Ser., 2005, 25, 9–11 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
