Transport and encapsulation of gold nanoparticles in carbon nanotubes†
Alessandro
La Torre
a,
Graham A.
Rance
a,
Jaouad
El Harfi
ad,
Jianing
Li
a,
Derek J.
Irvine
ad,
Paul D.
Brown
bc and
Andrei N.
Khlobystov
*a
aSchool of Chemistry, The University of Nottingham, University Park, Nottingham, NG7 2RD, UK. E-mail: andrei.khlobystov@nottingham.ac.uk
bDivision of Materials, Mechanics and Structures, Department of Mechanical, Materials and Manufacturing Engineering, Faculty of Engineering, The University of Nottingham, University Park, Nottingham, NG7 2RD, UK
cNottingham Nanotechnology and Nanoscience Centre, The University of Nottingham, University Park, Nottingham, NG7 2RD, UK
dDepartment of Chemical and Environmental Engineering, Faculty of Engineering, The University of Nottingham, University Park, Nottingham, NG7 2RD, UK
First published on 30th April 2010
Abstract
Nanoparticles confined in small volumes exhibit functional properties different from that of the bulk material. Furthermore, the smaller the volume available then the greater the effects of confinement are observed to be. Metallic nanoparticles encapsulated within carbon nanotubes have been proposed for many applications ranging from catalysis to quantum storage devices. In this study we examine encapsulation of discrete gold nanoparticles (AuNP) within multi-wall carbon nanotubes (MWNT), with internal diameter less than 10 nm. During the encapsulation process AuNP undergo Ostwald ripening allowing them to reach a diameter that precisely matches the internal diameter of MWNT (snug fit). The use of supercritical CO2 as a processing medium enables efficient transport and irreversible encapsulation of AuNP into narrow nanotubes. Once inside MWNT, the nanoparticles are unable to grow further and retain their spheroidal shape. This dynamic behaviour observed for AuNP differs significantly from the behaviour of molecular guest-species under similar conditions.
Carbon nanotubes with their chemically inert interiors and diameters ranging from ∼1 to 50 nm represent ideal nanoscopic containers for molecules and ions.1 The most important geometrical parameter that determines the efficiency of a host nanotube–guest molecule interaction is the ratio of the nanotube internal diameter to the diameter of the encapsulated species (Fig. 1).2 If the nanotube diameter is too small, the guest species is unable to enter the nanotube (Fig. 1a). Conversely, if the nanotube diameter is too big, the host–guest interaction may not be strong enough for the guest to stay inside the nanotube (Fig. 1c). The most efficient interaction is achieved when the van der Waals diameter of the guest molecule closely matches the internal diameter of the nanotube (Fig. 1b). For example, for the case of the fullerene C60 molecule (van der Waals diameter = 1.0 nm), an ideal geometric match, often referred to as a ‘snug fit,’ is achieved when the carbon nanotube exhibits an internal diameter of ∼1.3 nm (Fig. 1d). A snug fit ensures strong interaction between the encapsulated species and the nanotube sidewalls which can be exploited to control the chemical reactions of the encapsulated molecules2–4 or to tune the physical properties of the nanotube itself.5–7
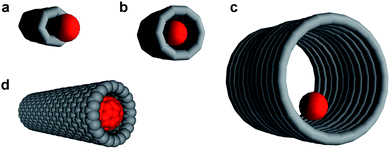 | ||
| Fig. 1 The ratio of guest-species diameter (red ball) to nanotube internal diameter determines the efficiency of nanotube–molecule interaction. If the nanotube is too narrow, the guest species cannot be encapsulated (a); if the nanotube is too wide, the host–guest interactions are weak (c). A ‘snug fit’ (b) enables efficient encapsulation of the guest species within the nanotubes, such as in the case of a fullerene C60 molecule and a 1.3 nm wide single-walled carbon nanotube (d—the van der Waals surfaces of the fullerene and nanotube are shown). | ||
When compared with the level of understanding that has been developed for the molecular guest-species, the understanding of how nanoparticles interact with the interior of nanotubes is still in its infancy. One of the key differences between nanoparticles (NP) and molecular species is that NP are intrinsically metastable and as such remain structurally and compositionally dynamic under the experimental conditions utilised for the filling of carbon nanotubes. In this context, Ostwald ripening,8 a thermodynamically driven process which minimises the fraction of surface atoms in a nanoparticle, causes larger NP to grow at the expense of smaller ones, producing a consequent increase in the mean diameter of NP in the sample over time (Fig. 2 and S1 in ESI†). These dynamic properties are characteristic only for nanoparticles, being distinct from molecular species which retain their unique size and shape during encapsulation within nanotubes. Accordingly, NP whose initial diameter does not match the internal diameter of a nanotube (Fig. 2b) can ripen to a size corresponding to a snug fit (Fig. 2c), thereby becoming irreversibly encapsulated (Fig. 2d). This opens up exciting new avenues for the construction of snug fit, host–guest nanostructures, where nanoparticles are confined within extremely small voids inside nanotubes.
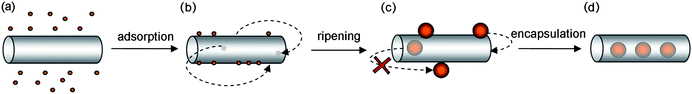 | ||
| Fig. 2 Schematic representation of the mechanism proposed for the filling of carbon nanotubes with gold nanoparticles. Nanoparticles adsorb onto the exterior surface of the carbon nanotubes at room temperature during solution-phase mixing (a and b). In (b), the two arrows indicate the reversible encapsulation of small nanoparticles. The nanoparticle size increases during treatment in supercritical carbon dioxide due to Ostwald ripening (c) reaching a snug-fit diameter that leads to irreversible encapsulation of nanoparticles (d). | ||
This article describes the first example of encapsulation of discrete gold nanoparticles (AuNP) within multi-wall carbon nanotubes (MWNT) which have an internal diameter less than 10 nm by the use of supercritical carbon dioxide (scCO2) as a processing medium.
A fundamental requirement for the transfer of guest-species, nanoparticle or otherwise, into the inner cavity of a carbon nanotube is the presence of accessible holes through which the guests can pass. These can either be the open ends of the nanotubes or defects in the sidewalls. Furthermore, the carbon nanotubes ideally should be as short as possible, to avoid the potential problem of ‘transport resistance’ which could inhibit the encapsulation of discrete nanoparticles. In this context, metallic silver generated by the pyrolitic decomposition of AgNO3 has been shown to be an efficient catalyst for the oxidative cutting of carbon nanotubes.9
The MWNT employed in our study were produced by chemical vapour deposition (CVD) and exhibited a mean length of 10–15 µm, and mean internal and external diameters of 8.7 ± 1.3 nm and 22.4 ± 3.8 nm respectively, as determined by transmission electron microscopy (TEM) (Fig. 4a and S2 in the ESI†). The catalyst for oxidative cutting of nanotubes was prepared in the form of dodecanethiolate-stabilised silver nanoparticles (AgNPs) with a mean core diameter of 7.4 ± 0.7 nm, being commensurate with the dimensions of the CVD grown MWNT (Fig. S3 in the ESI†). The adsorption of free-standing AgNP onto the MWNT directly from solution, rather than through the decomposition of silver salts, allowed for effective control of the density of adsorbed catalytic centres (Fig. 3a). The average length of the nanotubes after the oxidation step (Fig. 3b) was controlled simply by tuning the ratio of nanotubes and nanoparticles in solution.10
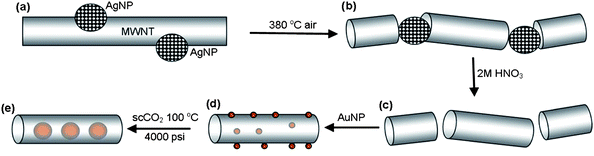 | ||
| Fig. 3 Schematic representation of the strategy used for the encapsulation of discrete Au nanoparticles inside short-section carbon nanotubes. MWNT shortened and opened by the oxidative cutting catalysed by AgNP (a and b); and followed by the dissolution of AgNP in nitric acid (c). Discrete AuNP (red balls) initially deposited on MWNT surface undergo Ostwald ripening and irreversible encapsulation into nanotubes in supercritical carbon dioxide (d and e). | ||
Initial investigation of the production of short-segment MWNT indicated that a mass ratio of 3 mg AgNP : 10 mg MWNT was optimal, to ensure the effective decoration of nanotubes with AgNP. TEM observation of the decorated MWNT revealed the presence of numerous AgNP located on the exterior surfaces of the nanotubes (Fig. 4b). The oxidation temperature of MWNT decorated with AgNP compared to undecorated MWNT decreased from 600 °C to 380 °C confirming that the thiol-stabilised AgNP acted as efficient catalytic centres for the oxidation of carbon nanotubes. The heat treatment of the MWNT in the presence of AgNP at 380 °C for 25 min in air led to the production of short-segment MWNT of mean length 324 ± 140 nm. Additionally, it was identified that many AgNP were present at the free ends of the resultant oxidatively cut nanotubes (Fig. 4c).
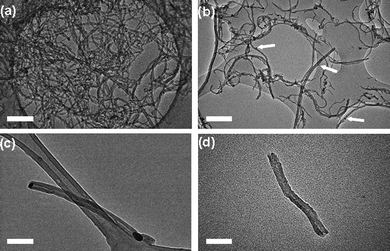 | ||
| Fig. 4 Bright field transmission electron micrographs of carbon nanotubes and silver nanoparticle decorated carbon nanotubes during the cutting and opening procedure: (a) long, closed MWNT exhibiting a mean length of ∼10 to 15 µm; (b) long MWNT with AgNP distributed regularly on the nanotube sidewalls, following solution-phase mixing (white arrows indicate the positions of adsorbed silver nanoparticles); (c) shorter MWNT with AgNP located at the nanotube termini, following thermal treatment; (d) shorter, open MWNT after AgNP catalyst was removed (mean length of sample batch: 324 ± 140 nm). Scale bars are 50 nm. | ||
The AgNP catalyst was then removed by washing the sample with dilute nitric acid solution (Fig. 4d). The resultant, short-segment, open nanotubes were then dried in vacuum, to remove water and volatile organic material from the inner cavities, making them viable for the encapsulation of guest-species (Fig. S4 in the ESI†).
Dodecanethiolate-stabilised gold nanoparticles (AuNP) which were largely spherical in morphology and with a mean core diameter of 2.3 ± 0.4 nm were selected for the encapsulation studies (Fig. S4 in the ESI†). Considering that the mean internal diameter of the MWNT was 8.4 nm, the initial diameter of the guest AuNP was far from an optimal snug fit. AuNP adsorbed onto the exterior surfaces of freshly opened MWNT (Fig. 3d and 5a) were then treated with scCO2 (Fig. 3d, e, and 5b).
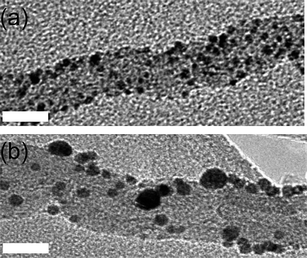 | ||
| Fig. 5 Bright field transmission electron micrographs of MWNT (a) with AuNP deposited on their surface before the treatment with scCO2 and (b) an intermediate sample before ripening has completed, taken after one hour of scCO2 treatment. Scale bar is 10 nm. | ||
Supercritical CO2 possesses a variety of unique properties intermediate between gas and liquid behaviour, specifically low viscosity, high diffusivity and zero surface tension.11 The very small size of CO2 molecules makes this a highly suitable medium for the transportation of guest species into narrow-bore carbon nanotubes to achieve a snug fit (Fig. 2c), as demonstrated for fullerene molecules in our early studies.2 The melting temperature of 2 nm AuNP used in our studies is expected to be ∼660 °C12 which ensures that AuNP remain solid and discrete under the scCO2 conditions (100 °C and 4000 psi) used for their insertion into the MWNT. An intermediate sample after one hour exposure to scCO2 shows that AuNP adsorbed onto MWNT (Fig. 5a) undergo Ostwald ripening on the nanotube surface (Fig. 5b): the total number of nanoparticles gradually decreases as the average nanoparticle size increases from 2.3 nm to 5.4 nm during the first hour of processing. After 24 h exposure to scCO2 the ripening process was complete and the resultant solid AuNP/MWNT composite was extensively characterised using TEM (Fig. 6).
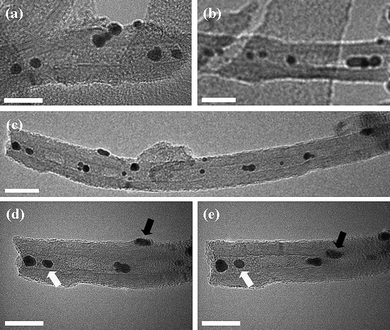 | ||
| Fig. 6 Bright field transmission electron micrographs of MWNT filled with gold nanoparticles: (a)–(c) MWNT filled with AuNP; (d) and (e) MWNT filled with AuNP, tilted at +27° and −27°, respectively, verifying internal encapsulation. The white arrows denote encapsulated nanoparticles; the black arrows indicate nanoparticles on the exterior MWNT surfaces. Scale bars are 20 nm. | ||
TEM investigation showed that a significant proportion of the AuNP were located in the internal cavity of the carbon nanotubes (Fig. 6a–c) post-scCO2 treatment. Furthermore, many AuNP positioned within the MWNT retained their position whilst the TEM specimen was tilted around the nanotube growth axis (e.g.Fig. 6d and e; nanoparticles highlighted with white arrows), confirming that they were indeed encapsulated. However, a number of nanoparticles were also found to move in and out of the line of the nanotube cavity during specimen tilting, indicating that they remained adsorbed onto the MWNT exterior surfaces (e.g.Fig. 6d and e: nanoparticles highlighted with black arrows). Further, statistical analysis showed that the nanotubes were filled to a level of 57 ± 5 NP µm−1, as compared with the filling rate of 1000 C60 µm−1 typically achieved for C60 fullerene under similar conditions in our previous studies.2
Of particular significance is the observation that, following the 24 h treatment with scCO2, the mean diameter of the AuNP increased from a value of 2.3 ± 0.4 nm prior to encapsulation to 7.3 ± 1.2 nm (AuNP inside the MWNT) and 7.9 ± 0.9 nm (AuNP outside the MWNT), respectively (Fig. 7a). Given that the mean internal diameter of the MWNT was 8.7 nm, most of AuNP observed inside the nanotubes corresponded to a near perfect snug fit. However, since AuNP matching the internal diameter of the MWNT were not present at the beginning of the encapsulation procedure, it is considered that they must have formed through Ostwald ripening during the scCO2 treatment.
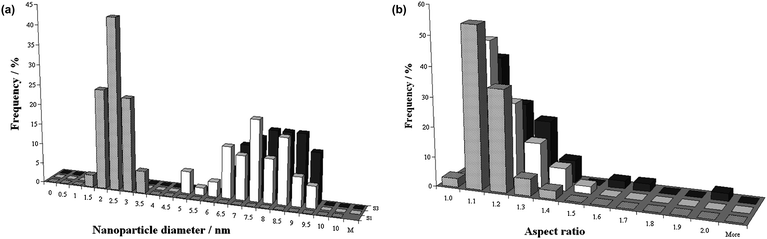 | ||
| Fig. 7 Statistical analysis of (a) the diameter distribution and (b) the aspect ratio of gold nanoparticles, based on TEM data. The nanoparticles were investigated prior to encapsulation (grey), and inside (white) and outside (black) the MWNT following encapsulation. | ||
Once the nanoparticles reached diameters of ∼7.3 nm, allowing snug fit within the MWNT, it became energetically more favourable for them to become situated inside the nanotubes. By analogy with spheroidal fullerene molecules,13 van der Waals interactions of nanoparticles with nanotube are significantly more effective when AuNP are positioned in MWNT cavity, which leads to their irreversible encapsulation (Fig. 2d). Subsequently, the AuNP trapped within the nanotubes were unable to grow further beyond the snug fit diameter because they were constrained and isolated from the supply of gold atoms. However, the AuNP adsorbed onto the nanotube exterior surfaces could continue to ripen, although it is recognised that further Ostwald ripening decreased sharply at the point of irreversible encapsulation because the amount of gold available for ripening in the system rapidly decreased (Fig. 7a). It is also noted that the shapes of nanoparticles located inside and outside the nanotubes were very similar, having virtually identical aspect ratios (Fig. 7b). If nanoparticles were to grow and ripen once inside a MWNT, the nanotube would be expected to have a templating effect, causing the aspect ratio of forming AuNP to deviate significantly from that of spherical (Fig. 6b). As our experimental observations show that both the encapsulated and adsorbed AuNP have the same aspect ratio, the ripening of encapsulated nanoparticles takes place in a similar isotropic environment to the adsorbed nanoparticles, i.e. outside the nanotubes. Thus, the experimental evidence indicates that AuNP ripen prior to insertion into the nanotube where they then become irreversibly encapsulated.
To evaluate the role of supercritical fluid in this process, a series of control experiments with MWNT and AuNP were carried out using conventional organic solvents (hexane and toluene) and without any solvent. As expected, nanoparticles undergo Ostwald ripening in the absence of scCO2 with their growth rate being controlled by temperature. However, significantly fewer AuNP were encapsulated in MWNT in the control experiments than in scCO2, which indicates the importance of supercritical medium for transport of nanoparticles into nanotubes. In the case of scCO2, nanoparticles exhibit a continual and uninhibited movement around the nanotube surface. Carbon nanotube surfaces are known to have a very low friction coefficient and CO2 molecules under high pressure may serve as an effective lubricant facilitating transport of AuNP into the middle of MWNT. Once a snug fit nanoparticle is encapsulated in the nanotube, CO2 molecules can vent out of the MWNT without dislodging nanoparticles owing their very small molecular size, which would be not possible for conventional solvents.
Gold nanoparticles encapsulated within carbon nanotubes are an interesting model system because AuNP/MWNT composite materials have shown promise for a variety of applications, specifically involving catalysis14 and optoelectronics.15 Here it has been shown that preformed, discrete, metallic gold nanoparticles can be encapsulated within carbon nanotubes with internal diameter less than 10 nm using scCO2. The filling process was preceded by a process of Ostwald ripening of the nanoparticles that allowed them to reach an optimum diameter suitable for snug fit encapsulation within the nanotubes. The nanoparticles remaining adsorbed onto the nanotube external surfaces provided a convenient reference for comparison with the AuNP encapsulated within the MWNT, giving valuable insight into the mechanisms of nanotube filling. This novel methodology for nanoparticle encapsulation is facile and can be potentially adopted for a wide variety of nanoparticle systems currently being investigated.
Experimental
Multi-walled carbon nanotubes (produced by chemical vapour deposition) were purchased from TimesNano, China and treated as described below prior to use. All other reagents and solvents were purchased from Sigma-Aldrich (UK) and used without further purification. Water was purified (>18.0 MΩ cm) using a Barnstead NANOpure II system. It should be noted that the preparation and use of silver and gold nanoparticles required glassware to be cleaned with ‘aqua regia’ (concentrated hydrochloric and nitric acids (3 : 1)), rinsed with deionised water, further cleaned with potassium hydroxide in methanol and finally rinsed thoroughly with deionised water prior to use. Ultraviolet-visible (UV-vis) spectra were recorded using 1 cm quartz cuvettes on a Perkin-Elmer Lambda 25 UV-vis spectrophotometer at a scan rate of 240 nm min−1 over the range 190–900 nm. High resolution imaging was performed using a Jeol 2100F transmission electron microscope (field emission electron gun source, information limit 0.19 nm) at room temperature. Samples were prepared by casting several drops of methanolic solution onto copper-grid mounted “holey” carbon films before drying under a stream of nitrogen. Statistical analysis was performed for each sample using Gatan Digital Micrograph software. The filling rate of encapsulated nanoparticles was determined by taking bright field micrographs (100 nm2 area) from multiple fields of view, estimating the proportion of filled nanotubes for each area and averaging across the entire sample. The syntheses of dodecanethiolate-stabilised silver and gold nanoparticles were performed using the methodologies outlined by Kang and Kim16 and Brust et al.,17 respectively. In a typical scCO2 experiment, MWNT (short and open) were added to a solution of dodecanethiolate-stabilised gold nanoparticles in hexane and the resulting black suspension was transferred into an autoclave and mixed with carbon dioxide for 24 h.Acknowledgements
This work was supported by the Nottingham Nanotechnology and Nanoscience Centre, The University of Nottingham, EPSRC and the Royal Society. The authors would like to thank Dr Mike Fay and Keith Dinsdale for technical assistance with TEM and technical support, respectively.References
- A. N. Khlobystov, D. A. Britz and G. A. D. Briggs, Acc. Chem. Res., 2005, 38, 901–909 CrossRef CAS.
- A. N. Khlobystov, D. A. Britz, J. Wang, S. A. O'Neil, M. Poliakoff and G. A. D. Briggs, J. Mater. Chem., 2004, 14, 2852–2863 RSC.
- T. Lu, E. M. Goldfield and S. K. Gray, J. Phys. Chem. B, 2006, 110, 1742–1751 CrossRef CAS.
- M. D. Halls and H. B. Schlegel, J. Phys. Chem. B, 2002, 106, 1921–1925 CrossRef CAS.
- M. Ashino, D. Obergfell, M. Haluska, S. Yang, A. N. Khlobystov, S. Roth and R. Weisendanger, Nat. Nanotechnol., 2008, 3, 337–341 CrossRef CAS.
- L.-J. Li, A. N. Khlobystov, J. G. Wiltshire, G. A. D. Briggs and R. J. Nicholas, Nat. Mater., 2005, 4, 481–485 CrossRef CAS.
- D. Obergfell, J. C. Meyer, M. Haluska, A. N. Khlobystov, S. Yang, L. Fan, D. Liu and S. Roth, Phys. Status Solidi B, 2006, 243, 3430–3434 CrossRef CAS.
- L. Zhu, G. Lu, S. Mao and C. Chen, NANO, 2007, 2, 149–156 CrossRef CAS.
- C. Wang, S. Guo, X. Pan, W. Chen and X. Bao, J. Mater. Chem., 2008, 18, 5782–5786 RSC.
- X. Peng and S. S. Wong, Chem. Mater., 2009, 21, 682–694 CrossRef CAS.
- X. R. Ye, Y. Lin, C. Wang, M. H. Engelhard, Y. Wang and C. Wai, J. Mater. Chem., 2004, 14, 908–913 RSC.
- T. Castro, R. Reifenberger, E. Choi and R. P. Andres, Phys. Rev. B: Condens. Matter, 1990, 42, 8548–8556 CrossRef CAS.
- H. Ulbricht, G. Moos and T. Hertel, Phys. Rev. Lett., 2003, 90, 095501 CrossRef.
- K. Jiang, A. Eitan, L. S. Schadler, P. M. Ajayan and R. W. Siegel, Nano Lett., 2003, 3, 275–517 CrossRef CAS.
- D. H. Marsh, G. A. Rance, M. H. Zaka, R. J. Whitby and A. N. Khlobystov, J. Mater. Chem., 2008, 18, 2249–2256 RSC.
- S. Y. Kang and K. Kim, Langmuir, 1998, 14, 226–230 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Chem. Commun., 1994, 801–802 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed description of nanotube processing and characterisation procedures. See DOI: 10.1039/c0nr00035c |
| This journal is © The Royal Society of Chemistry 2010 |
