Various self-assembled three-dimensional hierarchical architectures of La2(MoO4)3: controlled synthesis, growth mechanisms, luminescence properties and adsorption activities†
Lin
Xu
,
Chunliang
Lu
,
Zihui
Zhang
,
Xiaoyan
Yang
and
Wenhua
Hou
*
Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, P. R. China. E-mail: whou@nju.edu.cn; Fax: +86-25-83317761; Tel: +86-25-83686001
First published on 6th May 2010
Abstract
Tetragonal La2(MoO4)3 with various novel and complex 3D hierarchical architectures self-assembled from different building blocks were successfully synthesized by a hydrothermal method in EDTA-mediated processes. X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) were employed to characterize the as-obtained products. It was found that morphology modulation could be easily realized simply by changing the amount of EDTA introduced into the reaction system. The amount of EDTA not only affected the substructures of the hierarchical structures, but also determined the size distributions of the final products. The formation mechanisms for different hierarchical architectures were proposed on the basis of a series of time-dependent experiments. An investigation on the photoluminescence (PL) properties of La2(MoO4)3:Eu with different morphologies revealed that the optical properties were strongly correlated with the morphology and size. Furthermore, as highlighted by the fascinating hierarchical structures of La2(MoO4)3, the potential application of La2(MoO4)3 as an absorbent in water treatment was also investigated for the first time. Possible reasons responsible for the differences in photoluminescence behaviors and absorption activities of different hierarchical architectures were discussed.
1. Introduction
Recently, tremendous efforts in materials science have been progressively devoted to the design and construction of inorganic materials with unique and novel three-dimensional (3D) hierarchical structures, not only for the fundamental scientific interest but also for the technological applications and an approach toward the future realization of functional nanodevices.1 An increasing number of materials with 3D hierarchical structures exhibit superior properties to their 1D and 2D counterparts and have widespread potential applications in various fields such as electronics,2 catalysis,3 optics,4 photodegradation,5 water treatment6 and so forth. Self-assembly in solution-phase has proved to be a promising and reliable way for the fabrication of complicated 3D hierarchical architectures from substructures.7 Since the chemical and physical properties of inorganic materials usually have a very close correlation with the morphology, size distribution and dimensionality, the exploration of an efficient and simple way to elaborately organize the low dimensional (0D, 1D and 2D) primary building blocks into controllable 3D superstructures via self-assembly in solution-phase is of particularly great significance and thus becomes an intensive and hot research topic. Although considerable achievements have been made, it still remains a great challenge for chemists and materials scientists to develop a facile and controllable route for the construction of various hierarchical architectures. In the solution-based self-assembly process, through properly employing organic additives to the reaction system, the growth of inorganic nanocrystals can be rationally directed to obtain products with desirable morphologies and/or hierarchical structures.8 Particularly, the introduction of small chelating agents such as citric acid (CA) or ethylenediaminetetraacetic acid (EDTA) which act as “shape modifiers” plays an important role in determining the final product morphology.9,10 Very recently, we reported the successful synthesis of dandelion-like Y2(WO4)3:Eu with the aid of EDTA.11Rare earth molybdate compounds, R2(MoO4)3 and R2MoO6 (R = rare earth elements), have attracted extensive attention because of their potential technological applications as catalysts, high-performance phosphors, up-conversion materials, negative thermal expansion materials and so forth.12–15 Nevertheless, most of the previous works about rare earth molybdate compounds are mainly concentrated on the bulk materials obtained by conventional solid-state reaction, and little has been done on the fabrication of 3D hierarchical structures. Recently, several 3D La2(MoO4)3 microarchitectures obtained in the presence or absence of different kinds of surfactants have been reported.16 Nevertheless, to the best of our knowledge, up till now there is no systematic study on the shape control of 3D La2(MoO4)3 superstructures, especially hierarchical structures constructed by different building blocks.
Herein, for the first time we report an efficient method for the controlled fabrication of several different and novel hierarchical architectures of La2(MoO4)3 with a high yield and good uniformity via a facile and mild hydrothermal route in EDTA-mediated processes. The effects of the amount of EDTA introduced into the reaction systems and the pH value of the precursor solution on the product morphology were discussed in detail. The possible formation mechanisms of different hierarchical superstructures were put forward on the basis of a series of time-dependent experiments. To evaluate the potential applications of our products, the photoluminescent (PL) properties of the Eu-doped La2(MoO4)3 superstructures with different morphologies were measured. Furthermore, as highlighted by the fascinating hierarchical structures of La2(MoO4)3, the adsorption activities of La2(MoO4)3 as an absorbent in water treatment were also tested. The intensive relationship between microstructure and the property of the obtained products was discussed.
2. Experimental section
All reagents were of analytical grade and used as received without further purification. Distilled water was used throughout. In a typical procedure, 1 mmol of La2O3 was dissolved in 10% HNO3. Subsequently, a predetermined amount of EDTA was added to form the La3+-EDTA complex. After vigorous stirring for 20 min, 15 mL solution containing 0.43 mmol (NH4)6Mo7O24 was added dropwise into the above solution under continuous stirring. Then, the pH value of the white suspension was adjusted to 9 through the addition of 2 M NaOH solution. After additional agitation for 30 min, the as-obtained white colloidal precipitate was transferred into a 40-mL Teflon-lined autoclave (filled up to 80% of its total volume), and the autoclave was sealed and maintained at 180 °C for 24 h. After the autoclave was air-cooled to room temperature naturally, the final products were collected by filtration, washed with distilled water and absolute alcohol several times, dried at 60 °C and kept for further characterization. Eu-doped (2% mol) samples were prepared through a similar procedure by using 1.96 mmol La2O3 together with 0.04 mmol Eu2O3 as the starting materials.The phase purity and crystallinity of the products were identified by XRD on a Philip-X'Pert X-ray diffractometer equipped with a Cu-Kα radiation source (λ = 1.540562 Å) at a scanning rate of 0.02° s−1 in a 2θ range of 10–80°. The product morphology was investigated by scanning electron microscopy (SEM JEOL JEM-6300F) and transmission electron microscopy (TEM JEOL JEM-200CX, operated at an accelerating voltage of 200 kV). For TEM observation, the samples were dispersed in ethanol by ultrasonic treatment and dropped on carbon-copper grids. The nitrogen adsorption-desorption isotherms were measured on an ASAP 2020 apparatus at liquid nitrogen temperature. PL excitation and emission spectra were recorded on an Aminco Bowman luminescence spectrometer at room temperature.
The absorption activities of the La2(MoO4)3 samples with different morphologies were evaluated via the adsorption of Rhodamine B (Rh B) dye in an aqueous solution at ambient temperature. A beaker with capacity ca. 100 mL was used as the reaction vessel. 10 mg of the samples was added to the Rh B solution (10 mg L−1, 50 mL) under stirring. UV-vis absorption spectra were recorded at different time intervals to monitor the process.
3. Results and discussion
3.1 Structural study
The composition and phase purity of the products were characterized by XRD. The XRD patterns of the as-prepared La2(MoO4)3 products obtained in the presence of different amounts of EDTA are shown in Fig. 1. The diffraction peaks of the different samples can be readily indexed to the pure tetragonal phase of La2(MoO4)3 with the space group of I41/a, and coincide well with the literature values (JCPDS No. 45-0407). No other peaks were detected, revealing the high purity of the as-obtained products. It can be concluded that the well-crystallized tetragonal La2(MoO4)3 could be obtained under the present synthetic conditions.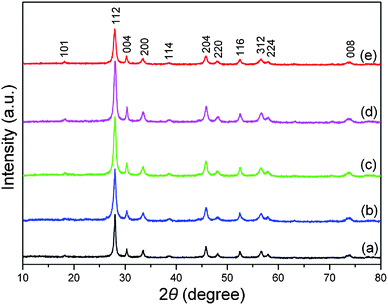 | ||
| Fig. 1 XRD patterns of the as-synthesized products obtained at pH = 9 in the presence of different amounts of EDTA. (a) 0.05 g EDTA. (b) 0.10 g EDTA. (c) 0.15 g EDTA. (d) 0.25 g EDTA. (e) 0.46 g EDTA. | ||
3.2 Morphology
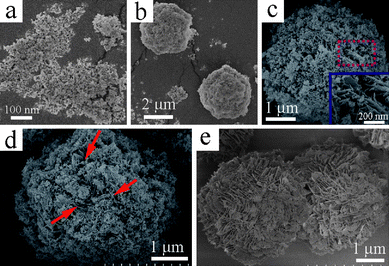
In the present work, it was found that the amount of EDTA introduced to the reaction system had a crucial effect on the morphology and size distribution of the final products. Varying the amount of EDTA could yield a series of La2(MoO4)3 microstructures with controlled size and morphology. Previous work has indicated that the products obtained in the EDTA-absence process are composed of micropompons with a diameter of ∼3.5 μm self-assembled from numerous nanoflakes through edge-to-flat-surface conjunction.16a However, the addition of EDTA, even a very small amount, can dramatically affect the final morphology of the products. The introduction of 0.05 g EDTA into the reaction system can bring on an intriguing morphology, as illustrated in Fig. 2. A panoramic SEM image shown in Fig. 2a demonstrates that the product is almost entirely composed of uniform microstructures with an average diameter of 7.5 μm. The magnified image displayed in Fig. 2b shows that the microflowers exhibit a hierarchical structure. Interestingly, the high-magnification SEM image shown in Fig. 2c vividly depicts that the as-obtained La2(MoO4)3 hierarchical microflowers in fact possess three structure levels. The La2(MoO4)3 hierarchical microflowers, acting as the tertiary structure, are constructed of dozens of 3D hyperbranched dendritic structures. The secondary structures of dendrites are densely interwoven with each other to form a microflower through self-assembly. A closer inspection further reveals that the dendrites are built of 2D nanoflakes. The primary structures of nanoflakes, with an average thickness of 30 nm, are highly ordered and symmetrically aligned on the opposite sides of the main trunks, as shown in Fig. 2d. As the amount of EDTA was increased to 0.10 g, the product morphology varied remarkably. As illustrated in Fig. 3a, the product consisted of a large quantity of nearly monodispersed microflowers with an average diameter of 4.8 μm and a high yield. A detailed surface observation (Fig. 3b) shows that the building blocks of each microflower are quite different from those of obtained in the presence of 0.05 g EDTA. It can be seen that these microflowers are actually built from a large number of 2D nanoplates with a thickness of ∼40 nm. The nanoplates are tightly aligned radially and intercrossed with each other to form hierarchical microflowers, as disclosed by Fig. 3c. Upon a further increase of the amount of EDTA up to 0.15 g, the product still exhibits a morphology of nearly monodispersed microspheres with an average diameter of 4.1 μm, as shown in Fig. 3d. However, a closer observation (Fig. 3e, 3f) indicates that there are two kinds of microspheres coexisting in the final product. One is constructed from numerous nanoparticles and has a coarse surface. Another is assembled by a great deal of interleaved nanoplates. As the amount of EDTA was increased successively from 0.15 to 0.26 g, although the morphology of the corresponding products was basically maintained, the number of microspheres constructed by nanoparticles increased continuously. Moreover, the average size of the product was reduced significantly from 4.1 to 2.5 μm. A further increase in the amount of EDTA to 0.30–0.50 g resulted in the formation of microspheres purely composed of smaller nanoparticles. Fig. 3g demonstrates the representative SEM images of the product obtained in the presence of 0.46 g EDTA. It is clearly shown that the uniform microspheres with an average diameter of 1.2 μm are the exclusive product. In addition, it can be seen from the surface of the spheres (Fig. 3h, 3i) that the microspheres are indeed constructed by nanoparticles and some of them are interconnected with each other.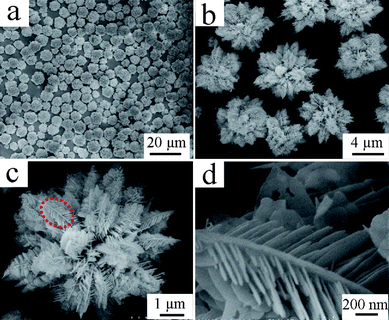 | ||
| Fig. 2 SEM images of the La2(MoO4)3 hierarchical microflowers obtained in the presence of 0.05 g EDTA at pH = 9. (a) Panoramic image; (b) magnified image; (c) image of an individual hierarchical microflower with 3 levels of structures; (d) high-magnification SEM image of the marked part in panel c. | ||
 | ||
| Fig. 3 SEM images of the La2(MoO4)3 microspheres built from different subunits obtained at pH = 9 with different amounts of EDTA. (a)–(c) 0.10 g EDTA. (d)–(f) 0.15 g EDTA. (g)–(i) 0.46 g EDTA. | ||
Besides the amount of EDTA introduced into the reaction system, the pH value of the precursor solution also had a significant influence on the product morphology. As shown in Fig. 4a and 4b, when the amount of EDTA was 0.35 g and the pH value was adjusted to 7, the product appeared as spindle-like microstructures with an average length of 2.2 μm and width of 900 nm, respectively. The micro-spindles are consisted of numerous nanoparticles with a mean size of 50 nm. When the pH value was increased to 9, the product was totally composed of microspheres with a diameter of ∼2.1 μm (Fig. 4c). A close look revealed that these microspheres with coarse surfaces were entirely constructed by nanoparticles (Fig. 4d). A further increase of pH value to 10 led to the formation of micro-flowers with an average size of 1.9 μm (Fig. 4e) and each microflower was constructed from nanoflakes with a thickness around 40 nm (Fig. 4f). The nanoflakes extended outwards from the center of the flower and were linked together through edge-to-edge and edge-to-surface conjunctions. As displayed in Fig. 4g, when the amount EDTA was 0.50 g and the pH value was 7, the as-obtained product was in the form of dumbbells with an average size of 5.0 μm. The high-magnification SEM image in Fig. 4h provides the detailed configuration of the dumbbells. The dumbbells are built from a large number of highly oriented nanoparticles and the dual fantails with rough surface take on a cauliflower-like appearance and are about 4.0 μm in diameter. Therefore, on the basis of the aforementioned SEM results, the amount of EDTA not only affects the product morphology, but also determines the size of product. By changing the amount of EDTA and the pH value, various self-assembled 3D La2(MoO4)3 hierarchical architectures such as microflowers, microspheres, microspindles and microdumbbells can be obtained and the building blocks of different hierarchical architectures vary from 3D dendrites to 2D nanoplates and eventually to 0D nanoparticles. Moreover, the greater the amount of EDTA, the smaller the average size of product. A brief summary of the detailed experimental conditions and the corresponding product morphologies is listed in Table 1.
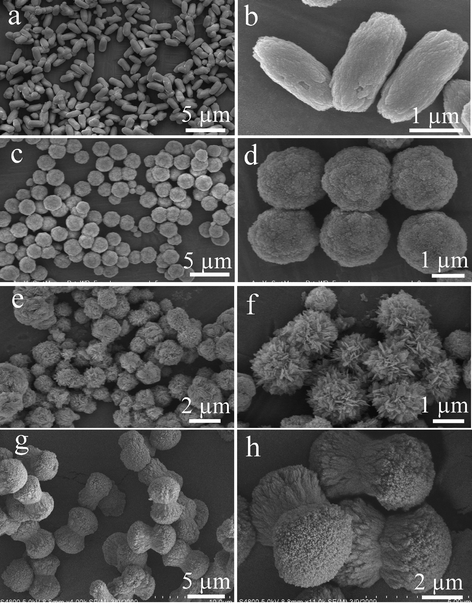 | ||
| Fig. 4 SEM images of the products obtained at different pH values with appropriate amount of EDTA. (a)–(b) pH = 7, 0.35 g EDTA. (c)–(d) pH = 9, 0.35 g EDTA. (e)–(f) pH = 10, 0.35 g EDTA. (g)–(h) pH = 7, 0.50 g EDTA. | ||
| Sample | pH value | Amount of EDTA/g | Average size/μm | Morphology |
|---|---|---|---|---|
| S1 | 9 | 0.05 | 7.5 | Microflowers assembled by hyperbranched 3D dendrites |
| S2 | 9 | 0.10 | 4.8 | Microflowers assembled by 2D nanoflakes |
| S3 | 9 | 0.15 | 4.1 | Mixture of microspheres constructed by 2D nanoflakes and 0D nanoparticles, respectively |
| S4 | 9 | 0.35 | 2.1 | Microspheres constructed by 0D nanoparticles |
| S5 | 9 | 0.46 | 1.2 | Microspheres constructed by 0D nanoparticles |
| S6 | 7 | 0.35 | 2.2 | Microspindles constructed by 0D nanoparticles |
| S7 | 10 | 0.35 | 1.9 | Microflowers built from 2D nanoflakes |
| S8 | 7 | 0.5 | 5.0 | Microdumbbells with two cauliflower-like fantails constructed by 0D nanoparticles |
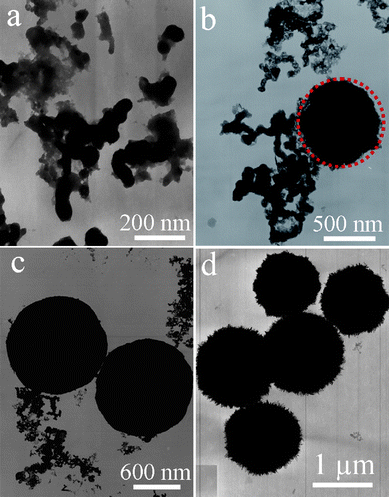
More details about the morphological and structural features were further investigated by employing TEM and HRTEM. Fig. 5a presents the representative TEM image of the La2(MoO4)3 microflowers obtained with the assistance of 0.05 g EDTA. It confirms that La2(MoO4)3 spherical architectures have an average diameter about 7.5 μm, which is consistent with the above SEM observation (Fig. 2a). The as-obtained La2(MoO4)3 superstructures were stable enough to withstand ultrasonic treatment for 30 min. They preserved their integrity and only some irregular nanoflakes were peeled from the flowers (Fig. 5b), indicating that strong chemical bonding existed between the building blocks. Fig. 5c displays the HRTEM image of the nanoflake performed on the marked area in Fig. 5b, the single-crystalline nature and the particular orientation of the nanoflake are clearly demonstrated and clarified. The interplanar lattice spacing was determined to be 0.329 nm, corresponding to (112) planes of La2(MoO4)3. The corresponding SAED pattern (the inset in Fig. 5c) further confirms the single-crystalline nature of the isolated nanoflake. Fig. 5d shows the typical TEM image of the microspheres obtained in the presence of 0.35 g EDTA. The uniform microspheres with obvious rough surfaces have an average diameter of 2.1 μm, which is in good accordance with the SEM image shown in Fig. 4d. The ED pattern recorded on an individual microsphere is inset in Fig. 5d, demonstrating the polycrystalline nature of the hierarchical structure. However, the symmetrical spot pattern also indicates that the hierarchical structure is built from single-crystalline building blocks. HRTEM image taken from the fringe of a microsphere marked by the red circle in Fig. 5e is shown in Fig. 5f. From the image, the lattice interplanar spacing was determined to be 0.332 nm, corresponding to the (112) planes of La2(MoO4)3. This result also confirms the single-crystalline nature of the nanoparticles. The EDX spectrum (Fig. 5g) was employed to determine the chemical composition of the as-obtained products. The result reveals that the product only consists of La, Mo, and O. The corresponding atomic ratio of is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.47
1.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.40, which is very close to the stoichiometric proportion of La2(MoO4)3.
7.40, which is very close to the stoichiometric proportion of La2(MoO4)3.
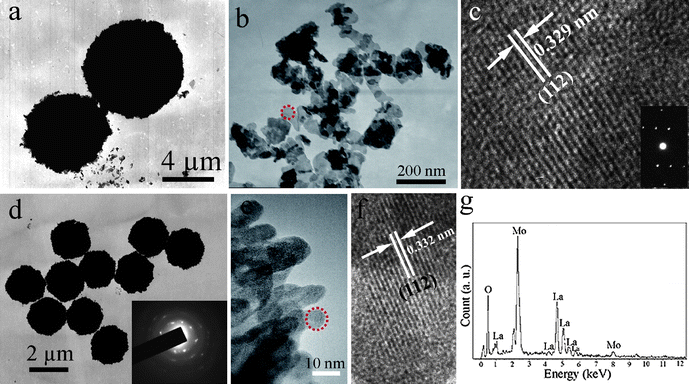 | ||
| Fig. 5 (a) TEM image of the microflowers obtained with the assistance of 0.05 g EDTA. (b) TEM image of the irregular nanoflakes peeled from integrated flowers after ultrasonic treatment for 30 min. (c) HRTEM image and ED pattern of a single nanoflake. (d) Representative TEM image of the microspheres obtained in the presence of 0.35 g EDTA (inset: ED pattern of an individual microsphere). (e) TEM image of the fringe of an individual microsphere. (f) HRTEM image of a nanoparticle marked in (e). (g) EDS spectrum of the microspheres. | ||
Concerning the thermal stability of the as-obtained products with different morphologies, a series of experiments were carried out to investigate the effect of calcination temperature on the crystallinity and morphology of the products. Three different hierarchical structures (S1, S2 and S5) were calcined at 350, 500, 650 and 800 °C in air for 3h, respectively. The crystallinity and morphology of the calcined products were characterized by XRD and SEM techniques.
Fig. SI1 in the Supporting Information† shows the XRD patterns of S1 calcined at different temperatures. It is evident that the product crystallinity is improved, as expected, with the increase of calcination temperature. Therefore, the calcined products exhibit higher diffraction intensity and narrower peaks due to the enhancement of crystallization. However, when the calcination temperature is increased to 800 °C, a second phase assigned to La2Mo2O9 (JCPDS 23-1145) can be occasionally detected to a certain extent, as marked by asterisks in Fig. SI1. Likewise, such a tendency is also observed for S2 and S5 (see Fig. SI2 and SI3), namely, the higher the calcination temperature, the higher the product crystallinity, and the impurity phase of La2Mo2O9 appears when the calcination temperature is raised to 800 °C.
Fig. SI4 displays a series of SEM images of S1 after calcination at different temperatures. It can be concluded that the hierarchical microstructures experience a great change upon calcination, although the external shape is almost maintained. It is noteworthy that the hierarchical microstructures can be successfully sustained and the characteristic morphology of the dendrites remains unchanged when the calcination temperature is below 500 °C, indicating that a rather high activation energy is needed for the collapse of these hierarchical structures (Fig. SI4a–d). As depicted in Fig. SI4e–f, the nanoflakes begin to fuse with each other after calcination at 650 °C for 3h, yet the main trunks of the dendritic structures still exist. However, when the calcination temperature is further increased to 800 °C, the characteristic morphology of dendrites no longer exists. Under this high-temperature condition, the relatively high surface energies of nanoflakes and main trunks cause the dendritic structures to further melt into each other, giving rise to the honeycomb-like structures made of numerous highly cross-linked nanoparticles (Fig. SI4g–h).
Similarly, S2 and S5 can also preserve their original morphology when the calcination temperature is below 500 °C (see Fig. SI5a–d and Fig. SI6a–b, respectively). Nevertheless, after calcination at 650 and 800 °C, respectively, the nanoflakes of S2 are fused with each other to different extents and evolve into a great many cross-linked nanoparticles, bringing out porous microspheres with different pore sizes (Fig. SI5e–h). However, the nanoparticles on the surface of the microspheres in S5 melt into each other to form bigger particles and the size of the microspheres is slightly shrunk compared with those calcined below 500 °C (Fig. SI6c–d).
3.3 Morphological evolution and growth mechanisms of different La2(MoO4)3 hierarchical structures
In order to reveal the morphological evolution and formation mechanisms of La2(MoO4)3 with different hierarchical architectures, a series of time-dependent experiments were carefully carried out and the intermediate products collected after different reaction intervals were then investigated by SEM. Fig. 6 shows the representative SEM images of the products obtained at different reaction intervals when the amount of EDTA was 0.05 g and the pH value of the precursor solution was 9. At the initial stage, the product collected after the completion of precipitation reaction between La3+-EDTA and (NH4)6Mo7O24 was only composed of the aggregates of amorphous nanoparticles, (Fig. 6a). Surprisingly, hydrothermal treatment for 45 min led to a significant change in morphology and gave rise to the formation of dendritic superstructures (Fig. 6b). As the treatment time was prolonged to 100 min, the dendrites aggregated together through self-assembly by sharing a common center, and thus a more complex hierarchical architecture was formed. From Fig. 6c, it can be seen that the dendrites sprout or emanate from the core of the flower. With the further increase in reaction time, dendrites aggregated into flowers which grew gradually and became more and more ripe and plump (Fig. 6d). Eventually, as the reaction proceeded to 6 h, well-assembled hierarchical microflowers constructed by numerous of interwoven dendrites were formed (Fig. 6e). Further prolonging the reaction time had no significant influence on the product morphology. It is worth noting that EDTA is indispensable for the formation of such intricate superstructures. Since EDTA possesses four carboxyl groups (–COOH) and two lone pairs of electrons on two nitrogen atoms as binding sites, it is endowed with strong chelating ability and excellent capping ability. Furthermore, the presence of the carboxyl functional group (–COOH) in EDTA results in the formation of hydrogen bonds and facilitates self-assembly or self-aggregation of complex superstructures. Therefore, the introduction of EDTA to a reaction system could always modulate the crystal growth and bring out various fascinating morphologies.10,11 On the basis of the aforementioned SEM observations, a reasonable formation mechanism of such intricate hierarchical architecture could be elucidated as follows. Initially, a simple precipitation reaction between La(NO3)3 and (NH4)6Mo7O24 with the assistance of 0.05 g EDTA at pH 9 could yield numerous amorphous particles of La2(MoO4)3 which acted as precursors of the crystal. Regarding to the formation of dendritic structure, the diffusion-limited aggregation (DLA) model can be employed to account for this.17 In principle, nonequilibrium growth and molecular anisotropy are the prerequisites for the formation of dendritic structure.18 The EDTA can coordinate with La3+ to form a stable La3+-EDTA complex, which decreases the concentration of free La3+ ions and leads to the slow generation of La2(MoO4)3 nanoparticles, thus facilitating the synthesis of high-quality crystals. These nanoparticles, driven by the stochastic diffusion and directive forces, grow to form La2(MoO4)3 nanoflakes and simultaneously, these randomly moving nuclei are accumulated with each other to form fractal structures. Since the dendrites possess considerable extended and exposed surfaces, the surface energy of the system is very high. Hence, from a thermodynamic point of view, the separate dendrites tend to aggregate together to minimize the surface energy through self-assembly via hydrogen bonds in the following growth stage. The imperfect superstructures continue to develop by combining with the remaining dendrites and finally expand into fully developed 3D spherical hierarchical architectures constructed by numerous intercrossed dendrites. On the basis of above analysis, the formation of spherical La2(MoO4)3 dendritic superstructures could be achieved through a two-tiered organization process: (1) mesoscale formation of dendrites from nanoflakes via self-assembly and (2) macroscopic reconstruction of dendrites into more complex hierarchal architectures. Such a growth process is analogous to the previous report on the formation of Co dendritic superstructures.19 | ||
| Fig. 6 A series of EM images of the products collected at different reaction time intervals in the presence of 0.05 g EDTA and a pH value of 9. (a) Before hydrothermal treatment, and after hydrothermal treatment for (b) 45 min, (c) 100 min, (d) 150 min, and (e) 6 h. | ||
The representative SEM images of the intermediate products obtained with the assistance of 0.10 g EDTA are presented in Fig. 7. As shown in Fig. 7a, the precursor is composed of irregular nanoparticles with a size about 30 nm. After hydrothermal treatment for 20 min, these nanoparticles aggregated together to form loose spherical aggregates with a rough surface and a diameter of ∼4 μm (Fig. 7b). When the reaction time was increased to 40 min, the spherical aggregates were still the dominant structure, but some nanoplates germinating on the surface of the microsphere (marked by a red rectangle in Fig. 7c) could be occasionally observed. The inset part in Fig. 7c reveals that the nanoplates with a thickness of ∼30 nm are perpendicular to the surface and interwoven with each other. Prolonging the reaction time to 1 h gave rise to more and more nanoplates sprouted from the microsphere (see red arrowheads in Fig. 7d). As a result of continuous growth, development and ripening, numerous 2D nanoplates grew from the microsphere and were arranged in a radical direction and eventually when the reaction was allowed to proceed to 4 h the La2(MoO4)3 microspheres with flower-like appearance were formed (Fig. 7e). Based on the above SEM observation, the possible formation mechanism of the flower-like La2(MoO4)3 hierarchical hyperstructures obtained in the presence of 0.10 g EDTA can be put forward as follows. At the beginning, La3+ and EDTA form stable La3+-EDTA complex, and the addition of molybdate leads to the competition of MoO42− for La3+ with EDTA to yield La2(MoO4)3 nanoparticles. With the aid of EDTA, the incipient nanoparticles tend to self-aggregate into large microspheres mediated by hydrogen bonds. The driving force for spontaneous self-aggregation is the elimination of high energy surfaces which will lead to the substantial minimization of the surface energy from the thermodynamic viewpoint.20 On the surface-roughened microspheres, there are numerous small protuberances, which provide many high energy sites for nanocrystal growth.21 With a further increase in reaction time, the small nanoparticles would dissolve and recrystallize on the protuberances spontaneously. Furthermore, owing to the intrinsic anisotropic feature of the product, these nanocrystals prefer to grow into 2D nanoplates, such a tendency has also been reported in the previous work.16a Therefore, as the reaction continues, more and more 2D nanoplates are germinated and developed from the surface-roughened microspheres, the spherical microparticles are gradually changed to spherical flower-like architectures. The smooth surface of the nanoplates is caused by Ostwald ripening.22 Herein, the formation process of the spherical flower-like architectures can be rationally expressed as a nucleation-dissolution-recrystallization growth mechanism.
 | ||
| Fig. 7 A series of SEM images of the products collected at different reaction time intervals in the presence of 0.10 g EDTA and a pH value of 9. (a) Before hydrothermal treatment, and after hydrothermal treatment for (b) 20 min, (c) 40 min, (d) 1h, and (e) 4 h. | ||
To reveal the growth process of La2(MoO4)3 microspheres constructed by nanoparticles in the presence of 0.46 g EDTA, the products were collected at different stages and their morphologies were then investigated by TEM. As displayed in Fig. 8a, the product was composed of amorphous nanoparticles without a discernable morphology when the precursor was treated for 5 min. As the reaction was prolonged to 15 min, it could be observed that some nanoparticles were organized into spherical aggregates (marked by red circle in Fig. 8b). As the reaction went on, more and more well-defined microspheres were generated though some nanoparticles still existed in the product (Fig. 8c). Eventually, when the reaction time was further extended to more than 3 h, the remaining nanoparticles disappeared and the product was entirely composed of uniform microspheres with an average diameter of 1.2 μm (Fig. 8d). On the basis of time-dependent morphological evolution, the growth mechanism of the La2(MoO4)3 microspheres constructed by nanoparticles can be illustrated as follows. First, the introduction of molybdate into the La3+-EDTA complex led to the formation of La2(MoO4)3 nanoparticles by the competition of MoO42− for La3+ with EDTA. The strong adsorption of released EDTA on the surface of La2(MoO4)3 nuclei prevented the growth of the fresh nanoparticles. Then, the primary nanoparticles self-aggregated together to form larger microspheres mediated by hydrogen bonds and thus minimize the interfacial energy. The EDTA here can be considered as a “cohesive agent”. The continuous self-aggregation accompanying with Ostwald ripening resulted in the formation of uniform microspheres built from numerous nanoparticles. Therefore, the formation of uniform La2(MoO4)3 microspheres here can be attributed to the self-aggregation and Ostwald ripening of nanoparticles.23
 | ||
| Fig. 8 TEM images of the products collected at different reaction times with the aid of 0.46 g EDTA. (a) 5 min, (b) 15 min, (c) 1 h and (d) 3h. | ||
Concerning the three reactions carried out in the presence of different amounts of EDTA, it can be concluded that the amount of EDTA is indispensable for the morphological modulation of the final products. Introducing appropriate amounts of EDTA could prominently change the growth pathway. As mentioned above, EDTA always acts as an efficient chelating agent and capping agent to tune the product morphology. In the current study, with the increase of the amount of EDTA, the capping ability of EDTA became more and more prevailing. Therefore, when the amount of EDTA was very small, the capping ability of EDTA was negligible and the anisotropic characteristic of La2(MoO4)3 was advantageous to the generation of 2D nanoflakes as primary building blocks. However, as the amount of EDTA reached a certain value, the capping ability of EDTA was dominant, thus preventing the further growth of nuclei. Meanwhile, hydrogen bonds also played an important role in self-assembling, which was helpful to the formation of 3D hierarchical architectures. The geometrical shapes of building blocks are responsible for the generation of spherical structures, since no surfactant or emulsion was introduced during the synthesis.24 With respect to the formation of microspindles, microdumbbells and microflowers at different pH values, the pH value of the precursor solution should also have played an important role. Generally, as the pH value is reduced from 9 to 7, the selective adsorption of EDTA onto different surfaces of growing La2(MoO4)3 crystallites changes significantly, leading to dramatic modification in the final product morphologies. When the pH value was adjusted to 7, the released EDTA might be preferentially adsorbed on the lateral side of the La2(MoO4)3 crystallites, thus the longitudinal growth direction was favored. In addition, as mediated by the hydrogen bond and electrostatic effects of EDTA, the initially formed nanoparticles tended to attach with each other in a parallel way and eventually self-organized into microspindles or microdumbbells. On the other hand, when the pH value of the initial solution was increased to 10, the presence of an excess amount of OH− and NH4+ adsorbed on the surfaces of the La2(MoO4)3 crystallites led to the linkage of nanoflakes through self-assembly by electrostatic attractions and repulsions, giving rise to the appearance of flower-like architectures.5a,16a Certainly, the actual formation processes are more complex and may still remain mysterious. However, it is understandable that the intrinsic structures of La2(MoO4)3 and the external factors (such as the amount of EDTA and the pH value of the precursor solution) have synergistic effects on the formation of different hierarchical structures. The possible formation mechanisms of La2(MoO4)3 3D hierarchical architectures with different shapes obtained under different experimental conditions are schematically illustrated in Scheme 1.
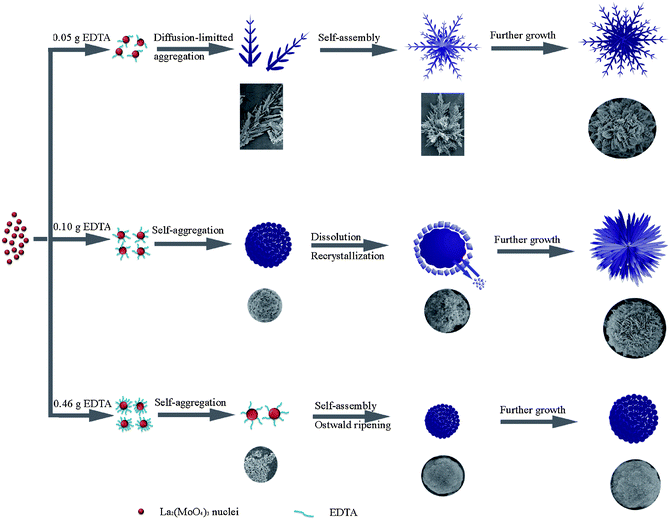 | ||
| Scheme 1 Schematic illustration of the morphological evolution processes of different hierarchical architectures obtained in the presence of different amounts of EDTA at pH = 9. | ||
3.4 Photoluminescence properties
La2(MoO4)3 has been demonstrated to be an efficient host for other lanthanide ions, bringing out various luminescence properties.14,16 Here, Eu3+ was selected as the doping ion to investigate the luminescence properties of the products with different morphologies obtained with the assistance of 0.05 g and 0.46 g EDTA, respectively. It is noteworthy that doping with Eu3+ alters neither the crystal structure nor the morphology of the host material due to the similarity of Eu3+ to La3+. After doping with Eu3+, the products obtained in the presence of 0.05 g and 0.46 g EDTA preserved the host morphologies, namely, microflowers with hyperbranched dendritic structures and microspheres constructed by numerous nanoparticles, respectively. Room temperature excitation and emission spectra of La2(MoO4)3:Eu microflowers (S1) and microspheres (S5) are presented in Fig. 9. The doping concentrations of Eu3+ in the two samples are 1.80% and 1.82%, respectively, as measured by EDS. The result reveals that the Eu3+ concentrations in two samples are almost the same. From Fig. 9, it can be clearly seen that the excitation and emission spectra of the two samples are similar in shape, but different in the intensity to some extent, indicating that the luminescence properties are closely correlated with the morphology and size of the products. As shown in Fig. 9a, the excitation spectra of the two samples both consist of the characteristic f–f transition lines within the Eu3+ 4f6 configuration between 320 and 440 nm. These transition lines can be assigned as the transitions from the 7F0 ground state to the different excited states of Eu3+, that is, 5D4 (362 nm), 5G2 (383 nm), 5L6 (396 nm), and 5D3 (417 nm), respectively. The PL emission spectra upon excitation into the strongest 7F0–5L6 transition of Eu3+ at 396 nm are shown in Fig. 9b. The obtained emission spectra are composed of a series of 5D0–7FJ (J = 1, 2, 3, 4) transition lines of Eu3+ as labeled in Fig. 9b, with the 5D0–7F2 transition centered at 614 nm being the most prominent group. It is noteworthy that the peak positions in the excitation and emission spectra of the two samples are almost the same since the 4f energy levels of Eu3+ are hardly affected by the crystal field due to the shielding effect of the 5s25p6 electrons.25 It is well-known that the intensities of the transitions between different J-number levels are dependent on the symmetry of the local environment of Eu3+ activators in terms of Judd–Ofelt theory.26 If Eu3+ is located in a site with an inversion center, the 5D0–7F1 magnetic dipole transition should be dominant, while in a site without an inversion center, the 5D0–7F2 electric dipole transition will be preponderant. In the present work, the most prominent red emission centered at 614 nm ascribed to 5D0–7F2 transition is evidently far stronger than the orange emission at 593 nm corresponding to the 5D0–7F1 transition in both samples, indicating that Eu3+ ions occupy the sites without inversion center and have low crystal field symmetry . This result also demonstrates that the as-synthesized La2(MoO4)3:Eu samples with the microflower and microsphere hierarchical architectures are a kind of efficient phosphor with high red color purity and may have potential applications in fluorescence lamps, field emission displays (FED) and plasma display panels (PDP).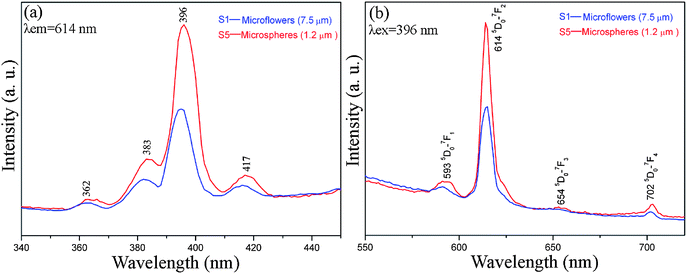 | ||
| Fig. 9 Room-temperature (a) excitation (λem = 614 nm) and (b) emission (λex = 396 nm) spectra of La2(MoO4)3:Eu microflowers with an average size of 7.5 μm (S1) and microspheres with an average size of 1.2 μm (S5). | ||
It is widely accepted that the luminescence properties of phosphors are strongly correlated with the morphology, size and crystallinity.27 In the present work, under identical measurement conditions, the relative emission intensities of the two samples are quite different, namely, the microspheres (S5) exhibit a much stronger emission than microflowers (S1). The relative intensity of the S5 is almost 2 times as high as that of S1. The possible reasons for the enhancement of emission intensities can be elucidated as follows. On the one hand, the microspheres have an average diameter of 1.2 μm, which is much smaller than the microflowers with an average diameter of 7.5 μm. The building blocks of the two samples vary from 3D dendrites to 0D nanoparticles, the quantum-confinement effect can be used to account for the improvement of emission intensities.28 Moreover, as the particle size is reduced, the ratio of surface Eu3+ is increased, lowering the local symmetry of the crystal field around the Eu3+ ions,29 and thus enhancing the emission intensity. On the other hand, the intrinsic geometry of the microspheres can efficiently minimize the scattering of light from the sample surface as compared with that of other morphologies, further leading to an improvement of emission intensity.30 Therefore, on the basis of the aforementioned explanations, the different luminescence behaviors can be reasonably attributed to the different morphologies and sizes of the two samples.
3.5 Adsorption activity of La2(MoO4)3 microstructures
Since rare earth elements are often lacking electrons in the 4f orbital, they have a strong affinity for electron-rich organics.31Accordingly, it is accepted that the obtained La2(MoO4)3 microstructures may also be used as adsorbents to remove organic wastes from water. Herein, as an example, Rhodamine B (Rh B), a widely used dye, was employed as model pollutant to evaluate the adsorption activity of La2(MoO4)3 microstructures. The characteristic absorption of Rh B at 553 nm was selected for monitoring the adsorption process. As shown by the photo images and UV-vis absorption curves at different time intervals in Fig. 10, when the initial concentration of Rh B in water is 10 mg L−1, the La2(MoO4)3 microflowers obtained with the assistance of 0.05 g EDTA could remove about 96.7% of the Rh B without any additives at room temperature. The adsorption capacity of the La2(MoO4)3 microflowers for Rh B was about 48.4 mg g−1. As a comparison, La2(MoO4)3 microspheres (S5) obtained with the aid of 0.46 g EDTA were also selected to detect the removal rate of Rh B. As shown in Fig. 11, the microflowers exhibit a relatively high adsorption rate compared to the microspheres, although the adsorption capacities of two samples are almost the same. Since the BET surface area of microspheres (34.28 m2 g−1) is higher than that of microflowers (22.00 m2 g−1), the enhancement of adsorption rate can be attributed to the novel hierarchical superstructure of microflowers assembled by numerous dendrites. As a result of the self-assembly of a large number of interwoven nanoflakes, the hierarchical microflowers have plenty of meso- and macro-pores (see Fig. SI7 and corresponding description in the Supporting Information†), providing efficient transport paths for adsorption.32 It was reported that chemical reactions can occur more easily when the transport paths through which molecules move into or out of the nanostructured materials are included as an integral part of the architectural design.33 Therefore, in the present work, the presence of efficient transport paths in La2(MoO4)3 microflowers benefits the enhancement of adsorption performance for the removal of Rh B.
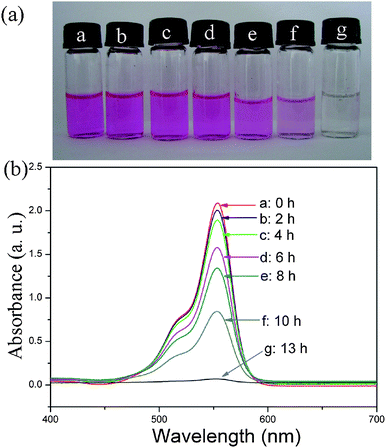 | ||
| Fig. 10 (a) Photo images of absorption of Rh B at different times by La2(MoO4)3 microflowers obtained with the assistance of 0.05 g EDTA. (b) Absorption spectra of a solution of Rh B (10 mg L−1, 50 mL) in the presence of 10 mg La2(MoO4)3 microflowers at different time intervals. | ||
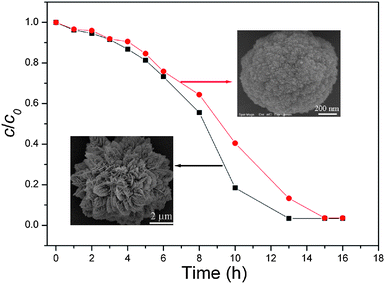 | ||
| Fig. 11 Adsorption rate of Rh B on La2(MoO4)3 microflowers with an average size of 7.5 μm (S1) and microspheres with an average size of 1.2 μm (S5). | ||
4. Conclusion
In conclusion, various novel and complex 3D La2(MoO4)3 hierarchical architectures self-assembled from different building blocks were successfully synthesized by a hydrothermal method in an EDTA-mediated processes. It was found that the product morphology and size distribution varied remarkably with the amount of EDTA introduced into the reaction system and the pH value of the precursor solution. By changing the amount of EDTA and the pH value, various self-assembled 3D La2(MoO4)3 hierarchical architectures such as microflowers, microspheres, microspindles and microdumbbells could be obtained and the building blocks of the different hierarchical architectures varied from 3D dendrites to 2D nanoplates and eventually to 0D nanoparticles. The possible formation mechanisms for different superstructures were carefully put forward on the basis of a series of time-dependent experiments. This work can be extended to the fabrication of other inorganic materials with complex hierarchical structures.As stimulated by the potential applications of the obtained products, the PL properties of the Eu-doped La2(MoO4)3 superstructures with different morphologies were measured. The results revealed that La2(MoO4)3:Eu microspheres constructed by nanoparticles displayed a stronger emission than La2(MoO4)3:Eu microflowers built from interwoven dendrites. Furthermore, to expand the application fields of La2(MoO4)3 hierarchical architectures, the adsorption activity of La2(MoO4)3 as an absorbent in water treatment was also investigated. It was found that the microflowers exhibited a relatively high adsorption rate compared to the microspheres, although the adsorption capacities of two samples are almost the same. Possible reasons responsible for the differences in PL properties and absorption activities of different hierarchical architectures were discussed. It was demonstrated that the PL properties and adsorption activities of the La2(MoO4)3 hierarchical architectures were strongly correlated with the morphology and size, further emphasizing the importance of rational shape control. This work could be of great significance for the exploration and expansion of potential applications of La2(MoO4)3, an outstanding advanced function material.
Acknowledgements
The authors greatly appreciate the financial support of the National Natural Science Foundation of China (Grant No. 20773065), National Basic Research Program (973 project) (Grant No. 2007CB936302) and Modern Analysis Center of Nanjing University.References
- H. Colfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576 CrossRef.
- (a) X. F. Duan, Y. Huang, Y. Cui, J. F. Wang and C. M. Lieber, Nature, 2001, 409, 66 CrossRef CAS; (b) H. Yan, R. He, J. Johnson, M. Law, R. J. Saykally and P. Yang, J. Am. Chem. Soc., 2003, 125, 4728 CrossRef CAS.
- (a) J. Perez-Ramirez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC; (b) G. Hu, D. Ma, L. Liu, M. Cheng and X. Bao, Angew. Chem., Int. Ed., 2004, 43, 3452 CrossRef CAS; (c) V. N. Shetti, J. Kim, R. Srivastava, M. Choi and R. Ryoo, J. Catal., 2008, 254, 296 CrossRef CAS.
- J. Yang, C. X. Li, X. M. Zhang, Z. W. Quan, C. M. Zhang, H. Y. Li and J. Lin, Chem.–Eur. J., 2008, 14, 4336 CrossRef CAS.
- (a) I. S. Cho, D. K. Kim, S. Lee, C. H. Kwark, S. T. Bae, J. H. Noh, S. H. Yoon, H. S. Jung, D. W. Kim and K. S. Hong, Adv. Funct. Mater., 2008, 18, 2154 CrossRef CAS; (b) D. Chen and J. H. Ye, Adv. Funct. Mater., 2008, 18, 1922 CrossRef CAS.
- (a) J. S. Hu, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 2977 CrossRef CAS; (b) J. Fei, Y. Cui, X. Yan, W. Qi, Y. Yang, K. Wang, Q. He and J. Li, Adv. Mater., 2008, 20, 452 CrossRef.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS.
- X. Wang, J. Zhuang, Q. Peng and Y. Li, Adv. Mater., 2006, 18, 2031 CrossRef CAS.
- (a) W. Di, M. G. Willinger, R. A. S. Ferreira, X. Ren, S. Lu and N. Pinna, J. Phys. Chem. C, 2008, 112, 18815 CAS; (b) L. Qian, J. Zhu, Z. Chen, Y. Gui, Q. Gong, Y. Yuan, J. Zai and X. Qian, Chem.–Eur. J., 2009, 15, 1233 CrossRef CAS; (c) D. Ma, S. Huang, W. Chen, S. Hu, F. Shi and K. Fan, J. Phys. Chem. C, 2009, 113, 4369 CrossRef CAS.
- (a) F. Cao, W. Shi, L. Zhao, S. Song, J. Yang, Y. Lei and H. Zhang, J. Phys. Chem. C, 2008, 112, 17095 CrossRef CAS; (b) H. Deng, C. Liu, S. Yang, S. Xiao, Z. K. Zhou and Q. Q. Wang, Cryst. Growth Des., 2008, 8, 4432 CrossRef CAS.
- L. Xu, J. M. Shen, C. L. Lu, Y. P. Chen and W. H. Hou, Cryst. Growth Des., 2009, 9, 3129 CrossRef CAS.
- F. D. Smet, P. Ruiz, B. Delmon and M. Devillers, J. Phys. Chem. B, 2001, 105, 12355 CrossRef CAS.
- H. Naruke and T. Yamase, Inorg. Chem., 2002, 41, 6514 CrossRef CAS.
- G. Yi, B. Sun, F. Yang, D. Chen, Y. Zhou and J. Cheng, Chem. Mater., 2002, 14, 2910 CrossRef CAS.
- J. S. O. Evans, T. A. Mary and A. W. Sleight, J. Solid State Chem., 1998, 137, 148 CrossRef CAS.
- (a) W. Bu, Y. Xu, N. Zhang, H. Chen, Z. Hua and J. Shi, Langmuir, 2007, 23, 9002 CrossRef CAS; (b) N. Zhang, W. B. Bu, Y. Xu, D. Jiang and J. Shi, J. Phys. Chem. C, 2007, 111, 5014 CrossRef CAS; (c) Z. Chen, W. Bu, N. Zhang and J. Shi, J. Phys. Chem. C, 2008, 112, 4378 CrossRef CAS; (d) N. Zhang, W. Bu, Y. Xu, D. Jiang and J. Shi, J. Nanosci. Nanotechnol., 2008, 8, 1468 CrossRef CAS.
- T. C. Halsey, B. Duplantier and K. Honda, Phys. Rev. Lett., 1997, 78, 1719 CrossRef CAS.
- Z. R. Tian, J. Liu, J. A. Voigt, H. F. Xu and M. J. Mcdermott, Nano Lett., 2003, 3, 89 CrossRef CAS.
- L. Zhu, H. Xiao, W. Zhang, Y. Yang and S. Fu, Cryst. Growth Des., 2008, 8, 1113 CrossRef CAS.
- Y. Chang, J. J. Teo and H. C. Zeng, Langmuir, 2005, 21, 1074 CrossRef CAS.
- (a) G. Xi, K. Xiong, Q. Zhao, R. Zhang, H. Zhang and Y. Qian, Cryst. Growth Des., 2006, 6, 577 CrossRef CAS; (b) L. Xu, C. Lu, J. Shen, Y. Chen, Z. Zhang and W. Hou, CrystEngComm, 2009, 11, 1323 RSC.
- Y. Cheng, Y. S. Wang, Y. H. Zheng and Y. Qin, J. Phys. Chem. B, 2005, 109, 11548 CrossRef CAS.
- (a) Y. Li, M. H. Cao and L. Y. Feng, Langmuir, 2009, 25, 1705 CrossRef CAS; (b) Q. Zhang, W. Yao, X. Chen, L. Zhu, Y. Fu, G. Zhang, L. Sheng and S. Yu, Cryst. Growth Des., 2007, 7, 1423 CrossRef CAS.
- (a) B. Liu and H. Zeng, J. Am. Chem. Soc., 2004, 126, 8124 CrossRef CAS; (b) B. B. Kale, J. O. Baeg, S. M. Lee, H. Chang, S. J. Moon and C. W. Lee, Adv. Funct. Mater., 2006, 16, 1349 CrossRef CAS.
- J. Yang, C. Li, Z. Quan, C. Zhang, P. Yang, Y. Li, C. Yu and J. Lin, J. Phys. Chem. C, 2008, 112, 12777 CrossRef CAS.
- (a) B. R. Judd, Phys. Rev., 1962, 127, 750 CrossRef CAS; (b) G. S. Ofelt, J. Chem. Phys., 1962, 37, 511 CrossRef.
- C. Li, J. Yang, Z. Quan, P. Yang, D. Kong and J. Lin, Chem. Mater., 2007, 19, 4933 CrossRef CAS.
- R. N. Bhargava, J. Lumin., 1996, 70, 85 CrossRef CAS.
- (a) H. Mai, Y. Zhang, R. Si, Z. Yan, L. Sun, L. You and C. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS; (b) Z. Wei, D. Sun, C. Liao, C. Yan and S. Huang, Appl. Phys. Lett., 2002, 80, 1447 CrossRef CAS.
- H. J. Zhou, Y. B. Mao and S. S. Wong, J. Mater. Chem., 2007, 17, 1707 RSC.
- F. Niu, A. M. Cao, W. G. Song and L. J. Wan, J. Phys. Chem. C, 2008, 112, 17988 CrossRef CAS.
- L. S. Zhang, W. Z. Wang, Z. G. Chen, L. Zhou, H. L. Xu and W. Zhu, J. Mater. Chem., 2007, 17, 2526 RSC.
- (a) D. R. Rolison, Science, 2003, 299, 1698 CrossRef CAS; (b) L. Zhang and J. Yu, Chem. Commun., 2003, 2078 RSC; (c) X. Wang, J. Yu, C. Ho, Y. Hou and X. Fu, Langmuir, 2005, 21, 2552 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XRD patterns, SEM images and adsorption-desorption isotherms. See DOI: 10.1039/b9nr00392d |
| This journal is © The Royal Society of Chemistry 2010 |
