A general corrosion route to nanostructured metal oxides†
Caixia
Xu
ab,
Rongyue
Wang
a,
Yan
Zhang
a and
Yi
Ding
*a
aSchool of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, China. E-mail: yding@sdu.edu.cn; Fax: +86-531-88366280; Tel: +86-531-88366513
bSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan, 250022, China
First published on 27th April 2010
Abstract
A general corrosion strategy was employed for the straightforward fabrication of a variety of metal oxide nanostructures, such as Fe3O4 octahedra, Co3O4 nanoplates, nanoporous TiO2, and Mn3O4 octahedra.
Metal oxide nanostructures are of tremendous fundamental and technological importance with wide applications in catalysis, magnetism, gas sensing, and so on.1 For instance, Co3O4 nanostructures were recently found to be highly catalytically active for low temperature CO oxidation.1a,d Consequently, considerable efforts have been devoted to the development of various synthesis methods for nanostructured metal oxides. In the past several decades, many methods have been proposed including a hydrothermal process,2 sol–gel,3 templating,4 and selective phase leaching.5 As most of these methods more or less involve high-temperature processing, excessive use of capping or organic agents, and multi-step procedures, it is therefore of practical importance to develop new method to fabricate metal oxide nanostructures in high through-put under mild conditions.
In this work, we present a general concept for the straightforward fabrication of metal oxide nanostructures by an unusually simple yet highly effective alloy corrosion method. It is quite well known that selective etching of active species from an alloy (also called dealloying) can produce very useful porous metal nanostructures such as nanoporous gold.6 This concept holds true for many noble metal containing materials. However, it is much less appreciated that this simple dealloying method can be extended to the fabrication of important transition metal oxide nanostructures, such as Fe3O4, Co3O4, TiO2, and Mn3O4, with intricate structural properties. Our approach mainly involves the alloying of the targeted transition metals with more active metal species such as Al, and a subsequent selective leaching of active metals in appropriate corrosion media. During this etching process, the transition metal atoms left behind will undergo spontaneous oxidation at the metal/electrolyte interface to form metal oxides. By carefully selecting source alloy compositions and etching conditions, this approach can in principle offer very flexible control to the structures of the resulted metal oxides. Meanwhile, compared with other methods, this strategy possesses evident advantages of simple processing, nearly absolute yield, and is applicable for large-scale synthesis.
We first choose Fe3O4 as an example to demonstrate the effectiveness of this dealloying method in the fabrication of nanostructured metal oxides. X-Ray diffraction (XRD) was first used to analyze the phase structure of Fe/Al source alloy. As shown in Fig. S1†, a majority of diffraction peaks can be ascribed to the Al13Fe4 alloy (JCPDS 50-0797), while two minor peaks at 2θ values of around 38° and 65° are indexed to Al (JCPDS 65-2869). The success in the formation of an Al/Fe alloy with a little excess of Al is actually expected because the nominal composition of source alloy is Al85Fe15. And the fact that the alloy is slightly rich in aluminium also benefits the corrosion process by accelerating the reaction rate in alkaline solution. Upon etching a piece of Fe/Al alloy foil in 5 mol L−1 NaOH solution for 48 h at room temperature, brownish precipitates were collected. As shown in Fig. 1a, all diffraction peaks for the resulting sample could be indexed to the cubic inverse spinal structure of Fe3O4 (JCPDS 65-3107). No additional diffraction peaks were observed, suggesting the high purity of the resulted Fe3O4. Fig. 1b shows a typical scanning electron spectroscopy (SEM) image for the sample. Interestingly, it was found that the product consists of regular Fe3O4 octahedra rather than a porous structure as often seen during dealloying of precious metal alloys.6 These octahedra show well dispersed, fully developed morphology with mean edge length of ∼800 nm (Fig. 1b). High resolution transmission electron spectroscopy (HRTEM) image in Fig. 1c indicates that the lattice fringes regularly occupy the whole structure of the particle, indicating a single-crystal nature of the Fe3O4 octahedra. The interplanar spacing was estimated to be 2.41 Å, which corresponds well to the (222) planes of the magnetite structure. The corresponding selected-area electron diffraction (SAED, inset in 1c) further reveals a single-crystal pattern recorded along the (111) zone axis direction. Composition analysis with energy dispersive spectroscopy (EDS) shows signals from Fe and O (Fig. S2†) alone, while the residual Al, if present, is below the detecting limit of the instrument, indicating the completion of the dealloying process.
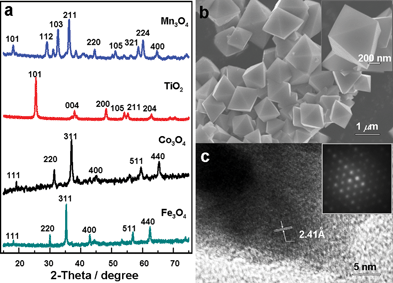 | ||
| Fig. 1 (a) XRD patterns of the resulted metal oxide nanostructures. (b, c) SEM, HRTEM images and SAED pattern of the Fe3O4 sample. | ||
To understand the formation of Fe3O4 octahedra, we also examined the roles of critical parameters in the size- and shape-guiding process. As illustrated in Fig. 2, a structural evolution of Fe3O4 nanostructures with increasing reaction time in 2 mol L−1 NaOH can be observed during the dealloying process. At the early reaction stage (8 h) the octahedral-like morphology can be clearly resolved in a majority of nanoparticles, although a perfect octahedral morphology is not yet developed. These particles have quite uniform structural dispersion with mean size around 300 nm. XRD analysis confirmed that the magnetite structure has already formed at this time (Fig. S3†), indicating that the removal of Al was rapidly completed at very early stage. Increasing the reaction time to 48 and 96 h led to regularization of octahedral nanoparticles and an increase in edge length to 500 and 1000 nm, respectively (Fig. 2). The size distribution of Fe3O4 nanoparticles for the 48 h sample is provided in Fig. S4†, from which an average size of ∼510 nm can be estimated for the obtained nano-octahedra. Changing the concentration of corrosion media (NaOH) can also affect the structure formation and evolution of Fe3O4 nanoparticles (Fig. S5†), and preliminary studies show that the edge length of Fe3O4 octahedra can be effectively tuned in a wide range from about 100 nm to several microns. Notably, this size range locates well within the reported dimensions for Fe3O4 nanoparticles required for biomedical applications due to their biological and structural compatibility with biomolecules such as proteins.7
 | ||
| Fig. 2 SEM images of the resulting Fe3O4 samples after dealloying for (a) 8, (b) 48, and (c) 96 h in 2 mol L−1 NaOH solution. | ||
Although our method looks like an up-down process governed by selective etching from a bulk material, the microstructure formation and evolution are actually bottom-up. Upon leaching Al atoms, the exposed Fe atoms will be quickly oxidized to form magnetite nuclei. During the dealloying process, these nuclei will subject to continuous growth as more and more iron atoms (or oxidized clusters) are released and land on them, generating relatively uniform micro-crystals (Fig. 2a). During the subsequent crystal growth process, the tendency to achieve a minimum total surface free energy favors the growth of larger particles at the expense of the smaller ones. Meanwhile, adopting a cubic structure, the general sequence of surface energies, γ(111) < γ (100) < γ (110),8 implies that Fe3O4 particles can exist in a low energy form of octahedron bounded by eight (111) planes. This speculation is supported by the fact that OH− ions can strongly adsorb on the Fe3O4 (111) surface9 and previous hydrothermal synthesis of iron oxides observed the preference for the formation of Fe3O4 phase and Fe3O4 octahedra under high pH values.10 In order to provide direct spectroscopic evidence for adsorbed OH on the nanoparticles, FTIR was used to analyze the obtained Fe3O4 octahedra which had been thoroughly washed and dried. As shown in Fig. S6†, the absorption peaks located at 585 and 440 cm−1 are attributed to the vibrations of Fe–O bonds from magnetite.11 The weak peak at 3739 cm−1 is assigned to a stretching vibration mode of isolated hydroxyl groups.12 The adsorption bands at 3333 and 3434 cm−1 are generally considered to be associated with hydroxyls which have an H bond to neighboring water molecules,13 indicating the presence of OH− strongly adsorbed on Fe3O4 surface. Meanwhile, the broad peak at 3150 cm−1 can be assigned to the O–H stretching vibration from water molecules, and the peak at 1630 cm−1 is ascribed to a low frequency scissor mode of the molecularly adsorbed water.12a Finally, control experiments were also carried out in dilute NaOH solution (0.05 mol L−1), and after dealloying, only needle-like Fe3O4 nanoaggregates were obtained (Fig. S7†). These nano-needles typically have a diameter around several tens of nanometres with a length slightly over one micron.
This simple corrosion method can be easily extended to fabricate other metal oxide nanostructures. We briefly discuss here three other examples to prove its versatility in making important oxide materials. By dealloying Co/Al, Ti/Al, and Mn/Al alloy precursors in NaOH solution (details provided in the electronic supplementary information†), Co3O4, TiO2, and Mn3O4 nanostructures can be effectively prepared. Structure determination with XRD proved the formation of cubic phase Co3O4 (JCPDS 65-3103), anatase structured TiO2 (JCPDS 21-1272) and tetragonal Mn3O4 (hausmannite, JCPDS 24-0734). No traces of other metal oxide polymorphs were detected, indicating the formation of high purity metal oxides. SEM observations revealed the corresponding micro-morphology for these structures. As shown in Fig. 3a, Co3O4 consists of a uniform dispersion of interconnected nano-plates with a diameter of several microns and a thickness around 100 nm. A closer inspection on an individual nanoplate shows interesting structure hierarchy with a large number of nano-needles grown on the surface. These nano-needles show relatively uniform dimensions of around 400 nm in length and 20 nm in diameter. This interesting structural feature endows the sample a with higher surface area, which is especially beneficial for catalysis1a and sensing applications. Similar to the synthesis of Fe3O4 octahedra, the structure and dimensions of Co3O4 nanoplates are also controllable by tailoring the reaction parameters (Fig. S8†). As to the dealloyed TiO2 sample, its microstructure is characterized by a three-dimensional bicontinuous network morphology with dramatically larger pore dimensions of ∼80 nm as compared with that of ligament size (10 nm). Finally, dealloying MnAl alloys produced uniform octahedral-like Mn3O4 nanoparticles. Compared to the Fe3O4 case, these Mn3O4 octahedra seem slightly elongated, with typical edge length around 300 nm. Based on the above observations, uniform metal oxide nanostructures with delicate micro-morphology can be generated by this simple corrosion approach.
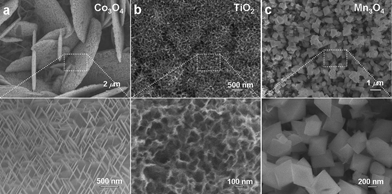 | ||
| Fig. 3 SEM images of (a) Co3O4, (b) TiO2, and (c) Mn3O4 nanostructures after dealloying Co/Al, Ti/Al and Mn/Al alloys in NaOH solutions at room temperature. | ||
As to the formation of the aforementioned metal oxide nanostructures, the actual corrosion conditions play a crucial role in determining their microstructure development. In the present case, upon immersing Al-based alloys into NaOH solutions, Al was rapidly dissolved and the remaining transition metal atoms became significantly less coordinated. These fresh metal sites are expected to be highly active and can undergo a natural oxidation with aqueous solution, while the presence of excessive OH− ions can work in a structure-directing way by preferentially adsorbing on certain crystal planes. To get further insight into the role of OH− for the structure formation, we have also carried out the dealloying process by tuning the operating conditions. Taking Fe/Al corrosion as an example, XRD and SEM analysis revealed that dealloying under the protection of high purity nitrogen gas does not affect the formation of Fe3O4 octahedra (Fig. S9†). Meanwhile, the presence of additional surface adsorbing reagents will strongly affect the morphology of the resulting metal oxides. As shown in Fig. S10a†, very thin (∼10 nm) nano-flakes were found uniformly covering the surface of the resulted precipitates after the Fe/Al alloy was etched in 0.2 mol L−1 NaOH solution in the presence of potassium citrate for 24 h. The addition of potassium citrate apparently led to the competition of different ions to the adsorption to the surface of nearly formed Fe3O4 nanoclusters, which in turn affected the development of the microstructure of the resulted sample. In addition, no Fe3O4 octahedra could be obtained if one tries the etching in acidic solutions. Fig. S10b† illustrates an iron oxide sample that was produced by dealloying in 50 mmol L−1 HCl for 10 h at room temperature. The resulting sample is characterized by a relatively uniform dispersion of spherical particles with diameter around 100 nm, in sharp contrast to those obtained from alkaline medium. These experimental observations further confirm the modulation role of OH− ions for the formation of metal oxide nanostructures. It should be mentioned that dealloying the above mentioned alloys generates distinct micro-structures for different metals, which may be related to a variety of factors such as the intrinsic chemical properties, the formation and adsorption of oxygenated species at the electrolyte/metal interface, and the subsequent nucleation and growth processes. It is considered that the Al component in alloys plays a crucial role in dispersing the targeted metals and forming particular alloy phases, which obviously affects the structure and morphology of the resulted metal oxides. More detailed investigation is needed to understand the micro-structure formation and evolution of individual metal oxides, especially when structure directing agents co-exist in the system during fabrication.
Finally, we take Fe3O4 octahedra as an example to characterize their structure-sensitive magnetic properties. We measured the magnetic hysteresis curves of three Fe3O4 octahedra samples with the typical edge lengths of 200, 400 and 800 nm. As shown in Fig. S11†, these samples exhibit a clear size-dependent ferromagnetic behavior. The saturation magnetization (Ms) of these samples is much lower than that of bulk Fe3O4 (∼90 emu g−1), and decreases in value as the characteristic crystalline size becomes smaller.14,15 As for the decrease of Ms with the reduction of structure dimension, it is considered that the higher surface to volume ratio in the smaller particles results in the existence of a nonmagnetized surface layer, which lead to the reduction of Ms of the nanocrystals.14 In contrast, the coercivity (Hc) of Fe3O4 octahedra shows a decreasing trend with an increase in the length. With the respective values of 273, 248, and 220 Oe for 200, 400 and 800 nm sized Fe3O4 octahedra samples, their Hc is evidently larger than that of bulk Fe3O4 (115 Oe).15 The enhancement of Hc may be related to the shape anisotropy of the octahedral structure. Geng et al. also observed high Hc for Fe3O4 dodecahedra, and they suggested that by reducing the crystal size, the anisotropy energy barrier will increase, which in turn results in the enhancement of Hc.14–17 The enhancement of Hc of the as-prepared Fe3O4 octahedra thus suggests their potential applications in the fields of ferrofluids and information storage.
In summary, we reported a simple alloy corrosion route for the facile fabrication of a series of nanostructured metal oxides. It is expected that many other metal oxides with tailored structural features can be made in a similar way by choosing the appropriate binary or multi-component alloy systems, screening the corrosion conditions, and/or introducing additional structure directing agents such as surfactants or polymers. Many topics remain unanswered at the present stage, and more detailed studies are required to understand the microstructure formation and evolution at the molecular level. Considering the importance of metal oxides, our findings offer new opportunities for the exploration of new metal oxide nanostructures and their shape-dependent properties, which are essential for their implementation in various nanoscale science and technology applications.
Acknowledgements
This work was supported by the National 973 (2007CB936602) Program Projects of China, the Key Project of Chinese Ministry of Education (108078) and the Natural Science Foundation of Shandong Province (Y2007B33). Y. D. is a Tai-Shan Scholar supported by the SEM-NCET Program and the Shandong Natural Science Fund for Distinguished Young Scholars.Notes and references
- (a) X. Xie, Y. Li, Z. Liu, M. Haruta and W. Shen, Nature, 2009, 458, 746 CrossRef CAS; (b) C. Yavuz, J. Mayo, W. Yu, A. Prakash, J. Falkner, S. Yean, L. Cong, H. Shipley, A. Kan, M. Tomson, D. Natelson and V. Colvin, Science, 2006, 314, 964 CrossRef; (c) Y. Wang, X. Jiang and Y. Xia, J. Am. Chem. Soc., 2003, 125, 16176 CrossRef CAS; (d) X. Xie and W. Shen, Nanoscale, 2009, 1, 50 RSC; (e) J. Chen, C. Li, W. Zhou, Q. Yan, L. Archerc and X. Lou, Nanoscale, 2009, 1, 280 RSC.
- B. Liu and E. S. Aydil, J. Am. Chem. Soc., 2009, 131, 3985 CrossRef CAS.
- N. Pinna, G. Neri, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2004, 43, 4345 CrossRef CAS.
- (a) B. Tian, X. Lui, L. Solovyov, Z. Liu, H. Yang, Z. Zhang, S. Xie, F. Zhang, B. Tu, C. Yu, O. Terasaki and D. Zhao, J. Am. Chem. Soc., 2004, 126, 865 CrossRef CAS; (b) F. Jiao, J. Jumas, M. Womes, A. Chadwick, A. Harrison and P. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS.
- E. S. Toberer and R. Seshadri, Chem. Commun., 2006, 3159 RSC.
- (a) J. Erlebacher, M. J. Aziz, A. Karama, N. Dimitrov and K. Sieradzki, Nature, 2001, 410, 450 CrossRef CAS; (b) Y. Ding and M. W. Chen, MRS Bull., 2009, 34, 569 CAS.
- S. Makhluf, R. Abu-Mukh, S. Rubinstein, H. Breitbart and A. Gedanken, Small, 2008, 4, 1453 CrossRef.
- Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153 CrossRef CAS.
- Y. Joseph, W. Ranke and W. Weiss, J. Phys. Chem. B, 2000, 104, 3224 CrossRef CAS.
- X. Liang, X. Wang, J. Zhuang, Y. T. Chen, D. S. Wang and Y. D. Li, Adv. Funct. Mater., 2006, 16, 1805 CrossRef CAS.
- J. L. Zhang, R. S. Srivastava and R. D. K. Misra, Langmuir, 2007, 23, 6342 CrossRef CAS.
- (a) C. Lemire, R. Meyer, V. E. Henrich, Sh. Shaikhutdinov and H.-J. Freund, Surf. Sci., 2004, 572, 103 CrossRef CAS; (b) M. A. Henderson, Surf. Sci. Rep., 2002, 46, 1 CrossRef CAS.
- (a) K. Jacobi, K. Bedürftig, Y. Wang and G. Ertl, Surf. Sci., 2001, 472, 9 CrossRef CAS; (b) S. Ahmad, U. Riaz, A. Kaushik and J. Alam, J. Inorg. Organomet. Polym. Mater., 2009, 19, 355 CrossRef CAS.
- M. H. Cao, T. F. Liu, S. Gao, G. B. Sun, X. L. Wu, C. W. Hu and Z. L. Wang, Angew. Chem., Int. Ed., 2005, 44, 4197 CrossRef CAS.
- B. Y. Geng, J. Z. Ma, X. W. Liu, Q. B. Du, M. G. Kong and L. D. Zhang, Appl. Phys. Lett., 2007, 90, 043120 CrossRef.
- B. Y. Geng, J. Z. Ma and J. H. You, Cryst. Growth Des., 2008, 8, 1443 CrossRef CAS.
- C. B. Rong, D. Li, V. Nandwana, N. Poudyal, Y. Ding, Z. L. Wang, L. H. Zeng and J. P. Liu, Adv. Mater., 2006, 18, 2984 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure, EDS, FTIR, XRD, SEM and magnetization curves, Figures S1-S11. See DOI: 10.1039/b9nr00351g |
| This journal is © The Royal Society of Chemistry 2010 |
