Preparation of nanoscale PbSe particles with different morphologies in diethylene glycol†
Zeping
Peng
ab,
Mingzhu
Liu
ab,
Chao
Yu
ab,
Zhanli
Chai
ab,
Hongjie
Zhang
*a and
Cheng
Wang
*a
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Changchun 130022, People's Republic of China. E-mail: hongjie@ns.ciac.jl.cn; cwang@ciac.jl.cn
bGraduate School of the Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 28th January 2010
Abstract
Nanoscale PbSe particles with different morphologies including octahedral, tetradecahedral and cubic shapes have been successfully prepared in diethylene glycol (DEG) at 240 °C in the presence of PVP-K30: poly(vinyl pyrrolidone), MW = 50 000. The formation of PbSe is believed to be an elemental recombination process of corresponding elements reduced from their precursors by the solvent. Experimental results showed that a prominent morphological variation was observed through varying the molar ratios of selenius acid to Pb2+ when Pb(Ac)2 was used as lead precursor, while the sizes of the final PbSe products tended to increase along with the increase of the molar ratios of selenius acid to Pb2+ when Pb(NO3)2 was used as lead precursor.
The physical properties and potential applications of nanoscale materials depend on their compositions, intrinsic structures, dimensional sizes as well as morphologies.1 In solution chemical synthetic processes, the morphological controls of these materials are usually achieved by tuning the kinetic experimental conditions such as precursor concentration, addition of inorganic or/and organic additive, reaction temperature, pH value and so on.2 Generally, isotropic growth along various directions results in the formation of spherical zero-dimensional (0D) nanoparticles while different growth rates of crystallographic planes cause anisotropic growths and lead to the formation of one-dimensional (1D) nanowires, two-dimensional (2D) nanoplates or even three-dimensional (3D) nanostructures. Recently, symmetrically polygonal 3D nanostructures including noble metals,3 cuprite4 and lead sulfide5 were obtained through delicately tuning those kinetic factors in solutions.
PbSe, one of the IV–VI semiconductors with a cubic structure, is a direct-gap semiconductor with a bandgap of 0.28 eV in its bulk phase.3a Compared with some II–VI semiconductors such as CdSe,4 it has a larger Bohr radius (46 nm). With its potential applications as infrared sensors,6 photocatalysts for solar cells,7 in telecommunications and thermoelectric fields,8 colloidal 0D and 1D PbSe with their sizes, in at least one dimension, less than the Bohr radius have been successfully prepared in liquid media by many research groups.9–11 In this work, several polyhedral 3D PbSe nanostructures have been successfully prepared in diethylene glycol (DEG) in the presence of PVP-K30.
Octahedral, tetradecahedral, cubic and cuboid PbSe nanoparticles were prepared in DEG at 240 °C using selenius acid and inorganic lead salts (Pb(Ac)2 or Pb(NO3)2) as precursors. Details of experiment are provided in the ESI.† The process was modified from our previous approach to PbS nanoparticles by substituting thiourea with selenius acid and ethylene glycol (EG) with DEG.5d The reason for changing solvent is only a mixture of PbSe and unknown impurities (see Fig. S1 of the ESI†) could be obtained in EG at its boiling point around 190 °C even with extended reaction time, e.g., 8 h. Phase-pure cubic PbSe products with high crystallinity as shown in XRD patterns (Fig. 1, JCPDS: 06-0354) could be obtained in DEG at this higher reaction temperature. Energy dispersive X-ray analysis (EDX) gives a molar ratio of selenium-to-lead close to its stoichiometric value for a cube-like product (see Fig. S2 of the ESI). The peak intensity ratios for (200) to (111) in Fig. 1 for the products prepared with various concentration ratios of selenius acid to Pb(Ac)2 (RSe/Pb) in the solution differ from each other (see Table S1) and that in the PDF card (3.33, PDF-06-0354) and suggest the anisotropic growth of PbSe products in this system. Due to the low solubility of Pb(Ac)2 within DEG, a higher concentration of 0.25 mol L−1 Pb(Ac)2 was prepared in mixed solvent containing 1.0 mL EG and 1.0 mL DEG and used for the synthesis of octahedral PbSe nanoparticles under similar conditions.
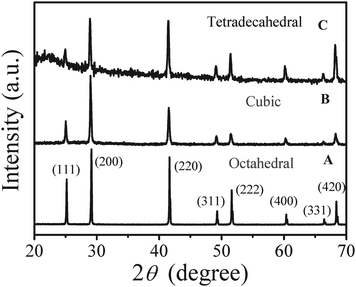 | ||
Fig. 1 XRD patterns of lead selenide with different morphologies. (A) 0.25 mol L−1 of Pb(Ac)2 in 1.0 mL EG + 1.0 mL DEG, RSe/Pb = 0.4, (B) 0.1 mol L−1 of Pb(Ac)2 in 2.0 mL DEG and RSe/Pb = 0.8, (C) 0.1 mol L−1 of Pb(Ac)2 in 2.0 mL DEG and RSe/Pb = 0.5. Concentration of PVP-K30 in 6.0 mL DEG![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) : :![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1.0 mol L−1, reaction temperature: 240 °C, duration time: 13 min. 1.0 mol L−1, reaction temperature: 240 °C, duration time: 13 min. | ||
FESEM results in Fig. 2 reveal that PbSe nanoparticles with the three dominant morphologies could be obtained at various RSe/Pb. The particle sizes of all the products are around 200 nm. Taking advantage of the deceleration mode of FESEM, it is possible to observe the surfaces of the as-prepared nanoparticles directly without interference from the sputtered conductive layer deposited during the FESEM sample preparation. Fig. 3 shows typical pictures of tetradecahedral PbSe nanoparticles. More than 90% of the products could be assigned to tetradecahedral products (Fig. 3A). At higher magnification (Fig. 3B), it can be seen that some smaller nanoparticles (<20 nm) were presented on both the square and hexagonal faces of the PbSe nanostructures. Under transmission electron microscopy (TEM) investigation (Fig. 3C and 3D), both rough surfaces and prominent contrasts between central and fringe of PbSe nanostructures could be observed. Electron diffraction (ED) results (Fig. 3E) show that the nanostrucures have a high crystallinity but the diffraction points are not symmetrically related. All these observations suggest that the as-prepared PbSe nanoparticles are polycrystalline in nature and the possible formation mechanism of these nanostructures could be based on the well known oriented attachment mechanism.12
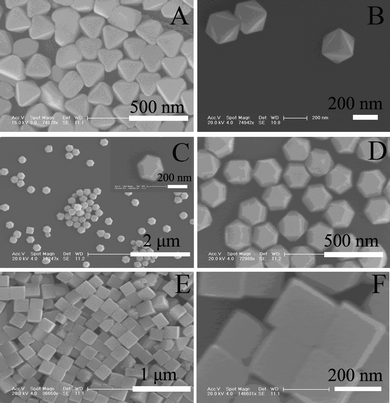 | ||
| Fig. 2 FESEM images of octahedral (A, B), tetradecahedral (C, D) and cubic (E, F) lead selenides. Experimental conditions are same as those shown in Fig. 1. | ||
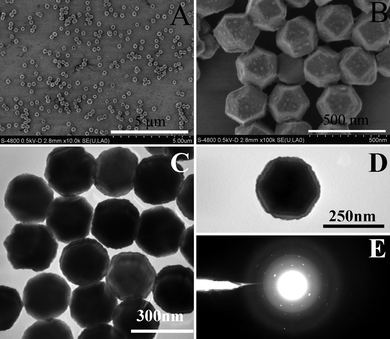 | ||
| Fig. 3 FESEM (A and B), TEM (C and D) images and ED (E) of tetradecahedral lead selenides. The FESEM images were obtained under deceleration mode. Experimental conditions are same as those for tetradecahedral particles shown in Fig. 1. | ||
At 240 °C, both inorganic lead salts and selenius acid could be reduced into elemental Pb and Se by DEG. The feasibility of reducing lead salts in a similar system has been demonstrated for the synthesis of nanocrystallined Pb.13 In our process, the reduction of 1.0 mmol Pb(Ac)2 in 6.0 mL DEG could only be completed when the reaction time is longer than 25 min. Prism-like and rod-like of micrometre size Pb nanoparticles (see Fig. S3A) were yielded under this condition. These Pb particles could be converted into PbSe upon the addition of selenius acid (see Fig. S3B–E). Meanwhile, the reduction of selenius acid could be completed within 20 min. So a plausible PbSe formation mechanism in this system might be the recombination of Pb and Se reduced from their source materials. We also noticed that the reaction time should not be too long to allow the complete reduction of the precursors as the morphologies of final products became less uniform for three typical shapes. With the faster reduction rate of selenius acid in DEG than lead salts, as reflected by their different reduction completion times, a smaller amount of selenius acid than lead salts was needed for preparing PbSe when the duration time (after additions of precursors) was set at 13 min.
It has been reported that star-like nuclei of PbSe could evolve into larger nanocubes or octahedra via oriented attachment of small PbSe nanoparticles either through a dynamic multi-injection process14 or in the presence of acetic acid.15 The advantage of the dynamic multi-injection is that it maintains a constant crystal growth rate while the added acetic acid might replace the organic additive at the Pb sites on the nanocrystals and alter the crystal growth rates of different planes. In our process, the use of a peristaltic pump to drain the chemicals into the reaction flask could offer even better control in terms of stable and continuous additions of the source materials.
The role of poly(vinyl pyrrolidone) (PVP) in preparing colloidal nanostructures has been well documented in the literatures.16 It acts as either a stabilizer or a capping agent to prevent the agglomeration of nanoscale particles. The morphological variations of PbSe nanoparticles (see Fig. S4) using Pb(Ac)2 as lead salt are fairly close to what we observed in the synthesis of PbS.5d Without the presence of PVP-K30, the shapes of the products are irregular and the size distribution is broad (see Fig. S4A). Gradually increasing its concentration to 0.2, 1.0 and 2.0 mol L−1, the products are cubic-like particles with rough surfaces and an average size around 180 nm (see Fig. S4B), uniform tetradecahedral (Fig. 2C and 2D), and mixtures consisting of octahdral, cubic and irregular particles with decreasing particle sizes (see Fig. S4C) accordingly. Therefore the presence of PVP-K30 at an appropriate concentration is essential for the morphological control of PbSe nanostructure in our process.
Pb(NO3)2 was used as a lead source to investigate the effect of inorganic anions on the morphology and particle size of PbSe. As shown in Fig. 4 and Table S2, the morphologies of the as-prepared PbSe nanopaticles are cubic when RSe/Pb is less than 0.5 and cuboid when RSe/Pb reaches 0.8. With the increase of RSe/Pb, the particle sizes of PbSe increased accordingly when the concentration of Pb(NO3)2 was kept at 0.2M. In other words, during the course of increasing RSe/Pb, morphological changes occurred when the acetate anion was used, while both particle sizes and morphological changes occurred when nitrate anion was used. Such a conspicuous difference might be attributed to the different intrinsic chemical natures of these two anions. Although both of them have less steric hindrance than that of organic additives such as PVP, the acetate ion might be a stronger ligand to bind the lead cation. This might imply that Pb(NO3)2 may have a higher degree of dissociation than that of Pb(Ac)2 in the solvents and that lead ions could be reduced more easily in the presence of selenius acid during the synthetic process.
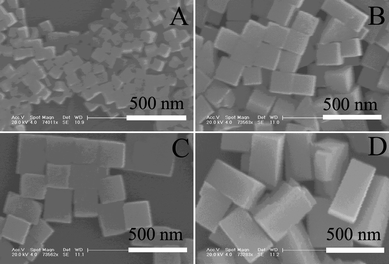 | ||
| Fig. 4 PbSe obtained with different molar ratios of selenius acid to Pb(NO3)2 (Se/Pb): (A) 0.1, (B) 0.2, (C) 0.5, (D) 0.8. Concentration of Pb(NO3)2: 0.2 mol L−1. Concentration of PVP-K30: 2.0 mol L −1, reaction temperature: 240 °C, duration time: 13 min. | ||
The synthesis of mixed morphologies with fixed ratio might be also achieved by controlling the experimental conditions. For example, PbSe nanoparticles with nearly 50% octahedral and 50% tetradecahedral (see Fig. S5) particles could be synthesized when the concentration of PVP-K30 in DEG was 2.0 mol L−1, the concentration of Pb(Ac)2 in a mixed solvent (0.4 mL EG + 1.6 mL DEG) is 0.2 mmol L −1, RSe/Pb is 0.6, the reaction temperature and duration time are 240 °C and 13 min respectively. This observation suggests that the synthesis of symmetric PbSe nanostructures is very sensitive to the deployed kinetic conditions in this system.
In conclusion, PbSe nanostructures with different morphologies and particle sizes could be prepared in DEG at 240 °C via an elementary recombination process in the presence of PVP-K30. The continuous addition of chemicals using a peristaltic pump was beneficial for the control of the morphologies and sizes of the final PbSe nanostructures. Due to the different affinities of inorganic anions toward lead cations, significant morphological variation could be observed through tuning the RSe/Pb when the acetate anion was used, while a size increase was observed with the increase of RSe/Pb when the nitrate anion was used. The experimental results also showed that both the morphology and size of PbSe nanostructures depend on the deployed kinetic conditions in a very susceptible manner in this system.
Acknowledgements
The authors are grateful to financial aid from the National Natural Science Foundation of China (20671087: C.W.,20631040: H.Z.), the MOST of China (Grant No.: 2006CB601103, H.Z.) and the National Basic Research Program of China (2007CB925103, C.W.).References
- (a) C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS; (b) X. Wang, J. Zhuang, Q. Peng and Y. Li, Nature, 2005, 437, 121 CrossRef CAS.
- (a) Y. W. Jun, J. S. Choi and J. Cheon, Angew. Chem., Int. Ed., 2006, 45, 3414 CrossRef CAS; (b) A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310 CrossRef CAS.
- (a) F. W. Wise, Acc. Chem. Res., 2000, 33, 773 CrossRef CAS; (b) A. Tao, P. Sinsermsuksakul and P. Yang, Nat. Nanotechnol., 2007, 2, 435 CrossRef CAS.
- (a) M. J. Siegfried and K. S. Choi, J. Am. Chem. Soc., 2006, 128, 10356 CrossRef CAS; (b) L. Gou and C. J. Murphy, Nano Lett., 2003, 3, 231 CrossRef CAS; (c) Y. Chang and H. C. Zeng, Cryst. Growth Des., 2004, 4, 273 CrossRef CAS; (d) Y. Tan, X. Xue, Q. Peng, H. Zhao, T. Wang and Y. Li, Nano Lett., 2007, 7, 3723 CrossRef CAS.
- (a) N. Zhao and L. Qi, Adv. Mater., 2006, 18, 359 CrossRef CAS; (b) Y. H. Zhang, L. Guo, P. G. Yin, R. Zhang, Q. Zhang and S. H. Yang, Chem.–Eur. J., 2007, 13, 2903 CrossRef CAS; (c) S. M. Lee, Y. W. Jun, S. N. Cho and J. Cheon, J. Am. Chem. Soc., 2002, 124, 11244 CrossRef CAS; (d) Z. Peng, Y. Jiang, Y. Song, C. Wang and H. Zhang, Chem. Mater., 2008, 20, 3153 CrossRef CAS.
- (a) S. Empedocles and M. Bawendi, Acc. Chem. Res., 1999, 32, 389 CrossRef CAS; (b) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2002, 124, 3343 CrossRef CAS.
- (a) G. Vergara, L. J. Gómez, V. Villamayor, M. Àlvarez, M. C. Torquemada, M. T. Rodrigo, M. Verdú, F. J. Sánchez, R. M. Almazán and J. Plaza, Proc. of SPIE, 2007, 6542, 654220 Search PubMed; (b) J. M. Pietryga, R. D. Schaller, D. Werder, M. H. Stewart, V. I. Klimov and J. A. Hollingsworth, J. Am. Chem. Soc., 2004, 126, 11752 CrossRef CAS.
- (a) R. D. Schaller and V. I. Klimov, Phys. Rev. Lett., 2004, 92, 186601–4 CrossRef CAS; (b) V. I. Klimov, Appl. Phys. Lett., 2006, 89, 123118–3 CrossRef.
- T. C. Harman, P. J. Taylor, M. P. Walsh and B. E. LaForge, Science, 2002, 297, 2229 CrossRef CAS.
- (a) S. J. Kim, W. J Kim, Y. Sahoo, A. N. Cartwright and P. N. Prasad, Appl. Phys. Lett., 2008, 92, 031107 CrossRef; (b) A. Lipovskii, Appl. Phys. Lett., 1997, 71, 3406 CrossRef CAS; (c) J. M. Harbold and F. W. Wise, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 125304 CrossRef; (d) J. S. Steckel, S. Coe-Sullivan, V. Bulovic and M. G. Bawendi, Adv. Mater., 2003, 15, 1862 CrossRef CAS.
- (a) W. Shi, Y. Sahoo, H. Zeng, Y. Ding, M. T. Swihart and P. N. Prasad, Adv. Mater., 2006, 18, 1889 CrossRef CAS; (b) W. W. Yu, J. C. Falkner, B. S. Shih and V. L. Colvin, Chem. Mater., 2004, 16, 3318 CrossRef CAS; (c) J. Xu, J. P. Ge and Y. D. Li, J. Phys. Chem. B, 2006, 110, 2497 CrossRef CAS; (d) J. S. Steckel, B. K. H. Yen, D. C. Oertel and M. G. Bawendi, J. Am. Chem. Soc., 2006, 128, 13032 CrossRef CAS; (e) E. Lifshitz, M. Bashouti, V. Kloper, A. Kigel, M. S. Eisen and S. Berger, Nano Lett., 2003, 3, 857 CrossRef CAS.
- (a) K. L. Hull, J. W. Grebinski, T. H. Kosel and M. Kuno, Chem. Mater., 2005, 17, 4416 CrossRef CAS; (b) K. S. Cho, D. V. Talapin, W. Gaschler and C. B. Murray, J. Am. Chem. Soc., 2005, 127, 7140 CrossRef CAS; (c) W. Lu, P. Gao, W. B. Jian, Z. L. Wang and J. Fang, J. Am. Chem. Soc., 2004, 126, 14816 CrossRef CAS; (d) K. T. Yong, Y. Sahoo, K. R. Choudhury, M. T. Swihart, J. R. Minter and P. N. Prasad, Nano Lett., 2006, 6, 709 CrossRef CAS.
- Y. Wang, X. Jiang, T. Herricks and Y. Xia, J. Phys. Chem. B, 2004, 108, 8631 CrossRef CAS.
- (a) W. Lu, J. Fang, Y. Ding and Z. L. Wang, J. Phys. Chem. B, 2005, 109, 19219 CrossRef CAS; (b) C. Qian, F. Kim, L. Ma, F. Tsui, P. Yang and J. Liu, J. Am. Chem. Soc., 2004, 126, 1195 CrossRef CAS.
- A. J. Houtepen, R. Koole, D. Vanmaekelbergh, J. Meeldijk and S. G. Hickey, J. Am. Chem. Soc., 2006, 128, 6792 CrossRef CAS.
- (a) Y. Xiong, I. Washio, J. Chen, H. Cai, Z. Y. Li and Y. Xia, Langmuir, 2006, 22, 8563 CrossRef CAS; (b) W. Z. Wang, B. Poudel, Y. Ma and Z. F. Ren, J. Phys. Chem. B, 2006, 110, 25702 CrossRef; (c) B. Wiley, Y. Sun, B. Mayers and Y. Xia, Chem.–Eur. J., 2005, 11, 454 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure; tables of calculated peak area ratio (200/111) of PbSe nanoparticle from Fig. 1 (Table S1) and Effect of RSe/Pb on the morphology and particle size of PbSe using Pb(NO3)2 as lead precursor (Table S2); XRD patterns of product obtained in EG at 190 °C (Fig. S1); EDX analysis result of cube-like PbSe obtained in DEG (Fig. S2); FESEM images and XRD patterns of Pb particles reduced by DEG and their conversion to PbSe with different amount of selenius acid added (Fig. S3); FESEM images of PbSe obtained with different concentration of PVP-K30 (Fig. S4); FESEM images of PbSe with nearly 50% octahedral and 50% tetradecahedral shapes (Fig. S5). See DOI: 10.1039/b9nr00305c |
| This journal is © The Royal Society of Chemistry 2010 |
