Nanostructured aligned CNT platforms enhance the controlled release of a neurotrophic protein from polypyrrole
Brianna C.
Thompson
,
Jun
Chen
,
Simon E.
Moulton
and
Gordon G.
Wallace
*
ARC Centre of Excellence for Electromaterials Science, Intelligent Polymer Research Institute, University of Wollongong, Wollongong, NSW 2522, Australia. E-mail: gordon_wallace@uow.edu.au; Fax: + 61 (2) 4221 3114; Tel: + 61 (2) 4221 3127
First published on 27th January 2010
Abstract
An aligned CNT array membrane electrode has been used as a nanostructured supporting platform for polypyrrole (PPy) films, exhibiting significant improvement in the controlled release of neurotrophin. In terms of linearity of release, stimulated to unstimulated control of NT-3 release and increased mass and % release of incorporated NT-3, the nanostructured material performed more favourably than the flat PPy film.
The inductive electrically-controlled release of several biologically significant ions from polypyrrole (glutamate,1 ATP2 and dexamethasone3) has previously been described. Incorporation and release of larger biomolecules such as proteins is more difficult to achieve. However, successful strategies have recently been reported.4 The release of the neurotrophic protein neurotrophin-3 (NT-3) from polypyrrole (PPy)5 was subsequently shown to enhance the survival of auditory nerves in vitro,6 and has potential applications in enhancing neural interfacing with conducting materials.
The ability of a material to release therapeutic molecules in a controlled manner provides the basis for a drug-delivery device. Many of the commonly used biodegradable materials [e.g. poly(lactic acid) and poly(lactide-co-glycolide)] are suitable for delivery of drugs over defined periods in vivo. However, they do not provide the ability to initiate and modify drug delivery on command.7 In order to achieve externally controllable release, it is desirable for a material to exhibit good on–off control and a linear release profile. Release of therapeutics from PPy films should provide these characteristics by using electrical stimulation to control the rate of release. As the process driving drug delivery is electrochemical, nanostructuring of the material should improve the release characteristics of PPy materials. For example, nanostructuring of the PPy surface is expected to increase the surface area exposed to the solution, therefore increasing the available area for release. Additionally, nanostructured conducting polymers have been shown to have higher conductivity and faster switching speeds,8 both of which are important in achieving good controllability in the controlled release profile.
Aligned carbon nanotube (ACNT) substrates have been used as the nanostructured platform for PPy growth,9 materials for lithium ion battery applications10 and electrocatalysis.11 It is expected that the high surface area offered by the ACNT substrates could be used to provide low impedance organic electrodes for interfacing with biological systems, and particularly for integration of electronics with the nervous system. The ability to release nerve growth factors, such as neurotrophin-3 (NT-3), could potentially enhance the integration of the electrode with the nervous system. We present here studies demonstrating the controlled release of NT-3 from PPy films deposited on ACNT substrates, with significantly enhanced performance compared to previously published work.
Aligned multi-walled carbon nanotubes were produced on a quartz plate by pyrolysis of iron(II) phthalocyanine as described by Dai.12 A thin PPy film was then deposited onto the ACNT arrays by a vapour-phase polymerisation method using ferric p-toluenesulfonate (Fe(III) pTS) as the oxidant. Fe(III) pTS was coated onto the ACNT arrays via spin-coating and the resultant Fe(III) pTS-modified ACNT arrays were then exposed to pyrrole monomer vapour for 15 min to deposit PPy at room temperature. After PPy film deposition, a thin Pt film (∼120 nm) was sputter-coated onto the ACNT/PPy arrays for 10 min at 30 mA to improve the electronic connection among individual ACNTs. A poly(vinylidene fluoride) (PVDF) layer was then cast onto the sample, providing sufficient mechanical support to enable the structure to be peeled off from the quartz plate. Finally, a wash step was performed to shrink the expanded PPy structure and entrap the protein, as described in our previous studies.13 The ACNT/PPy/Pt-PVDF membrane electrode was washed in a sufficient volume of 125I-labelled NT-3 ethanol solution and left to dry overnight, and then soaked in Milli-Q water for 5 h to remove surface-adsorbed NT-3. As “flat film” controls, NT-3-incorporated PPy films were synthesised on cleaned Au-coated mylar sheets under identical vapour-phase polymerisation conditions to those described above.
The configuration of the composite material was characterised by scanning electron microscopy (SEM) (Fig. 1). The SEM images of ACNT/PPy-NT-3/Pt-PVDF show an array structure, with the individual aligned multi-walled carbon nanotubes being coated by a PPy layer (approximately 75 nm diameter), and supported by an underlying Pt/PVDF composite layer. The PPy layer provides the matrix for incorporation and release of NT-3, while the Pt and PVDF layers respectively provide the conductive link between the ACNTs and sufficient mechanical strength for handling. The nanostructured composite array produced was robust with no obvious loss of the aligned carbon nanotube layer structure observed throughout the handling required for experimental procedures.
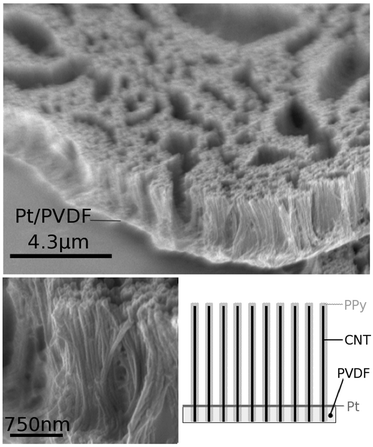 | ||
| Fig. 1 SEM images and schematic of composite ACNT/PPy/Pt/PVDF material with NT-3 incorporated into the PPy layer. | ||
The average mass of NT-3 incorporated into composite ACNT/PPy-NT-3/Pt-PVDF structures was found to be 90 ± 20 ng cm−2 (geometric surface area). Release studies used sample areas of 1 cm2 and were performed in 1 mL 0.9% NaCl solution to mimic the ionic concentration of biological fluids. This 1 mL solution was removed and replaced with fresh 0.9% NaCl at various intervals for 7 days. Electrically stimulated samples received 100 μs biphasic current pulses at 250 Hz and at a current density of ± 1 mA cm−2 to mimic the electrical stimulation optimised for neural stimulation by the cochlear implant.5 The mass of NT-3 in the polymer and all saline samples was determined using a gamma-counter to detect the 125I label on the neurotrophin.4
The electrically stimulated and unstimulated (passive) release of NT-3 from the ACNT/PPy-NT-3/Pt-PVDF electrode was compared to analogous release from the control flat PPy film and shown in Fig. 2. A summary of the rates of release, the R2 values for the linear regression analysis of the data (to provide a measure of linearity, with 1.0 being perfectly linear), and the stimulated:unstimulated release ratios are provided in Table 1. The stimulated flat film showed a small increase (1.9-fold) in the mass of NT-3 released compared with the unstimulated film (as indicated by the ratio of the stimulated to unstimulated NT-3 release). However, the major effect of stimulation occurred in the first hour of release. This high initial rate of release is undesirable for a controlled release material, where a more linear release over the entire period of release is required.
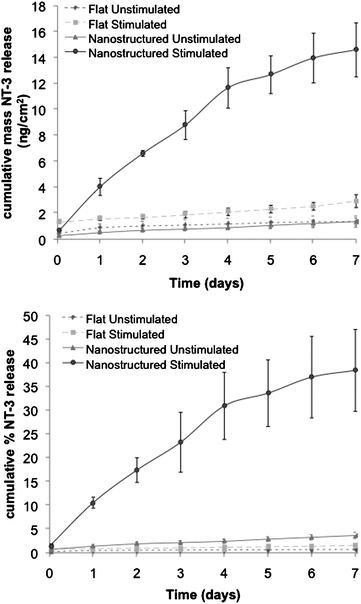 | ||
| Fig. 2 Release of NT-3 from flat and nanostructured PPy films over 7 days. (a) shows mass of NT-3 released, while (b) shows % of total NT-3 incorporated released from each PPy film. Each point represents the mean ± s.e. measured across 3 samples. | ||
| Unstimulated | Stimulated | Ratio stim:unstim release | |||
|---|---|---|---|---|---|
| Rate of NT-3 release/ng cm−2 day−1 (%/day) | R 2 | Rate NT-3 release/ng cm−2 day−1 (%/day) | R 2 | ||
| Flat film | 0.11 (0.05%) | 0.92 | 0.21 (0.11%) | 0.99 | 1.9 |
| Nanostructured film | 0.15 (0.39%) | 0.99 | 2.00 (5.3%) | 0.98 | 13.8 |
The release of NT-3 from the nanostructured ACNT/PPy-NT-3/Pt-PVDF electrode was significantly enhanced (14-fold) by electrical stimulation over 7 days (Fig. 2). Both stimulated and unstimulated release were found to be quite linear over the 7 day release period (R2 = 0.98 for stimulated; 14.5 ng cm−2 or 38% release, and 0.99 for unstimulated; 1.5 ng cm−2 or 3.5% release). Compared with flat films, the nanostructured ACNT/PPy-NT-3/Pt-PVDF electrode shows significant improvement in terms of burst release of NT-3, which is reflected in the fact that only 4.6% of the total NT-3 released over the 7 day period was released in the first hour, compared to 45% for flat PPy films for stimulated electrodes.
The mass and percentage of NT-3 release were also enhanced significantly using the nanostructured ACNT/PPy-NT-3/Pt-PVDF electrode, when compared to results obtained using flat PPy films (Table 1). The percentage of incorporated NT-3 that was released increased to 38% over 7 days (5.3% of incorporated NT-3 per day) with electrical stimulation, compared to 0.39% per day for unstimulated release. Additionally, the ratio of stimulated:unstimulated release was improved to 13.8-fold compared to 1.9-fold for the flat PPy film, indicating better performance as a controlled-release material. The mass of electrically stimulated NT-3 release from the nanostructured electrode (2.0 ng cm−2 day−1) was nearly 10 times higher than that (0.21 ng cm−2 day−1) from the flat film, while the unstimulated release was only 1.4 times greater (0.15 and 0.11 ng cm−2 day−1, respectively).
Overall, the nanostructured ACNT/PPy film showed a great improvement in the properties desirable for controlled release compared to the flat PPy film. In terms of linearity of release, stimulated to unstimulated control of NT-3 release and increased mass and % release of incorporated NT-3, the nanostructured material performed more favourably than the flat PPy film. This is likely to be due to the better performance of the ACNT array electrode due to the greatly enhanced effective surface area (more than 10 times higher than the geometric area) exposed to the electrolyte. Additional improvements may arise from the more efficient electrical connection to the polypyrrole provided by the more highly conducting carbon nanotubes,14 and investigation into de-coupling these effects is currently underway. As observed during the stimulated release, the individual aligned CNT could act as a nano-electrode to stimulate the NT-3 release from the PPy film deposited on the CNT. Nanostructuring of polypyrrole films has therefore been shown to be an effective method for improving the controlled release characteristics of the conducting polymer for electrically stimulated drug release.
Conclusions
Nanostructure is found to improve the controlled release characteristics of PPy for the release of NT-3. This improvement, combined with the higher surface area that can be achieved with nanostructured organic electrodes, suggests that better electrical connections with the nervous system could be achieved with such a composite material. The improvement of release characteristics with nanostructure has implications for the electrochemically-driven release of other drugs and therapeutic molecules from PPy.Acknowledgements
The authors wish to acknowledge the continued financial support of the Australian Research Council.References
- B. Zinger and L. L. Miller, J. Am. Chem. Soc., 1984, 106, 6861 CrossRef CAS
.
- Y. J. Li and S. J. Dong, J. Chem. Soc., Chem. Commun., 1992, 827 RSC
.
- R. Wadhwa, C. F. Lagenaur and X. T. Cui, J. Controlled Release, 2006, 110, 531 CrossRef CAS
.
- J. N. Barisci, A. J. Hodgson, L. Liu, G. G. Wallace and G. Harper, React. Funct. Polym., 1999, 39, 269 CrossRef CAS
.
- B. C. Thompson, S. E. Moulton, J. Ding, R. Richardson, A. Cameron, S. O'Leary, G. G. Wallace and G. M. Clark, J. Controlled Release, 2006, 116, 285 CrossRef CAS
.
- R. T. Richardson, B. C. Thompson, S. E. Moulton, A. Cameron, G. G. Wallace, R. Kapsa, G. M. Clark and S. O'Leary, Biomaterials, 2007, 28, 513 CrossRef CAS
.
- W. M. Saltzman and W. L. Olbricht, Nat. Rev. Drug Discovery, 2002, 1, 177 CrossRef CAS
.
- G. G. Wallace and P. C. Innis, J. Nanosci. Nanotechnol., 2002, 2, 441 CrossRef CAS
.
- M. Gao, L. M. Dai and G. G. Wallace, Electroanalysis, 2003, 15, 1089 CrossRef CAS
.
- J. Chen, Y. Liu, A. I. Minett, C. Lynam, J. Wang and G. G. Wallace, Chem. Mater., 2007, 19, 3595 CrossRef CAS
.
- Y. Liu, J. Chen, W. M. Zhang, Z. Ma, G. F. Sweigers, C. O. Too and G. G. Wallace, Chem. Mater., 2008, 20, 2603 CrossRef CAS
.
- Y. Yang, S. Huang, H. He, A. W. H. Mau and L. Dai, J. Am. Chem. Soc., 1999, 121, 10832 CrossRef CAS
; L. Dai, A. Patil, X. Gong, Z. Guo, L. Liu, Y. Liu and D. Zhu, ChemPhysChem, 2003, 4, 1150 CrossRef CAS
.
- B. Winther-Jensen, J. Chen, K. West and G. Wallace, Polymer, 2005, 46, 4664
.
- M. Gao, L. Dai and G. G. Wallace, Synth. Met., 2003, 137, 1393 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2010 |
