Mixed metal nanoparticle assembly and the effect on surface-enhanced Raman scattering†
Fiona
McKenzie
,
Karen
Faulds
and
Duncan
Graham
*
Centre for Molecular Nanometrology, Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL, UK. E-mail: Duncan.Graham@strath.ac.uk
First published on 5th November 2009
Abstract
Here we report the assembly of mixed metal nanoparticles using an oligonucleotide-templated approach. Substitution of one of the gold nanoparticle probes with an analogous silver probe to produce a hetero-metal duplex permitted surface-enhanced Raman scattering of a dye label, exploiting the improved surface enhancement properties of silver nanoparticles whilst maintaining the surface chemistry benefits of gold nanoparticles.
Surface-enhanced Raman scattering (SERS) is a phenomenon which has been investigated by a wide variety of techniques and employs the use of a range of surfaces to provide the surface enhancement component of the technique. One of the most favoured surfaces is that of metallic nanoparticles due to their ease of synthesis and handling in a variety of different experimental formats. Individual nanoparticles will give rise to significant SERS signals when an appropriate SERS-active molecule is immobilised on their surface. Larger signals can be obtained from these molecules by aggregating the nanoparticles into discrete clusters to improve the electromagnetic enhancement of the Raman tags and SERS can thus be used to follow cluster formation.1,2 Aggregation can be achieved by a number of non-specific methods involving alteration of surface charges, and ultimately leads to precipitation of the nanoparticles.3–5 An alternative method to provide greater control over the aggregation and assembly state of the nanoparticles is to use a DNA-templated approach. This has previously been used to assemble nanoparticles in a number of different studies6–9 but only recently to investigate the enhancement of the SERS effect.10,11 The main advantage of employing SERS for signal transduction of the assembly process is the capability to identify multiple assembly events simultaneously. The narrow and molecularly specific emission features of SERS spectra make SERS significantly more favourable than other optical spectroscopies such as extinction or fluorescence.
To date, most of these studies have involved single metal nanoparticle assemblies, however, in order to probe the effectiveness of different types of metallic nanoparticles we have devised an approach to allow comparisons between the most commonly used metal nanoparticles, namely gold and silver. This approach has also allowed us to demonstrate the phenomenon of using a nanoparticle purely as an enhancing species rather than containing a molecular probe of interest. Here we report the assembly of mixed metal nanoparticle conjugates induced by hybridisation of oligonucleotidenanoparticle conjugates to the complementary target sequence in a split-probe assembly (Fig. 1).
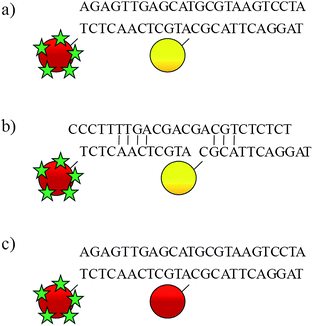 | ||
| Fig. 1 Head-to-tail assembly of oligonucleotide probes: a) mixed metal nanoparticle assembly; b) mixed metal probes in presence of non-complementary DNA; c) gold nanoparticle assembly. The red and yellow spheres represent gold and silver nanoparticles, respectively. The green stars represent malachite green isothiocyanate. | ||
One of the probes is based on a gold nanoparticle which in addition to being functionalised with oligonucleotides is also labelled with the strongly Raman-active species, malachite green isothiocyanate. The second probe incorporates a silver nanoparticle that is only functionalised with oligonucleotides and bears no SERS-active label. It is necessary to ensure that the probe-to-target concentration is in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, however since there are multiple DNAoligonucleotides per nanoparticle, the nanoparticle concentration needs to be adjusted accordingly. Furthermore, the number of oligonucleotides adsorbed onto 35 nm silver nanoparticles is approximately ten times greater than for 13 nm gold nanoparticles due to the increased surface area of silver compared with gold nanoparticles and so the gold nanoparticle concentration will be different to that of the silver nanoparticles. An approximate 1
1 ratio, however since there are multiple DNAoligonucleotides per nanoparticle, the nanoparticle concentration needs to be adjusted accordingly. Furthermore, the number of oligonucleotides adsorbed onto 35 nm silver nanoparticles is approximately ten times greater than for 13 nm gold nanoparticles due to the increased surface area of silver compared with gold nanoparticles and so the gold nanoparticle concentration will be different to that of the silver nanoparticles. An approximate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was achieved by using the gold nanoparticle probes, silver nanoparticle probes and target DNA at 0.5 nM, 50 pM, and 50 nM, respectively.
1 ratio was achieved by using the gold nanoparticle probes, silver nanoparticle probes and target DNA at 0.5 nM, 50 pM, and 50 nM, respectively.
The UV–visible spectra of the mixed metal assembly are illustrated in Fig. 2. Before the addition of the target sequence, the plasmon resonance peak of the silver nanoparticles can be clearly identified at 410 nm exhibiting a shoulder at 520 nm which corresponds to the gold nanoparticles. Although the silver nanoparticle probes are used at a lower concentration, their larger extinction coefficient makes the silver peak the most dominant. Addition of the target and subsequent hybridisation to the probes resulted in aggregation of the nanoparticles. This gives rise to a red-shift and broadening of the plasmon resonances due to the change in dielectric experienced by each nanoparticle.12
 | ||
| Fig. 2 Extinction spectra of mixed metal probes before (—) and after (- - -) the addition of the complementary target sequence. | ||
Upon aggregation, the electromagnetic fields surrounding each nanoparticle couple to produce ‘hot spots’. These are regions of high electromagnetic field intensity and are responsible for the large surface enhancement effects of the Raman signals.13 Furthermore, the plasmon resonance wavelength of the nanoparticles is tuned to that of the laser excitation, promoting plasmon excitation and improved surface enhancement effects. Since the addition of the DNA target results in nanoparticle aggregation, it can be used to generate increased SERS intensities. This is illustrated in Fig. 3. The SERS intensity of the mixed metal assembly is more than a factor of 3 greater than the monodisperse nanoparticles in the absence of the target sequence. Oligonucleotide-functionalised gold nanoparticles have been shown to yield optimum SERS spectra at longer excitation wavelengths due to the red-shifted plasmon resonance wavelength compared with silver. It has been suggested that electronic transitions associated with the bulk metal which occur at shorter wavelengths than the plasmon excitation peak are responsible for the poor behaviour of gold nanoparticle-based analyses at 514.5 nm.14 As such, the mixed metal assembly was analysed at 632.8 nm and the SERS label was chosen so that it was in resonance with this excitation wavelength.
 | ||
| Fig. 3 SERS spectra before (—) and after (- - -) the addition of a) the complementary and b) a non-complementary target sequence to the mixed metal probes using a 632.8 nm excitation wavelength. | ||
To demonstrate that the observed increase in SERS intensity is the result of specific Watson–Crick hydrogen bonding, a non-complementary target was added to the nanoparticle probes. Only a slight increase was observed which is likely to be the result of a few base-pairing interactions, of which the most energetically favourable are shown in Fig. 1b.
Silver nanoparticles are known to yield significantly greater surface enhancement of Raman signals than gold nanoparticles,14 although it is advantageous to utilise gold nanoparticles due to their more established surface chemistry which often exploits the high affinity thiols have for gold surfaces. Gold nanoparticles are renowned for having improved stability compared with silver and so are often preferred for use in solution-based experiments. We have found that surface modification of silver nanoparticles for labelling purposes often resulted in irreversible aggregation as a result of the hydrophobic nature of many commercially available fluorophores. A mixed metal assembly profits from the greater stability of gold nanoparticles and the exceptional enhancement effects provided by the silver nanoparticles. The requirement for using silver is emphasised in Fig. 4 which presents the spectra when the silver nanoparticle probe is substituted so that the assembly consists of two gold nanoparticle probes. Although a colour change from red to blue was observed and there was a significant shift in the plasmon resonance, the increase in SERS intensity was negligible. This indicates that the plasmon resonance coupling is not as effective as for the gold–silver scenario, or indeed a silver–silver scenario.10 This is a highly significant result as it proves that silver has a larger enhancing capacity than gold and provides evidence that in terms of electromagnetic enhancement of SERS, silver is the optimal metal to use.
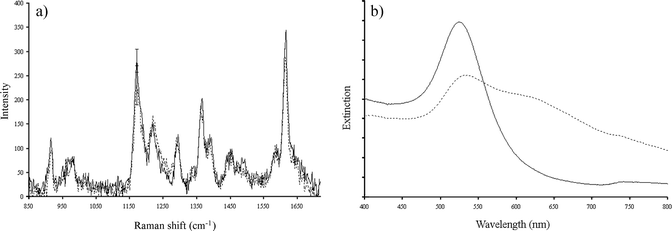 | ||
| Fig. 4 (a) SERS spectrum and (b) UV–visible spectrum of the gold nanoparticle assembly before (—) and after (- - -) the addition of the target DNA. The SERS spectra were recorded using a 632.8 nm excitation wavelength. | ||
In conclusion, we have shown that we can study the effect of different types of metal nanoparticles on an assembly-based enhanced SERS effect using oligonucleotide templating. Silver nanoparticles provide greater enhancement compared to gold nanoparticles and this has also allowed us to demonstrate that only one species needs to be modified to contain the Raman-active tag rather than all species as in previous studies. This is a significant step forward in the understanding of assembly processes of nanoparticles in relation to surface-enhanced Raman scattering and offers significant opportunities for exploitation in terms of using this data to better control and understand nanoparticle assemblies and advanced optical spectroscopies .
References
- I. Khan, D. Cunningham, R. E. Littleford, D. Graham, W. E. Smith and D. W. McComb, Anal. Chem., 2006, 78, 224–230 CrossRef CAS.
- K. Faulds, R. E. Littleford, D. Graham, G. Dent and W. E. Smith, Anal. Chem., 2004, 76, 592–598 CrossRef CAS.
- J. C. Jones, C. McLaughlin, D. Littlejohn, D. A. Sadler, D. Graham and W. E. Smith, Anal. Chem., 1999, 71, 596–601 CrossRef CAS.
- C. H. Munro, W. E. Smith, M. Garner, J. Clarkson and P. C. White, Langmuir, 1995, 11, 3712–3720 CrossRef CAS.
- D. Cunningham, R. E. Littleford, W. E. Smith, P. J. Lundahl, I. Khan, D. W. McComb, D. Graham and N. Laforest, Faraday Discuss., 2006, 132, 135–145 RSC.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS.
- A. P. Alivisatos, K. P. Johnsson, X. G. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez and P. G. Schultz, Nature, 1996, 382, 609–611 CrossRef CAS.
- C. J. Loweth, W. B. Caldwell, X. H. Peng and A. P. Alivisatos, Angew. Chem., Int. Ed., 1999, 38, 1808–1812 CrossRef CAS.
- R. C. Jin, G. S. Wu, Z. Li, C. A. Mirkin and G. C. Schatz, J. Am. Chem. Soc., 2003, 125, 1643–1654 CrossRef CAS.
- D. Graham, D. G. Thompson, W. E. Smith and K. Faulds, Nat. Nanotechnol., 2008, 3, 548–551 CrossRef CAS.
- X. Qian, X. Zhou and S. Nie, J. Am. Chem. Soc., 2008, 130, 14934–14935 CrossRef.
- D. G. Thompson, R. J. Stokes, R. W. Martin, P. J. Lundahl, K. Faulds and D. Graham, Small, 2008, 4, 1054–1057 CrossRef CAS.
- J. M. McMahon, A.-I. Henry, K. L. Wustholz, M. J. Natan, R. G. Freeman, R. P. Van Duyne and G. C. Schatz, Anal. Bioanal. Chem., 2009, 394, 1819–1825 CrossRef CAS.
- R. J. Stokes, A. Macaskill, P. J. Lundahl, W. E. Smith, K. Faulds and D. Graham, Small, 2007, 3, 1593–1601 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b9nr00211a |
| This journal is © The Royal Society of Chemistry 2010 |
