Small organic molecule templating synthesis of organic–inorganic hybrid materials: their nanostructures and properties
Hong-Bin
Yao
,
Min-Rui
Gao
and
Shu-Hong
Yu
*
Division of Nanomaterials and Chemistry, Hefei National Laboratory for Physical Sciences at Microscale, Department of Chemistry, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: shyu@ustc.edu.cn; Fax: +0086 551 3603040
First published on 1st December 2009
Abstract
The design and synthesis of organic–inorganic hybrid materials has developed over the last two decades as chemists and materials engineers have maintained their attention on these materials. In the synthetic process of organic–inorganic hybrid materials, the organic components usually act as templates for directing the connectivity and arrangement of inorganic building blocks. Specifically, according to the size and scale of inorganic building blocks, these organic–inorganic hybrid materials can be divided into molecular scale and nanoscale organic–inorganic hybrid materials. In this review, we highlight the recent advances in using small organic molecules as the templates for the synthesis of organic–inorganic hybrid nanomaterials with interesting properties. The synthetic techniques, hybrid crystal structures, templating roles, crystal growth mechanism, control of sizes and morphologies, and novel properties of organic–inorganic hybrid materials will be discussed.
 Hong-Bin Yao | Hong-Bin Yao received his BSc degree in chemistry from University of Science and Technology of China in 2006. He started his PhD studies on the project of organic–inorganic semiconductor hybrid materials under the supervision of Prof. Shu-Hong Yu in 2006. During 2007–2008, he attended the joint-training program for studying crystal structure analysis in Prof. Jing Li's group in Rutgers University. He is currently interested in organic–inorganic semiconductor hybrid materials, and lightweight, ultrastrong, stiff, and tough organic–inorganic nanocomposite materials. |
 Min-Rui Gao | Min-Rui Gao received his BSc degree in chemistry from Hefei University in 2006. He started his PhD studies under the supervision of Prof. Shu-Hong Yu in 2008, at the University of Science and Technology of China. His research interests are focused on the synthesis of mesostructured inorganic–organic hybrid nanocrystals and their potential applications. |
 Shu-Hong Yu | Shu-Hong Yu received his BSc degree from Hefei University of Technology, and his PhD from the University of Science and Technology of China (USTC). He then joined Prof. Masahiro Yoshimura's Lab, at the Tokyo Institute of Technology, as a post-doctoral Fellow. After that, he was as an Alexander von Humboldt Research Fellow at the Max Planck Institute of Colloids and Interfaces, Germany, working with Prof. Dr Markus Antonietti and Prof. Dr Habil Helmut Cölfen. He joined the Department of Chemistry USTC as a full professor in 2002, and was appointed the Cheung Kong Professorship in 2006 by the Chinese Ministry of Education. He is now leading the Division of Nanomaterials and Chemistry at the Hefei National Laboratory for Physical Sciences at the Microscale, USTC. His research focuses on bio-inspired synthesis and self-assembly of new nanostructured materials and nanocomposites, carbon-related materials, and their related properties. He has authored and co-authored more than 188 refereed journal publications, and nine invited book chapters. He serves as an associate editor for the international journal Materials Research Bulletin, and is a board member of the journals Crystal Engineering Communications, Nano Research, and Current Nanoscience. |
1. Introduction
Organic–inorganic hybrid materials have emerged as a class of novel materials over the last two decades, as they combine functional organic components and inorganic building blocks into unique materials through various chemical or physical interactions. These organic–inorganic hybrid materials are not the simply sum of two components but their synergic combination. The functionalities of hybrid material can be tuned or tailored from the atomic or molecular scale to the nanoscale which greatly expands the applications of organic–inorganic hybrid materials through incorporation of different functional organic and inorganic components. In fact, the applications of organic–inorganic hybrid materials penetrate nearly every scientific field e.g. optics, electronics, membranes, protective coatings, catalysts, sensors, barriers, and biology.1–7In the design and synthesis of organic–inorganic hybrid materials, the organic templates play a key role which mainly determines the morphologies, structures and properties of the hybrid materials. For example, different surfactants or copolymers used in the synthesis of mesostructured hybrid materials result in different meso-architectures, pore sizes and other parameters;8–10 different organic ligands used in the synthesis of zeolites and metal–organic frameworks yield different connectivity styles and crystalline structures.11–13 In other words, the choice and use of the organic templates are essential parts in the design and synthesis of organic–inorganic hybrid materials.
Recently, in addition to the numerous traditional organic templates used in the synthesis of organic–inorganic hybrid materials, many small organic molecules with amine or alcohol functional groups have been used in the synthesis of novel organic–inorganic hybrid materials. These small organic molecules act both as solvents and structure-directing agents in the design and synthesis of hybrid materials.14 Moreover, because of their simple structures, high efficiency, and low cost, these small organic molecules display more advantages than traditional organic templates.
Here, we define small organic molecules as those molecules in which the number of carbon atoms is equal to or fewer than 10, in order to distinguish them from conventional long-chain alkanesurfactants, polymers, and macromolecules. These small organic molecules usually are liquid phases hence they could act as solvents and structure-directing agents during the synthetic process, including many amine and alcohol molecules such as hydrazine, methylamine, ethylamine, propylamine, butylamine, ethylenediamine, diethylenetriamine, triethylenetetraamine, benzyl alcohol and so on. Some of these organic molecules have been intercalated between the inorganic molecular sheets forming unique organic–inorganic single crystalline structures; the others could stabilize the inorganic nano-building blocks and connect them as nanoscale or mesostructured organic–inorganic hybrid materials.
In this review, organic–inorganic hybrid materials prepared by the use of small organic molecules will be divided into molecular scale organic–inorganic hybrid materials and nanoscale organic–inorganic hybrid materials according to the size and scale of the inorganic building blocks. Furthermore, based on the inorganic components, these organic–inorganic hybrid materials will be further categorized as organic–metal chalcogenide and organic–metal oxide hybrid materials. We will focus on the synthetic techniques, hybrid crystal structures, templating roles, crystal growth mechanism, and morphology control in small organic molecule templating synthetic processes of organic–inorganic hybrid materials. The optical, electronic, and magnetic properties, of these novel hybrid materials will also be discussed. Finally, the conclusions and outlooks will be given.
2. Molecular scale organoamine–inorganic hybrid materials
Molecular scale organic–inorganic hybrid materials are namely a class of hybrid materials that consists of organic molecules hybridized with metal ions or inorganic frameworks to form the hybrid framework compounds, including both metal–organic coordination polymers and systems that contain extended inorganic connectivity. This kind of hybrid material contains numerous compounds, from the view of crystalline structures, their diversity extending from 1D chains to 3D frameworks; from the view of constituents, the diversity of the second building units and organic ligands determines their variety. About the structural diversity and chemical trends in hybrid organic–inorganic framework materials, Rao et al. has given a very comprehensive review.15 There have also been several excellent reviews about metal–organic frameworks by Férey,16–22 and Yaghi23–27et al. As an aspect of this review, we only focus on several small amine molecules as the structure-directing agents in the synthesis of small organoamine–inorganic hybrid framework materials.2.1. Hydrazine-metal chalcogenide hybrid materials
Among these small organic amine molecules, hydrazine is the simplest and has the highest solubility and reducing capability to chalcogenide elements. In 2004, based on the low-temperature decomposition of highly soluble hydrazinium precursors, Mitzi et al. reported a technique for spin-coating ultrathin (50 Å), crystalline and continuous metal chalcogenide films.28 Actually the hydrazinum chalcogenide precursors are the hydrazine-based metal chalcogenide hybrid materials. More concretely, the synthesis, structures, and thermal properties of the soluble hydrazinium germanium(IV) and tin(IV) selenide salts were investigated by Mitzi.29The template role of hydrazine in the synthetic process of hydrazine-based metal chacolgenide hybrid materials could be illustrated from 1D chains to 3D frameworks. By using an ambient-temperature hydrazinesolvent approach for dissolving ZnTe, neutral hydrazine directly coordinates to the ZnTe framework and forms extended inorganic chains that interact with each other through van der Waals and hydrogen-bonding interactions resulting in one-dimensional (1D) ZnTe polymorphs α-and β-(N2H4)2ZnTe.30Fig. 1 shows the structures of α- and β-(N2H4)2ZnTe chains viewed along different directions, illustrating the structure-directing role of the hydrazine coordinated to the ZnTe chains. 2D infinite layered connectivity of metal chalcogenides also could be synthesized in the hydrazinesolvent. Li's group have reported the layered monohydrazine compound (N2H4)ZnTe prepared by reacting Zn(NO3)2·6H2O, tellurium, and hydrazine under solvothermal conditions (110 °C).31Hydrazine acts as the structure-directing agent to guide the growth of the 2D ZnTe slices. Moreover, novel 2D extended Cu7S4− sheets could be constructed under the template and stabilization role of a mixture of hydrazinium and hydrazine molecules.32 The structure of N4H9Cu7S4 consists of well-defined Cu7S4− sheets separated by hydrazinium cations and hydrazine molecules (Fig. 2).The extended two-dimensional (2D) inorganic anion of N4H9Cu7S4 is distinct from the three-dimensional (3D) frameworks found in the binary copper sulfides because of the templating role of the hydrazine molecules. Another excellent example illustrating the solvating, ligating, and reducing abilities of hydrazine at room temperature is a series of structures of hydrazine metal chalcogenide hybrids spanning from 0D discrete molecules (SnSe4Mn2(N2H4)10), to 1D chains (SnS4Mn2(N2H4)6), 2D layers (SnSe4Mn2(N2H4)7), to 3D networks (SnS4Mn2(N2H4)5) (Fig. 3) reported by Yuan and co-workers.33 The coordination and connectivity styles of hydrazine could be tuned by the changing the concentration of hydrazine in the reaction systems. Lately, Kanatzidis's group have reported a new hydrazine–metal chalcogenide hybrid framework material, Mn2SnS4(N2H4)2, in which the hydrazine acted as an integral part of the framework as opposed to being located in the pores.34 This framework further expand the structural diversity of the hydrazine–metal chalcogenide hybrid materials.
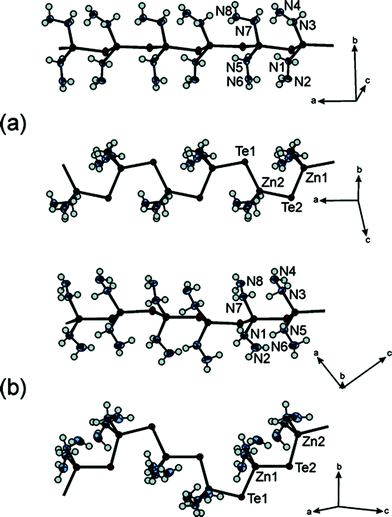 | ||
| Fig. 1 Detailed structure (two views) of the (N2H4)2ZnTe chains in (a) α-(N2H4)2ZnTe and (b) β-(N2H4)2ZnTe, with atom labeling shown. The thermal ellipsoids for Zn, Te, and N atoms are drawn at 50% probability. For clarity, hydrogen atoms are represented as spheres. Reproduced with permission from ref. 30. Copyright 2005 American Chemical Society. | ||
![Detailed structure of N4H9Cu7S4 viewed down [100], with the unit cell shown (---). For clarity, atoms are represented as spheres, with uniform sizes selected for each atom type. Reproduced with permission from ref. 32. Copyright 2007 American Chemical Society.](/image/article/2010/NR/b9nr00192a/b9nr00192a-f2.gif) | ||
| Fig. 2 Detailed structure of N4H9Cu7S4 viewed down [100], with the unit cell shown (---). For clarity, atoms are represented as spheres, with uniform sizes selected for each atom type. Reproduced with permission from ref. 32. Copyright 2007 American Chemical Society. | ||
 | ||
| Fig. 3 Crystal structures of compounds SnSe4Mn2(N2H4)10(1), SnS4Mn2(N2H4)6(2), SnSe4Mn2(N2H4)7(3), and SnS4Mn2(N2H4)5(4) showing how the metal chalcogenide building units are organized into zero, one, two, and three dimensions by the hydrazine linkers. All hydrogen atoms are omitted for clarity. Reprinted with permission from ref. 33. Copyright 2007 American Chemical Society. | ||
2.2. Organic mono/diamine–metal chalcogenide hybrid materials
Organic mono- or diamine molecules also can be used as solvents and structure-directing agents for constructing amine–metal chalcongenide hybrid materials under mild solvothermal reaction conditions. For example, the ethylenediamine (en) is an excellent solvent for the solvothermal synthesis of metal chalcogenides. Its relatively low critical pressure makes it possible to perform many reactions under mild conditions (e.g.T< 180 °C). Many inorganic species have reasonable solubility in en, such as alkali–metal chalcogenides (e.g. A2Q, A–K, Rb, Cs; Q–S, Se, Te) and metal chlorides, which are common reagents used in the solvothermal synthesis of metal chalcogenides. A comprehensive review on en–metal chalcogenide hybrid materials has been given by Li and co-workers.35 Herein, we only introduce two recently developed typical classes of mono/diamine–metal chalcogenide hybrid materials, i.e.diamine–IB –VA–VIA hybrid materials and mono/diamine–II –VI hybrid materials.Li's group firstly reported the diamine–IB–VA–VIA semiconductor hybrid materials, Cu2SbSe3·0.5en and Cu2SbSe3·en, which were prepared by soft solvothermal reactions of CuCl, SbCl3, and Se with A2Se (A = Na, K) in the presence of ethylenediamine.36 A mixed-valent copper–antimony sulfide, [H2NCH2CH2NH2]0.5[Cu2SbS3], has been solvothermally synthesized from binary metal sulfides in the presence of ethylenediamine by Chippindale et al.37 In both hybrid materials, the valence of copper were assumed to be a mixed valence of +1 and +2, this was confirmed by magnetic susceptibility measurements. In other cases, the novel copper(I)-thioantimonates(III) (enH2+2)0.5Cu2SbS3, (1,3-DAPH2+2)0.5Cu2SbS3, (1,3-DAP = 1,3 diaminopropane) and (1,4-DABH22+)0.5Cu2SbS3 (1,4-DAB = 1,4-diaminobutane) were synthesized under solvothermal conditions by the reaction of Sb2S3, CuCl2·2H2O, and S with the diamines.38 The monovalence of copper(I) in these hybrid materials was also demonstrated by magnetic measurement. However, no matter what valence of copper existed in the hybrid materials, the structures of these hybrid materials are similar sandwiched structures where the diamine molecules were intercalated between the layered Cu2SbS/Se3− clusters. Fig. 4 illustrates the crystal structure of [H2NCH2CH2NH2]0.5[Cu2SbS3]. In particular, these layered Cu2SbS/Se3− clusters also displayed similar structures. All this evidence indicates that the diamine molecules play a similar structure-directing role in the synthesis of diamine–copper–thio/selenoantimonates hybrid materials.
![View along the [101] direction showing the location of neutral ethylenediamine template molecules between Cu2SbS3 slabs. Key: copper, large black circles; antimony, large shaded circles; sulfur, large open circles; carbon, small black circles; nitrogen, small open circles. Hydrogen atoms of the template are omitted for clarity. Reprinted with permission from ref. 37. Copyright 2000 Royal Society of Chemistry.](/image/article/2010/NR/b9nr00192a/b9nr00192a-f4.gif) | ||
| Fig. 4 View along the [101] direction showing the location of neutral ethylenediamine template molecules between Cu2SbS3 slabs. Key: copper, large black circles; antimony, large shaded circles; sulfur, large open circles; carbon, small black circles; nitrogen, small open circles. Hydrogen atoms of the template are omitted for clarity. Reprinted with permission from ref. 37. Copyright 2000 Royal Society of Chemistry. | ||
By substituting silver for copper in similar solvothermal reactions, Powell et al. synthesized the first diamine-templated silver thioantimonates, [C2H9N2][Ag2SbS3] and [C2H9N2]2[Ag5Sb3S8].39 Both materials consist of complex silver thioantimonate layers, separated by ethylenediamine molecules. The structure of [C2H9N2][Ag2SbS3] is closely related to those of the copper–antimony chalcogenides. However, [C2H9N2]2[Ag5Sb3S8] provides an entirely new structure type related to Li3Bi, in which simultaneous occupation of half of the tetrahedral and all of the octahedral interstitial sites between pairs of close-packed anion layers is seen for the first time. This example illustrates the templating role of organodiamine in the synthesis of hybrid materials as well.
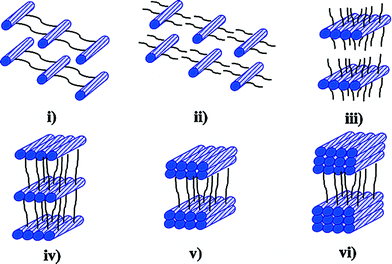 | ||
| Fig. 5 The designed possible structural models of mono- or diamine–II –VI semiconductor hybrid materials. (i) MQ as 1D chains, interconnected via organic spacers; (ii) MQ as isolated 1D chains, coordinated to, but not interconnected by, organic groups; (iii) MQ as isolated monatomic layers; (iv) MQ as monatomic layers, interconnected; (v) MQ as bilayers, interconnected; and (vi) MQ as trilayers, interconnected. Reprinted with permission from ref. 31. Copyright 2003 American Chemical Society. | ||
In 2003, Li et al. reported a number of 1D, 2D, and 3D hybrid structures synthesized in various mono/diaminesolvents such as [ZnTe(pda)] (pda = propanediamine), a 1D crystal structure containing single ZnTe chains (Type ii); [ZnTe(ma)] (ma = Methylamine), 2D crystal structures containing monatomic ZnTe slabs (Type iii); and [CdSe(en)0.5](en = ethylenediamine) and [CdSe(pda)0.5], and 3D crystals structures containing CdSe monolayers (Type iv).31 These organic mono/diamine–II –VI hybrid materials were produced by solvothermal reactions using metal ions, chalcogenide elements, and mono/diaminesolvents. The mono/diamine molecules directed the growth of II–VIinorganic building blocks and linked them forming organic–inorganic hybrid materials. The diversity of the organic mono/diamines expanded the variety of these organamine–II–VI hybrid materials.
In their next work on organic amine–II –VI hybrid materials, Li et al. expanded the single slab (MQ) of II–VI to the bilayer (M2Q2) of II–V (from type ii to type v) by slightly modifying the reaction conditions and using monoamine as the structure directing agents and spacers.41
The systematic work and detailed investigation of the mono/diamine–II –VI semiconductor hybrid materials demonstrated the structure-directing role (templating role) of the organic amines in the design and synthesis of organic–inorganic hybrid materials. By just conveniently adopting the metal salts and chalcongenide elementary substances in solvothermal reactions with mono/diamine as solvents, various structures of small organic amine–II –VI semiconductor hybrid materials can be obtained, from 1D to 2D and to 3D hybrid structures, from monolayers of II–VI slabs to bilayers of II–VI slabs. Moreover, no matter what the dimensionalities and layers of II–VI slabs, the hybrid materials have, they all show similar uniform and periodic crystal structures.
2.3. Organoamine–metal oxide hybrid materials
Small organoamine molecules are also excellent structure-directing agents and spacers in the synthesis of organoamine–metal oxide hybrid materials. A comprehensive and detailed review about the synthesis and crystal structures of organodiamine–molybdenum oxide hybrid materials has been given by Zubieta et al.42Here, we exemplify organoamine–tungsten oxide hybrid materials to illustrate the small organoamine molecule templating synthesis of organic–inorganic hybrid materials. A series of organoamine–tungsten oxide hybrid materials were obtained by solvothermal reactions of Na2WO4·2H2O, MCl2·nH2O (M = Cu, Zn), organoamines, and deionized water under mild conditions (140–160 °C).43 The crystal structures of the organoamine–tungsten oxide hybrid materials are shown in Fig. 6. The structure topologies of [WO3(pyz)0.5] (pyz = pyrazine) and [WO3(bpy)0.5] (bpy = 4,4′-bipyridyl) are similar. They both consist of corner-sharing [WO5N] octahedra parallel to the ab-plane linked through pyz/bpy molecules into a 3D pyz/bpy-tungsten oxide hybrid framework. In contrast to the three-dimensional covalent/coordination hybrid frameworks of [WO3(pyz)0.5] and [WO3(bpy)0.5], the structure of [W3O10(enH2)] consists of [W3O10]22− layers, separated by interlamellar enH2+2 cations. The success in synthesizing amine–tungsten oxide hybrid materials provides innovative examples of the utility of the template-mediated hydrothermal method for the construction of versatile tungsten oxide hybrid solids. It further demonstrates the multifunctional roles of organoamines as ligands bound to the tungsten oxide skeleton, as in the compounds [WO3(pyz)0.5] and [WO3(bpy)0.5], and as a simple counter-ion in the compound [W3O10(enH2)], in the cooperative assembly of organic/tungsten oxide hybrid materials.
![Crystal structure of [WO3(pyz)0.5] viewed along the b-axis. (b) Crystal structure of [WO3(bpy)0.5] viewed along the [110] direction. (c) A polyhedral and ball-and-stick view of [W3O10(enH2)]. Reprinted with permission from ref. 43. Copyright 2000 Royal Society of Chemistry.](/image/article/2010/NR/b9nr00192a/b9nr00192a-f6.gif) | ||
| Fig. 6 Crystal structure of [WO3(pyz)0.5] viewed along the b-axis. (b) Crystal structure of [WO3(bpy)0.5] viewed along the [110] direction. (c) A polyhedral and ball-and-stick view of [W3O10(enH2)]. Reprinted with permission from ref. 43. Copyright 2000 Royal Society of Chemistry. | ||
2.4. Control of crystal growth and morphologies of molecular scale small organic amine–inorganic hybrid nanomaterials
As discussed in the above section, small organic amine molecules can efficiently intercalate into inorganic frameworks to form novel organic–inorganic hybrid materials. These novel hybrid materials have totally new crystal structures, in which the amine molecules are linked with inorganic slabs at atomic/molecular scale.On the other hand, with the extensive demand for micro/nanoscale devices and the development of nanoscience, the controlled growth of crystals at the micro/nanoscale has attracted much attention.44–52 In particular, one-dimensional nanomaterials have been intensively investigated because they provide ideal systems to study the dependence of transport properties on size confinement and their electronic, magnetic, optical properties on the nanoscale.53–59
Hence, it is important to control the crystal growth and morphologies of these molecular-scale organic amine–inorganic hybrid materials to fabricate novel one-dimensional organic–inorganic hybrid nanomaterials. One aspect is that totally new hybrid crystal structures offer new systems to study the crystal growth; the other aspect is that organic–inorganic hybrid nanomaterials could display novel properties and be applied in nanodevices.
The control of crystal growth and morphologies of organic amine–inorganic hybrid materials actually depends on the templating role of the small organic amine molecules. In early work, solvothermal reactions of organoamines, metal salts, and chalcogenides were used for the synthesis of metal-chalcogenide nanocrystals.60–62 Then, some organic amine–inorganic hybrid materials were found as the metastable phases during the synthetic processes of nanocrystalline metal chalcogenides and the phase transformation from hybrid to pure inorganic was investigated, which also demonstrated that the hybrid materials are excellent precursors for the preparation of pure nanocrystalline metal chalcogenides.63–65
In recent years, our group has developed a mixed-solvent system, in which uniform 1D nanobelts, nanowires, and nanofibers of small organic amine–inorganic hybrid materials can be synthesized.66–68 The mixed-solvent medium plays a key role on the control of crystal growth and the nanomorphologies of these hybrid materials. In the synthesis of uniform and well defined [ZnSe](DETA)0.5 (DETA = diethylenetriamin) nanobelts, we realized for the first time that carefully tuning the compositions of ternary solvents made of DETA, hydrazine hydrate, and deionized water allows us to control the morphology.66 A suitable volume ratio of solvents (VN2H4·H2O/VDETA/VH2O = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16) is essential for the formation of elegant and uniform [ZnSe](DETA)0.5 nanobelts (Fig. 7).
16) is essential for the formation of elegant and uniform [ZnSe](DETA)0.5 nanobelts (Fig. 7).
0.5 nanobelts prepared in a mixed solution with a volume ratio of VN2H4·H2O/VDETA/VH2O = 5 : 14 : 16. (b) Schematic illustration of the [ZnSe](DETA)0.5 structure. Reprinted with permission from ref. 66. Copyright 2005 Wiley-VCH.](/image/article/2010/NR/b9nr00192a/b9nr00192a-f7.gif) | ||
Fig. 7 (a) TEM image of [ZnSe](DETA)0.5 nanobelts prepared in a mixed solution with a volume ratio of VN2H4·H2O/VDETA/VH2O = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16. (b) Schematic illustration of the [ZnSe](DETA)0.5 structure. Reprinted with permission from ref. 66. Copyright 2005 Wiley-VCH. 16. (b) Schematic illustration of the [ZnSe](DETA)0.5 structure. Reprinted with permission from ref. 66. Copyright 2005 Wiley-VCH. | ||
Later, we extended this mixed-solvent system to synthesize small organic amine–ferrosulfide hybrid nanomaterials. [Fe18S25](TETAH)14 (TETA = triethylenetetramine) nanoribbons with a width of 100–250 nm, a thickness of 10–30 nm, and a length up to 10 μm (Fig. 8a) were synthesized by a mixed-solvent strategy using FeSO4·7H2O and C2H5NS as the reactants.67 We also observed that the volume ratio of TETA to deionized water had an important effect on the morphologies of the product. A possible crystal structure model of [Fe18S25](TETAH)14 was proposed, in which the TETAH was assumed to intercalate between the Fe18S254− layers (Fig. 8b). In addition, well-defined [Fe18S25](TETAH)14nanoribbons were demonstrated to be excellent precursor to Fe7S8nanowires and porous Fe2O3nanorods as shown in Fig. 8c and d.
14. (b) Proposed structure of [Fe18S25](TETAH)14. The yellow balls are sulfur; dark purple balls, Fe; blue balls, N; gray balls, C; white balls, H. (c) SEM image of Fe7S8nanowires and (d) SEM image of porous Fe2O3nanorods. Reproduced with permission from ref. 67. Copyright 2008 American Chemical Society.](/image/article/2010/NR/b9nr00192a/b9nr00192a-f8.gif) | ||
| Fig. 8 (a) SEM image of [Fe18S25](TETAH)14. (b) Proposed structure of [Fe18S25](TETAH)14. The yellow balls are sulfur; dark purple balls, Fe; blue balls, N; gray balls, C; white balls, H. (c) SEM image of Fe7S8nanowires and (d) SEM image of porous Fe2O3nanorods. Reproduced with permission from ref. 67. Copyright 2008 American Chemical Society. | ||
The mixed-solvent system can be extended to reflux reactions at ambient conditions, and a new kind of blue-light-emitting ultralong Cd(L)(TeO3) (L = ethylenediamine, diethylenetriamine) nanofibre bundles were prepared by refluxing.68 These uniform nanobundles with lengths ranging from several to a few hundred micrometres consist of many uniform nanofibers with a diameter of about 50 nm and seem very flexible (Fig. 9).
 | ||
| Fig. 9 SEM images of Cd(L)(TeO3) nanofibre bundles. (a,c) SEM images of Cd(en)(TeO3). (b,d) SEM images of Cd(DETA)(TeO3). Reproduced with permission from ref. 68. Copyright 2009 Wiley-VCH. | ||
As the above evidence shows, mixed-solvent media offer a very efficient one-pot synthesis of 1D nanostructured molecular scale organic amine–inorganic hybrid nanomaterials. The key point is how these elegant and uniform 1D nanostructured hybrid materials form in the mixed solvents. We propose that the organoamine, organoammonium, and hydroxyl groups contained in this mixed system displayed bi-functionalities that direct the hybrid crystal structure growth and control the 1D morphologies. The crystal-structure-directing role has been illustrated above. Here, a possible mechanism has been proposed to show how the mixed solvents control the morphologies of these hybrid materials as illustrated in Fig. 10. At the first stage, inorganic slabs were formed under the structure-directing role of the organoamine. These inorganic slabs were linked by the organoamine forming the initial crystal nucleus. The organoammonium or hydroxyl groups were assumed to be adsorbed on the side surfaces which impeded the further growth of inorganic slabs. With more and more inorganic slabs aligned together through organoamine linkages, the 1D nanostructured hybrid materials grew along the direction perpendicular to the inorganic slabs.
 | ||
| Fig. 10 Illustration of the growth mechanism of 1D nanostructured molecular scale hybrid materials in mixed-solvent media. | ||
3. Nanoscale organic–inorganic hybrid materials
Small organic molecules with functional groups have been demonstrated as useful and efficient surfactants to stabilize nanocrystals, such as thiol groups for the capping of II–VI semiconductor nanocrystals,69–72 alcohol groups for synthesizing metal oxidenanocrystals73–75 and so on. Herein, we prefer to show that the small organic molecules act not only as capping agents for stabilizing nanoscale inorganic building blocks, but also as 1D nanostructure-directing agents for the alignment of inorganic building blocks under some reaction conditions. We define the nanoscale organic–inorganic hybrid materials as a kind of material that consists of nanoscale inorganic building blocks and organic molecules. For example nanosheets of clays, natural nanoscale building blocks, have been combined with polymers to form nanoscale organic–inorganic hybrid materials with high performance mechanical properties and other novel properties.76–81The main difference between molecular scale organic–inorganic hybrid materials and nanoscale organic–inorganic hybrid materials is the size of the inorganic building blocks.3.1. Nanoscale organic amine–metal chalcogenide hybrid materials
The traditional method for preparing organic–metal chalcogenide hybrid materials is through the non-aqueous surfactant-templated assembly of chalcogenide cluster precursors first developed by Ozin et al.82 This method was further extended and used to fabricate more organic–metal chalcogenide hybrid materials by Kanatzidis et al.83–85Here, we introduce the small organoamine solvothermal reactions as a convenient one-pot synthetic method for the fabrication of nanoscale organic amine–metal chalcogenide hybrid materials. The highly aligned mesostructured amine–ZnS nanowire hybrid materials were fabricated by a mild solution reaction with the use of different amines such as n-butylamine, ethylamine, and tetraethylenepentamine.86Fig. 11A shows the small-angle X-ray diffraction pattern of the as-prepared products obtained in n-butylamine at different temperatures. From room temperature to 160 °C, obvious diffraction peaks located at low angles which can be indexed as (001), (002), (003) reflections were observed, revealing the formation of nanostructures. With increasing reaction temperature from 80 °C to 160 °C, the diffraction peaks become stronger and the d-spacing of the (001) diffractions linearly increased with temperature as shown in Fig. 11B. The nanostructured bundles consist of parallel ultrathin ZnS nanowires (Fig. 11C). These nanowires with well-defined lattice structures are packed along the c-axis and are linked by a layer of amine molecules (Fig. 11D).
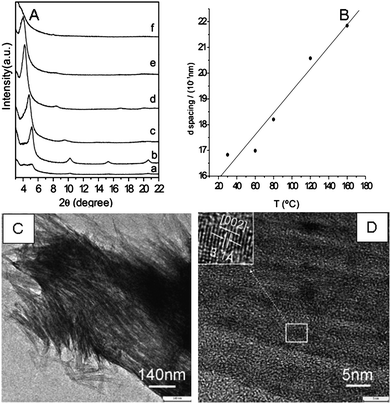 | ||
| Fig. 11 (A) Small-angle X-ray diffraction patterns of mesostructured n-butylamine–ZnS nanowire hybrid materials at different temperatures. (a) 30 °C, Na2S ·9H2O, 12 h. (b) 60 °C, Na2S ·9H2O, 12 h. c) 80 °C, CS(NH2)2, 12 h. d) 120 °C, CS(NH2)2, 12 h. e) 160 °C, CS(NH2)2, 12 h. f) 180 °C, CS(NH2)2, 48 h. (B) Plot of the d-spacing versus temperature (30–160 °C) in the n-butylamine system. (C) A typical TEM image of a nanowire array. (D) Lattice-resolved HRTEM image showing clear (002) lattice fringes. Reproduced with permission from ref. 86. Copyright 2007 Wiley-VCH. | ||
Another example showing the template role of small organic amines for the synthesis of nanoscale organic amine–metal chalcogenide hybrid materials has been recently reported.87 A typical amine–CoSe2 hybrid nanobelt was synthesized by solvothermal reaction of Co(Ac)2 ·H2O and Na2SeO3 in a mixed solution with a volume ratio of Vamine/VDIW = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [amine = diethylenetriamine (DETA), triethylenetetramine (TETA), or tetraethylenepentamine (TEPA)] at 180 °C for 16 h. The PXRD patterns of these obtained hybrid nanobelts are shown in Fig. 12A, in which the peaks of 00l′ can be indexed as (001) and (002) with lattice spacings of 1.10 and 0.55 nm respectively, and the wide-angle reflections can be readily indexed to a pure primitive cubic phase of CoSe2. The SEM and TEM images (Fig. 12B and C) show that these nanobelts with widths of 100–500 nm and lengths up to several tens of micrometres are flexible, smooth, thin, and almost transparent. Interestingly, the lateral view of the as-obtained nanobelts along the thickness direction clearly shows well-defined multilayer (Fig. 12D and E).
1 [amine = diethylenetriamine (DETA), triethylenetetramine (TETA), or tetraethylenepentamine (TEPA)] at 180 °C for 16 h. The PXRD patterns of these obtained hybrid nanobelts are shown in Fig. 12A, in which the peaks of 00l′ can be indexed as (001) and (002) with lattice spacings of 1.10 and 0.55 nm respectively, and the wide-angle reflections can be readily indexed to a pure primitive cubic phase of CoSe2. The SEM and TEM images (Fig. 12B and C) show that these nanobelts with widths of 100–500 nm and lengths up to several tens of micrometres are flexible, smooth, thin, and almost transparent. Interestingly, the lateral view of the as-obtained nanobelts along the thickness direction clearly shows well-defined multilayer (Fig. 12D and E).
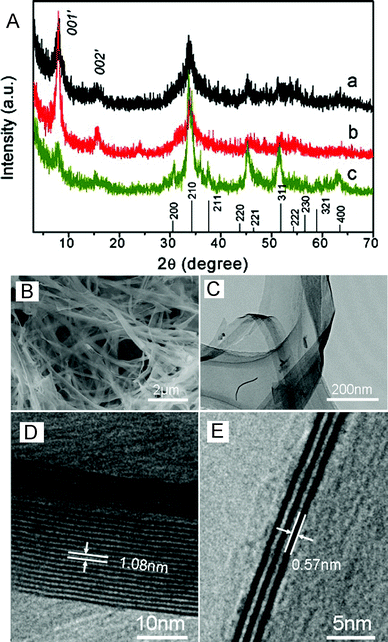 | ||
| Fig. 12 (A) PXRD patterns of (a) DETA–CoSe2 nanobelts, (b) TEPA–CoSe2 nanobelts, and (c) TETA–CoSe2 nanobelts. (B, C) SEM and TEM images of lamellar mesostructured DETA–CoSe2 nanobelts, respectively. (D, E) Typical HRTEM images viewed along the lateral thickness direction. Reproduced with permission from ref. 87. Copyright 2009 American Chemical Society. | ||
The formation mechanism of the above unique nanobelts has been proposed87 as illustrated in Fig. 13. First, some amine molecules are protonated by reaction with water under solvothermal conditions at 180 °C and form positively charged ammonium ions. Then, the protonated amine molecules are incorporated into neighboring CoSe2 layers by coordination with Se. The amine with a linear configuration acts as a template molecule, which not only leads to the new mesostructured nanobelts but also induces anisotropic growth of 1D nanostructures. Furthermore, the interlamellar distances remains constant although other amines with different chain lengths were used, because the protonated amine molecules are incorporated into the inorganic framework by taking a horizontal configuration from the viewpoint of energy minimization.87
 | ||
| Fig. 13 The proposed formation mechanism of the DETA–CoSe2 lamellar nanobelts. Red, white, blue, gray, pink, and yellow balls correspond to O, H, N, C, Co, and Se atoms, respectively. Hydrogen atoms are omitted for clarity in the structure. Reproduced with permission from ref. 87. Copyright 2009 American Chemical Society. | ||
3.2. Nanoscale organic amine/alcohol–metal oxide hybrid materials
Nanoscale organic amine/alcohol–metal oxide hybrid materials could also be obtained by taking advantage of the role of small organic molecules in connecting or expanding nanoscale inorganic building blocks. Tang et al. have fabricated en-GeOx hybrid nanowires with a periodic arrangement of sub-nanoscale GeOx building blocks via an Fe2O3-assisted liquid-phase hydrothermal method.88 The as-obtained hybrid materials possess a uniform, wirelike morphology with a diameter of 50–120 nm and a length of several tens of micrometres [Fig. 14(a)]. The transmission electron microscopyTEM image in Fig. 14(b) shows a clearly visible set of periodic black/white strips occurring across the whole nanowire; the period was ∼1.10 nm wide. The en molecules act as both nanostructure directing agents and linkers between GeOx building blocks [Fig. 14(c)]. | ||
| Fig. 14 (a) SEM image of en-GeOx hybrid nanowires. (b) TEM image of en-GeOx hybrid nanowires. (c) Schematic representation of the structure of organic–inorganic hybrid en-GeOxnanowires. Reproduced with permission from ref. 88. Copyright 2008 Wiley-VCH. | ||
Similarly nanostructured n-alkylamine–tungsten oxide hybrid materials were obtained through the reaction of H2W2O7·xH2O (x = 3.49) with n-alkylamines in a system of heptane/n-alkylamine/H2W2O7·xH2O under ambient conditions, reported by Sugahara et al.89
It has been demonstrated that the “benzyl alcohol route” plays a crucial role, giving access to various oxide-based hybrid nanostructures including rare earth oxide hybrid materials,90–92tungsten oxide-based hybrid materials,93 and titanium dioxide-based hybrid materials.94 This so called “benzyl alcohol route” has been comprehensively reviewed by Antonietti, Niederberger and Pinna et al.74,75,95–98 Here, synthesis of tungsten oxide-based hybrid materials has been chosen as an example to illustrate the templating role of benzyl alcohol and its derivatives in forming the semiconducting organic–inorganic hybrid materials. In pure anhydrous benzyl alcoholsolvent, only inorganic nanocrystallites were obtained.73,93 When a small quantity of a specific ligand, deferoxamine mesylate or 4-tert-butylcatechol, was introduced into this reaction system, the supermolecular arrangement of tungsten oxide nanobuilding blocks stabilized by benzaldehyde molecules was achieved.74,93 As shown in Fig. 15(a), the 1D supernanostrucutred tungsten oxide based hybrid materials are consisted of independent inorganic nanoplatate building blocks via π–π interactions between benzaldehyde molecules adsorbed at their surfaces [Fig. 15(b)]. The parallel alignment of the nanowires with respect to each other in the as-synthesized product indicates the presence of an ordered, supramolecular arrangement of the intercalated organic molecules. The thickness of the organic layer, that is, the distance between the surfaces of two nanowires, is about 0.9 nm.
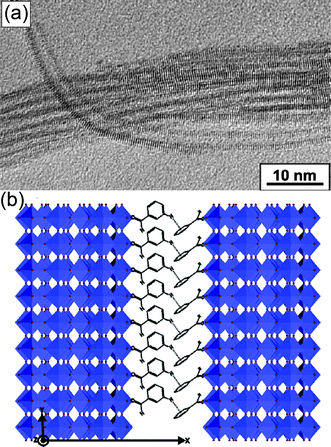 | ||
| Fig. 15 (a) HRTEM image of linear and curved tungsten oxide-based hybrid nanowires. (b) The proposed model for the tungsen oxide based-nanohybrid structure. Reproduced with permission from ref. 93. Copyright 2005 American Chemical Society. | ||
4. Properties of organic–inorganic hybrid materials
With the synergetic combination of organic molecules and inorganic parts, some novel optical, electrical, magnetic, etc. properties have been realized in organic–inorganic hybrid materials.28,29,39–41,99–110 Here, these novel properties will be summarized and discussed separately to illustrate how the organic molecules tune and influence the properties. The potential functionalities of these organic–inorganic hybrid materials will also be prospected.4.1. Optical properties of organic–inorganic hybrid materials
The organic–inorganic [MQ(L)1/0.5](M = Mn, Zn, Cd; Q = S, Se, Te; L = mono/diamine) hybrid structures, possessing uniform and periodic crystal structures, exhibit a very large blue shift in their optical adsorption edge due to a strong quantum confinement effect (QCE) induced by the internal sub-nanostructures.40,103,104,107 Interestingly, the bandgap of these organic–II–VIinorganic hybrid materials could not only be tuned by organic molecules but also by the thickness of the inorganic layer.41 The band-edge shifts in the double-layer 2D-[(M2Q2)(L)] structures are significantly smaller than those of the corresponding single-layer 2D-[(MQ)(L)] compounds because the extent of QCE is less in the former than in the latter. As shown in Fig. 16, the strongest confinement is achieved in the single-layer (n = 1) structures such as 2D-[(MQ)(L)]. As the thickness of inorganic layer decreases, the extent of QCE increase, as does the blue-shift in their band-edge absorption.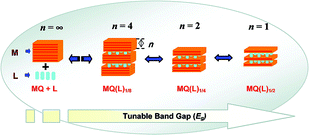 | ||
| Fig. 16 Illustration of how the variation in thickness of the inorganic layer (shown in orange), can lead to systematic tuning of the bandgap. Reproduced with permission from ref. 41. Copyright 2007 American Chemical Society. | ||
The novel luminescence of Mn-substituted organic amine–inorganicII–VI semiconductor hybrid materials has been extensively studied. Qian et al. have reported a series of Zn1−xMnxSe(L)0.5 and Cd1−xMnxSe(L)0.5 (L = diamine) materials and illustrated their luminescence at 2.12 eV (584 nm) which can be assigned to a Mn2+ internal transition (4T1 → 6A1) and its excitation peak overlaps with the photoemission peak of the 2D exciton ground state which indicates that the Mn2+ emission is driven by the 2D exciton ground-state transition.111,112 The influence of chain length of L on the luminescence intensity has also been investigated; longer L chain lengths lead to higher luminescence intensities. The temperature- and pressure-dependent luminescence properties of Mn-substituted [Zn1−xMnxSe](DETA)0.5 (x = 0–0.3) inorganic–organic hybrid nanobelts have been studied very recently.113 In an interesting application, Mn-substituted 2D-[(M2Q2)(L)] (L = monoamine) frameworks were used as semiconducting bulk materials that emit direct white light (Fig. 17).110
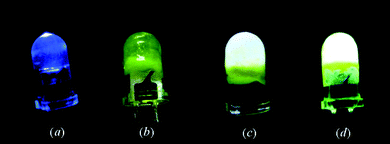 | ||
| Fig. 17 (a) A 5 mm reference UV LED (360 nm) illuminating blue light. (b) The same LED coated with a thin layer of sample before illumination, and (c) after illumination. (d) The same LED illuminating a coated thin layer of Mn-doped sample (0.1 mol%). Reproduced with permission from ref. 110. Copyright 2008 American Chemical Society. | ||
Moreover, the influence of organic molecules on the luminescence of a europium-doped yttria-based inorganic–organic nanohybrid have been studied, and it was found that red-shift of the luminescence peak is caused by the benzyl rings of the organic layer and subsequent energy transfer from organic molecules to the europium centers.90 Interestingly, tungsten oxide-based organic–inorganic hybrid microflowers have been used as a novel visible-light-driven photochromic material with high-reversibility recently.114
4.2. Electronic properties of organic–inorganic hybrid materials
Because of the low electrical conductivities of organic amines organic amine–inorganic hybrid materials are usually insulators under ambient conditions. There has been no study on the experimental measurement of the electronic properties of hybrid materials. Much research work has been focused on the theoretical calculation of the electronic properties of hybrid organic–inorganic semiconductors that are desirable for photonic applications.104,105,107However, organic–inorganic hybrid materials have been demonstrated to be efficient precursors for the fabrication of pure inorganic semiconducting thin-film field-effect transistors, which exhibit n-type transport, large current densities (>105 A cm−2) and mobilities greater than 10 cm2V−1s−1 —an order of magnitude higher than the previously reported values for spin-coated semiconductors.28 Low-voltage semiconducting transistors have also been fabricated by a similar technique.100
4.3. Magnetic properties of organic–inorganic hybrid materials
Organic–inorganic hybrid framework materials offer broad platform for studying molecular magnets. There have been many research works about molecular magnets such as single-chain magnets,115,116 2D spin-half antiferromagnets,117 and so on. Mn-substitued organic amine–inorganicII–VI semiconductor hybrid framework materials have also been demonstrated to be dilute magnetic semiconductors.102,111,112 Moreover, the magnetic properties of 1D [Fe18S25](TETAH)14nanoribbons have been studied, and found to be different from those of pure inorganic iron sulfide.675. Conclusions and outlook
In conclusion, the latest advances on the templating synthesis of molecular-scale or nanoscale organic–inorganic hybrid materials and their nanostructures and properties have been overviewed. The small molecules can act as both efficient solvents for the synthesis of chalcogenides and structure-directing agents for the crystal growth of molecular organic–inorganic hybrids. The syntheses and crystal structures of hydrazine–metal chalcogenids hybrid materials, diamine–IB –VA–VIA hybrid materials, mono/diamine–II –VI hybrid materials, and organoamine–metal oxide hybrid materials were exemplified to illustrate the templating roles of small organic molecules in directing the growth of inorganic slabs. Furthermore, mixed-solvent media has been introduced to control the crystal growth and shapes of molecular-scale organic–inorganic hybrid materials. Interestingly, the present advances in this field have demonstrated that small organic molecules can act not only as capping agents for stabilizing nanoscaled inorganic building blocks but also as 1D nanostructured directing agents for the alignment of inorganic building blocks. The novel optical, electronic and magnetic properties of organic–inorganic hybrid materials have been summarized and discussed. The potential functionalities and applications of these hybrid materials have been considered as luminescent materials, transistors, and molecular magnets.Although small organic molecule template synthesis offers a novel approach for the design and synthesis of organic–inorganic hybrid materials, exact control over the crystal structures and nanostructures of these hybrid materials is still hard to achieve. More and more such hybrid materials are expected to be synthesized and it is also necessary for more detailed formation mechanisms to be investigated in the future.
Beside the synthesis of organic–inorganic hybrid materials, their properties and potential functionalities also need more attention. These hybrid materials have been used as very efficient precursors for preparing nanostructured inorganic semiconductors and ultra-thin-film transistors. More nanodevices could be fabricated by using these novel nanostructured hybrid materials as building blocks and then transferring to inorganic semiconductor devices such as optoelectronic devices, transistors, and so on. As for the novel luminescence of Mn-substituted organic–inorganic hybrid materials, they could be potentially used to fabricate LEDs based on these hybrid materials.
Moreover, new nanocomposites based on these novel hybrid materials and other nanomaterials such as QDs, or gold or silver nanoparticles will be very interesting. It will be possible to modify or attach nanoparticles on the hybrid materials because of the amino groups on their surfaces. The properties of the nanocomposites could be tuned by the hybrid materials and the attached nanoparticles, and these nanocomposites would bring in new multi-functionalities.
Acknowledgements
S.-H. Yu acknowledges funding support from the National Basic Research Program of China (2010CB934700), the Natural Science Foundation of China (Grants Nos. 50732006, 20671085), and the Partner-Group of the Chinese Academy of Sciences-the Max Planck Society.References
- C. Sanchez, B. Julian, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559 RSC.
- L. L. Welbes and A. S. Borovik, Acc. Chem. Res., 2005, 38, 765 CrossRef CAS.
- J. Allouche, M. Boissiere, C. Helary, J. Livage and T. Coradin, J. Mater. Chem., 2006, 16, 3120 RSC.
- G. Andrade, E. F. Barbosa-Stancioli, A. A. P. Mansur, W. L. Vasconcelos and H. S. Mansur, Biomed. Mater., 2006, 1, 221 Search PubMed.
- A. Arvinte, A. M. Sesay, V. Virtanen and C. Bala, Electroanalysis, 2008, 20, 2355 CrossRef CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS.
- D. W. Hatchett and M. Josowicz, Chem. Rev., 2008, 108, 746 CrossRef CAS.
- G. J. d. A. A. Soler-Illia, C. Sanchez, B. Lebeau and J. Patarin, Chem. Rev., 2002, 102, 4093 CrossRef.
- Y. Wan and Zhao, Chem. Rev., 2007, 107, 2821 CrossRef CAS.
- H. Zou, S. S. Wu and J. Shen, Chem. Rev., 2008, 108, 3893 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour and I. Margiolaki, Angew. Chem., Int. Ed., 2004, 43, 6296 CrossRef CAS.
- N. W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2005, 38, 176 CrossRef CAS.
- W.-T. Yao and S.-H. Yu, Adv. Funct. Mater., 2008, 18, 3357 CrossRef.
- A. K. Cheetham, C. N. R. Rao and R. K. Feller, Chem. Commun., 2006, 4780 RSC.
- C. Livage, C. Egger, M. Nogues and G. Férey, J. Mater. Chem., 1998, 8, 2743 RSC.
- D. Riou and G. Férey, J. Mater. Chem., 1998, 8, 2733 RSC.
- F. Serpaggi and G. Férey, J. Mater. Chem., 1998, 8, 2737 RSC.
- F. Serpaggi and G. Férey, J. Mater. Chem., 1998, 8, 2749 RSC.
- G. Férey, J. Solid State Chem., 2000, 152, 37 CrossRef CAS.
- F. Millange, C. Serre, J. Marrot, N. Gardant, F. Pelle and G. Férey, J. Mater. Chem., 2004, 14, 642 RSC.
- G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef CAS.
- O. M. Yaghi, H. L. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS.
- M. O'Keeffe, M. Eddaoudi, H. L. Li, T. Reineke and O. M. Yaghi, J. Solid State Chem., 2000, 152, 3 CrossRef CAS.
- M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS.
- O. Delgado-Friedrichs, M. D. Foster, M. O'Keeffe, D. M. Proserpio, M. M. J. Treacy and O. M. Yaghi, J. Solid State Chem., 2005, 178, 2533 CrossRef CAS.
- M. O'Keeffe and O. M. Yaghi, J. Solid State Chem., 2005, 178 DOI:10.1016/S0022-4596(05)00368-3.
- D. B. Mitzi, L. L. Kosbar, C. E. Murray, M. Copel and A. Afzali, Nature, 2004, 428, 299 CrossRef CAS.
- D. B. Mitzi, Inorg. Chem., 2005, 44, 3755 CrossRef CAS.
- D. B. Mitzi, Inorg. Chem., 2005, 44, 7078 CrossRef CAS.
- X. Y. Huang, J. Li, Y. Zhang and A. Mascarenhas, J. Am. Chem. Soc., 2003, 125, 7049 CrossRef CAS.
- D. B. Mitzi, Inorg. Chem., 2007, 46, 926 CrossRef CAS.
- M. Yuan, M. Dirmyer, J. Badding, A. Sen, M. Dahlberg and P. Schiffer, Inorg. Chem., 2007, 46, 7238 CrossRef CAS.
- M. J. Manos and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 4658 CrossRef CAS.
- J. Li, Z. Chen, R.-J. Wang and D. M. Proserpio, Coord. Chem. Rev., 1999, 190–192, 707 CrossRef CAS.
- Z. Chen, R. E. Dilks, R.-J. Wang, J. Y. Lu and J. Li, Chem. Mater., 1998, 10, 3184 CrossRef CAS.
- A. V. Powell, S. Boissiere and A. M. Chippindale, J. Chem. Soc., Dalton Trans., 2000, 4192 RSC.
- V. Spetzler, H. Rijnberk, C. Näher and W. Bensch, Z. Anorg. Allg. Chem., 2004, 630, 142 CrossRef CAS.
- P. Vaqueiro, A. M. Chippindale, A. R. Cowley and A. V. Powell, Inorg. Chem., 2003, 42, 7846 CrossRef CAS.
- X. Y. Huang, J. Li and H. X. Fu, J. Am. Chem. Soc., 2000, 122, 8789 CrossRef CAS.
- X. Y. Huang and J. Li, J. Am. Chem. Soc., 2007, 129, 3157 CrossRef CAS.
- P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef.
- B. Yan, Y. Xu, N. K. Goh and L. S. Chia, Chem. Commun., 2000, 2169 RSC.
- J. T. Hu, M. Ouyang, P. D. Yang and C. M. Lieber, Nature, 1999, 399, 48 CrossRef CAS.
- H. Gleiter, Acta Mater., 2000, 48, 1 CrossRef CAS.
- J. D. Holmes, K. P. Johnston, R. C. Doty and B. A. Korgel, Science, 2000, 287, 1471 CrossRef CAS.
- M. H. Huang, Y. Y. Wu, H. Feick, N. Tran, E. Weber and P. D. Yang, Adv. Mater., 2001, 13, 113 CrossRef CAS.
- M. S. Gudiksen, L. J. Lauhon, J. Wang, D. C. Smith and C. M. Lieber, Nature, 2002, 415, 617 CrossRef CAS.
- P. D. Yang, H. Q. Yan, S. Mao, R. Russo, J. Johnson, R. Saykally, N. Morris, J. Pham, R. R. He and H. J. Choi, Adv. Funct. Mater., 2002, 12, 323 CrossRef CAS.
- H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350 CrossRef.
- L. Vayssieres, Adv. Mater., 2003, 15, 464 CrossRef CAS.
- X. Y. Kong, Y. Ding, R. Yang and Z. L. Wang, Science, 2004, 303, 1348 CrossRef CAS.
- Y. Li, G. W. Meng, L. D. Zhang and F. Phillipp, Appl. Phys. Lett., 2000, 76, 2011 CrossRef CAS.
- Y. Huang, X. F. Duan, Q. Q. Wei and C. M. Lieber, Science, 2001, 291, 630 CrossRef CAS.
- G. R. Patzke, F. Krumeich and R. Nesper, Angew. Chem., Int. Ed., 2002, 41, 2446 CrossRef.
- X. F. Duan, Y. Huang, R. Agarwal and C. M. Lieber, Nature, 2003, 421, 241 CrossRef CAS.
- J. Goldberger, R. R. He, Y. F. Zhang, S. W. Lee, H. Q. Yan, H. J. Choi and P. D. Yang, Nature, 2003, 422, 599 CrossRef CAS.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and Y. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- Z. L. Wang, Annu. Rev. Phys. Chem., 2004, 55, 159 CrossRef CAS.
- Y.-D. Li, H.-W. Liao, Y. Ding, Y.-T. Qian, L. Yang and G.-E. Zhou, Chem. Mater., 1998, 10, 2301 CrossRef CAS.
- S.-H. Yu, Y.-S. Wu, J. Yang, Z.-H. Han, Y. Xie, Y.-T. Qian and X.-M. Liu, Chem. Mater., 1998, 10, 2309 CrossRef CAS.
- J. Yang, C. Xue, S.-H. Yu, J.-H. Zeng and Y.-T. Qian, Angew. Chem., Int. Ed., 2002, 41, 4697 CrossRef CAS.
- Z.-X. Deng, C. Wang, X.-M. Sun and Y.-D. Li, Inorg. Chem., 2002, 41, 869 CrossRef CAS.
- S.-H. Yu and M. Yoshimura, Adv. Mater., 2002, 14, 296 CrossRef CAS.
- Z.-X. Deng, L. Li and Y. Li, Inorg. Chem., 2003, 42, 2331 CrossRef CAS.
- W.-T. Yao, S.-H. Yu, X.-Y. Huang, J. Jiang, L.-Q. Zhao, L. Pan and J. Li, Adv. Mater., 2005, 17, 2799 CrossRef CAS.
- Z. A. Zang, H. B. Yao, Y. X. Zhou, W. T. Yao and S. H. Yu, Chem. Mater., 2008, 20, 4749 CrossRef CAS.
- H.-B. Yao, X.-B. Li and S.-H. Yu, Chem.–Eur. J., 2009, 15, 7611 CrossRef CAS.
- N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmuller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS.
- H. Zhang, D. Y. Wang and H. Möhwald, Angew. Chem., Int. Ed., 2006, 45, 748 CrossRef CAS.
- H. Zhang, D. Wang, B. Yang and H. Mohwald, J. Am. Chem. Soc., 2006, 128, 10171 CrossRef CAS.
- Y. He, L.-M. Sai, H.-T. Lu, M. Hu, W.-Y. Lai, Q.-L. Fan, L.-H. Wang and W. Huang, Chem. Mater., 2007, 19, 359 CrossRef.
- M. Niederberger, M. H. Bartl and G. D. Stucky, J. Am. Chem. Soc., 2002, 124, 13642 CrossRef CAS.
- J. Polleux, M. Antonietti and M. Niederberger, J. Mater. Chem., 2006, 16, 3969 RSC.
- N. Pinna and M. Niederberger, Angew. Chem., Int. Ed., 2008, 47, 5292 CrossRef CAS.
- P. B. Messersmith and E. P. Giannelis, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 1047 CrossRef CAS.
- Z. Wang and T. J. Pinnavaia, Chem. Mater., 1998, 10, 3769 CrossRef CAS.
- L. M. Liu, Z. N. Qi and X. G. Zhu, J. Appl. Polym. Sci., 1999, 71, 1133 CrossRef CAS.
- E. Manias, A. Touny, L. Wu, K. Strawhecker, B. Lu and T. C. Chung, Chem. Mater., 2001, 13, 3516 CrossRef CAS.
- Z. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413 CrossRef CAS.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim, J. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80 CrossRef CAS.
- M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 397, 681 CrossRef CAS.
- P. N. Trikalitis, K. K. Rangan, T. Bakas and M. G. Kanatzidis, Nature, 2001, 410, 671 CrossRef CAS.
- P. N. Trikalitis, N. Ding, C. Malliakas, S. J. L. Billinge and M. G. Kanatzidis, J. Am. Chem. Soc., 2004, 126, 15326 CrossRef CAS.
- M. G. Kanatzidis, Adv. Mater., 2007, 19, 1165 CrossRef CAS.
- W. T. Yao, S. H. Yu and Q. S. Wu, Adv. Funct. Mater., 2007, 17, 623 CrossRef CAS.
- M.-R. Gao, W.-T. Yao, H.-B. Yao and S.-H. Yu, J. Am. Chem. Soc., 2009, 131, 7486 CrossRef CAS.
- Q. S. Gao, P. Chen, Y. H. Zhang and Y. Tang, Adv. Mater., 2008, 20, 1837 CrossRef CAS.
- D. Chen and Y. Sugahara, Chem. Mater., 2007, 19, 1808 CrossRef CAS.
- N. Pinna, G. Garnweitner, P. Beato, M. Niederberger and M. Antonietti, Small, 2005, 1, 112 CrossRef CAS.
- M. Karmaoui, R. A. Sa Ferreira, A. T. Mane, L. D. Carlos and N. Pinna, Chem. Mater., 2006, 18, 4493 CrossRef CAS.
- M. Karmaoui, L. Mafra, R. A. Sa Ferreira, J. Rocha, L. D. Carlos and N. Pinna, J. Phys. Chem. C, 2007, 111, 2539 CrossRef CAS.
- J. Polleux, N. Pinna, M. Antonietti and M. Niederberger, J. Am. Chem. Soc., 2005, 127, 15595 CrossRef CAS.
- R. Rahal, S. Daniele, L. G. Hubert-Pfalzgraf, V. Guyot-Ferréol and J.-F. Tranchant, Eur. J. Inorg. Chem., 2008, 980 CrossRef CAS.
- N. Pinna, J. Mater. Chem., 2007, 17, 2769 RSC.
- M. Antonietti, M. Niederberger and B. Smarsly, Dalton Trans., 2008, 18 RSC.
- G. Garnweitner and M. Niederberger, J. Mater. Chem., 2008, 18, 1171 RSC.
- I. Djerdj, D. Arčon, Z. Jagličić and M. Niederberger, J. Solid State Chem., 2008, 181, 1571 CrossRef CAS.
- D. B. Mitzi, J. Mater. Chem., 2004, 14, 2355 RSC.
- D. B. Mitzi, M. Copel and S. J. Chey, Adv. Mater., 2005, 17, 1285 CrossRef CAS.
- D. J. Milliron, D. B. Mitzi, M. Copel and C. E. Murray, Chem. Mater., 2006, 18, 587 CrossRef CAS.
- H. R. Heulings, X. Y. Huang, J. Li, T. Yuen and C. L. Lin, Nano Lett., 2001, 1, 521 CrossRef CAS.
- X. Y. Huang, H. R. Heulings, V. Le and J. Li, Chem. Mater., 2001, 13, 3754 CrossRef CAS.
- B. Fluegel, Y. Zhang, A. Mascarenhas, X. Huang and J. Li, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 205308 CrossRef.
- H. X. Fu and J. Li, J. Chem. Phys., 2004, 120, 6721 CrossRef CAS.
- C. R. Kagan, D. B. Mitzi and C. D. Dimitrakopoulos, Science, 1999, 286, 945 CrossRef CAS.
- Y. Zhang, G. M. Dalpian, B. Fluegel, S. H. Wei, A. Mascarenhas, X. Y. Huang, J. Li and L. W. Wang, Phys. Rev. Lett., 2006, 96, 026405 CrossRef.
- J. Li, W. H. Bi, W. Ki, X. Y. Huang and S. Reddy, J. Am. Chem. Soc., 2007, 129, 14140 CrossRef CAS.
- Y. Zhang, Z. Islam, Y. Ren, P. A. Parilla, S. P. Ahrenkiel, P. L. Lee, A. Mascarenhas, M. J. McNevin, I. Naumov, H. X. Fu, X. Y. Huang and J. Li, Phys. Rev. Lett., 2007, 99, 215901 CrossRef CAS.
- W. Ki and J. Li, J. Am. Chem. Soc., 2008, 130, 8114 CrossRef CAS.
- J. Lu, S. Wei, Y. Peng, W. Yu and Y. Qian, J. Phys. Chem. B, 2003, 107, 3427 CrossRef CAS.
- J. Lu, S. Wei, W. Yu, H. Zhang and Y. Qian, Chem. Mater., 2005, 17, 1698 CrossRef CAS.
- M. Zhang, C. Shi, T.-K. Zhang, M. Feng, L. Chang, W.-T. Yao and S.-H. Yu, Chem. Mater., 2009, 21, 5485 CrossRef CAS.
- Z.-G. Zhao and M. Miyauchi, Chem. Commun., 2009, 2204 RSC.
- T. F. Liu, D. Fu, S. Gao, Y. Z. Zhang, H. L. Sun, G. Su and Y. J. Liu, J. Am. Chem. Soc., 2003, 125, 13976 CrossRef CAS.
- S. Wang, J. L. Zuo, S. Gao, Y. Song, H. C. Zhou, Y. Z. Zhang and X. Z. You, J. Am. Chem. Soc., 2004, 126, 8900 CrossRef CAS.
- X. Y. Wang, L. Wang, Z. M. Wang and S. Gao, J. Am. Chem. Soc., 2006, 128, 674 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
