Assembly of a magnetic polyoxometalate on SWNTs†
Gaëlle
Charron
*a,
Anna
Giusti
,
Sandra
Mazerat
,
Pierre
Mialane
,
Alexandre
Gloter
,
Frédéric
Miserque
,
Bineta
Keita
,
Louis
Nadjo
,
Arianna
Filoramo
,
Eric
Rivière
,
Wolfgang
Wernsdorfer
,
Vincent
Huc
,
Jean-Philippe
Bourgoin
and
Talal
Mallah
*b
aSchool of Chemical Sciences - Energy Material Lab, University of East Anglia, Norwich NR4 7TJ, UK. E-mail: gaellecharron@gmail.com
bUMR 8182, Université Paris-Sud, 15 rue Georges Clémenceau, 91405 Orsay Cedex, France. E-mail: mallah@icmo.u-psud.fr
First published on 8th October 2009
Abstract
Recently, the organisation of magnetic molecules on carbon nanotubes has raised much interest due to their possible interesting contribution to molecular spintronics. In this paper, we describe the assembly on SWNTs of a magnetic polyoxometalate encompassing a single cobalt ion (CoPOM) and its isostructural diamagnetic zinc analogue (ZnPOM). The simple magnetic behaviour of CoPOM and the availability of its diamagnetic counterpart render these POM@NTs systems interesting model compounds for the study of molecular electronics devices based on carbon nanotubes and magnetic molecules. The success and rate of the grafting have been investigated by electron microscopy, electron energy loss spectroscopy, X-ray photoelectron spectroscopy, cyclic voltammetry, Raman scattering and magnetisation measurements. These characterisations altogether demonstrate the preservation of the structural and magnetic properties of the molecules upon functionalisation and the existence of an electronic communication between the molecules and the nanotubes.
Introduction
Molecular electronics and more recently molecular spintronics have attracted much attention since they can provide an interesting contribution to nanoscaled functional electronics.1–5Over the past ten years, carbon nanotubes (NTs) have proven to be exciting candidates for molecular electronics.6–8Single-wall carbon nanotubes (SWNTs) in particular present tunable electronic properties due to their large responsiveness to the surrounding chemical environment.9,10 In addition, the carbon-only composition of NTs leads to very low spin-orbit and hyperfine couplings and hence to high spin coherence length.4,11 These characteristics are ideal for spintronics devices. Recently, entire arrays of NTs have been assembled therefore opening the door to carbon-based nanocircuitry.12
The tailored organisation on surfaces of molecules possessing bistable behaviour could lead to ultimate nanosized memory dots, logic gates or electrically driven oscillators and resonators.5,13 The crucial step to reach these goals is to achieve the assembly of bistable molecules with nanoscale accuracy while maintaining their structure integrity and properties. Polyoxometalates (POMs) have especially retained our attention since they consist of a family of compounds with a rich diversity of properties, encompassing luminescence, catalysis and magnetism.14–17 Moreover, their robustness allow for a preservation of their properties upon assembling, as already reported.18–21
In 2006, Fei et al. assembled Keggin-type phosphotungstic acid POM on pristine multi-wall carbon nanotubes in order to impart them water solubility by electrostatic repulsion.22 In the same year, Pan et al. grafted phosphotungstic acid on Pt-coated NTs with the aim of building a modified electrode with enhanced performance for methanol oxidation.23
Recently, our group reported the first direct assembly of a single-molecule magnet (SMM) polyoxometalate of formula [Fe4(H2O)2(FeW9O34)2]10− (Fe6POM) on SWNTs with preservation of the SMM behaviour upon assembly.24 In the present work, we aim to demonstrate the successful grafting of another magnetic POM together with the retention of its magnetic properties once assembled on SWNTs. We thus extended the grafting procedure of the Fe6POM to the paramagnetic POM of formula [As2W20O68Co(H2O)]8− (CoPOM) and its isostructural diamagnetic analogue [As2W20O68Zn(H2O)]8− (ZnPOM) (Fig. 1). With its single CoII ion, the CoPOM allows for checking the possibility of detecting the magnetic signal of a molecule containing only one paramagnetic centre when assembled on SWNTs. It possesses a characteristic magnetic signal (due to its large magnetic anisotropy) that acts as an intrinsic signature and thus facilitates the characterisation of the assembly. The diamagnetic ZnPOM was used as a reference for the magnetic studies (see below). Moreover, the couple of isostructural CoPOM and ZnPOM molecules is particularly relevant for future spintronic applications: the diamagnetic analogue ZnPOM could allow for the investigation of the coupling of the host POM matrix alone to the current (since no magnetic ion is present), whereas there is no diamagnetic analogue to the Fe6POM@NTs system we previously reported. This would be advantageous to ultimately design in a rational way molecular systems for spintronics.
Control of the grafting procedure while maintaining magnetic properties paves the way to the production of model devices based on nanotubes and magnetic molecules, such as the NanoSQUID.25 Here, the success and yield of the grafting, the intrinsic behaviour of the POM within the nanoassemblies, as well as the influence of the molecules on the electronic structure of the SWNTs were investigated by high-resolution transmission electron microscopy (HRTEM), high-angular annular dark-field scanning transmission electron microscopy (HAADF-STEM), electron energy loss spectroscopy (EELS), XPS, resonant Raman spectroscopy, surface-enhanced Raman spectroscopy (SERS), cyclic voltammetry and magnetisation measurements.
Results and discussion
Assembly of the POM molecules
SWNTs were purified from their catalyst and carbonasceous impurities following a procedure already reported by some of us.26 Details of the functionalisation of the purified carbon nanotubes (pNTs) by the MPOM molecules (M = CoII or ZnII) are provided in the ESI.† Briefly, pNTs were added to an acidic solution of the MPOM. The mixture was stirred in an ultrasonic bath for 20 h while maintaining the temperature of the bath below 12 °C to prevent damaging of the pNTs. This led to a dark suspension that was subsequently centrifugated. The supernatant was isolated, filtered on a nanoporous membrane and washed with water, giving rise to a grey glassy mat of MPOM@NTs. The sediments contained both poorly functionalised pNTs (as large bundles) and MPOM@NTs assemblies precipitated because of the high ionic strength of the reaction mixture. The sediments could be redispersed in pure water and led after centrifugation to identical grey glassy mats.We checked the stability of the molecules by subjecting the CoPOM solution to the same reaction conditions (i.e. 20 h in the ultrasonic bath), precipitating the molecules by counter-ion exchange and recording the FTIR spectrum of the obtained green solid (data not shown).
Electron microscopy and EELS
HAADF-STEM experiments were performed on both pNTs and CoPOM@NTs assembly. The pNTs sample gives rise to low-contrasted bundles, while the nanotubes in the CoPOM@NTs assembly are heavily loaded with bright spots corresponding to heavy elements (Fig. 2). EELS analysis at the M4,5 W edge unravels the presence of tungsten within these bright spots (Fig. 3 and Fig. S2 of the ESI† ), which hence correspond to POM molecules. The analysis of the density profile allows for a determination of the size of the grafted objects. Along one isolated SWNT, it reveals a regular necklace of objects of about 1 to 2 nm width, consistent with the dimensions of the CoPOM molecules (Fig. 4). Quantitative information about the thickness of objects has been previously extracted from the intensity of the density profile.27 This analysis is precluded in this case due to the experimental setup. However, similar information emerges from the HRTEM images, with pNTs revealing bundles with a smooth surface and CoPOM@NTs displaying bundles decorated by dark spots of about 1 nm thickness (Fig. S1 of the ESI† ). This thickness matches the dimensions of the molecules and demonstrates that CoPOM molecules assemble on SWNTs as a monolayer. Finally, low-magnification images on different areas indicate that the grafting of CoPOM is homogeneous over the whole sample (Fig. 2).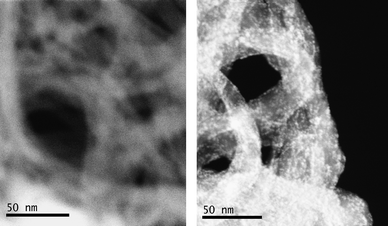 | ||
| Fig. 2 HAADF-STEM images of pNTs (left, scale bar: 50 nm) and CoPOM@NTs (right, scale bar: 50 nm). | ||
 | ||
| Fig. 3 EELS spectrum on a selected area (see also Fig. S2). | ||
 | ||
| Fig. 4 HAADF-STEM image of CoPOM@NTs along an isolated SWNT (scale bar: 5 nm) and corresponding profile of density. | ||
XPS
Prior to the analysis of the CoPOM@NTs assembly, the spectrum of the CoPOM was recorded by drop-casting the acidic solution on a silicon wafer. It displays the W(4f) spin-orbit doublet (4f7/2, 4f5/2) and the Co 2p3/2 and shake-up peaks.28 The intensity of Co peaks is weak due to the presence of one metallic centre only in each molecule (Fig. 5). The XPS spectra of the CoPOM@NTs assembly display the W(4f) peaks as well as the Co(2p) signals, even if weak and poorly resolved because of the dilution effect (Fig.5). Each peak of the CoPOM@NTs sample is shifted by about 0.2 eV towards high-binding energies with respect to the corresponding peaks in the molecule. This is due to a charging effect of the organic membrane used as a support. The ZnPOM@NTs assembly was also measured and revealed a successful grafting of ZnPOM (Fig. S3 of the ESI† ). | ||
| Fig. 5 XPS spectra of CoPOM and CoPOM@NTs at the W(4f) and Co(2p) core levels. | ||
Electrochemistry
Electrochemistry measurements have been performed on both the CoPOM molecule free in solution and CoPOM@NTs deposited on an electrode (for the preparation of the modified electrode, see the ESI† ). The cyclic voltammogram of pure SWNTs was also recorded for comparison.For clarity, the redox processes of the W-centres and the Co-centre will be described separately, in a pH = 5 medium (0.4 M CH3COONa + CH3COOH).
In solution, the W-centres exhibit three closely spaced reduction waves in the potential domain explored, associated, on potential reversal, with two largely separated oxidation waves (Fig. 6). Such behaviour was observed previously for the W-waves of the analogous complex [Co(H2O)2(γ-SiW10O35)2]10−.29 Compared to this cyclic voltammogram, the pattern displayed by the CoPOM@NTs-modified electrode (Fig. 6a) shows an overall shift of the W-reduction-waves towards positive potentials, with a slight splitting of the first wave. In addition, the second oxidation wave is now much closer to the first one. Moreover, a linear variation of reduction peak currents as a function of scan rate is observed individually for all the waves (Fig. 6b shown for the last reduction wave of Fig. 6a), a feature that testifies that CoPOM is grafted on the SWNTs.30 The two following reasons, at the very least, might justify the observed overall trends between the solution and the surface electrochemistry behaviours of CoPOM: i) fast electron-transfer kinetics usually associated with the exceptional electronic properties of SWNTs; ii) large changes in surface pKa values of the molecules once adsorbed on the nanotubes,31,32 bearing in mind the important influence of the acid–base properties of reduced W-centres on the voltammetric pattern observed for these addenda centres.
 | ||
| Fig. 6 (a) Comparison of the cyclic voltammograms (CVs) restricted to the W-electroactivity domain. Curve 1: SWNTs deposited on glassy carbon (GC) and used in the pure pH = 5 electrolyte; curve 2: polished GC electrode in the presence of 5.10−4 M CoPOM freely diffusing in the pH = 5 solution; curve 3: CoPOM@NTs-modified GC electrode used in the pure pH = 5 electrolyte. The pH = 5 medium contained (0.4 M CH3COONa + CH3COOH), the reference electrode was a KCl saturated calomel electrode (SCE) and the counterelectrode a platinum gauze of large surface area. The scan rate was 10 mV s−1. The CVs are recorded from positive to negative potentials (cathodic branch) and back. (b) Peak current intensity variations of the last tungsten reduction wave as a function of the scan rate. | ||
Finally, a precise comparison of the appropriate voltammograms (Fig. S4 of the ESI) reveals the electroactivity of the Co2+ centre. It is worth pointing out the usual difficulty in cleanly characterizing Co2+ centres of polyoxometalates by electrochemistry.29 The voltammetric pattern exhibited by the CoPOM@NTs-modified electrode was stable during prolonged potential cycling. All these observations, together, indicate the successful grafting of CoPOM onto SWNTs and also the integrity and stability of the resulting material.
Raman spectroscopy and SERS
We performed a Raman spectroscopy analysis of CoPOM, pNTs and CoPOM@NTs with the dual goal of further evidencing the success of the assembly of CoPOM molecules on the pNTs as well as probing their influence on the electronic structure of the nanotubes. The resonant Raman spectra have been recorded under irradiation at 647, 568, 514 and 488 nm. Details regarding the acquisition of the spectra, especially the calibration, and the processing of the data are described in the ESI.As expected, the spectra of the pNTs are intense and display the three characteristic RBM (Radial Breathing mode), D (Defect mode) and G modes (longitudinal and transverse modes respectively) of SWNTs (Fig. S5), thanks to the resonant effect with the electronic transitions between van Hove singularities.33 The spectra of CoPOM@NTs also display these three modes but no vibrational feature of the POM molecule, due to its weak Raman cross section.
To probe the vibrational structure of the molecule on the nanotubes, we exploited the enhancement of the Raman signal on metallic surfaces (surface-enhanced Raman spectroscopy or SERS).34 Silver-coated substrates having adequate roughness were prepared by depositing silver colloids on glass microscope slides. The CoPOM@NTsSERS spectrum under irradiation at 514 nm displays several vibrational features within the NT-silent range (Fig. 7). These have been fitted and further assigned to POM modes, according to previous studies on Keggin POMs (Table S1).34–37 The presence of normally forbidden vibrational modes in the SERS spectrum is not surprising since the near-field aspect of SERS is expected to cause a relaxation of the selection rules and thus to give rise to otherwise inactive Raman transitions.38
 | ||
| Fig. 7 SERS spectra under irradiation at 514 nm of CoPOM and CoPOM@NTs. | ||
The influence of the molecules on the electronic structure of the nanotubes can be evidenced by analysing the G modes, whose profiles are modified at 647, 568 and 514 nm. The spectra of the purified nanotubes in this domain present three peaks corresponding to the G−metallic, G−semiconductor and G+ modes (Fig. 8 and S6).33 In the spectra of the CoPOM@NTs assembly, the intensity of the G−metallic mode has decreased, which could be attributed to a modification of resonance conditions upon functionalisation. In addition, at 647, 514 and 488 nm, the G+ modes are coherently translated by 2–3 cm−1 towards higher wavenumbers after functionalisation. Such shifts have been previously reported as evidences of charge-doping of the nanotubes.39 Here, these shifts confirm the interaction of nanotube sidewalls with the highly charged CoPOM molecules. Although several models for this interaction have been proposed, based on either an electrostatic interaction with protonated nanotubes or on an interaction with defects on the sidewalls,22,40,41 the precise nature of this interaction yet remains to be determined.
 | ||
| Fig. 8 Resonant Raman spectra focused on the G-mode area of pNTs and CoPOM@NTs (recorded under irradiation at 514 nm). | ||
Valuable information can be obtained from the analysis of the RBM mode. Indeed, each RBM peak uniquely correlates with one nanotube of given chirality.33 Its display therefore probes the presence of this nanotube within the sample and its resonance with the used irradiation wavelength.
The RBM mode of the pNTs at 514 nm contains more peaks than the mode of the CoPOM@NTs sample (Fig. 9a). This may either indicate that some nanotubes have been discarded during the functionalisation process or that some have been brought off-resonance. The distribution of the RBM intensity according to the chirality of both purified and functionalized nanotubes has been estimated from the RBM modes at each wavelength (see the ESI for the method) (Fig. 9b).42,43 The differences observed between the chirality maps of pNTs and CoPOM@NTs are not compatible with the loss of some particular nanotubes upon functionalisation only. Hence, they are likely to arise from a tuning of the resonance condition by interaction with the adsorbed molecules: some nanotubes have been brought off-resonance while others have been brought on-resonance.44 Interestingly, one can note that all the nanotubes whose signal changes belong to the semi-metallic type. This result further demonstrates the occurrence of an electronic communication between the grafted molecules and the nanotubes, the interaction mainly affecting the semi-metallic nanotubes. However, complementary experiments need to be performed to fully understand the nature of this interaction.
 | ||
| Fig. 9 (a) Spectra of pNTs and CoPOM@NTs samples under irradiation at 514 nm focusing on the RBM region (the stars indicate vibrational modes of the support); (b) chirality maps of the RBM intensities in the pNTs and CoPOM@NTs samples (black and green circles respectively) obtained from the multifrequency analysis of the RBM modes. | ||
Magnetisation measurements
Magnetisation vs. field curves have been recorded for the CoPOM@NTs assembly as well as for its diamagnetic counterpart ZnPOM@NTs (Fig. 10a). The magnetisation curve of ZnPOM@NTs presents, at saturation, a weak magnetic signal (0.3 emu g−1), due to the remaining ferromagnetic catalyst particles used in the synthesis of SWNTs. Since both samples have been prepared under the same conditions, their amounts of magnetic impurities are similar. Hence, the ZnPOM@NTs curve has been thereafter used as a reference signal for the study of the magnetic behaviour of CoPOM@NTs, As expected, the saturation magnetisation of CoPOM@NTs is higher (0.9 emu g−1) than that of ZnPOM@NTs. This detection of a net magnetic moment is reproducible upon using different NT batches (Fig. S7). | ||
| Fig. 10 (a) Magnetisation vs field plots of CoPOM@NTs and ZnPOM@NTs recorded at 2K; (b) net magnetisation arising from {CoPOM@NTs – ZnPOM@NTs} as a function of the field. | ||
Moreover, substraction of the magnetic background arising from the remaining catalyst impurities (i.e. substraction of the ZnPOM@NTs signal from that of CoPOM@NTs) leads to a curve that is homothetic to the curve of the pure CoPOM molecule (Fig. 10b). Importantly, this demonstrates that the magnetic characteristics of the CoPOM molecules have been preserved upon assembling on the NTs, as previously observed with the Fe6POM SMM. This confirms that the use of polyoxometalates in spintronic devices based on carbon nanotubes is indeed a relevant strategy.
This further allows for a titration of the CoPOM molecules within the CoPOM@NTs sample. An average grafting rate of 8.10−4 POM molecules per C atom is obtained, which is equivalent to one POM molecule every 10 nm segment of a NT with a 1 nm diameter (an (8,8) NT for example) on average. It is noteworthy that examples of titration of grafted molecules on NTs are quite rare. Interestingly, these magnetisation measurements have led to a quantitative, convenient and non-destructive measure of the amount of molecules grafted.
Concluding remarks
This paper has described the functionalisation of nanotubes by simple stirring of SWNTs in the presence of POM molecules. The retention of the structural integrity of the molecules, and in particular the presence of the transition metal ions CoII and ZnII , has been assessed by both microscopic (HAADF-STEM and EELS) and macroscopic (XPS, cyclic voltammetry and SERS) measurements. In addition, resonant Raman spectroscopy experiments have shown that there is an electronic communication between POMs and NTs, although the nature of the interaction is still to be determined.In addition to the survival of the structural integrity of the CoPOM complexes upon the assembly process, we demonstrated that their magnetic behaviour was retained, thanks to the comparison with the diamagnetic ZnPOM@NTs assembly. Therefore, the use of magnetic ions encapsulated in polyoxometalate matrices holds great promise for the design of robust spintronic devices based on carbon nanotubes and magnetic molecules. This could ultimately lead to the organisation and electrical control of switchable POM molecules in nanotube-based nanocircuitry.
References
- C. Joachim, J. K. Gimzewski and A. Aviram, Nature, 2000, 408, 541–548 CrossRef CAS.
- S. Das Sarma, Am. Sci., 2001, 89, 516–523.
- A. R. Rocha, V. M. Garcia-Suarez, S. W. Bailey, C. J. Lambert, J. Ferrer and S. Sanvito, Nat. Mater., 2005, 4, 335–339 CrossRef CAS.
- S. Sanvito and A. R. Rocha, J. Comput. Theor. Nanosci., 2006, 3, 624–642 CAS.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS.
- S. J. Tans, A. R. M. Verschueren and C. Dekker, Nature, 1998, 393, 49–52 CrossRef CAS.
- T. Rueckes, K. Kim, E. Joselevich, G. Y. Tseng, C. L. Cheung and C. M. Lieber, Science, 2000, 289, 94–97 CrossRef CAS.
- P. Avouris, Acc. Chem. Res., 2002, 35, 1026–1034 CrossRef CAS.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801–1804 CrossRef CAS.
- B. L. Allen, P. D. Kichambare and A. Star, Adv. Mater., 2007, 19, 1439–1451 CrossRef CAS.
- L. E. Hueso, J. M. Pruneda, V. Ferrari, G. Burnell, J. P. Valdes-Herrera, B. D. Simons, P. B. Littlewood, E. Artacho, A. Fert and N. D. Mathur, Nature, 2007, 445, 410–413 CrossRef CAS.
- Q. Cao and J. A. Rogers, Adv. Mater., 2009, 21, 29–53 CrossRef CAS.
- J. Camarero and E. Coronado, J. Mater. Chem., 2009, 19, 1678–1684 RSC.
- M. T. Pope and A. Muller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- E. Coronado and C. J. Gomez-Garcia, Chem. Rev., 1998, 98, 273–296 CrossRef CAS.
- T. Yamase, Chem. Rev., 1998, 98, 307–325 CrossRef CAS.
- J. M. Clemente-Juan and E. Coronado, Coord. Chem. Rev., 1999, 193–195, 361–394 CrossRef CAS.
- M. Clemente-León, E. Coronado, C. L. Gómez-García, C. Mingotaud, S. Ravaine, G. Romualdo-Torres and P. Delhaès, Chem.–Eur. J., 2005, 11, 3979–3987 CrossRef CAS.
- J. Kasai, Y. Nakagawa, S. Uchida, K. Yamaguchi and N. Mizuno, Chem.–Eur. J., 2006, 12, 4176–4184 CrossRef CAS.
- W. Qi, H. L. Li and L. X. Wu, J. Phys. Chem. B, 2008, 112, 8257–8263 CrossRef CAS.
- B. B. Xu, L. Xu, G. G. Gao, Y. B. Yang, W. H. Guo, S. P. Liu and Z. Z. Sun, Electrochim. Acta, 2009, 54, 2246–2252 CrossRef CAS.
- B. Fei, H. F. Lu, Z. G. Hu and J. H. Xin, Nanotechnology, 2006, 17, 1589–1593 CrossRef CAS.
- D. W. Pan, J. H. Chen, W. Y. Tao, L. H. Nie and S. Z. Yao, Langmuir, 2006, 22, 5872–5876 CrossRef CAS.
- A. Giusti, G. Charron, S. Mazerat, J.-D. Compain, P. Mialane, A. Dolbecq, E. Rivière, W. Wernsdorfer, R. Ngo Biboum, B. Keita, L. Nadjo, A. Filoramo, J.-P. Bourgoin and T. Mallah, Angew. Chem., Int. Ed., 2009, 48, 4949–4952 CrossRef CAS.
- J. P. Cleuziou, W. Wernsdorfer, V. Bouchiat, T. Ondarcuhu and M. Monthioux, Nat. Nanotechnol., 2006, 1, 53–59 CrossRef CAS.
- G. Charron, S. Mazerat, M. Erdogan, A. Gloter, A. Filoramo, J. Cambedouzou, P. Launois, E. Rivière, W. Wernsdorfer, J.-P. Bourgoin and T. Mallah, New J. Chem., 2009, 33, 1211–1223 RSC.
- D. B. Williams, C. B. Carter, Transmission Electron Microscopy – A Textbook for Material Science, Plenum Press, New York, 1996 Search PubMed.
- T. J. Chuang, C. R. Brundle and D. W. Rice, Surf. Sci., 1976, 59, 413–429 CrossRef CAS.
- B. S. Bassil, M. H. Dickman, M. Reicke, U. Kortz, B. Keita and L. Nadjo, Dalton Trans., 2006, 4253–4259 RSC.
- E. Laviron, in Electroanalytical chemistry, ed. A. J. Bard, Dekker, New York, 1983 Search PubMed.
- A. T. Masheter, P. Abiman, G. G. Wildgoose, E. Wong, L. Xiao, N. V. Rees, R. Taylor, G. A. Attard, R. Baron, A. Crossley, J. H. Jones and R. G. Compton, J. Mater. Chem., 2007, 17, 2616–2626 RSC.
- C. Y. Lee and M. S. Strano, J. Am. Chem. Soc., 2008, 130, 1766–1773 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus and M. Hofmann, Vib. Spectrosc., 2007, 45, 71–81 CrossRef CAS.
- G. C. Lica, K. P. Browne and Y. Y. Tong, J. Cluster Sci., 2006, 17, 349–359 CrossRef CAS.
- A. J. Bridgeman, Chem. Phys., 2003, 287, 55–69 CrossRef CAS.
- C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Inorg. Chem., 1983, 22, 207–216 CrossRef CAS.
- M. Tsubaki and N. T. Yu, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 3581–3585 CAS.
- M. Moskovits, J. Raman Spectrosc., 2005, 36, 485–496 CrossRef CAS.
- S. Gupta and J. Robertson, J. Appl. Phys., 2006, 100, 9 CrossRef.
- A. Cuentas-Gallegos, R. Martinez-Rosales, M. Baibarac, P. Gomez-Romero and M. E. Rincon, Electrochem. Commun., 2007, 9, 2088–2092 CrossRef CAS.
- Z. H. Kang, Y. B. Wang, E. B. Wang, S. Y. Lian, L. Gao, W. S. You, C. W. Hu and L. Xu, Solid State Commun., 2004, 129, 559–564 CrossRef CAS.
- H. Son, A. Reina, G. G. Samsonidze, R. Saito, A. Jorio, M. S. Dresselhaus and J. Kong, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 073406 CrossRef.
- R. Bhowmick, B. M. Clemens and B. A. Cruden, Carbon, 2008, 46, 907–922 CrossRef CAS.
- P. T. Araujo and A. Jorio, Phys. Status Solidi B, 2008, 245, 2201–2204 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Sample preparation and XPS, Raman spectroscopy, and cyclic voltammetry details. See DOI: 10.1039/b9nr00190e |
| This journal is © The Royal Society of Chemistry 2010 |

