Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision†
Dongping
Sun
,
Jiazhi
Yang
and
Xin
Wang
*
Key Laboratory for Soft Chemistry and Functional Materials (Nanjing University of Science and Technology), Ministry of Education, Nanjing 210094, China. E-mail: wxin@public1.ptt.js.cn
First published on 11th November 2009
Abstract
Bacterial cellulose (BC) nanofibers were biosynthesized by Acetobacter xylinum NUST5.2, and displayed a remarkable capability for orienting TiO2 nanoparticle arrays. Large quantities of uniform BC nanofibers coated with TiO2 nanoparticles can be easily prepared by surface hydrolysis with molecular precision, resulting in the formation of uniform and well-defined hybrid nanofiber structures. The mechanism of arraying spherical TiO2 nanoparticles on BC nanofibers and forming well-defined, narrow mesopores are discussed in this paper. The BC/TiO2 hybrid nanofibers were used as photocatalyst for methyl orange degradation under UV irradiation, and they showed higher efficiency than that of the commercial photocatalyst P25.
1. Introduction
Environmental pollution and destruction on a global scale have attracted much attention to the necessity of new, safe and clean chemical technologies and processes.1Photocatalysis continues to develop as a promising alternative technology for the renewal of our environment.2 Among various oxidesemiconductor photocatalysts , titanium oxide (TiO2) has so far proven the most promising material for fundamental research and practical applications. This is due to its high photoreactivity, biological and chemical inertness, cost effectiveness, non-toxicity, and long-term stability against corrosion .3,4 However, the practical applications of TiO2 are still challenging in terms of reaction efficiency with visible light and sufficient use without degradation of the supporting materials.5,6 To increase the effective utilization of solar energy, TiO2photocatalysts have been doped with nonmetallic atoms (namely S, N, P, etc.), transition metals (namely Pt, Cu, Mg, etc.), polymers or co-doped with several ions.7,8 Among those studied, nitrogen-doped photocatalysts have become one of the hottest topics in the field of visible photocatalysis .9To improve photocatalytic activity, many researchers have attempted to decrease the particle size and increase the surface-to-volume ratio of anatase particles. Thus, the design and preparation of TiO2nanorods or nanofibers has attracted great attention during the last decade because of their potential for enhancing photocatalytic activity with high surface area.10,11 Using different methods, fibrous structures of TiO2, and TiO2-coated electrospunpolymernanofibers have been prepared. These one dimensional nanostructured TiO2 fibers have shown interesting results.12–14 With growing environmental problems caused by the end-of-life disposal of several organic polymeric matrices, hunting for renewable polymers to replace synthetic ones has become a hot topic.15
Bacterial cellulose (BC) fibers produced by Acetobacter xylinum were found to be superior, on many accounts, to plant fibers. The structure of BC is similar to that of plant cellulose but consists of microfibrillar ribbons with a width less than 100 nm, i.e. it is of nanoscale size and has an extremely developed specific surface.16,17 These fibers have a wide range of applications due to their mechanical properties and their stability towards chemicals and high temperatures.18,19 Recently BC membranes used as a kind of green matrix for palladium catalysts were studied, and results show that palladium catalysts have excellent catalytic activity. However, no reports have highlighted BC nanofiber’s unique structure and large specific surface area or its potential as a supporting catalyst.20,21 In the work reported here, bacterial cellulose (BC) was biosynthesized and used as a substrate for the fabrication of BC/TiO2 hybrid nanofibers. The hybrid nanofibers were prepared by a controlled surface hydrolysis method. Herein we put forward a plausible growth mechanism for the hybrid nanofibers, for verification we utilized scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In addition, the structure was characterized by Fourier transform infrared (FTIR) spectroscopy.
2. Experimental
2.1. Preparation of BC fibers
Acetobacter xylinum NUST5.2 was incubated in an agitated culture at 29 °C for 7 days. This culture was comprised of D-glucose, sucrose, yeast extract, (NH4)2SO4, KH2PO4, and MgSO4 dissolving in deionized water (DI) to form concentrations of 20, 21, 10, 4, 2, and 0.4 g L−1. The pH of the medium was adjusted to 6.0–6.2 by 2.5 M NaOH. BC fibers were purified by soaking in DI at 70 °C for 3 h and then 1 M NaOH in DI at 70 °C for 90 min. Samples were rinsed with DI to pH = 7 and stored in refrigerator at 4 °C prior to use.2.2. Preparation of hybrid TiO2/BC fibers
To make a non-doped TiO2/BC fiber composite, the BC fibers were dipped into an ethanol–water (50/50 v/v) solution. A solvent exchange process was adopted by increasing the ethanol content up to 99% stepwise to remove the disordered water. The treated BC fibers were collected by centrifugation and added to 200 mL ethanolic solution of Ti(OBu)4. The reaction was carried out for 2 h at 30 °C with mechanical stirring (200 rpm). Subsequently, the mixture was transferred into a 200 mL (internal volume) stainless steel autoclave with a Teflon tube attached. After that the autoclave was placed in a preheated oven (150 °C), and the mixture was allowed to react for 5 h under closed conditions. The final fiber composite was centrifuged, washed with distilled water and ethanol three times separately, and finally dried in vacuo at 60 °C overnight. For N-doped TiO2/BC fiber composite, the same preparation procedure was used, however, urea was added.62.3. Characterization of the hybrid fibers
The morphology of the samples was investigated by means of SEM (JEOL JSM-6380LV) and TEM (JEM-2100). The crystallinity and the phase composition of samples were characterized by XRD (Bruker D8 ADVANCE). UV–vis spectra was recorded on a Cary 5000 spectrophotometer equipped with an integrated sphere accessory for diffusive reflectance UV–vis spectroscopy. BaSO4 was used as a reference for the measurements. N2 adsorption–desorption experiments were carried out at 77 K to determine the Brunauer–Emmett–Teller (BET) surface area and micropore volume (t-plot method). Before the surface area determination, the samples were always degassed at 150 °C for 12 h. The spectroscopic properties of the samples was investigated by FTIR (Bomen MB154S) and XPS (PHI-5300).2.4. Photocatalytic activity
P25 was used as a reference TiO2 source and methyl orange (MO) was used as a model pollutant. A set of photocatalytic degradation experiments was performed according to the following procedure: suspensions of the samples (200 mg) in a MO solution (20 ppm) were placed in a quartz reactor (200 mL) with a 300W UV lamp. Prior to the photoreaction, the solution was magnetically stirred in the dark for 60 min to reach adsorption–desorption equilibrium; then the reaction was irradiated by the UV light from the top with stirring. During the photoreaction, samples were taken from the reaction mixture at 5-min intervals for assessment of photocatalytic activity.3. Results and discussion
SEM images of bare BC nanofibers and TEM images of BC/TiO2 hybrid nanofibers are presented in Fig. 1. The SEM image of Fig. 1a shows a side view of the BC nanofibers biosynthesized by Acetobacter xylinum NUST5.2, with an average diameter of about 30 nm and a length ranging from micrometres up to dozens of micrometres. Fig. 1c and Fig. 1d clearly show TiO2 particles deposited on the surface of BC fibers. The average diameter for BC/TiO2 is 7.8 ± 0.2 nm, with a relatively narrow size distribution (4.3–8.5 nm). In addition, from the TEM images, it can be observed that a relatively ordered nanoporous morphology can be observed, originating from the regular package of the nanoparticles. | ||
| Fig. 1 SEM and TEM images of BC nanofibers. (a) A general view of the nanofibers. (b) A TEM image of the nanofibers. (c–d) TEM images of BC/TiO2 hybrid nanofibers. | ||
To evaluate the interactions between BC and TiO2 nanoparticles in hybrid fibers, FTIR was applied. The recorded spectra for pure BC and BC/TiO2 hybrid fibers are shown in Fig. 2. The peaks at around 1000–1300 cm−1 for BC/TiO2 hybrid fibers, due to C–OH stretching (1060 cm−1) and C–O–C bending vibrations (1163 cm−1), are weakened in comparison to the peaks in BC because the TiO2 nanoparticles grow on the surface of the BC.22 A characteristic IR band at 1642 cm−1 corresponding to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibration of the BC moved slightly downfield as a result of the Ti–O–C vibration (from 1642 cm−1 to 1630 cm−1), which indicates that a strong interaction occurs at the interface of BC and TiO2 nanoparticles.23 A similar phenomenon was observed for the C–H stretching vibration (2910 cm−1). Due to coverage by thin layer of TiO2, this C–H in-plane stretching was blocked as result of steric hindrance (as shown in Fig. 2).
C vibration of the BC moved slightly downfield as a result of the Ti–O–C vibration (from 1642 cm−1 to 1630 cm−1), which indicates that a strong interaction occurs at the interface of BC and TiO2 nanoparticles.23 A similar phenomenon was observed for the C–H stretching vibration (2910 cm−1). Due to coverage by thin layer of TiO2, this C–H in-plane stretching was blocked as result of steric hindrance (as shown in Fig. 2).
 | ||
| Fig. 2 FTIR spectra of BC nanofibers and BC/TiO2 hybrid nanofibers. | ||
Based on the morphology results and FTIR spectra, we can state that the arrays of spherical TiO2 nanoparticles on BC nanofibers are produced through a surface hydrolysis process. Scheme 1 illustrates the entire procedure of the fabrication of BC/TiO2 hybrid nanofibers. Since it is widely accepted that ordered water molecules are always present in a cellulose–water system, a layer of water molecules covers the BC fibers via H-bonding,24 and the disordered water layers are located outside (Scheme 1a). BC nanofibers with an ordered water layer were prepared by solvent exchange (Scheme 1b) (as shown in section 2.2), and the BC nanofibers with an ordered water layer were then dispersed in a mixture of titanium butoxide (Ti(OBu)4) in an ethanol/urea solution. Ti(OBu)4 hydrolyzes at the ordered water layer of the BC nanofibers, as the reactivity of Ti(OBu)4 is mild upon exposure to moisture (Scheme 1c). With Ti(OH)4 dehydrating in the hydrothermal process, TiO2 nanoparticles with regulated size can be produced in situ (Scheme 1d).
 | ||
| Scheme 1 Schematic illustration of the preparation of BC/TiO2 hybrid nanofibers. | ||
If the hypothesis of fabrication of BC/TiO2 hybrid nanofibers is accurate, the ordered interstice between the arrays of the TiO2 nanoparticles will be formed when Ti(OH)4 dehydrates in the hydrothermal process. The average diameter of the interstice can be calculated through plane of the closer-packing of spheres method (Scheme 2.) by using the following equation,
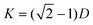 | (1) |
 | ||
| Scheme 2 Model of the close-packing of spheres. | ||
To confirm that pores of the hybrid fiber were formed, the BC fibers and the BC/TiO2 hybrid nanofibers were examined by N2 adsorption–desorption experiments and the isotherms are shown in Fig. 3a. The slope of the N2 adsorption–desorption isotherm together with the area of hysteresis increases significantly with the BC/TiO2 hybrid fibers, indicating an increase in the surface area and the total pore volume. On the other hand, the nitrogen uptake at low pressure also rises with the loading of TiO2, indicating an enhancement of the overall surface area. The corresponding quantitative data are summarized in Table 1. In comparison with the BC fibers, the BC/TiO2 hybrid fibers showed an increase in surface area of much as 156-fold and a 25-fold increase of pore volume. These values reached 208.17 m2g−1 and 0.151 cm3g−1 respectively. The pore size distribution plot was calculated using the Barrett–Joyner–Halenda (BJH) equation from the adsorption branch of the isotherm and is shown in Fig. 3b. For the BC fibers, a broad distribution of both mesopores and macropores is found (ranging from 2 to 100 nm). Interestingly, with the loading of TiO2, a relatively unique size distribution of the fibers is produced. Specifically, the BC/TiO2 hybrid fibers exhibit a well-defined, narrow mesopore population centered at 3.5 nm, which agrees quite well with the theoretical value of 3.3 nm.
| Sample | S BET | Total pore volume/cm3g−1 | Mesopore volume (%) | Average pore diameter (nitrogen desorption)/nm |
|---|---|---|---|---|
| BC fibers | 1.37 | 0.006 | 63.1 | 35.4 |
| BC/TiO2 fibers | 208.17 | 0.151 | 96.5 | 3.5 |
 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms of BC nanofibers and BC/TiO2 hybrid nanofibers. (a) pore size distribution for the BC nanofibers and BC/TiO2 hybrid nanofibers (b) and (c) are the adsorption–desorption isotherms at 0–120 nm and 0–10 nm, respectively. | ||
It is well known that anatase-type TiO2 show better photocatalytic activities than the other types of TiO2. So insights on the nature of the formed TiO2 phases were obtained from the XRD diffraction pattern. As seen from the upper pattern (Fig. 4), the three characteristic peaks located at the 14.5, 16.6, and 22.5 can be assigned to the BC fiber. After the BC fiber is coated with TiO2, the intensities of the peaks mentioned above become weaker and inconspicuous. In addition, several new strong peaks at 25.3, 38.1, 47.8, 54.1, 62.2, 69.3 and 75.0 are observed. Comparing peak positions with crystallographic databases identifies the material as the anatase phase of TiO2. It is noted that the characteristic peaks of the TiO2 are broad, which is an indication of the small size of the TiO2 particles formed on the BC fibers. The crystallite size as calculated from the Debye–Scherrer equation is 7.2 nm which is in agreement with particle size estimates from TEM.
 | ||
| Fig. 4 XRD patterns of BC and BC/TiO2 hybrid nanofibers. | ||
Fig. S1 of the ESI† displays the TG curves of pure BC and the BC/TiO2 hybrid nanofibers. A maximum degradation for both pure BC and BC/TiO2 hybrid nanofibers at around 300 °C is observed. With increasing emperature, pure BC degrades completely, leaving only minute amounts, while 75% of the sample still remains in BC/TiO2. White ashes were obtained after the measurement of BC/TiO2.
Fig. 5 displays the electronic absorption spectra of N-doped BC/TiO2 hybrid nanofibers and BC/TiO2 hybrid nanofibers. A clear shift of absorption in the visible light region can be observed for the N-doped BC/TiO2 hybrid nanofibers in comparison with BC/TiO2 hybrid nanofibers. The band gap energy (Eg) of these materials can be estimated by the formula:25Eg = 1240/λg, where λg is the wavelength corresponding to the intersection point of the vertical and horizontal parts of the spectrum. The calculated Eg of N-doped BC/TiO2 (2.99 eV) is lower than that of the BC/TiO2 (3.1 eV). This may be caused by the varying degree of N-doping in BC/TiO2 hybrid nanofibers. The N-doped BC/TiO2 hybrid nanofibers were examined by X-ray photoelectron spectroscopy (XPS). Fig. S2 of the ESI† shows the high-resolution XPS spectra of the N 1s region on the surface of N-doped BC/TiO2 hybrid nanofibers. The N 1s spectrum of N-doped BC/TiO2 hybrid nanofibers contains one peak at about 399 eV. It has been reported that a peak at about 400 eV is a sign of nitrogen doping in the lattice of the titanium, which can be attributed to N–O.26 It should be noted that a long absorption tail of (400–600 nm) is observed for both hybrid nanofibers (Fig. 5) and this may be attributed to the BC substrate.
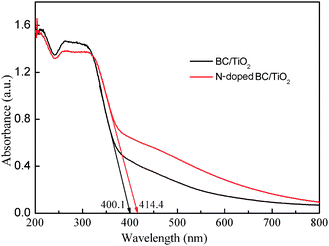 | ||
| Fig. 5 UV–vis diffuse reflectance spectra of BC/TiO2 and N-doped BC/TiO2nanofibers. | ||
The photocatalytic activities of the N-doped BC/TiO2 and undoped BC/TiO2 hybrid nanofibers were evaluated by photocatalytic degradation of methyl orange under UV irradiation. As a control, the activity of the commercial photocatalyst P25 was also tested under the same conditions. Results of the photocatalytic evaluation are shown in Fig. 6. The photocatalytic activities of N-doped BC/TiO2 and BC/TiO2 hybrid nanofibers are much higher than that of P25, which may be attributed to their larger specific surface areas and smaller crystallite size. (The specific surface areas and crystallite size of P25 are usually about 50 m2g−1 and 30 nm, respectively.27). Recently A. Ghicov et al. reported that nitrogen-doped TiO2 nanotube layers increased the conversion efficiency in photocatalysis because of the combination of a high surface area with a photoresponse in visible light.9 This is also a suitable explanation for the higher photocatalytic activity of N-doped BC/TiO2 relative to BC/TiO2.
 | ||
| Fig. 6 Comparison of the photocatalytic activity of BC/TiO2nanofibers, N-doped BC/TiO2 nanfibers, and P25. | ||
4. Conclusion
In summary, a simple approach to fabricate BC/TiO2 hybrid nanofibers with high surface area and high photocatalytic activity has been reported. To do this, the oriented anatase TiO2 nanoparticle arrays were implanted on a BC fibers using a molecular imprinting technique. The BC/TiO2 hybrid nanofibers are mesoporous and consist of partially cemented anatase particles with diameters in the 4.3–8.5 nm range and a surface area of 208.17 m2g−1. The BC/TiO2 hybrid nanofibers show high photocatalytic activity and exceed that of Degussa P25. Compared with BC/TiO2 hybrid nanofibers, BC/TiO2 hybrid nanofibers doped with nitrogen show much higher photocatalytic ability. Therefore, a promising way to optimize photocatalytic activity is to deposit nitrogen-doped TiO2 nanoparticles on the renewable BC nanofibers.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China and China Academy of Engineering Physics (No.10776014), and the Jiangsu Provincial Department of Science and Technology (No. BE2009159).References
- K. Nagaveni, G. Sivalingam, M. S. Hegde and G. Madras, Environ. Sci. Technol., 2004, 38, 1600 CrossRef CAS.
- W. Y. Wang, A. Irawan and Y. Ku, Water Res., 2008, 42, 4275.
- A. Selloni, Nat. Mater., 2008, 7, 613 CrossRef CAS.
- H. B. Yu, S. Chen, X. Quan, H. M. Zhao and Y. B. Zhang, Environ. Sci. Technol., 2008, 42, 3791 CrossRef CAS.
- F. Cesano, S. Bertarione, A. Damin, G. Agostini, S. Usseglio, J. G. Vitillo, C. Lamberti, G. Spoto, D. Scarano and A. Zecchina, Adv. Mater., 2008, 20, 3342 CrossRef CAS.
- T. G. Schaaff and D. A. Blom, Nano Lett., 2002, 2, 507 CrossRef CAS.
- S. Usseglio, A. Damin, D. Scarano, S. Bordiga, A. Zecchina and C. Lamberti, J. Am. Chem. Soc., 2007, 129, 2822 CrossRef CAS.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis and B. J. Marinas, Nature, 2008, 452, 301 CrossRef CAS.
- A. Ghicov, J. M. Macak, H. Tsuchiya, J. Kunze, V. Haeublein, L. Frey and P. Schmuki, Nano Lett., 2006, 6, 1080 CrossRef CAS.
- C. R. Xiong, M. J. Kim and K. J. Balkus, Small, 2006, 2, 52 CrossRef CAS.
- S. Z. Zhang, W. H. Ni, X. S. Kou, M. H. Yeung, L. D. Sun, J. F. Wang and C. H. Yan, Adv. Funct. Mater., 2007, 17, 3258 CrossRef CAS.
- M. Jin, X. T. Zhang, S. Nishimoto, Z. Y. Liu, D. A. Tryk, A. V. Emeline, T. Murakami and A. Fujishima, J. Phys. Chem. C, 2007, 111, 658 CrossRef CAS.
- J. A. Lee, K. C. Krogman, M. l. Ma, R. M. Hill, P. T. Hammond and G. C. Rutledge, Adv. Mater., 2009, 21, 1252 CrossRef CAS.
- J. H. Jang, K. S. Jeon, S. Oh, H. J. Kim, T. Asahi, H. Masuhara and M. Yoon, Chem. Mater., 2007, 19, 1984 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358 CrossRef CAS.
- J. Juntaro, M. Pommet, G. Kalinka, A. Mantalaris, M. S. P. Shaffer and A. Bismarck, Adv. Mater., 2008, 20, 3122 CrossRef CAS.
- Y. Z. Wan, L. Hong, S. R. Jia, Y. Huang, Y. Zhu, Y. L. Wang and H. J. Jiang, Compos. Sci. Technol., 2006, 66, 1825 CrossRef CAS.
- H. Yano, J. Sugiyama, A. N. Nakagaito, M. Nogi, T. Matsuura, M. Hikita and K. Handa, Adv. Mater., 2005, 17, 153 CrossRef CAS.
- G. Guhados, W. K. Wan and J. L. Hunter, Langmuir, 2005, 21, 6642 CrossRef CAS.
- B. R. Evans, H. M. O'Neill, V. P. Malyvanh, I. Lee and J. Woodward, Biosens. Bioelectron., 2003, 18, 917 CrossRef CAS.
- U. D. Patel and S. Suresh, J. Colloid Interface Sci., 2008, 319, 462 CrossRef CAS.
- H. S. Qian, M. Antonietti and S. H. Yu, Adv. Funct. Mater., 2007, 17, 637 CrossRef CAS.
- C. Wang, E. Y. Yan, Z. H. Huang, Q. Zhao and Y. Xin, Macromol. Rapid Commun., 2007, 28, 205 CrossRef CAS.
- S. Kaewnopparat, K. Sansernluk and D. Faroongsarng, Aaps Pharmsci, 2008, 9, 70 Search PubMed.
- G. S. Shao, X. J. Zhang and Z. Y. Yuan, Appl. Catal., B, 2008, 82, 208 CrossRef CAS.
- S. K. Joung, T. Amemiya, M. Murabayashi and K. Itoh, Chem.–Eur. J., 2006, 12, 5526 CrossRef CAS.
- A. R. Liu, S. M. Wang, Y. R. Zhao and Z. Zheng, Mater. Chem. Phys., 2006, 99, 131 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Thermogravimetric analysis curves for BC and BC/TiO2 hybrid nanofibers and XPS spectrum of an N-doped BC/TiO2nanofiber sample. See DOI: 10.1039/b9nr00158a |
| This journal is © The Royal Society of Chemistry 2010 |
