Preparation, characterization and applications of low-molecular-weight alginate–oligochitosan nanocapsules
Ting
Wang†
and
Nongyue
He
*
Chien-Shiung Wu Laboratory, Southeast University, Nanjing, MD 210096, China. E-mail: nyhe@seu.edu.cn; Fax: +86-25-83790885; Tel: +86-25-83790885
First published on 4th November 2009
Abstract
The development of drug-delivering nanoparticles from natural materials for various biomedical applications is an area of great promise. However, the contradictory data on their uncontrollable diameter, unstable structure and toxic effects, highlight the need to study their preparation, characterization and cytotoxic effects in cells. In this work, nanocapsules are made from a type of W/O microemulsion system with low-molecular-weight alginate (LMWALG) and oligochitosan (OCS). The particles possess excellent biocompatibility and good biodegradability. The size of capsules is controlled and optimized by carefully adjusting the molecular weight and concentration of LMWALG and OCS. We found, from orthogonal experiments, the encapsulation time leading to a uniform size distribution with an average diameter of 136 nm. Furthermore, we found that molecular weights of LMWALG and OCS significantly influence the stability and size of capsules. The optimized nanocapsules are further used to study the drug release of BSA. Results show that the efficiency of encapsulation approximately reaches 88.4% and the concentration of BSA in phosphate-buffered solution (PBS, pH = 7.4) is well maintained at a level of 35 to 40% from 12 h to 48 h, due to the stable and slow degradation of the nanocapusules. The biocompatibility of LMWALG/OCS nanocapsules is cross-examined by cytotoxicity experiments and acute systemic toxicological tests, and they were found to enhance the survival rate of the cells from 80.30 to 95.39% in 7 days. The synthesized nanocapsules exhibit high biocompatibility, non-toxicity, biodegradation, and uniform size, providing a new potential candidate for drug releases in clinic experiments.
Introduction
Capsules based on natural materials have attracted much attention for many important potential applications in the fields of drug delivery, biomedical and tissue engineering due to their unique structural properties, biodegradation, and biocompatibility.1 Over the last two decades, many groups have been involved in the development of polymeric biomaterials for the immobilization of various biologically-active species. However, one of the most promising preparation methods of stable microcapsules, which has attracted considerable attention, is the creation of polyelectrolyte complexes based on the interaction between natural polyelectrolytes with excellent biocompatibility such as chitosan and chitosan derivatives, alginate,2,3 carboxymethyl cellulose,4chondroitin sulfate,5 gellan,6hyaluronic acid7 and carrageenans.8 However there are some disadvantages associated with the capsule’s carrier prepared by natural polyelectrolytes, including high biomolecule leakage, low mechanical strength, and serious swelling due to their open structure, large porosity, and high hydrophilicility.10–12 In most cases, the reactions have stoichiometric character and strongly depend on charge density and the degree of ionization of the ionizable sites on the polyanion and polycation.Nowadays, improvement in the stability and control of biomolecule release of the microcapsules still receives great focus. Some researchers have coated the surface of alginate gel beads with other reagents, such as poly(L-lysine) and glutaraldehyde.13–16 Although these fabrication methods have often suffered from low efficiency, high toxicity, and complexity.17 We selected alginate and oligochitosan (OCS, a derivative of chitosan) among the possible biomaterials. Alginate has many wonderful qualities, for example, good biocompatibility and biodegradation. It has been widely used in bioreactors , drug carriers and biomaterial scaffolds.18Chitosan is a biocompatible and slowly biodegrading polymer that has been widely used in controlled drug delivery.19–24 With chains of different charge for these two types of biomaterials, the control of the molar weight is a key parameter in the formation of stable, elastic capsules with high modulus.9 Therefore, recent studies in the field of OCS have attracted more and more interest, because OCS is not only water-soluble but also possesses versatile functional properties such as antitumor activity,25,26 immunostimulating effects,27antimicrobial activity,28–30 free-radical scavenging activity,31 and angiotensin converting enzyme (ACE) inhibitory activity.32
Bartkowiak and Hunkeler studied the mechanistic resistance for membrane permeability of the capsules prepared from alginate and OCS within the diameter of 2.9–3.2 mm,33,34 and discussed the polyelectrolyte complexation reaction between two oppositely charged polysaccharides. They showed that the ionic strength and the pH of the solution applied during the capsule formation strongly influenced the structure of the alginate–oligochitosan membrane where the relative number of interchain ionic bonds, which determines the interpolymer complex density and membrane properties, can be controlled by either the pH or ionic strength. Based on LMWALG and OCS, a new generation of nanocapsules was prepared with a novel method using ternary microemulsions. Existing as a thermodynamically kinetic stable system, microemulsions have recently attracted considerable attention because of their potential application as a system to prepare microcapsules for medical implants. Many investigations related to microemulsions have been done. Researchers have studied another type of microemulsion system for chitosan–alginatenanoparticles with a core–shell morphology for gene delivery.35 This method has also been used for the encapsulation of cells that release cytokines, hormones, and other agents for gene therapy.36–41
For the eminent characters of microemulsions, the inverse water/oil (W/O) microemulsion system was applied for the oligochitosan (OCS) and low-molecular-weight alginate (LMWALG).
As a material, the OCS and LMWALG drug delivery system can be broadly used in the field of medical technology and gene delivery. Compared to polymers and liposomes, polysaccharides are especially important in the domain of water-soluble polymers, where they play an important role as thickening, gelling and emulsifying, hydrating and suspending polymers. In particular, the fact that some polysaccharides give physical gels under well-defined thermodynamic conditions is important.42,43 These constitute a very important class of materials in food, cosmetic, biomedical or pharmaceutical applications.
By plotting the ternary phase diagrams of LMWALG, the optimal composition was obtained. At the same time, the composition of the OCS and LMWALG system was also studied. The microemulsion system was stable when LMWALG gelatum in the W/O system was less than 30 wt%, and OCS less than 35.71 wt%. In this microemulsion system, nanocapsules were obtained with an average diameter of 136 nm.
It was reported that the potential toxicity of surfactants and co-surfactants in microemulsion systems has to be noticed,44 in our investigation cytotoxicity experiments and the acute systemic toxicological tests have been done for the obtained nanocapsules. It was demonstrated that during 7 days the survival rate of the cells ascended to 95.39% (the seventh day) from 80.30% (the second day) compared with the control group. For the acute toxicity test, it was found that the abnormal symptom was not found in the experimental group. Moreover, the encapsulation efficiency (EE) was optimized by checking different preparation processes with the model protein of bovine serum albumin (BSA), the results demonstrated that the encapsulation rate could reach 88.4% and the release characteristics of the capsules sustained well during 24 h.
Experimental section
Preparation of LMWALG
Sodium alginate was obtained from Shanghai Chemical Products Corporation. An intrinsic viscosity was measured in deionized (DI) water at 25 °C in a capillary viscosimeter (Viscologic TI 1, SEMA Tech, France). This reflects a molecular weight (Mw) of 425 kDa. After degradation by UV irradiation, alginate samples with molecular weights ranging from 200–130 kDa were obtained and called ‘low-molecular-weight alginate’ (LMWALG).Preparation of oligochitosan
OCS samples with various Mw (1–9 kDa) were obtained by controlled degradation via continuous adding of 30% H2O2 solution to 3.0 g of chitosan powder (Mw = 90 kDa, degree of deacetylation >90%. Com. Haidebei. Shanghai, China) at 75 °C for 45 min until a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (w/w) of H2O2 solution to polysaccharide was reached. The Mw of OCS samples was 3 kDa. The molecular weight was determined by triple-detection size-exclusion chromatography. The weight-average molecular weights (Mw) were determined as described previously.45
1 ratio (w/w) of H2O2 solution to polysaccharide was reached. The Mw of OCS samples was 3 kDa. The molecular weight was determined by triple-detection size-exclusion chromatography. The weight-average molecular weights (Mw) were determined as described previously.45
BSA (Mw = 70 kDa, pI = 4.9, Shanghai Chemical company, China) as a type of model medicine was encapsulated in nanocapsules.
For the toxicity test, 10 healthy Kunming mice weighing from 23 to 25 g were obtained from Zhongshan hospital and bred for 3 weeks.
Fibroblasts (L-929), obtained from Zhongshan Hospital were proliferated in the extracting solution of the capsules as experimental group, and then estimated by MTT [3-(4, 5-dimethylthiazol-2-yl) -2, 5 -diphenyl tetrazolium bromide] assay . All other reagents were of analytical grade.
Preparation of microemulsions
LMWALG and OCS were dissolved in DI water respectively with a concentration of 1–3% and 1–4% in aqueous phase. A mixture of cyclohexane, Triton X-100 and n-pentanol (Tianjin Chemical Company, China.) was added to the aqueous phase, to obtain a transparent solution. The microemulsion phase regions were optimized via investigating the ternary phase diagram of microemulsions.For the ternary phase diagrams of LMWALG and OCS, Triton X-100 (S) was mixed with n-pentanol (A) at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w) of surfactant, then cyclohexane (OIL) was dissolved in the above surfactant at a ratio ranging from 0–100% (v/v), followed by the addition of OCS (4 wt%) solution dropwise until the solution turned transparent. The amount of OCS solution in the ternary phase diagram was obtained. The ternary phase diagram of OCS was obtained as shown in Fig. 1. In the same way, the ternary phase diagram of LMWALG (3 wt%) was obtained.
2 (w/w) of surfactant, then cyclohexane (OIL) was dissolved in the above surfactant at a ratio ranging from 0–100% (v/v), followed by the addition of OCS (4 wt%) solution dropwise until the solution turned transparent. The amount of OCS solution in the ternary phase diagram was obtained. The ternary phase diagram of OCS was obtained as shown in Fig. 1. In the same way, the ternary phase diagram of LMWALG (3 wt%) was obtained.
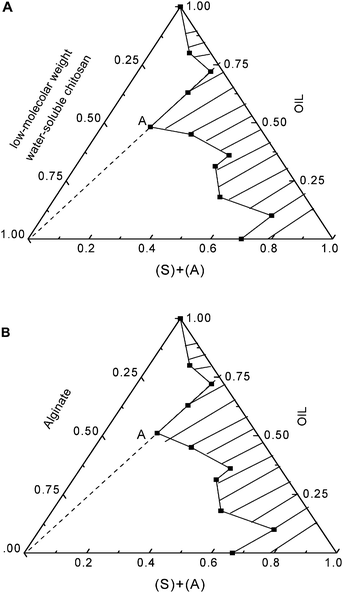 | ||
| Fig. 1 The phase diagram of micelles for two different microemulsions under the temperature of 30 °C. A: A microemulsion of Triton X-100/n-pentanol/cyclohexane/OCS, B: A microemulsion of Triton X-100/n-pentanol/cyclohexane/alginate. | ||
Preparation of nanocapsules
In the quaternary W/O inverse microemulsions, LMWALG aqueous solutions with different concentrations were slowly added into the above surfactant solution using a syringe pump, and then churned up using a high-speed blender to disperse them in the emulsion solution. Following that, a quantity of cyclohexane was added into the solution, completing the preparation of the LMWALG microemulsions. The composing rate of the microemulsions should fall into the shadow region of the ternary phase diagram. Then OCS aqueous solutions with different concentrations were slowly injected into the LMWALG microemulsions, and nanocapsules were obtained after stirring for several minutes. Finally, the nanocapsules were collected by centrifugal separation at 4000 rpm followed by washing in ethanol three times.Conditions for preparing the nanocapsules were optimized using a nine-run orthogonal experiment with 4 factors and 3 levels. The four different investigated factors shown in Table 1 are Mw of chitosan, Mw of alginate, concentration of OCS, and encapsulation time.
| Factors | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A: Mw of alginate | 130 kDa (A1) | 155 kDa (A2) | 200 kDa (A3) |
| B: Mw of OCS | 5 kDa (B1) | 3 kDa (B2) | 2 kDa (B3) |
| C: Concentration of OCS/W V−1 | 4% (C1) | 2% (C2) | 1% (C3) |
| D: Encapsulate time/min | 60 (D1) | 30 (D2) | 10 (D3) |
The value of span in the following formulation was used as an assessment to optimize the factors and levels in Table 1 listed for the synthesized nanocapsules. Span is the proportional factor of size distribution of diameter, calculated from the formula:
| Span = (D90 − D10)/D50 |
Preparation of BSA-loading nanocapsules
BSA was dissolved in the 2% (w/v) alginate solution at concentrations of 0.2, 0.5, and 1.0 mg mL−1, respectively, under slow magnetic stirring. The next steps followed the approach mentioned in the above section. A nine-run orthogonal experiment with 3 factors and 3 levels (Table 2) was designed to optimize the drug encapsulation.| Factors | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A: Concentration of alginate/W V−1 | 1% (A1) | 2% (A2) | 3% (A3) |
| B: Concentration of OCS/W V−1 | 1% (B1) | 2% (B2) | 4% (B3) |
| C: Concentration of BSA/mg mL−1 | 0.2 (C1) | 0.5 (C2) | 1.0 (C3) |
Preparation of extraction solution
The extraction solution containing nanocapsules, used to culture cells was prepared using the following procedure. The nanocapsules were first washed three times with an aseptic solution of sodium chloride with a concentration of 0.9 wt%. 0.1 g of nanocapsules (dried under vacuum) were then emerged in 1 mL of the same aseptic solution. After vibration in the horizontal of the solution using a jerking table (Biotech, KYC) for 48 h at 37 ± 0.15 °C, the extract solution was ready.Transmission electron microscopy (TEM)
TEM images were taken with a Philips CM-100 transmission electron microscope equipped with a Hamamatsu digital camera ORCA-HR operated using AMT software at 50 kV (Advanced Microscopy Techniques Corp, Danver, MA). Samples were prepared by depositing a diluted particle suspension (5 μL) onto a carbon-coated copper grid which was then air-dried before measurement.Scanning electron microscopy (SEM)
SEM images were recorded on an AMRAY 1910 FE field-emission scanning electron microscope (Philips CM-100, Holand) equipped with a backscattered electron detector at 15–30 kV. Samples were sputter-coated with about 15 nm Au using a Polaron sputter coating system.Particle size analysis
A Particle Size Analyzer (LS-3000, COM. CILAS USA) was used to measure the size distribution (D90, D50, and D10) of the nanocapsules dispersed in ethanol at the percentage of 8 wt%.The delivery and release of BSA
The BSA-loading nanocapsules were deliquesced in citrate to check the drug loading amount and the drug release. Enzyme calibration (SLT-210, COM. DYNEX U.S.A) and BSA kits (Pharmaceutical Factory of Shanghai, China) were used to detect the amount of BSA in nanocapsules. The encapsulation efficiency (EE) of BSA can be obtained from the formula below:where WC is the encapsulated BSA quantity and WG is the gross quantity of BSA.
In the release measurement, phosphate-buffered saline (PBS) solution (pH = 7.4) was used as the release medium. BSA-loaded capsules were emerged in PBS solution at a velocity of 100 rpm at 37 °C for 24 h. The EE was tested at the points of 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 36, and 48 h by extracting 5 mL of the BSA solution while adding the same volume of PBS to keep a constant volume. The release-time curve was obtained based on the readings from enzyme calibration.
Acute toxicity test of nanocapsules on Kunming mice
Ten healthy Kunming mice weighing from 23 to 25 g were selected for the experiment. After being fed using the same conditions for 3 days no abnormal cases in weight, actions, and appetite were found. The mice were divided into two groups. An aseptic solution of 0.9 wt% sodium chloride was injected into five of them as a negative control group in the ratio of 50 mL kg−1. The remaining mice made up the test group and were injected with the nanocapsule extracts in the same ratio. After being fed for 3 days, the mice were weighed and the activities of them were observed at 24 h, 48 h, 72 h, and one week respectively. The protocols are shown in Table 3.| Control group: aseptic solution of 0.9 wt% sodium chloride solution |
| Experiment group: extracts of nanocapsules/50 mL kg−1 |
| Evaluation viain vivo experiment |
| Inject of extracts |

|
Cell culture in the extraction solution
The L-929 fibroblasts of the Kunming mice were cultured in a medium containing 10% fetal bovine serum (FBS) at 37 ± 1 °C in 5% CO2. A concentration of 1 × 104 cells mL−1 was attained for the experimental group. Equal volumes of extraction solution to the control group were added and the culture lasted for more than 7 days, while the solution without extracts was used for a negative control group.Cytotoxicity assays of nanocapsules
The MTTassay was used to measure the viability of the fibroblast cells. The MTTassay was performed as described previously with modifications. Briefly, 20 μL of MTT solution (5 mg mL−1, Sigma) was added to each well of a 96-well culture plate possessing 100 μL of FBS and incubated at 37 °C for 24 h, then the MTT solution and FBS were removed from the wells, replaced with dimethyl sulfoxide (DMSO, Sigma) to determine the absorbance at 490 nm using a plate reader (Multiskan MK3 Thermo Labsystems UK). The results were evaluated by the formulation of the relative growth rate (RGR) shown as follows. The morphology of cells was observed with a polarizing light microscope (E200POL Nikon Japan).where ODe is the absorbance of the experimental group and ODn is the absorbance of the negative control group.
Results and discussion
Diagram of micelles for microemulsions
The morphology of three-phase emulsions was examined in the two binary mixtures of cyclohexane/n-pentanol and Triton X-100/water at the same temperature (303 K) but different concentrations. Fig. 1 illustrates the ternary phase diagram in OCS and LMWALG solution phases, respectively. In order to determine the concentration of different components in the microemulsions, the OCS and LMWALG concentrations were measured while the concentration ratio of oil to surfactant changes from 0.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to 1.0
1.0 to 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0 changing by 0.25 every time.
0.0 changing by 0.25 every time.
As shown in Fig. 1A and B, the three-phase diagram was divided into two regions, the shadow region represents a steady microemulsion system, the other region represents a unsteady demulsification emulsion phase.
Point “A” was found to be the maximum solubility of the W/O system, at which the surfactant weight ratio (Triton X-100vs.n-pentanol) was fixed at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The weight ratio of water phase to cyclohexane to surfactant in the microemulsions was 35.71
1. The weight ratio of water phase to cyclohexane to surfactant in the microemulsions was 35.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.21
48.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.79 in the 4 wt% OCS (Fig. 1A) and 30
16.79 in the 4 wt% OCS (Fig. 1A) and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48.21
48.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.79 (Fig. 1B) in the 3 wt% LMWALG solution, respectively. It can be seen that the maximum solubility of 4 wt% OCS and 3 wt% LMWALG distributed in the microemulsion phases appears at the same position of 48
16.79 (Fig. 1B) in the 3 wt% LMWALG solution, respectively. It can be seen that the maximum solubility of 4 wt% OCS and 3 wt% LMWALG distributed in the microemulsion phases appears at the same position of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.79, at which the amount of OCS and LMWALG has comparable values of ∼30–35%. Since the molecular structures of OCS and LMWALG are similar, it is not surprising that similar concentration trends of oil to water to surfactant at the equilibrium state were observed.
16.79, at which the amount of OCS and LMWALG has comparable values of ∼30–35%. Since the molecular structures of OCS and LMWALG are similar, it is not surprising that similar concentration trends of oil to water to surfactant at the equilibrium state were observed.
Characterizations of OCS/LMWALG nanocapsules
The self-assembly procedure of OCS and LMWALG is shown in Fig. 2.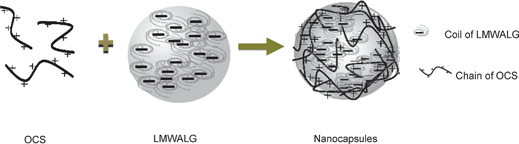 | ||
| Fig. 2 The self-assembly of OCS and LMWALG is driven by electrostatic interactions. The positive chain-like OCS interacts with a micelle of LMWALG liquid with spherical random coils to form a stable network. | ||
During the self-assembly process, anion chain diffusion at the surface of an alginate solution droplet will cause OCS with positive short chains (n < 100) to interlace with many spherical-shaped micelles of LMWALG liquid with amorphous LMWALG chains34,35 to form a network associated by electrostatic interactions. As shown in Fig. 2, the electrostatic interaction occurs between the carboxyl groups in LMWALG and the amido group in OCS.
Furthermore, Fig. 2 illustrates that the chain-like OCS interacts with the spherical random coil of LMWALG and generates of a stable capsule with an amorphous network surface. Ionic interactions between the polyelectrolyte molecules are demonstrated in the FT-IR spectra of Fig. 3.
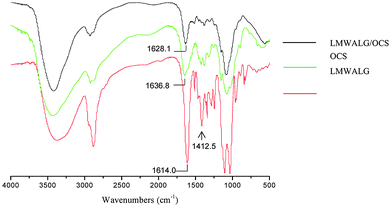 | ||
| Fig. 3 FT-IR difference curves of LMWALG/OCS capsules, OCS and LMWALG. The characteristic peaks of the carboxyl groups in LMWALG and the amido groups in OCS are pointed out. | ||
The characteristic peak at 1650 cm−1 of the carboxyl groups in LMWALG, shifts as shown in the FT-IR spectra in Fig. 3, which proves the reaction between the carboxyl groups of LMWALG and amido groups of OCS. The ∼1400 cm−1 band attributed to the –COO group of LMWALG is much weaker in the curve of the complexed nanocapsules than that of the LMWALG, accompanied by a shift from 1636.8 to 1628.1 cm−1 for the vibration band of N–H after the reaction between –NH and –COO. This result implies that the electrostatic interaction between OCS and LMWALG is based on the association between –NH and –COO groups as illustrated in Fig. 2.
An orthogonal experiment with 3 factors and 4 levels, comprising nine-run tests, was designed to determine the optimal conditions for preparing nanocapsules. The experimental conditions of the nine-run tests are shown in Table 4, while the corresponding standard deviations of nanocapsule size were shown in Table 5. According to the range method, the larger the R value, the more important the effect of the factor in determining the size distribution of nanocapsules. As shown in Table 4, the descending order of important factors is as follows: Mw of alginate > Mw of OCS > encapsulation time > concentration of OCS. The good control of the first two factors is able to generate the uniform size distribution of nanocapsules within 229 ± 19 nm. Although the encapsulation time and concentration of OCS play a less important role in size distribution, they affect the aggregation of nanocapsules obviously.
| Run | A | B | C | D | Median/μm | Span |
|---|---|---|---|---|---|---|
| a Four parameters were optimized: A (A1, A2, A3) is LMWALG with different Mw; B (B1, B2, B3) is OCS with different Mw; C (C1, C2, C3) is the concentration of OCS; D (D1, D2, D3) is the encapsulation time. Midian is the average diameter of capsules. | ||||||
| 1 | A1 | B1 | C1 | D1 | 0.134 | 1.8264 |
| 2 | A1 | B2 | C2 | D2 | 0.185 | 0.8556 |
| 3 | A1 | B3 | C3 | D3 | 0.229 | 3.1408 |
| 4 | A2 | B1 | C2 | D3 | 25.32 | 2.2882 |
| 5 | A2 | B2 | C3 | D1 | 22.78 | 2.8671 |
| 6 | A2 | B3 | C1 | D2 | 16.52 | 2.8975 |
| 7 | A3 | B1 | C3 | D2 | 144.1 | 1.7250 |
| 8 | A3 | B2 | C1 | D3 | 146.4 | 1.7479 |
| 9 | A3 | B3 | C2 | D1 | 90.59 | 2.7058 |
| I | 1.657 | 2.231 | 2.157 | 2.466 | — | — |
| II | 2.968 | 1.824 | 2.234 | 1.826 | — | — |
| III | 2.060 | 2.631 | 2.293 | 2.392 | — | — |
| R | 1.311 | 0.807 | 0.136 | 0.640 | — | — |
| Resours | A | B | C | D |
|---|---|---|---|---|
| a S: sum of square, F: degree of freedom, f: the average of variance, F(creitial): error effect all creitial functions. | ||||
| S | 2.709 | 0.977 | 0.028 | 0.736 |
| F | 2 | 2 | 2 | 2 |
| S/f | 1.00 | 0.361 | 0.010 | 0.272 |
| f | 2.709 | 2.706 | 2.8 | 2.705 |
| F(creitial) | F0.05(2.71) = 19.0 | F0.01(2.2) = 99.0 | — | — |
Analysis of relative variance was used to determine the optimal conditions in Table 4. The best condition of A should be “I” which is the minimum number among I, II and III, which means Mw of LMWALG should be 130 kDa. In the same way, the Mw of OCS should be 3 kDa, the concentration of OCS and encapsulation time should be 4 wt% and 30 min. The analysis showed that the group of A1B2C1D2 should be the best condition for preparing the nanocapsules. Since in the L9 (34) orthogonal experiment no run followed the results obtained in the analysis, supplementary experiments were conducted.
The size distribution was measured using a particle size analyzer (PSA). The results of A1B2C2D2 and A1B2C1D2 are showed in Fig. 4A and B. Fig. 4A shows the size distribution of capsules of A1B2C2D2 in the L9 (34) orthogonal experiment which displays a smaller diameter and more narrow distribution compared with the other obtained capsules made from the nine-run orthogonal experiment. However, from the PSA experiment the nanocapsules corresponding to Fig. 4A are not the best ones because of the appearance of a mixed peak, as shown in Fig. 4A. This result was in accordance with the analysis of the orthogonal design experiment.
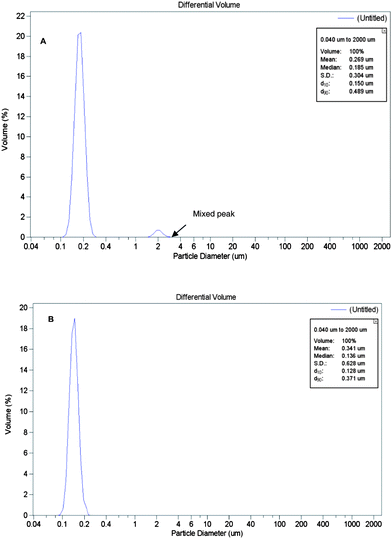 | ||
| Fig. 4 Particle size analyzer (PSA) curves. The mixed peak is indicated with an arrow. A: The best one A1B2C2D2 in the nine-run orthogonal experiment; B: The optimal product A1B2C1D2. | ||
In order to obtain the optimal conditions to prepare nanocapsules with a small diameter, high dispersion and narrow size distribution, a supplementary experiment following the condition of A1B2C1D2 was conducted.
From Fig. 4B for the size distribution of the sample A1B2C1D2, no other peaks except the main one which is narrower were found. The result was in accordance with the analysis of orthogonal design experiment.
It was found that nanocapsules of A1B2C1D2 displayed the smallest diameter, the highest degree of dispersion and the narrowest size distribution (as shown in Fig. 4B). The results demonstrated that the optimal condition for product was A1B2C1D2, the nanocapsules with the average diameter of 136 nm and span of 0.5597 were obtained (as shown in Fig. 6A). While the others not only displayed large diameters and wide size distribution, but some of them are also agglomerated as shown in Fig. 5 where the SEM image shows capsules with diameter from 500 nm to 100 μm.
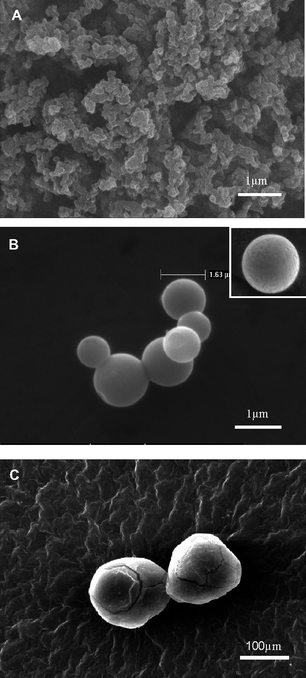 | ||
| Fig. 5 SEM images of capsules prepared under different conditions of the nine-run orthogonal experiment. A: the agglomerated nanocapsules of A1B3C3D3; B: The microcapsules of A2B3C1D2, the surface details of this kind of capsules can be found in the insert image; C: The microcapsules of A3B1C3D2. | ||
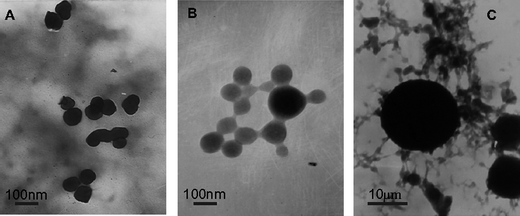 | ||
| Fig. 6 TEM images of OCS/LMWALG capsules. A: The microcapsules of the optimal product A1B2C1D2; B: The smallest product A1B1C1D1 of the nine-run orthogonal experiment; C: The product A2B2C3D1 with the value of span > 2.1 and value of diameter > 20 μm. The average span value of the nine-run orthogonal experiment is 2.178, and span is one of the most important factors we analyzed. | ||
From the SEM analysis we suggest that the span value has its own significant influence on the dispersion of nanocapsules. From our study, the capsules with high span values are always agglomerated, as shown in Fig. 5A where the capsules of A1B3C3D3 having a span value of 3.1408 are severely agglomerated and the size is around 200 nm. At the same time the microcapsules of A2B3C1D2 with a span value of 2.8975 were shown to be only slightly agglomerated. Furthermore, the capsules of A3B1C3D2 with the span value of 1.7250 did not agglomerate and the median diameter is around 144 μm, as show in Table 4, which is much bigger than the other two.
TEM was used to investigate the microstructure and the morphologies of the nanoparticles. As shown in Fig. 6A and B, the nanocapsules prepared from the same Mw of alginate possess similar diameters. However the ones using different concentrations of OCS and different times of encapsulation are clustered as shown in Fig. 6C. This result is in good agreement with the SEM analysis and demonstrates that the Mw of OCS and the concentration of OCS are associated with the agglomeration of capsules. Comparing Fig. 6A with C, the Mw of alginate greatly influenced the size of the capsules.
Combined TEM and SEM analyses found that the Mw of alginate significantly influenced the size of the particles. The other conditions such as the concentration of OCS influenced the distribution, monodispersion and other characteristics of the nanocapsules. From this result we suggest that low-molecular-weight alginate would form smaller micelles in the microemulsion solution.
The data from the orthogonal design experiment listed in Table 4 was in good agreement with the information shown in the SEM and TEM images. In this investigation the optimal Mw of alginate is 130 kDa. When the Mw increased to 200 kDa the average diameter of the nanocapsules increased significantly from 130 nm to 147.6 μm as listed in Table 4. When the Mw of alginate is 130 kDa, the average diameters of the capsules are always in the range 100 to 300 nm; when the Mw increased to 155 kDa, the diameter of nanocapsules is around 10 to 30 μm, and when the Mw of alginate increased to 200 kDa, the lowest diameter capsule is 90.59 μm and most of the capsules diameters fall around 100 μm. This result means that the other conditions were not as important for the diameter and size distribution of nanocapsules as the Mw of alginate.
From above changes of the diameter of capsules, we suggest that as the size decreased the electric charge should have greater influence on encapsulation. Alginate possessing a lower molecular weight forms micelles more easily, thus with the decrease of the Mw of alginate, the core size should decrease.
From Fig. 5C we found that the bigger capsules present split, which was not observed for the smaller capsules shown in Fig. 5B, suggesting that the LMWALG with a small Mw can more tightly associate with OCS than those with larger molecular weights. Due to this, the degree of reaction should be influenced by the pyranoside of alginate and van der Waals forces between the shell and core of the nanocapsules. We found that when the chain of pyranoside was shorter, the ionic bond would form more easily, and the van der Waals forces between alginic acid and oligochitosan should be stronger, which may lead to the increased thickness of the shell. From the above analysis we deduce that a polyelectrolyte complexation membrane would be formed more easily between LMWALG and OCS.
Release and loading rates of BSA in nanocapsules
The conditions for loading BSA in the nanocapsules were optimized by a L9 (33) orthogonal experiment as shown in Table 6.| Run | A | B | C | Loading (%) |
|---|---|---|---|---|
| a A: concentration of LMWALG (W V−1) with the molecular weight of 130 kDa; B: concentration of OCS (W V−1); C: concentration of BSA (mg mL−1); R: range of factors. | ||||
| 1 | A1 | B1 | C1 | 68.24 |
| 2 | A1 | B2 | C2 | 77.38 |
| 3 | A1 | B3 | C3 | 48.66 |
| 4 | A2 | B1 | C2 | 79.44 |
| 5 | A2 | B2 | C3 | 49.92 |
| 6 | A2 | B3 | C1 | 48.52 |
| 7 | A3 | B1 | C3 | 58.28 |
| 8 | A3 | B2 | C1 | 40.66 |
| 9 | A3 | B3 | C2 | 74.89 |
| I | 194.28 | 177.48 | 165.83 | — |
| II | 205.96 | 167.96 | 172.07 | — |
| III | 157.42 | 231.71 | 156.86 | — |
| R | 48.54 | 63.75 | 15.39 | — |
Analysis of variance of the data in Table 6 was used to determine the best conditions for BSA loading. The concentrations of alginate (A) and OCS (B) were optimized, and were determine to be 2% and 4%, respectively, while the concentration of BSA (C) should be 0.5 mg mL−1. However, the optimal result according to the analysis of variance could not be obtained in any of the test runs show in Table 6, therefore a supplementary experiment was carried out with the concentration of 2% LMWALG (130 kDa), 4% OCS and BSA 0.5 mg mL−1, and microcapsules with the EE of 88.4 wt% were obtained.
The concentration of OCS has the greatest range (R = 63.75) as shown in Table 6. Therefore, it should be the most important factor for preparing the BSA-loaded nanocapsules. When the concentration of OCS was increased, a high reaction rate was gained, owing to the fact that OCS has less steric hindrance compared with chitosan. This kind of character makes the reaction of OCS with alginate take place more easily to form the shells of the capsules while alginate–BSA micelles act as the cores. From our study we determined the optimal concentration of OCS should be 4%. This produced thicker and tighter shells, a greater mechanical resistance of the microcapsule and the increased loading of BSA in the capsules.
The surface of the microcapsules was observed by SEM and the image is shown in Fig. 7. The diameter of the microcapsules was around 1.5 μm, the size distribution was uniform, and the surface of the microcapsules was cracked. This kind of structure was beneficial to the release of BSA.
 | ||
| Fig. 7 SEM image of microcapsules (A2B3C1) with BSA loading. | ||
To investigate the stability of release, the release rate of BSA was determined at the time of 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 36, 48 h respectively in a PBS medium (pH = 7.4) and the diffused concentration–time curve is shown in Fig. 8.
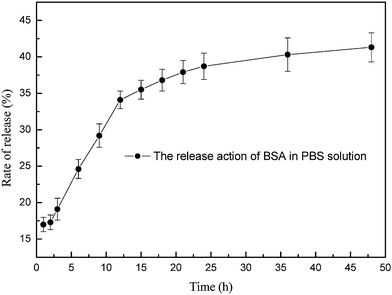 | ||
| Fig. 8 In vitro release of BSA from the microcapsules in the PBS solution with a pH of 7.4. | ||
The PBS solution (pH = 7.4) was chosen as the medium for microcapsule loading of BSA because of the following reasons: OCS, which is almost completely composed of glucose pyranoside in the microcapsules is not completely deacetylated. Therefore, the crosslinking density of the polyelectrolyte was reinforced when the amino group was ionized into an amido group in the solution at pH<7.0, which increased the compactness of the shell. However, making the PBS solution more alkaline decreased the amount of cationic amido, which influenced the crosslinking density of the polyelectrolyte complexes produced, and brought about the incompactness of the shell and the release of BSA from the microcapsules.
Fig. 8 showed that the release of BSA from the microcapsules was abrupt in the first hour, then the curve become gentle from 1 to 2 h. The change of the curve is due to two reasons: first, some BSA absorbed in the shell of the microcapsules quickly released into the PBS solution; second, during 1 to 2 h the penetration of PBS into the shell slowly brought a change in the cross-linking density of the polyelectrolyte complexes, which lead to a slow release of BSA. After that, a burst effect occurred during 2 to 12 h because the shell was destroyed and BSA in the exterior of the core was released. After 12 h the BSA deep in the core slowly diffused into solution because the degradation speed of LMWALG was slow, so the concentration–time curve become gentle again and the release rate of BSA was 42.2% at 48 h. This result demonstrated that the microcapsules have a good characterization for drug loading and releasing.
In this report, the shell of the microcapsules was composed of permeable and degradable membrane, so the thickness, porosity and degradation speed of the electrical membrane and solubility of the drug all influenced the delivery and release of drugin vivo and in vitro (i.e., the intestine membrane transfer).
In vivo experiment
Acute toxicity test of nanocapsules on Kunming mice was carried out. The evaluation criteria for the acute toxicity experiment following the Medical Devices Quality Management Systems Requirements for Regulatory Purposes of China 2000.6 is shown in Table 7. The group of mice shown in the range of middle toxicity were abandoned and the experiment repeated. In the repeated experiment the extract solution was fresh and the number of animals was doubled. The result of the repeat experiment was in accordance with the first rule.| Test | Result |
|---|---|
| 1. The symptoms of the experimental group should be similar to or milder than that of the control group during 7 days | Non-toxicity |
| 2. Two or more mice of the experimental group possess pathology character hypokinesia, breathing difficulties or abdominal pain in 7 days | Middle toxicity |
| 3. All mice lose weight or one of the mice was obviously poisoned or died even though the other mice of the experimental group were not affected. | Middle toxicity |
| 4. Two or three of the mice in the experimental group were severely acutely poisoned or died. | Acute toxicity |
In our experiments, at 24, 48 and 72 h to one week, the toxicity symptoms were not found in the experimental group. After feeding for 7 days under the same conditions all the mice were weighed and found to be at the same level ranging from 23 to 25 g. In comparison with the control group, the experiment group exhibited no breathing difficulties, hypokinesia or other abnormal cases. This result indicates that the extract of nanocapsules is non-toxic to mice.
In vitro experiments
Fibroblast cells (L-929) were used to evaluate the nanoparticles' compatibility on the cellular level. Cell viability was assessed using an MTTAssay . The Medical Devices Quality Management Systems Requirements for Regulatory Purposes of China 2000.6 was used as the criteria for evaluation of nanocapsules cellular compatibility. The results are shown in Table 8.| Standard | Relative growth rate of cell (RGR) |
|---|---|
| a Medical Devices Quality Management Systems Requirements for Regulatory Purposes of China 2000.6. | |
| 0 | ≥100 |
| 1 | 75–99 |
| 2 | 50–74 |
| 3 | 25–49 |
| 4 | 1–24 |
| 5 | 0 |
If the relative growth rate (RGR) of cells was at the level of 0 and 1, the experimental materials were considered to be non-toxic. If the RGR was at the level of 2, the morphology of the cells were investigated. At the level of 3 to 5 the experimental materials were considered to be toxic.
The polarizing microscope images of cells cultured in the extract solution for 4 days and 7 days are shown in Fig. 9(A) and (B). The values of relative growth rate of cells on the 2nd, 4th, and 7th day are shown in Table 9, and it is indicated that fibroblast cells cultured in the extract solution of nanocapsules grew well and formed cell clusters. So the value of the RGR increased from 80.30% to 95.39% during the 7 days, and the degree of standard for RGR was at level 1, meaning that the extract solution of nanocapsules was safe to use.
| Date | ODe | ODn | RGR (%) | Standard of cytotoxicity |
|---|---|---|---|---|
| 2nd | 0.106 | 0.132 | 80.30% | 1 |
| 4th | 0.162 | 0.191 | 84.99% | 1 |
| 7th | 0.207 | 0.217 | 95.39% | 1 |
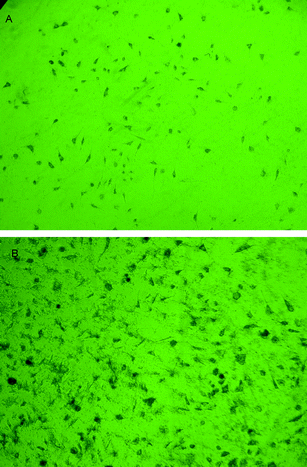 | ||
| Fig. 9 Polarizing microscope images of the cells cultured in the extract of the capsules (1 × 104 cells every 50 μL extract of the capsules) A: 4 days culture B: 7 days culture. | ||
The above results demonstrate that the nanocapsules prepared from LMWALG and OCS have a good biocompatibility and can be used in vivo and in vitro.
Conclusion
A microemulsion system of LMWALG and OCS was established to obtain nanocapsuels with the diameter around 130 nm using two kinds of natural materials: LMWALG and OCS. From the three-phase diagram of the W/O microemulsion we found that maximum solubilities of 4 wt% OCS and 3 wt% LMWALG in the microemulsion phases appeared at the same point of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.79 (w:w, oil:surfactant) respectively, at which the amounts of OCS and LMWALG have comparable values of ∼30–35%, and we attribute the above phenomenon to the similarity between the molecular structure of OCS and that of LMWALG. Orthogonal experiments based on the molecular weights of alginate and OCS, concentration of OCS, and encapsulation time were used to control and optimize the size and monodispersivity of the nanocapsules. By controlling the molecular weight within 130 kDa for LMWALG and 3 kDa for OCS, a uniform size distribution of nanocapsules within 136 ± 19 nm could be generated. The encapsulation time and concentration of OCS should be 30 min and 3%, and will effect the aggregation of nanocapsules; though it play a less important role in size distribution. From the FT-IR analysis we suggest that in this reaction positive OCS with short chains (n < 100) will interlace with many spherical-shaped random coils of LMWALG to form an amorphous network associated through electrostatic interactions. With the SEM observation we found that the bigger capsules present split, but this was not found for smaller capsules. This result suggested that the low molecular weight of these two types of polysaccharides caused them to associate with each other more tightly than those with high molecular weights. With the shorter chain of pyranoside, low-molecular-weight alginate will form ionic bonds more easily, and the van der Waals forces between alginic acid and oligochitosan will be stronger, which may lead to an increase in thickness and tightness of the shell.
16.79 (w:w, oil:surfactant) respectively, at which the amounts of OCS and LMWALG have comparable values of ∼30–35%, and we attribute the above phenomenon to the similarity between the molecular structure of OCS and that of LMWALG. Orthogonal experiments based on the molecular weights of alginate and OCS, concentration of OCS, and encapsulation time were used to control and optimize the size and monodispersivity of the nanocapsules. By controlling the molecular weight within 130 kDa for LMWALG and 3 kDa for OCS, a uniform size distribution of nanocapsules within 136 ± 19 nm could be generated. The encapsulation time and concentration of OCS should be 30 min and 3%, and will effect the aggregation of nanocapsules; though it play a less important role in size distribution. From the FT-IR analysis we suggest that in this reaction positive OCS with short chains (n < 100) will interlace with many spherical-shaped random coils of LMWALG to form an amorphous network associated through electrostatic interactions. With the SEM observation we found that the bigger capsules present split, but this was not found for smaller capsules. This result suggested that the low molecular weight of these two types of polysaccharides caused them to associate with each other more tightly than those with high molecular weights. With the shorter chain of pyranoside, low-molecular-weight alginate will form ionic bonds more easily, and the van der Waals forces between alginic acid and oligochitosan will be stronger, which may lead to an increase in thickness and tightness of the shell.
The efficiency of encapsulation of the optimized nanocapsules was approximately 88.4%. The concentration of BSA in PBS is well maintained at the level of 35% to 40% from 12 h to 48 h due to the slow degradation of the nanocapsules. After cross-examination by a cytotoxicity experiment and acute systemic toxicological test, the biocompatibilities of the capsules were proved.
The microemulsion process of oligochitosan/alginate is interesting. Based on polysaccharides materials, the capsules own excellent biocompatibilities. Furthermore, they also presents high efficiency of encapsulation and smoothly biodegradable characters, and are potentially applicable in the field of drug release and clinical experiments.
Acknowledgements
We gratefully acknowledge the support for research from the National Natural Science Foundation (NO. 60571032, 90606027, 60927001), the National Key Program for Developing Basic Research (NO. 2007CB936104, 2010CB933903) and the 863 High Tech Project (NO. 2007AA022007), as well as the Scientific Research Foundation of Graduate School of Southeast University.References
- T. A. Read, D. R. Sorensen, R. Mahesparan, P. Ø. Enger, R. Timpl, B. R. Olsen, M. H. B. Hjelstuen, O. Haraldseth and R. Bjerkvig, Nat. Biotechnol., 2001, 19, 29–34 CrossRef CAS.
- A. D. Friedman, S. J. Triezenberg and S. L. McKnight, Nature, 1988, 335, 452–454 CrossRef CAS.
- A. Polk, B. Amsden, K. De Yao, T. Peng and M. F. A. Goosen, J. Pharm. Sci., 1994, 83, 178–185 CrossRef CAS.
- T. Yoshioka, Y. Hirano, T. Shioya and M. Kako, Biotechnol. Bioeng., 1990, 35, 66–72 CrossRef CAS.
- H. W. Matthew, S. O. Salley, W. D. Peterson and M. D. Klein, Biotechnol. Prog., 1993, 9, 510–519 CrossRef CAS.
- M. Amaike, Y. Senoo and H. Yamamoto, Macromol. Rapid Commun., 1998, 19, 287–289 CrossRef CAS.
- A. Denuziere, D. Ferrier and A. Domard, Carbohydr. Polym., 1996, 29, 317–323 CrossRef CAS.
- A. Hugerth, N. Caram-Lelham and L. O. Sundeloef, Carbohydr. Polym., 1997, 34, 149–156 CrossRef CAS.
- K. Yao, T. Peng, Y. Tin, M. Xu and M. Goosen, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 1995, 1, 155 Search PubMed.
- A. Blandino and D. Cantero, Enzyme Microb. Technol., 2000, 27, 319–324 CrossRef CAS.
- A. Blandino and D. Cantero, Process Biochem., 2001, 36, 601–606 CrossRef CAS.
- S. K. Bajpai and S. Saxena, React. Funct. Polym., 2004, 59, 129–129 CrossRef CAS.
- G. W. Vandenberg, C. Drolet, S. L. Scott and J. Noue, J. Controlled Release, 2001, 77, 297–307 CrossRef CAS.
- E. Taqieddin and M. Amiji, Biomaterials, 2004, 25, 1937–1945 CrossRef CAS.
- A. Tanriseven and S. Dogan, Process Biochem., 2002, 38, 27–30 CrossRef CAS.
- F. Cellesi, N. Tirelli and J. A. Hubbell, Biomaterials, 2004, 25, 5115–5124 CrossRef CAS.
- S. S. Betigeri and S. H. Neau, Biomaterials, 2002, 23, 3627–3636 CrossRef CAS.
- T. Wang, Z. Feng, N. He, Z. Wang, S. Li, Y. Guo and L. Xu, J. Nanosci. Nanotechnol., 2007, 7, 4571–4574 CrossRef CAS.
- W. Y. Lee et al, Arzneimittel-Forschung, 1998, 48, 300–304.
- T. J. Aspden, J. D. T. Mason, N. S. Jones, J. Lowe, O. Skaugrud and L. Illum, J. Pharm. Sci., 1997, 86, 509–513 CrossRef CAS.
- R. Bodmeier and H. G. Chen, Paeratakul O. Pharm Res, 1989, 6, 413–417 Search PubMed.
- L. Illum, N. F. Farraj and S. S. Davis, Pharm. Res., 1994, 11, 1186–1189 CrossRef CAS.
- S. Miyazaki, A. Nakayama, M. Oda, M. Takada and D. Attwood, Biol. Pharm. Bull., 1994, 17, 745–747 CAS.
- H. Tozaki, T. Odoriba, N. Okada, T. Fujita, A. Terabe, T. Suzuki, S. Okabe, S. Muranishi and A. Yamamoto, J. Pharm. Sci., 1997, 86, 1016–1021 CrossRef CAS.
- K. Suzuki, T. Mikami, Y. Okawa, A. Tokorom, S. Suzuki and M. Suzuki, Carbohydr. Res., 1986, 151, 403–408 CrossRef CAS.
- Y. J. Jeon and S. K. Kim, J. Microbiol. Biotechnol., 2002, 12, 503–507 CAS.
- S. Suzuki, T. Watanabe, T. Mikami, T. Matsumoto, J. Suzuki, P. A. Sanford and J. P. Zikakis, Advances in Chitin and Chitosan, 1992, 12, 277–316 Search PubMed.
- L. A. Hadwiger and J. M. Beckman, Plant Physiol., 1980, 66, 205–211 CrossRef CAS.
- S. Hirano and N. Nagao, Agrical Biology and Chemistry, 1989, 53, 3065–3066 Search PubMed.
- Y. J. Jeon, P. J. Park and S. K. Kim, Carbohydr. Polym., 2001, 44, 71–76 CrossRef CAS.
- P. J. Park, J. Y. Je and S. K. Kim, Carbohydr. Polym., 2004, 55, 17–22 CrossRef CAS.
- P. J. Park, J. Y. Je and S. K. Kim, J. Agric. Food Chem., 2003, 51, 4930–4934 CrossRef CAS.
- Artur Bartkowiak and David Hunkeler, Chem. Mater., 1999, 11, 2486–2492 CrossRef.
- Artur Bartkowiak and David Hunkeler, Chem. Mater., 2000, 12, 206–212 CrossRef CAS.
- J. O. You, Y. C. Liu and C. A. Peng, Int. J. Nanomed., 2006, 1173–180.
- N. Ismail, G. Hortelano and A. Al-Hendy, in Cell encapsulation technology and therapeutics, Birkhäuser, Boston, 1999, 15, pp. 343–350 Search PubMed.
- M. Machluf, A. Orsola and A. Atala, World J. Urol., 2000, 18, 80–83 CrossRef CAS.
- R. L. Coffman and A. Oudenaren, J. Immunol. Methods, 1994, 17, 185–196.
- M. Machluf, O. Regev, Y. Peled, J. Kost and S. Cohen, J. Controlled Release, 1997, 43, 35–45 CrossRef CAS.
- S. Prakash and T. M. S. Chang, Nat. Med., 1996, 2, 883–887 CrossRef CAS.
- T. Mossman, J. Immunol., 1983, 65, 55–63.
- M. Rinaudo, Macromol. Biosci., 2006, 6, 590 CrossRef CAS.
- M. Rinaudo, J. Intell. Mater. Syst. Struct., 1993, 4, 210 CrossRef.
- H. Uludag and M. V. Sefton, Biomaterials, 1990, 11, 708–712 CrossRef CAS.
- M. X. Weinhold, J. C. M. Sauvageau, B. Tartsch, P. Clarke, B. Jastorff and J. Thoming, Adv. Chitin Sci., 2007, 10, 66–71 Search PubMed.
Footnote |
| † Present address: Department of Chemistry, 930 North University Avenue University of Michigan, Ann Arbor, MI 48109. E-mail: E-mail: echo2165@163.com |
| This journal is © The Royal Society of Chemistry 2010 |