Triterpenoids
Joseph D.
Connolly
and
Robert A.
Hill
*
Department of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK
First published on 26th November 2009
Abstract
Covering: January 2007 to December 2008. Previous review: Nat. Prod. Rep., 2008, 25, 794
This review covers the isolation and structure determination of triterpenoids including squalene derivatives, protostanes, lanostanes, holostanes, cycloartanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, lupanes, oleananes, friedelanes, ursanes, hopanes, isomalabaricanes and saponins; 574 references are cited.
1 Introduction
Interest in the pharmaceutical activities of triterpenoids continues to increase.1 Reviews have appeared on the triterpenoid constituents of Boswellia serrata,2Lantana camara,3Lysimachia species4 and Maytenus species.5 The classification and occurrence6 and extraction7 of plant triterpenoid saponins have been surveyed. Further reviews include the pharmaceutical activities of pentacyclic triterpenoid saponins,8 the biological activities of triterpenoid saponins containing monoterpenoid moieties9 and central nervous system activities of triterpenoid saponins.102 The squalene group
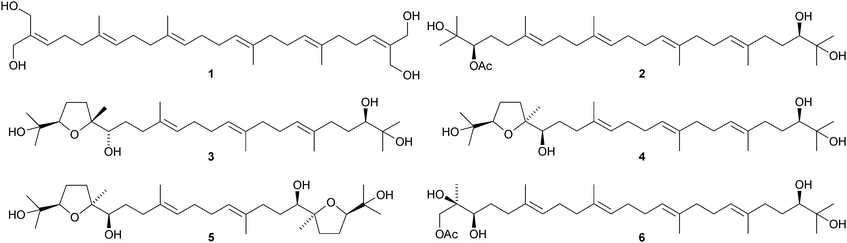
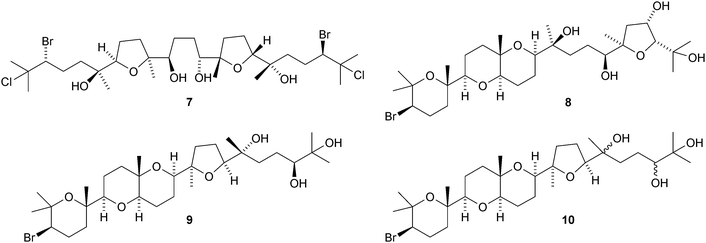
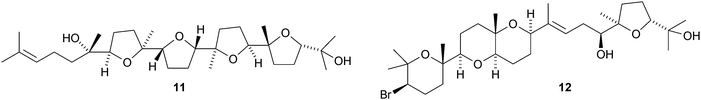
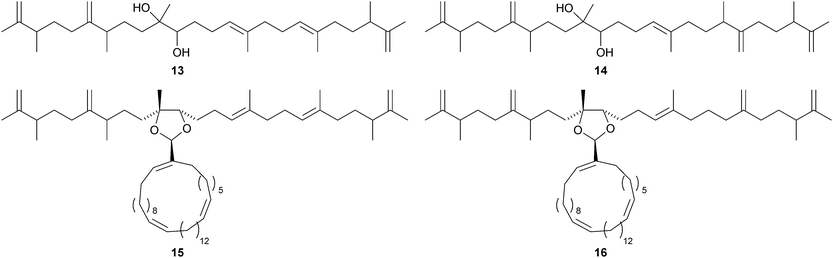
Tetrahydroxysqualene 1, from the leaves and twigs of Rhus taitensis, is active against Mycobacterium tuberculosis.11 Ekeberins D1–D52–6 are antiplasmodial squalene derivatives from the stem bark of Ekebergia capensis.12 The absolute configuration of intricatetraol 7, from Laurencia intricata, has been determined by total synthesis.13 Several squalene-derived polyethers have been reported from marine organisms. These include aplysiols A 8 and B 9 from the mantle of the sea hare Aplysia dactylomela,14 laurenmariannol 10, together with compound 8, from the red alga Laurencia mariannensis15 and omaezakianol 11 and 15,16-anhydrothyrsiferol 12 from Laurencia omaezakiana.16The condensation of C32 and C34 macrocyclic aldehydes with the methylated squalene diols 13 and 14 gives rise to the braunicetals (e.g.15 and 16), an inseparable mixture of compounds from the green microalga Botryococcus braunii.17 An enantioselective total synthesis of achilleol B 17 has led to the revision of its C-18 configuration.18
Oxidosqualene cyclase homologues from Arabidopsis thaliana continue to attract attention. At1g78955 (CAMS1) converted oxidosqualene almost entirely (98%) into the monocyclic product camelliol C 18, with only traces of achilleol A (2%) and β-amyrin (0.2%) being formed.19 This enzyme appears to have evolved from the enzymes which lead to pentacyclic products. A lanosterol synthase-deficient yeast strain At1g78500 afforded the two 8,14-seco-derivatives 19 and 20 of α-amyrin and β-amyrin.20 The C-14 configuration of arabidiol 21, produced by At4g15340, has been established as R.21 The stereochemistry of the addition of water to triterpenoid cationic intermediates is also discussed. Replacement of Phe699 by threonine in the oxidosqualene-lanosterol cyclase ERG7 from Saccharomyces cerevisiae resulted in the formation of protosta-13(17),24-dien-3β-ol 22 instead of lanosterol.22 Calculations suggest that the biosynthesis of friedelin is a non-stop process which involves pentacyclisation of squalene oxide to the lupanyl cation followed by ten suprafacial 1,2-shifts of methyls and hydrogens.23 Several reviews have appeared covering aspects of oxidosqualene-lanosterol cyclase,24 engineering squalene cyclising enzymes25 and the properties of oxidosqualene cyclases.26,27
3 The lanostane group
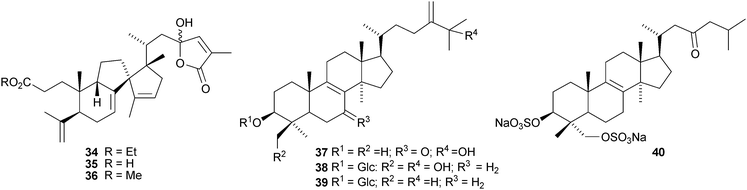
The marine-derived fungus Aspergillus sydowii produces the new protostane triterpenoid 23, a hydrate of the known helvolic acid.28 In the original paper this compound is described as a nordammarane. 1,2-Dihydrohelvolic acid 24 has been found in the entomopathogenic fungus Metarhizium anisopliae.29 Alisolide 25, alisol O 26 and alisol P 27 are new compounds from the rhizome of Alisma orientale, a rich source of protostanes.30 Unfortunately the name alisol O has already been used. The X-ray crystal structure of the known alisol B 23-acetate is also reported in this paper. Other new compounds from Alisma orientale include 25-anhydroalisol F 28 and 11-anhydroalisol F 2931 and 11,25-bisanhydroalisol F 30.32 Compound 29 has also been named 24-deacetylalisol O, referring to the original alisol O structure.33Abiesanolides E 31, F 32, I 33 and J 34 are new lanostane and mariesane derivatives from Abies sachalinensis.34,35 The free acid 35 and methyl ester 36 analogues of abiesanolide J have also been isolated from the same source.36 Marianine 37 and marianosides A 38 and B 39 are chymotrypsin-inhibitory constituents of the whole plant of Silybum marianum.37 The structure of a lanostane disulfate 40, from the green microalga Tydemania expeditionis, was confirmed by X-ray crystallographic analysis.38
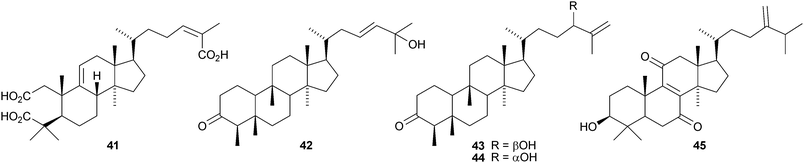
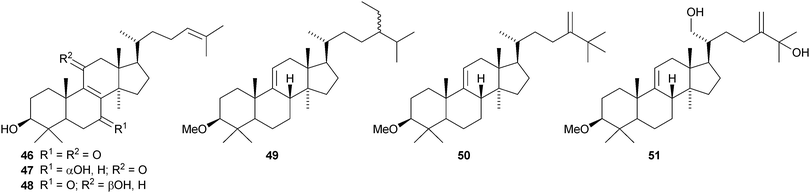
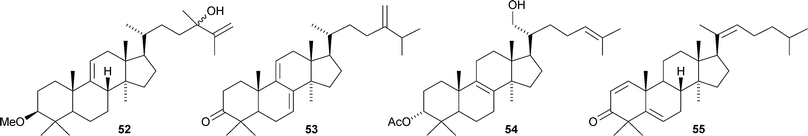
Lanopropic acid 41 is a 2,3-secolanostane from Schisandra propinqua var. propinqua.39 Other new lanostanes include the nigrumane derivatives 42–44 from the mangrove plant Hibiscus tiliaceus,4045–48 from Euphorbia humifusa,4149–52 from Diospyros discolor,4253 from the stems of Artabotrys uncinatus,43 the acetate 54 from Fomitopsis pinicola44 and nigralanostenone 55 from the leaves of Solanum nigrum.45
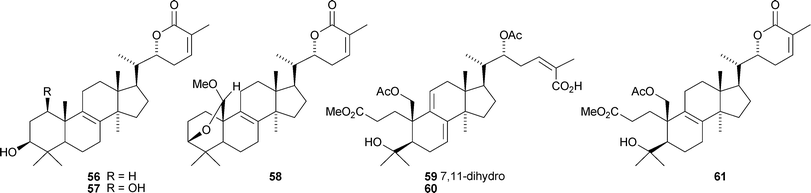
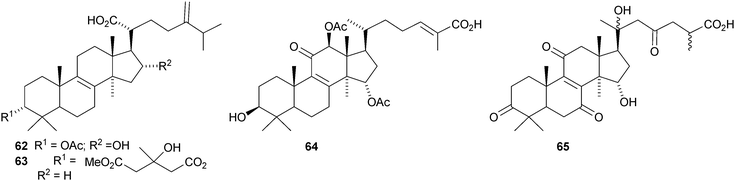
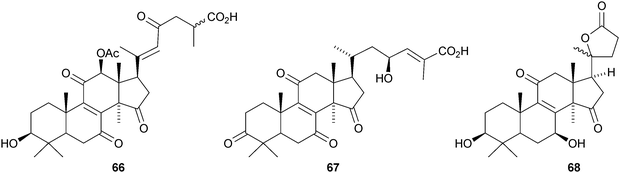
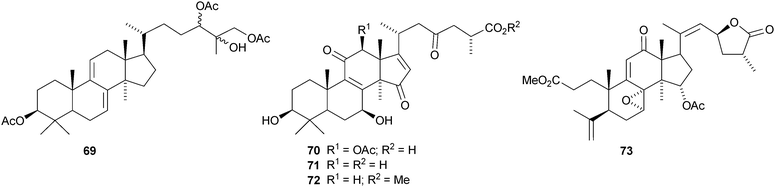
Colossolactones I 56, II 57, III 58, V 59, VI 60 and VII 61 are new constituents of the Vietnamese mushroom Ganoderma colossum.46,47 New compounds from other Ganoderma species include 3-epi-pachymic acid 62 and 63 from Ganoderma resinaceum,48 ganoderic acids AP2 64 and AP3 65 from Ganoderma applanatum,4966 and 67 from Ganoderma lucidum50 and ganolactone B 68 and ganoderiol A triacetate 69 from Ganoderma sinense.51 Three unusual Δ16-lanostanes 70–72 have been reported from Ganoderma lucidum.52 Methyl australate 73, from Ganoderma australe, shows antimicrobial activity.53 The pharmacological activities of the Ganoderma triterpenoids, including the hepatoprotective54 and anti-cancer55 effects have attracted a lot of interest.56,57,58
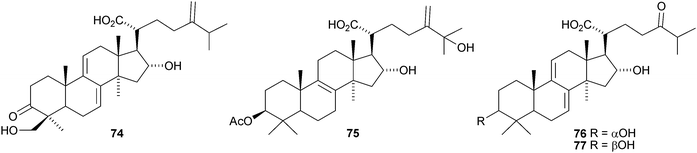
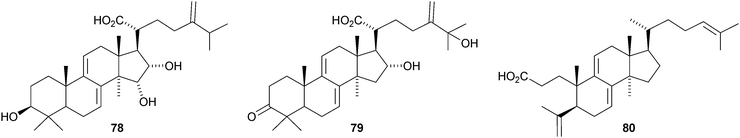
More lanostane derivatives have been reported from Poria cocus. These include 29-hydroxypolyporenic acid C 74 and 25-hydroxypachymic acid 75,59,60 poriacosones A 76 and B 7761 and 15α-hydroxydehydrotumulosic acid 78, 16α,25-dihydroxydehydroeburicoic acid 79, 16-deoxyporicoic acid B 80, poricoic acid CM 81, the endoperoxide 82 and 25-hydroxyporicoic acid H 83.62
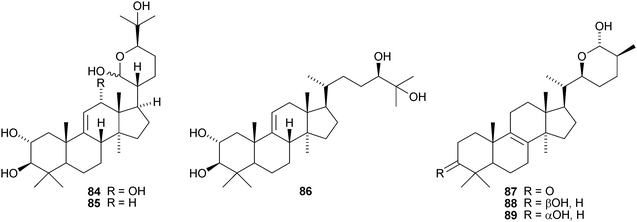
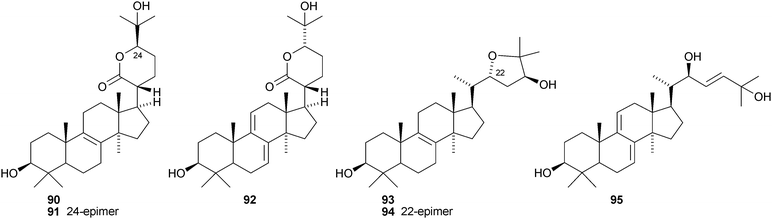
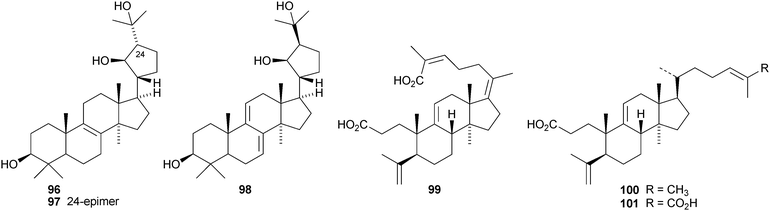
Aeruginosols A 84, B 85 and C 86 are constituents of the fruiting bodies of Stropharia aeruginosa.63 Astrapteridiol 87, astrapteridone 88 and 3-epi-astrapteridiol 89 are from the mushroom Astraeus pteridis.64 3-epi-Astrapterdiol 89 shows anti-tuberculosis activity. The structure of astrapteridone 88 was confirmed by X-ray crystallographic analysis. Inonotsulides A 90, B 91 and C 92,65 inonotsuoxides A 93 and B 94,66 the triol 9567 and inonotsutriols A 96, B 97 and C 9868 are all constituents of Inonotus obliquus. The structure of inonotsuoxide A 95 was confirmed by X-ray analysis of the corresponding diacetate.
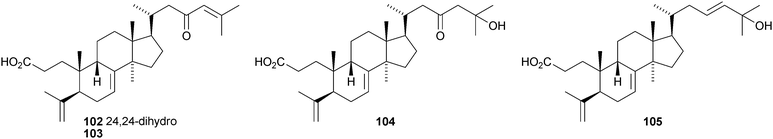
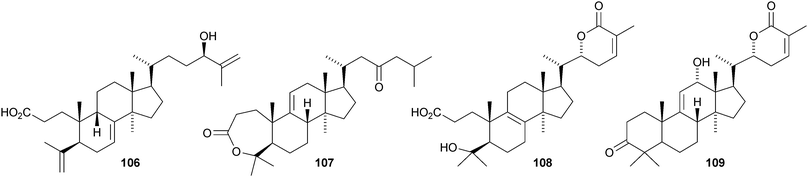
Kadsura coccinea is a rich source of lanostane derivatives. The new compounds reported include kadsuracoccinic acids A 99, B 100 and C 101 from the rhizomes,69 secococcinic acids A–E 102–106, F 101 and coccinilactone A 107 from the rhizomes70 and kadcoccilactone R 108 from the stems.71 The structure of kadsuracoccinic acid A 99 was confirmed by X-ray analysis. Kadsuracoccinic acid C and secococcinic acid F have the same structure 101. Schisanlactone E 109 is a constituent of Kadsura longipedunculata.72 The name schisanlactone E has been previously used for a seco-cycloartane derivative.
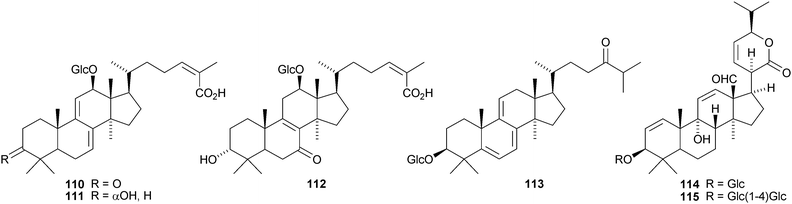
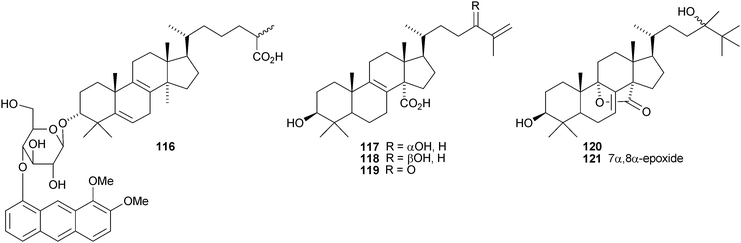
Oligoporins A 110, B 111 and C 112 are new lanostane saponins from Oligoporus tephroleucus.73 Three new saponins, sativalanosteryl glucoside 11374 and orizalanosterolides A 114 and 11575 have been reported from rice hulls of Oryza sativa. The unusual compound 116 is a proposed metabolite of Catharanthus roseus hairy root cultures.76 Eylosides F1–F7 and M–V are lanostane saponins from the Caribbean sponges Erylus formosus and Erylus goffrilleri. The saponins from Erylus formosus include the new genins 117–11977 while erylosides T and U from Erylus goffrilleri have the new genins 120 and 121.78
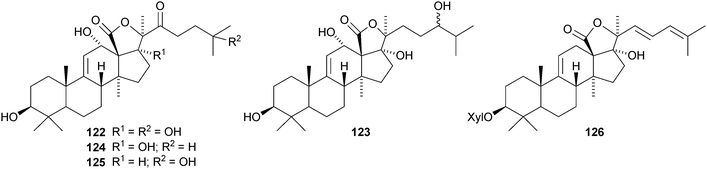
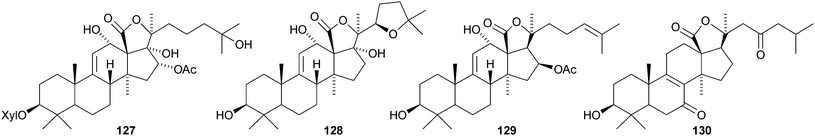
Leucospilotaside A, a new holostane glycoside from the sea cucumber Holothuria leucospilota, has the genin 122.79 The same genin is present in holothurin A3 from the Vietnamese sea cucumber Holothuria scabra.80 It is accompanied by holothurin A4, based on genin 123. 17-Hydroxyfuscocineroside B and 25-hydroxyfuscocineroside B, from the sea cucumber Bohadschia marmorata, have the genins 124 and 125 respectively.81 Another sea cucumber, Holothuria hilla, contains hillasides A 126 and B 127.82 Axilogoside, the 22-epimer of holothurin B, with the new genin 128, has been isolated from Holothuria axiloga.83 Arguside A, a cytotoxic glycoside from Bohadschia argus, has the new genin 12984 and synaptoside A1, from Synapta maculata, has the new genin 130 while the co-occurring synaptoside A has a known genin.85 Five new oligoglycosides, frondosides A7-1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) –
–![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) A7-4 and isofrondoside C, have been reported from Cucumaria frondosa, all with known genins.86 Other new holostane saponins with known genins include argusides B–E from Bohadschia argus,87,88 hillaside C (ananaside D) from Holothuria hilla,89 impatienside A from Holothuria impatiens,90 lecanorosides A and B from Actinopyga lecanora,91 leucospilotasides A92,93 and C94 from Holothuria leucospilota, liouvillosides A1–A3, B1 and B2 from Staurocumis liouvillei,95 marmoroside C from Bohadschia marmorata,96 okhotosides A1-1, A2-1,97 and B1–B398 from Cucumaria okhotensis and saponins without trivial names from Actinopyga lecanora99 and Pseudocolochirus violaceus.100 The biological activities of the holostane saponins have been surveyed.101,102
A7-4 and isofrondoside C, have been reported from Cucumaria frondosa, all with known genins.86 Other new holostane saponins with known genins include argusides B–E from Bohadschia argus,87,88 hillaside C (ananaside D) from Holothuria hilla,89 impatienside A from Holothuria impatiens,90 lecanorosides A and B from Actinopyga lecanora,91 leucospilotasides A92,93 and C94 from Holothuria leucospilota, liouvillosides A1–A3, B1 and B2 from Staurocumis liouvillei,95 marmoroside C from Bohadschia marmorata,96 okhotosides A1-1, A2-1,97 and B1–B398 from Cucumaria okhotensis and saponins without trivial names from Actinopyga lecanora99 and Pseudocolochirus violaceus.100 The biological activities of the holostane saponins have been surveyed.101,102
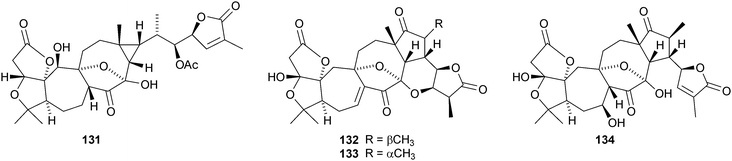
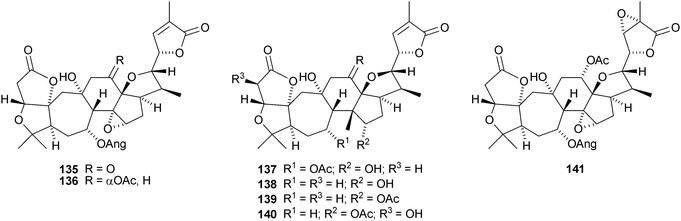
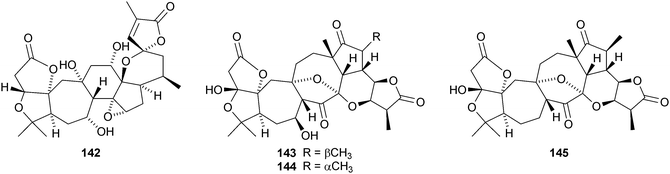
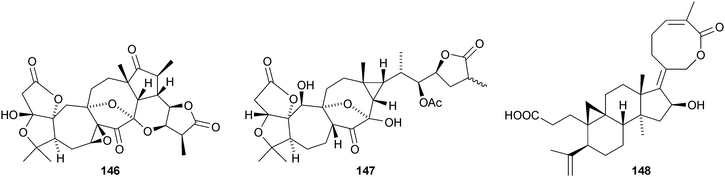
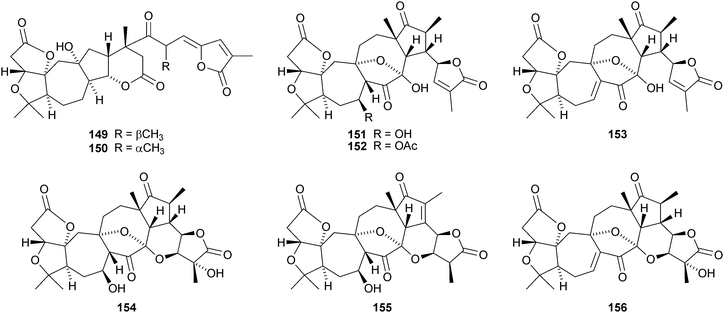
The amazing skeletal diversity of complex cycloartane-derived products from Schisandra and Kadsura species continues to impress.103 Preschisanartanin 131 and schindilactones A 132, B 133 and C 134 are constituents of Schisandra chinensis.104 The structures of 131 and 132 were confirmed by X-ray analyses. The same source afforded wuweizidilactones A–F 135–140105 and wuweizidilactones G 141, H 142, schindilactones D–G 143–146, preschisanartanin B 147 and wuweizilactone acid 148.106 The structure of 135 was confirmed by X-ray analysis. In solution, schintrilactone A 149, from Schisandra chinensis, is in equilibrium with its 20-epimer, schintrilactone B 150.107 Extraction of the leaves and stems of Schisandra lancifolia afforded lancifodilactones I–N 151–156.108 The structures of 151 and 154 were confirmed by X-ray analysis.
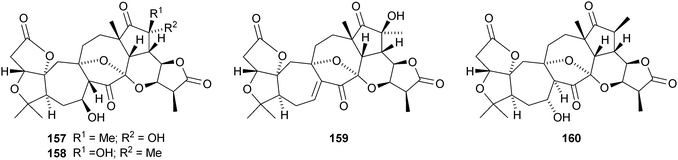
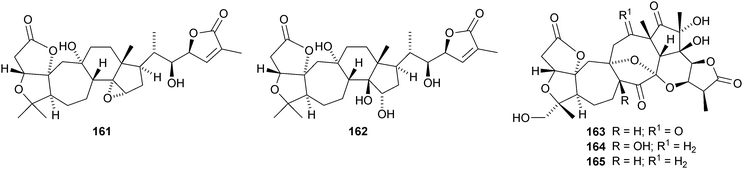
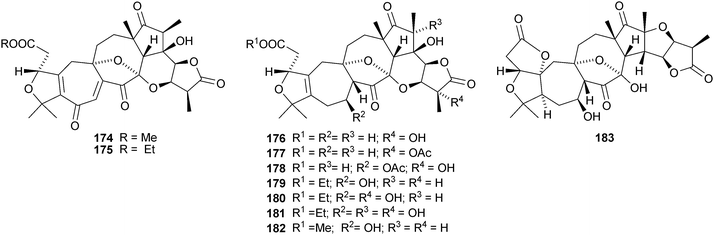
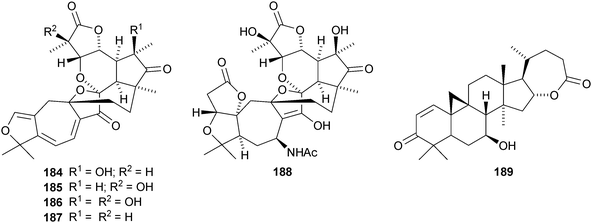
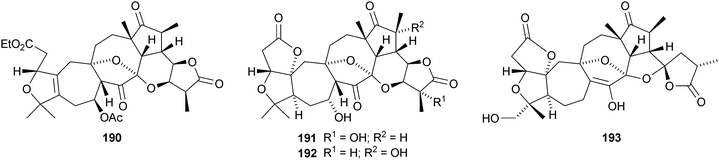
Micrandilactones D–G 157–160 are further constituents of the leaves and stems of Schisandra micrantha.109 The structures and stereochemistry of micrandilactones B 161 and C 162 have been confirmed by X-ray analyses.110 New compounds from Schisandra propinqua var. propinqua include propindilactones A–D 163–166111 and propindilactones E–J 167–172.112 Rubriflorins A–J 173–182 are constituents of Schisandra rubriflora.113,114 Lignans from this plant have also been named rubriflorins A and B. The structure of rubrifloradilactone 183, from Schisandra rubriflora, has been confirmed by X-ray analysis.115Schisandra sphenanthera is the source of sphenalactones A–D 184–187116 and sphlenadilactone C 188 and sphenasin A 189.117 Wilsonianadilactones A–C 190–192 have been reported from Schisandra wilsoniana.118 Calculations show that the naturally-occurring enol lancifodilactone G 193 is more stable than its keto tautomer.119
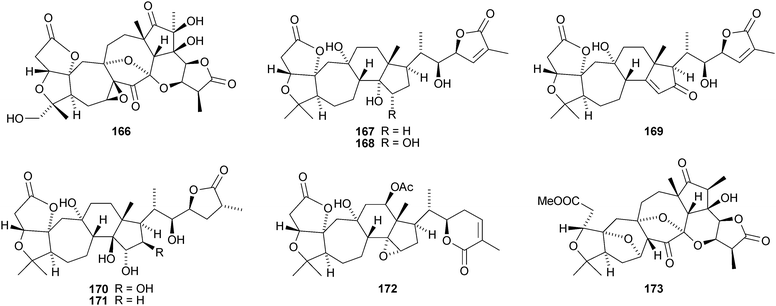
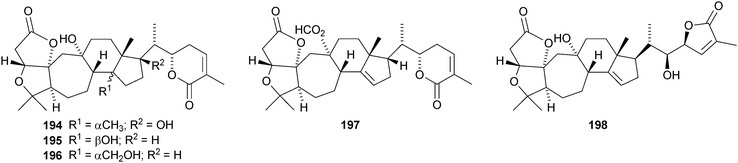
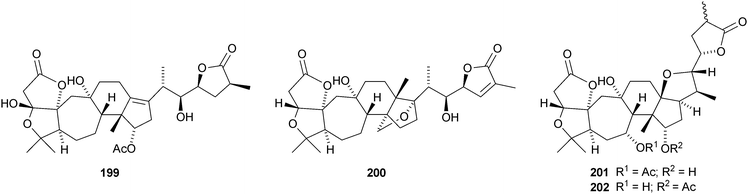
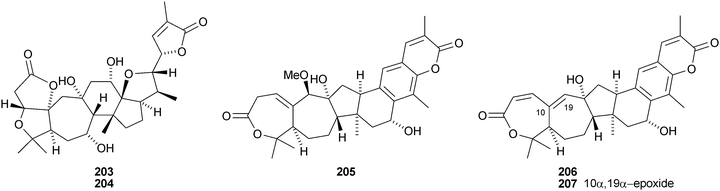
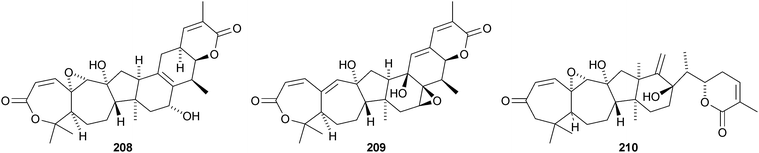
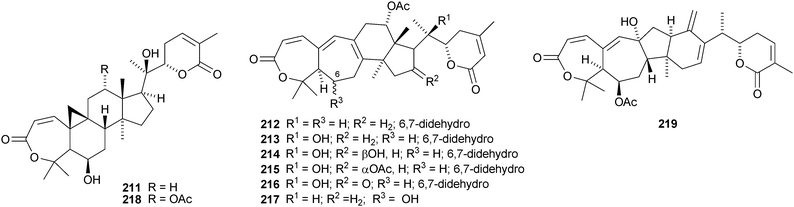
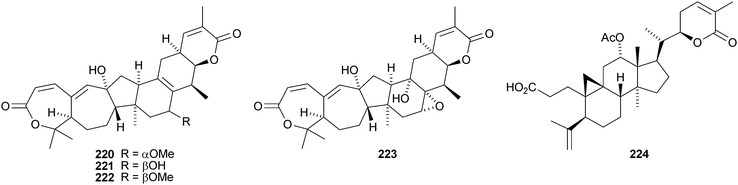
Kadcoccilactones A–Q 194–211 have been isolated from Kadsura coccinea.71,120 The structure of 194 was confirmed by X-ray analysis. Kadsura heteroclita is the source of heteroclitalactones G–M 212–218121 and longipedlactone J 219.122 Other compounds in this series include kadlongilactones C–F 220–223 from Kadsura longipedunculata,123 polysperlactones A 224 and B 212 (same as heteraclitalactone G) from Kadsura polysperma124 and renchanglactone A 211 (same as kadcoccilactone Q) from Kadsura renchangiana.125
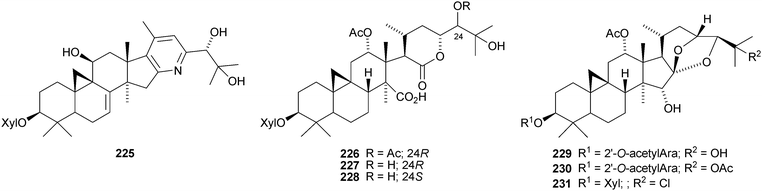
Two groups have reported a new cycloartane alkaloid 225 from the rhizomes of Cimicifuga foetida,126,127 which has two names, cimicifine A and cimicifugadine. Three new ring-D cleaved glycosides 226–228 have been isolated from Cimicifuga rhizome.128 Cimicifoetisides A 229 and B 230 are cytotoxic glycosides from Cimicifuga foetida.129 Chlorodeoxycimigenol 3-O-β-D-xyloside 231 has been identified in extracts of Cimicifuga racemosa.130 It appears to be an artefact of the extraction process.
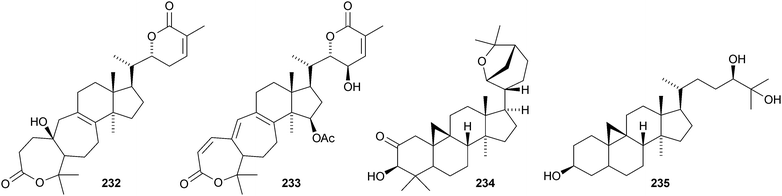
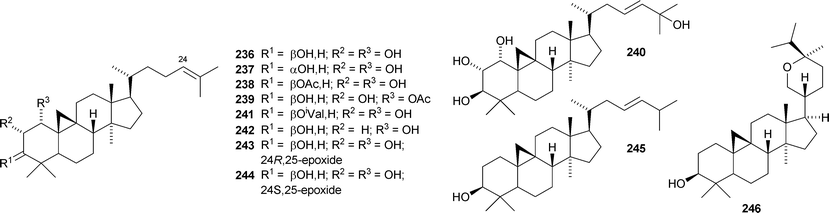
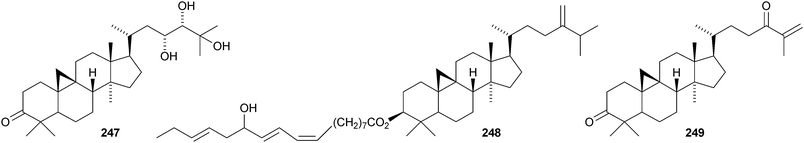
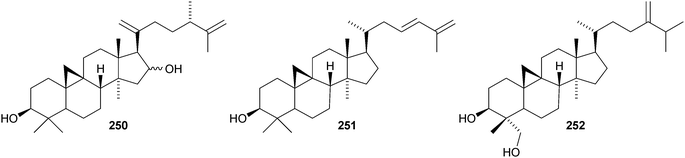
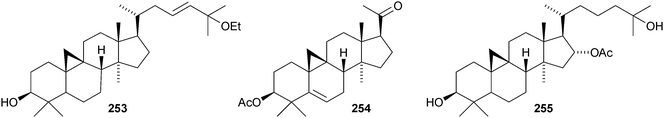
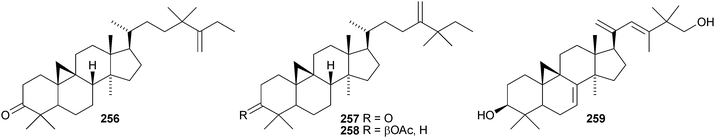
Colossolactones IV 23246 and VIII 23347 are rearranged cycloartane ring-A lactones from the Vietnamese mushroom Ganoderma colossum. Monocarpinin 234 and the 28,29-dinor-derivative 235 are constituents of Monocarpia marginalis131 and Chukrasia tabularis var. velutina,132 respectively. Commiphora opobalsamum contains nine new cycloartanes 236–244.133,134 Other simple new cycloartanes include 245 from Senefelderopsis chiribiquetensis,135 berenjenol 246 from Oxandra cf. xylopioides,136247 from Aglaia forbesii,137248 from Gnetum pendulum,138249 from Derris laxiflora,139250 from Skimmia laureola,140251 from the marine green alga Cladophora fascicularis,141252 from Euphorbia guyoniana,142253 from Euphorbia humifusa,143 artocapuates A 254 and B 255 from Artocarpus nobilis,144256–258 from Cocos nucifera,145259 and 260 from Nigella sativa,146261 from Fritillaria hupehensis,147 and the chloro-derivative 262 from Ligularia stenocephala.148 Reference NMR data from synthetic 23E- and 23Z-cycloart-23-ene-3β,25-diols indicate that structural revision is required for several related triterpenoids.149
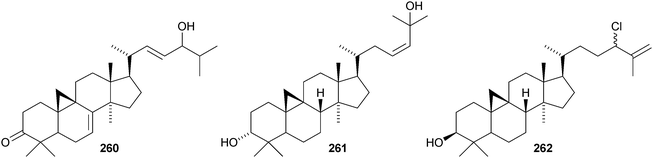
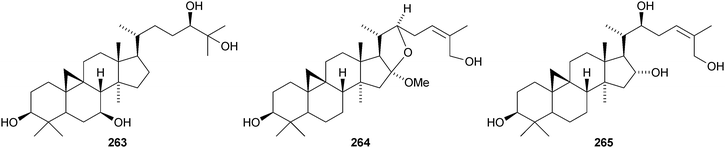
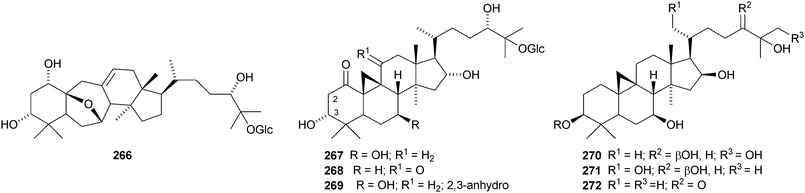
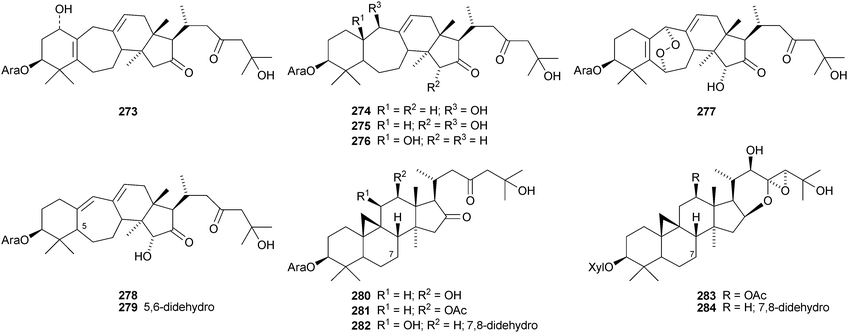
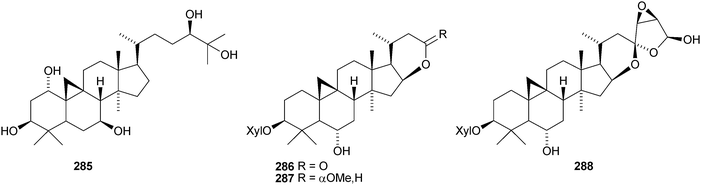
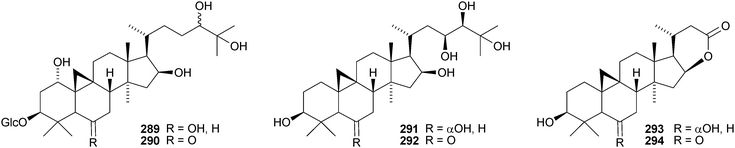
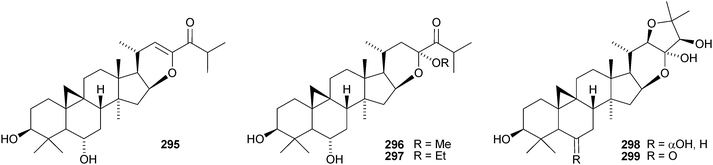
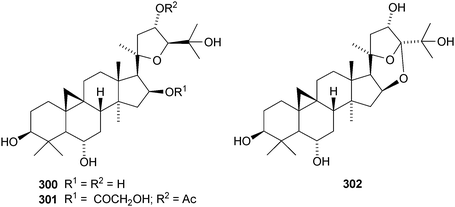
The cycloartane-3β,7β,24R,25-tetraol 263 is a new genin of the cycloartane glycosides from Camptosorus sibiricus.150 Aquilegiosides K and L, from Aquilegia vulgaris, also have new genins 264 and 265.151 Sutherlandiosides A–D 266–269 are new glucosides from Sutherlandia frutescens.152 The structure of 266 was confirmed by X-ray analysis. Tareciliosides A–G have been reported from the leaves of Tarenna gracilipes and include the new genins 270–272.153 Podocarpasides A–J 273–282 are constituents of the roots of Actea podocarpa.154,155 The structure of podocarpaside E 277 was revised on the basis of an X-ray analysis.156 Two new cycloartane xylosides 283 and 284 have been reported from the roots of Actea pachypoda, together with the known 12β-acetoxycimigenol.157 Cyclomacrogenin B 285 is cycloartane-1α,3β,7β,24R,25-pentaol, which was obtained from Astragalus macropus.158 A variety of saponins with new genins has been reported from Astragalus species, including bicusposides A 286, B 287 and C 288 from Astragalus bicuspis,159 mongholicosides A 289 and B 290 from Astragalus membranaceus var. mongholicus,160 eremophilosides A–K from Astragalus eremophilus161 (eremophilosides C–K have the new genins 291–299) and three saponins from Astragalus campylosema ssp. campylosema with the new genins 300–302.162
New cycloartane saponins with known genins include astramembranosides A and B from Astragalus membranaceus,163 cimiaceroside C, cimifosides A–D164 and cimifoetisides VI and VII165 from Cimicifuga foetida, cycloascauloside B from Astragalus caucasicus,166 cyclochivinosides B,167 C168 and D169 from Astragalus chivensis, cyclosophoside A from Cassia sophera,170 cyclotrisectoside from Astragalus dissectus171 and saponins without trivial names from Camptosorus sibiricus172,173,174 and Thalictrum fortunei.175
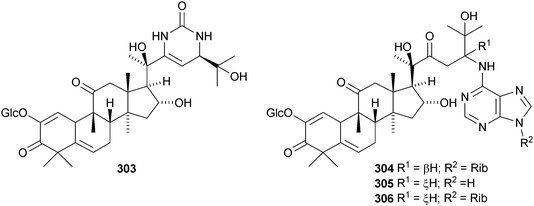
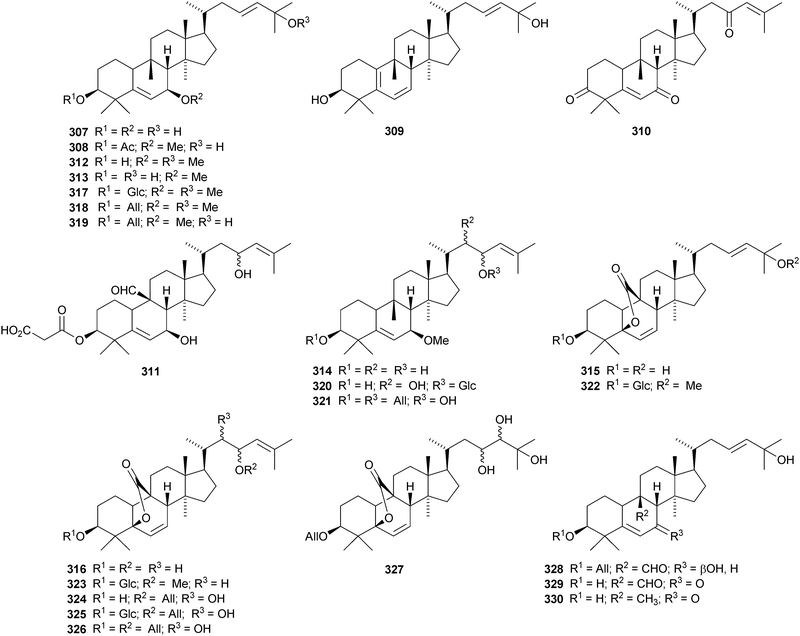
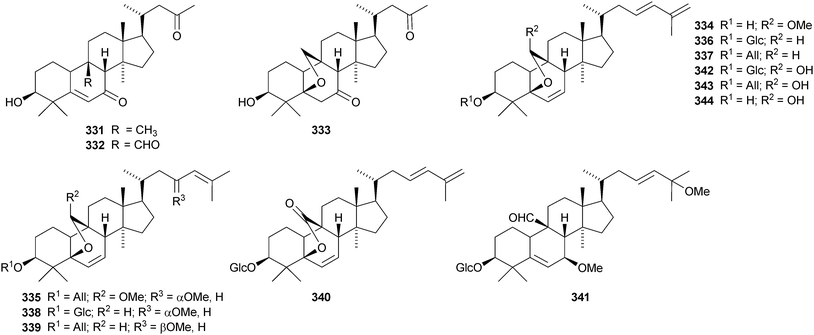
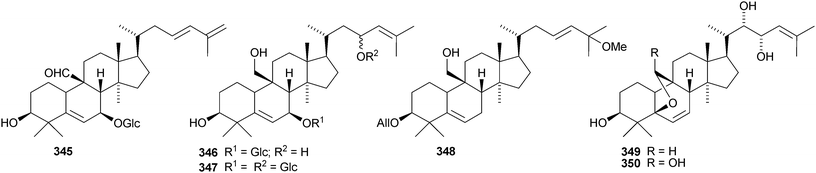
Machilaminosides A 303 and B 304 are unusual nitrogen-containing cucurbitacins from the stem bark of Machilus yaoshansis.176 Cucurbitaglycosides A 305 and B 306, with unspecified stereochemistry at C-24, have been isolated from the fruit of Cucurbita pepo cv. dayangua.177 Many new cucurbitacins and their glycosides have been reported from Momordica charantia. They include 307–310 from the stems,178 the known momordicin I and its 3-malonyl derivative 311,179 karavilagenins A–E 312–316 and karavilosides I–IX 317–327 from the dried fruit,180,181 karavilagenins A 312 and B 313 and 328 from the dried gourds,182 kuguacins A–E 329–333 from the roots,183 charantosides I–VIII 334–341 from the fruit,184 glycosides 342. and 343 together with their genin 344,185 kuguaglycosides A–H, of which C–E have new genins 345–347, from the root,186 two new glycosides from the fruit, one with the new genin 348,187 momordicosides M–O with two new genins 349–350188 and momordicoside P189 and momordicine V,190 both with known genins.
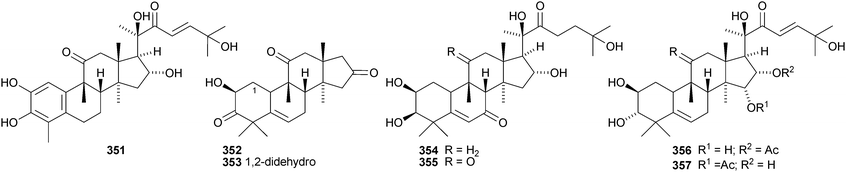
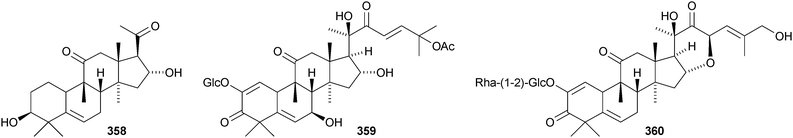
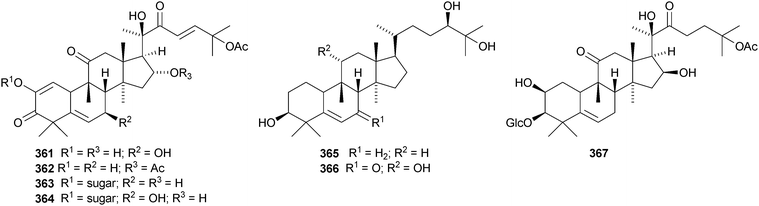
Desacetylfevicordin A 351, from the Indonesian medicinal plant Phaleria macrocarpa, is the same as fevicordin C and has been identified as a natural product for the first time.191 Endecaphyllacins A 352 and B 353 are octanor-cucurbitane constituents of the tubers of Hemsleya endecaphylla.192 The structure of 352 was confirmed by X-ray analysis. Other new cucurbitacins include 354 and 355 from Cayaponia racemosa,193356 and 357 from Physocarpus capitatus,194 a saponin with a new hexanorcucurbitane genin 358195 from the fruit of Cucurbita pepo cv. dayangua and colocynthosides A 359 and B 360 from Citrullus colocynthis.196 Bacobitacins A–D 361–364 have been isolated from Bacopa monnieri.197 The fruit of Siraitia grosvenorii yielded several new glycosides,198,199 two of which, 11-deoxymogroside III and 7-oxomogroside II E, have the new genins 365 and 366, respectively. Glucoside 367 is a constituent of Hintonia latiflora.200 Delavanosides A–E, all with known genins, have been reported from the tubers of Hemsleya delavayi.201
4 The dammarane group
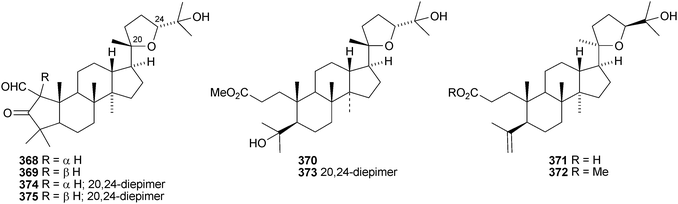
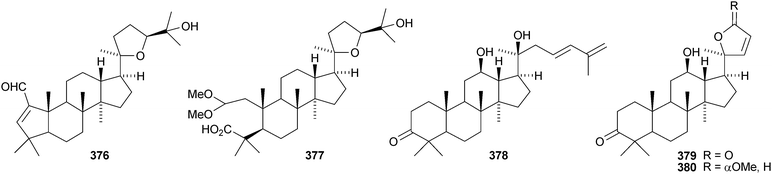
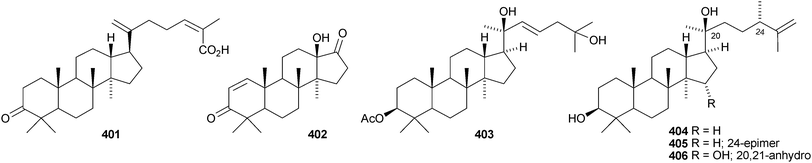
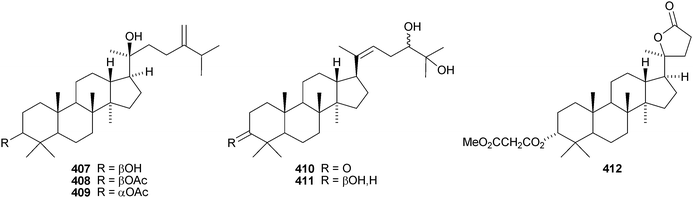
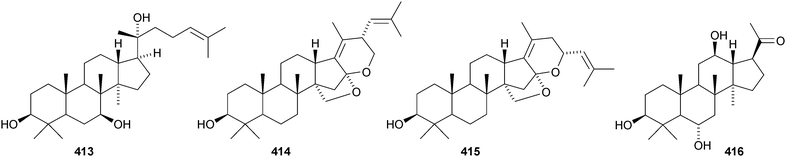
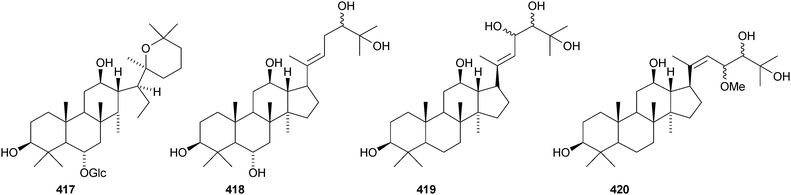
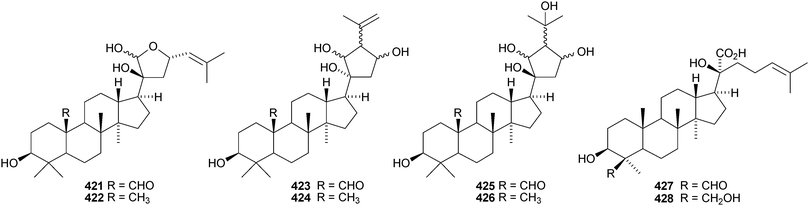
Aglaia sylvestris is a rich source of dammaranes. New compounds include silvaglins A 368 and B 369, methyl isofoveolate B 370, isoeichlerianic acid 371 and its methyl ester 372, methyl foveolate B 373, isosilvaglins A 374 and B 375, deoxysilvaglin 376 and aglasilvinic acid 377.202,203 Also the stereochemistry of the known foveolin B (free acid of 373) has been revised. Six new dammaranes, cylindrictones A–F 378–383, have been isolated from the leaves of Viburnum cylindricum.204 The two dammarane derivatives 384 and 385, from Aglaia perviridis, have an unusual rearranged side-chain.205 Other reports of new dammaranes include santolins A–C 386–388 from Salvia santolinifolia,206 oliganthas A 389 from Saussurea oligantha,207 gentirigenic acid 390 and gentirigeosides A–E 391–395 from the roots of Gentiana rigescens,208396–398 from Cabralea canjerana,209 the hydroperoxides 399 and 400 from Ligustrum lucidum,210401 and the octanor-derivative 402 from Maytenus macrocarpa,211403 from radix Ranunculus ternati,212 foliasalacins A1–A4404–407 from the leaves of Salacia chinensis,213408 and 409 from Phyllanthus polyanthus,214 ailexcelone 410 and ailexcelol 411 from the heartwood of Ailanthus excelsa215 and the methyl malonate 412 from the floral spikes of Betula platyphylla var. japonica.216Sapinmusaponins O and P are new saponins from the fruit and galls of Sapindus mukurossi.217 Sapimnusaponin P has the new genin 413. Bacopasaponin G and bacoside A6, from Bacopa monniera, are glycosides of 17,20-anhydro-derivatives 414 and 415 of jujubogenin and pseudojujubogenin, respectively.218 The aglycone 416 of notoginsenside R10 has been obtained from the leaves of Panax ginseng.219 The new glucoside 417 has been isolated from the roots of Panax notoginseng.220 Notoginsenosides ST-1, ST-2, ST-3 and ST-5 are constituents of steamed Panax notoginseng.221 The first three have the new genins 418–420 respectively. New dammarane saponins from Gymnostemma pentaphyllum include six new genins 421–426222 while those from Gymnostemma pubescens have two new genins 427 and 428.223 New dammarane saponins with known genins include bacopaside VI from Bacopa monnieri,224 floralginsenosides A–F,225 G–K, La, Lb226 and M–P227 from Panax ginseng flower buds, jujuboside G from seeds of Zizyphus jujuba,228 notoginsenosides Rw1, Rw2,229 FP1 and FP2230 from Panax notoginseng, quinquefolosides La and Lb from Panax quinquefolium231 and saponins without trivial names from Panax ginseng232 and Panax quinquefolium.233 A review covering the biosynthesis of the ginsenosides has been produced.234
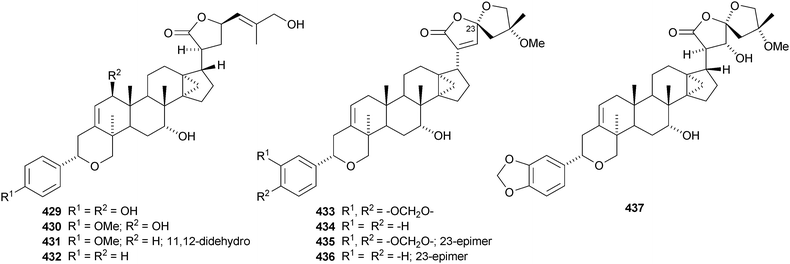
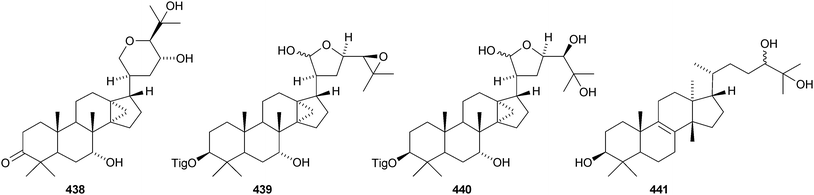
Dichapetalins I–J 429–432 are constituents of the stem bark of Dichapetalum gelonoides.235 Five new dichapetalins, acutissimatriterpenes A–E 433–437, have been reported from the aerial parts of Phyllanthus acutissima.236 The structures of 433 and 437 were confirmed by X-ray analyses. Aglaiaglabretols A 438, B 439 and C 440 are cytotoxic constituents of Aglaia crassinervia.237
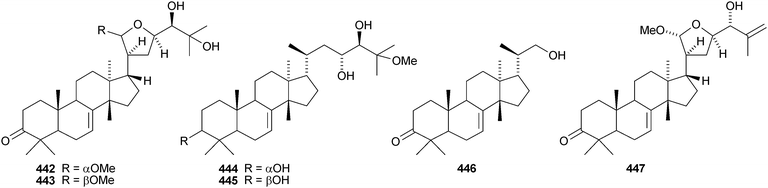
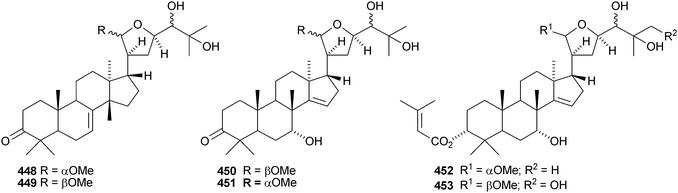
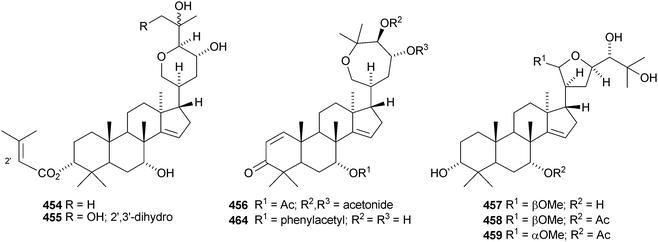
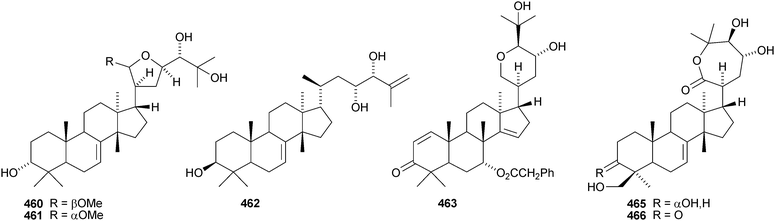
The simple euphane 441 derivative has been obtained from Clusia columnaris.238 The fruit of Poncirus trifoliata yielded four tirucallane derivatives, 21α-O-methylmelianodiol 442, 21β-O-methylmelianodiol 443, hispidiol A 25-methyl ether 444 and hispidiol B 25-methyl ether 445,239 while the fruit of Phellodendron chinense var. glabriusculum gave the pentanor-derivative phellogin 446240 and compound 447.241Cedrela sinensis contains the tirucallanes 448 and 449 and the apotirucallanes 450–456.242 The structure of 456 was confirmed by X-ray analysis. A similar mixture of apotirucallanes, agladupols A–C 457–459, and tirucallanes, agladupols D 460 and E 461, was found in the leaves and stems of Aglaia duperreana.243 Other new compounds include turrapubesols A–C 462–464 from Turrea pubescens,244 munronosides I–IV from Munronia delavayi245 with two new genins 465 and 466, and sapinmusaponins Q and R, with known genins, from the galls of Sapindus mukorossi.246
4.1 Tetranortriterpenoids
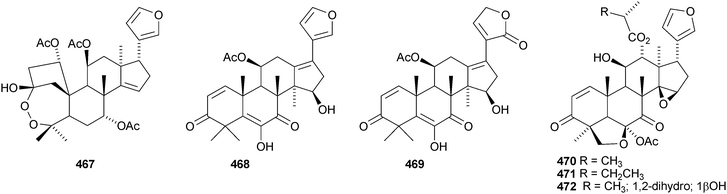
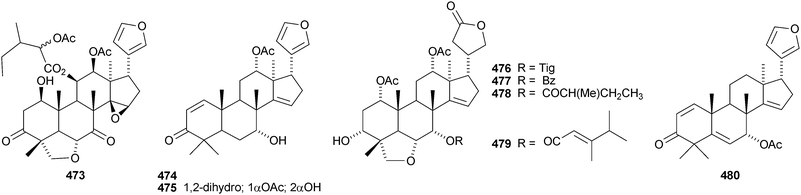
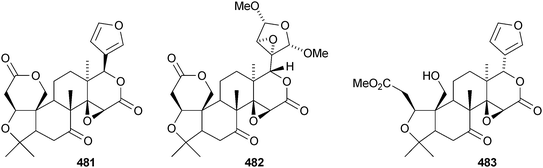
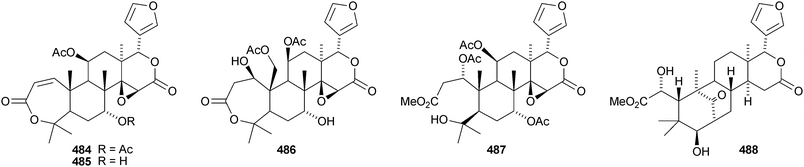
Walsuronoid A 467 is a peroxide derivative from Walsura robusta.247 It is accompanied by walsuronoids B 468 and C 469, which apparently are rare tetranor-dammarane derivatives. The authors suggest that they are formed by methyl migration following acid-catalysed 14,15-epoxide ring opening of a limonoid. The structure of walsuronoid A 467 was confirmed by X-ray analysis. A Malleastrum species from the Madagascar rainforest afforded malleastrones A–C 470–472.248 The structures of malleastrones A and B were confirmed by X-ray analyses. Munronin G 473 is a new compound from Munronia delavayi.249 Other new compounds include the azadirone derivatives 474 and 475 from Turraea cornucopia,250 dysoxylins A–D 476–479 from Dysoxylum gaudichaudianum251 and chisosiamensin 480 from the seeds of Chisocheton siamensis.252 X-ray structure analyses of the known epoxyazadiradione253 and 6α-acetoxyazadirone254 have been published.A review covering the biological activities of the citrus limonoids has been published.255 17-epi-Limonin 481 has been reported from a Citrus species.256 Evolimorutanin 482 from the unripe fruit of Evodia rutaecarpa257 and methyl uguenenoate 483 from Vepris uguenensis258 are presumably transformation products of limonin. The obacunol derivatives 484 and 485, the nomilin derivatives 486 and 487 (odoralide) and 8β,14α-dihydroswietenolide 488 are constituents of the stem bark of Cedrela odorata.259
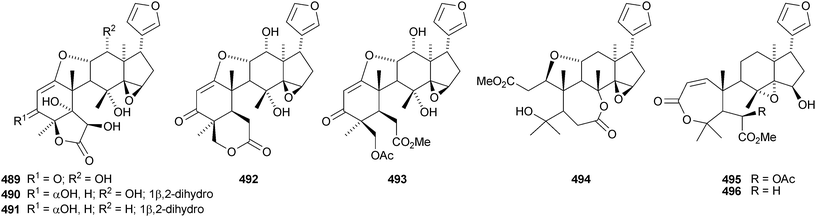
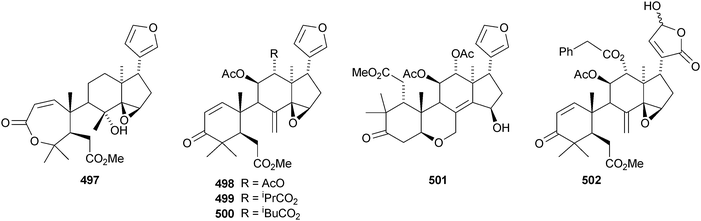
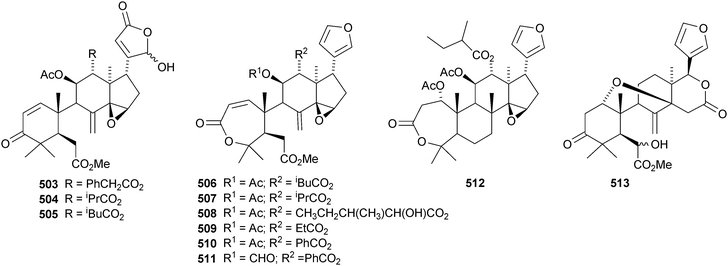
The leaves and stems of Toona ciliata contain a range of limonoids including the nor-derivatives toonaciliatins A 489, F 490 and G 491 and toonaciliatins B–E 492–495,H 496 and I 497.260 Toonaciliatins H and I are reported for the first time as natural products. Turraea pubescens261 and Amoora tsangii262 are also rich sources of limonoids. The former produced turrapubesic acids A–C 498–500 and turrapubescins C–G 501–505 while the latter yielded amotsangins A–G 506–512. The C-17 epimer 513 of methyl 6-hydroxyangolensate (the structure is wrongly drawn in the paper) has been isolated from Cichorium intybus.263
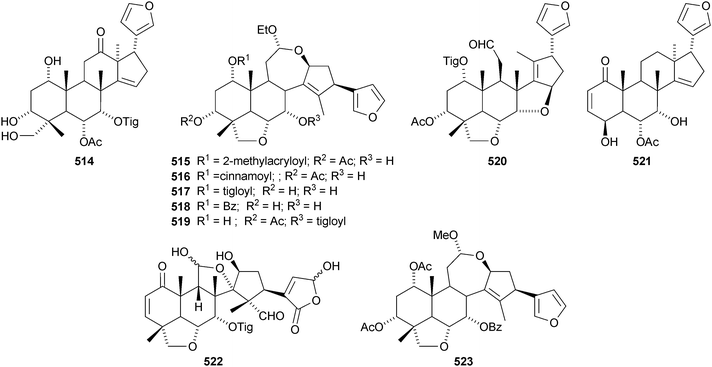
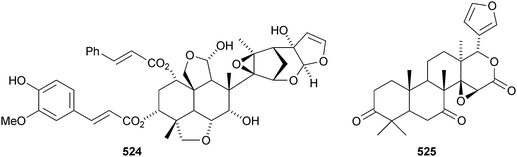
A review covering the biological effects of toosendanin has been published.264 New compounds from Melia toosendan include the 12-ketone toosendone 514 and the ring-C-cleaved acetals 12-ethoxynimbolinins A–D 515–518.265 Two other ring-C-cleaved derivatives, 519 and 520, have also been isolated from Melia toosendan.266 Two unusual limonoids, ceramicine A 521, which lacks methyl groups at C-4, and the ring-C-seco walsogyne A 522, have been reported from Chisocheton ceramicus and Walsura chrysogyne respectively.267 12-O-Methylnimbolinin A 523 and the meliacarpin derivative 524 have been identified in fruits of Melia azedarach.268 The gedunin derivative ekeberin C1525 is found in the stem bark of Ekebergia capensis.12
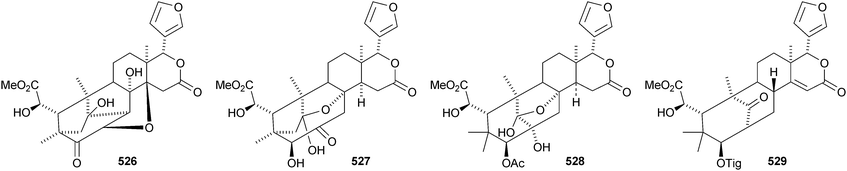
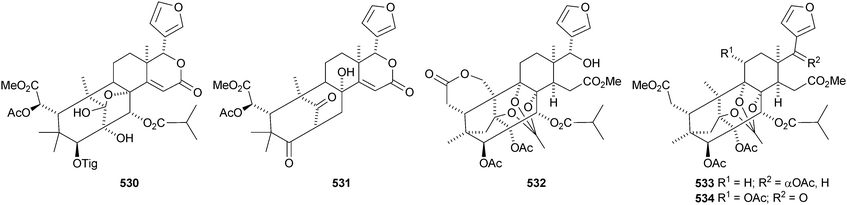
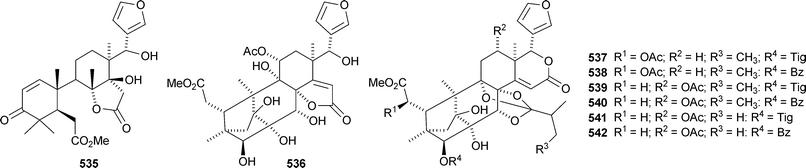
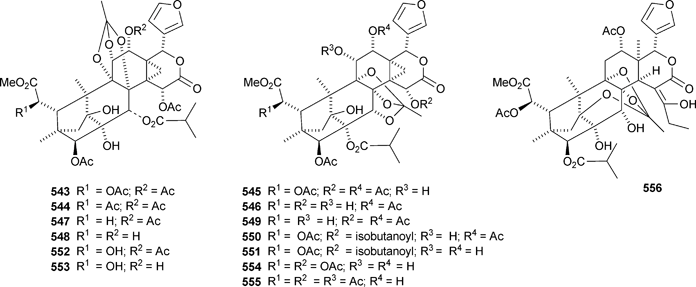
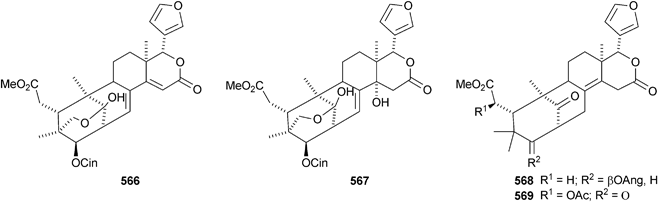
There seems to be no end to the list of new bicyclononanolides and their variants from the Meliaceae family – more than fifty compounds have been reported in the period of review. These include deacetylkhayanolide E 526, 6S-hydroxykhayalactone 527 and grandifolide A 528 from the stem bark of Khaya grandifoliola,269 angolensins A–C 529–531 from the root bark of Entandrophragma angolense,270 kotschins A–C 532–534 from the roots of Pseudocedrela kotschyi,271 swiemahogins A 535 and B 536 from Swietenia mahogani,272 six phragmalin derivatives 537–542 from Swietenia macrophylla,273 the cyclopropylphragmalin derivatives tabularisins A–D 543–546 from the seeds of Chukrasia tabularis,274 tabularisins E–N 547–555 and tabularisin P 556, which is in equilibrium with its non-enolic form tabularisin O, from the twigs and leaves of Chukrasia tabularis,38,275 the cyclic carbonate chuktabrin A 557 and the unusual chuktabrin B 558 also from the twigs and leaves of Chukrasia tabularis,276 the acetals chuktabularins A–D 559–562 from the stem bark of Chukrasia tabularis277 and erythrocarpines A–E 563–567 from Chisocheton erythrocarpus.278 Erythrocarpine A is seneganolide A 3-benzoate. Ekeberins C2568 and C3569 have been isolated from the stem bark of Ekebergia capensis.12
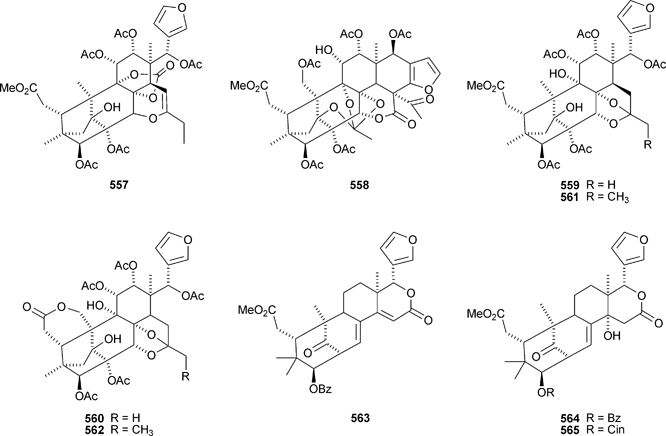
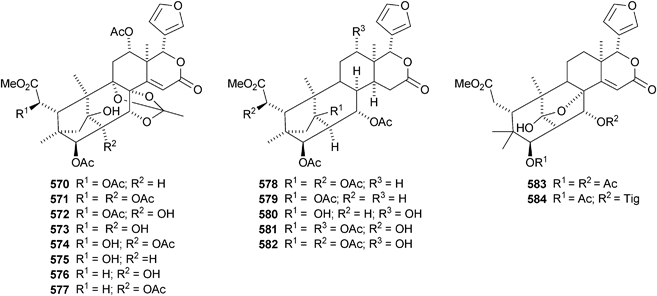
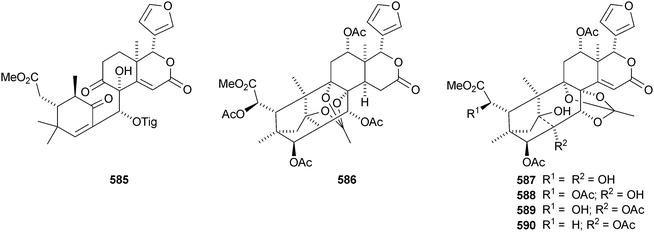
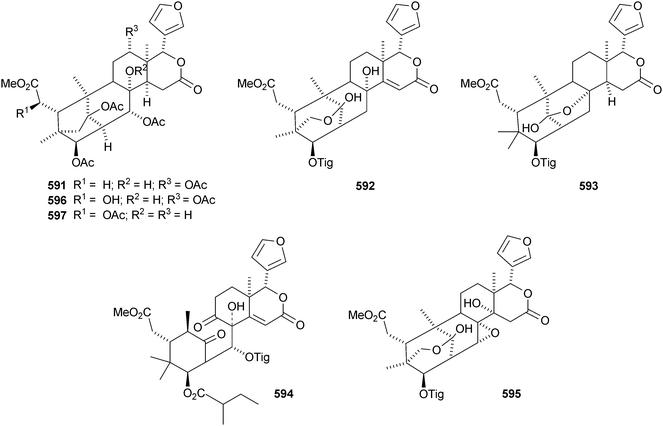
The Chinese mangrove Xylocarpus granatum is also a good source of bicyclononanolides related to phragmalin. There is some duplication in the published trivial names. Several groups of compounds have been reported including xyloccensins Q–V 570–577279 (the structure of xyloccensin Q 570 was confirmed by X-ray analysis and in the original reference the structures are drawn with the wrong absolute configuration), xylocarpins A–I 578–586,280 xyloccensins Q–U 587–591281 (duplicate names), xylogranatin E 592,282 an equilibrium mixture of the hemiacetal xylocarpin A 593 and its ketol isomer xylocarpin B283 (duplicate names) and granaxylocarpins A 594 and C–E 595–597 from the seeds of Xylocarpus granatum.284 Granaxylocarpin B, from the same source, appears to be the same as xylocarpin H 585. The structure of xylocarpin U 591 was revised in this paper.
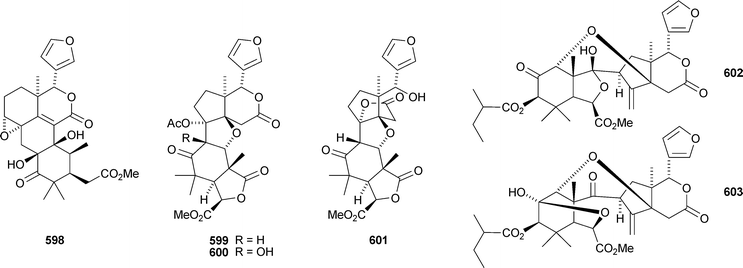
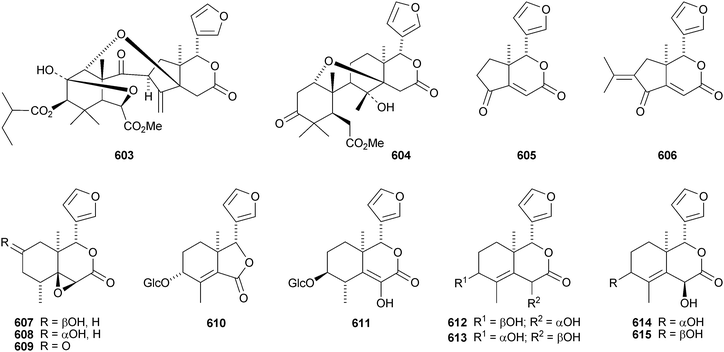
seco-Dukunolide F 598 is a new limonoid from Lansium domesticum.285 Trijugins D–H 599–603, the methyl angolensate derivative 604 and the two degraded limonoids 605 and 606 are constituents of Trichilia connaroides.286 Other degraded limonoids include dysodensiols A–C 607–609 from Dysoxylum densiflorum,287 9α-hydroxyfraxinellone 9-O-β-D-glucopyranoside 610, dictamnusine 611, dictamdiol 612 and dictamdiol B 613 from the root bark of Dictamnus dasycarpus,288 and isodictamdiol 614 and the known dictamdiol 615 from the root of Dictamnus radicis.289,290 The structures of both 614 and 615 were confirmed by X-ray analyses. The authors suggest that these compounds have opposite absolute configurations to other limonoids, but this would be very unusual and requires confirmation.
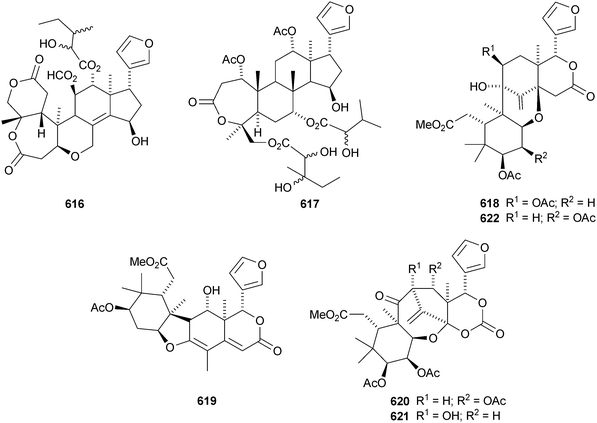
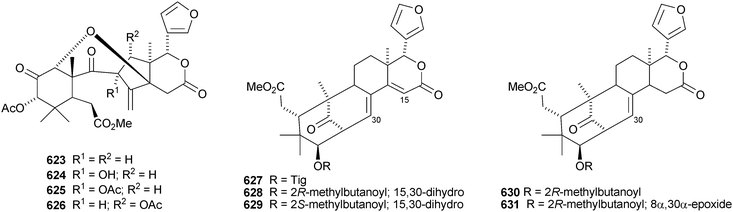
Plants of the Cipadessa genus contain a varied range of limonoids. Cipadessalide 616 and rubralin D 617 are constituents of Cipadessa baccifera,291 while Cipadessa cinerascens contains cipadesins D 618 and E 619.292 Another group has published cipadesins D–F 620–622 (duplicate names) from Cipadessa cinerascens.293 Cipatrijugins A–D 623–626 are constituents of the leaves of Cipadessa cinerascens.294 Five simple mexicanolide derivatives 627–631 have been isolated from the seeds of Cipadessa baccifera.295
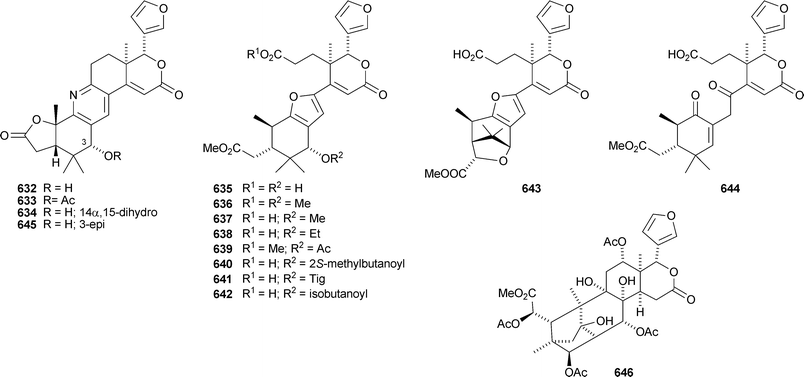
Undoubtedly the most interesting limonoids to be reported during this period are xylogranatins F–R 632–644 from the Chinese mangrove Xylocarpus granatum.296 They are based on a highly-cleaved carbon skeleton 644 which can cyclise to form a furan ring (635–643) or incorporate nitrogen to form the pyridine derivatives xylogranatins F–H 632–634. A second group has published another alkaloid, granatoine 645, apparently the C-3 epimer of xylogranatin F, and the 12-acetate 646 of xyloccensin Y from the same source.297 A review covering the occurrence, biosynthesis and biological activity of ring-D- and ring-B,D-seco-limonoids from the Meliaceae has been published.298
5 The lupane group
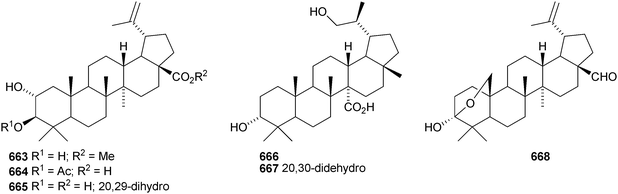
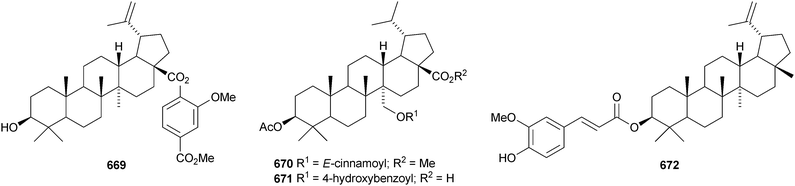
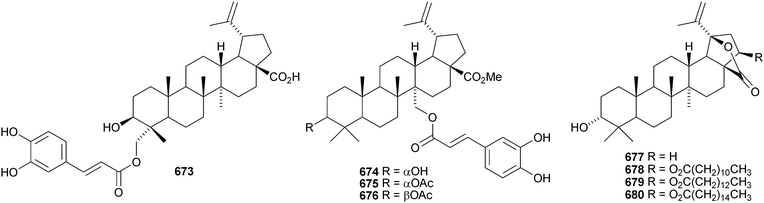
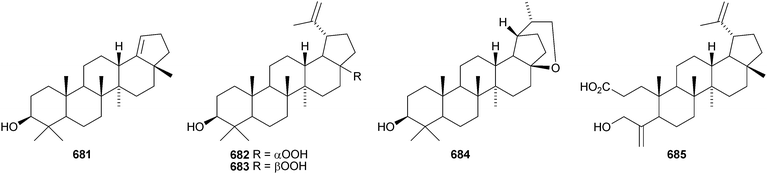
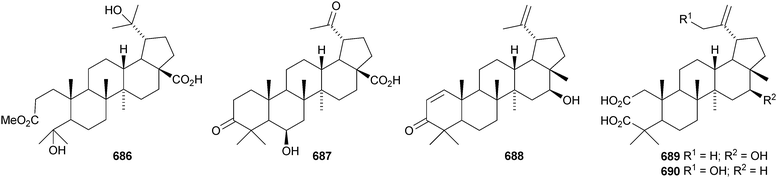
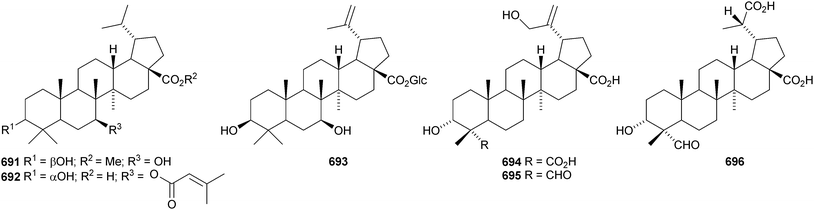
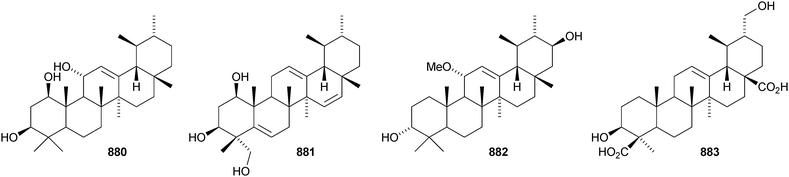
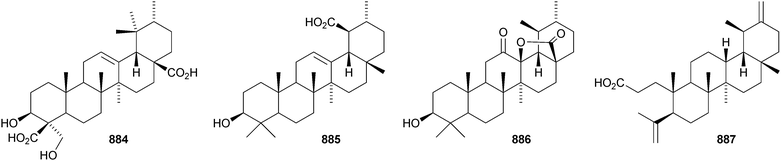
A constituent of Drypetes tessmanniana has been identified as lup-20(29)-ene-3β,6α-diol 647,299 which has previously been claimed from Perilpoca aphylla.300 Due to differences in NMR data the authors suggest that the Perilpoca aphylla constituent should be reassigned as the corresponding 6β-isomer. Sorbinal B 648, from Sorbus cashmariana, has been assigned the structure lupa-12,22(29)-diene-2α,3β,23,28-tetrol.301 The structure of sorbinol B 648 is incorrectly drawn in the reference with a 5,6-double bond. Other new lupane alcohol derivatives include lup-20(29)-ene-3β,22β-diol 649 from Moldenhawera nutans,302 lup-20(30)-ene-1β,3β,29-triol 650 from Salvia sclareoides303 and the acetates 651 and 652 from Salvia macrochlamys.304 Foliasalacins B1–B3653–655 are new lupane derivatives from the leaves of Salacia chinensis.213 The lupan-29-al derivatives 656–658 from Acacia mellifera show cytotoxic activity,305 and the lupan-28-oic acid derivatives 659 and 660 from Fagara tessmannii have α-glucosidase inhibitory properties.306Gypsophila repens is the source of the unusual lupane sulfate gypsophilin 661 and its glucosyl ester gypsophilinoside 662.307 The methyl ester 663 and the 3-acetate 664 of alphitolic acid have been isolated from seeds of Ziziphus jujuba var. spinosa308and the resin of Garcinia hanburyi309 respectively, and the related 20,29-dihydro derivative 665 has been found in Eugenia grandis stem bark.310 Other simple lupane derivatives include the 27-carboxylic acids 666 and 667 from Potentilla discolor311and the hemiacetal lantabetulal 668 from roots of Rhus javanica var. roxburghiana.312 Ocimol 669 from Ocimum basilicum is the ester of betulinic acid with methyl vanillate.313 Other lupane aromatic esters include the cinnamate 670 and the 4-hydroxybenzoate 671 from Helicteres angustifolia,314 3-ferruloyllupeol 672 from Ceriops tagal315 and the caffeoyl esters 673 from Xanthoceras sorbifolia316 and 674–676 from Peganum nigellastrum.317 3α-Hydroxylup-20(29)-en-28,19β-olide 677 is a constituent of Perrottetia arisanensis, where it occurs with the esters 678–680.31820,29,30-Trinorlup-18-en-3β-ol 681 from Arabidopsis thaliana has been given the misleading name trinorlupeol.319 The 17-hydroperoxy-28-norlup-20(29)-en-3β-ols 682 and 683 have been isolated from Melaleuca ericifolia together with the 17,29-epoxide 684 which has an impossible stereochemistry.320 It is possible that 684 has the opposite stereochemistry at C-17. The 3,4-secolupane 685 has been reported from Euphorbia humifusa.143 The related 3,4-secolupane derivative 686 is a constituent of Viburnum awabuki together with the 30-norlupane 687.321 16β-Hydroxylupa-1,20(29)-dien-3-one 688 and 16β-hydroxy-2,3-secolup-20(29)-ene-2,3-dioic acid 689 have been found in Stauntonia obovatifolia ssp. intermedia.322 The related 2,3-secolupane 690 has been isolated from Microtropis fokiensis together with the 7-oxygenated derivatives 691 and 692.294 The lupane saponin 693 from fruits of Polygonum orientale has a new genin.323 Acankoreosides F–H, from Acanthopanax koreanum, also have new lupane genins 694–696, respectively.324 Lupane saponins with known genins include stallatoside B and erucasaponin A from the cactus Stenocereus eruca325 and a saponin from roots of Carthamus tinctorius.326
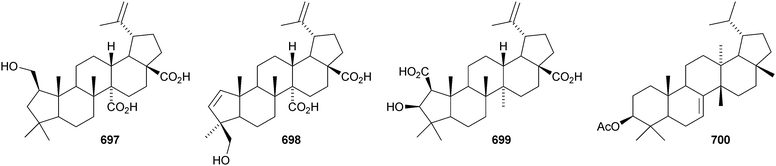
Gouanic acids A 697 and B 698 from aerial parts of Gouania ulmifolia327 and 1-epi-ceanothic acid 699 from Gleditsia sinensis328 are 3(2→1)-abeo-lupane derivatives. Elephanmollen 700 is a D:C-friedolupane derivative from Elephantopus mollis that shows cytotoxic properties.329
6 The oleanane group
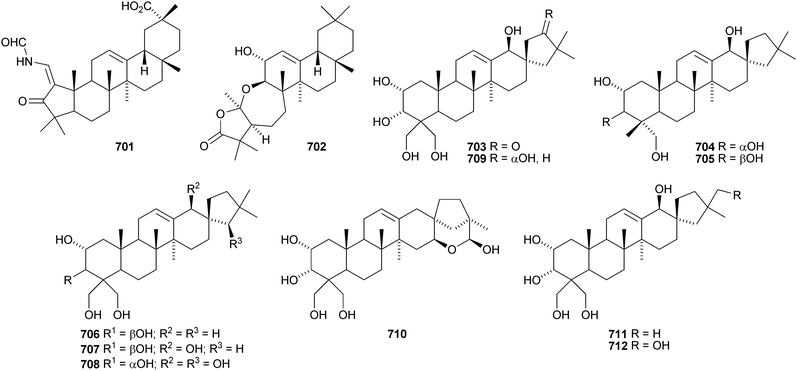
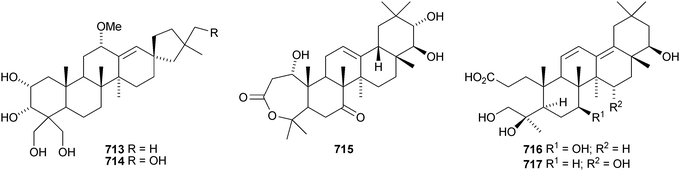
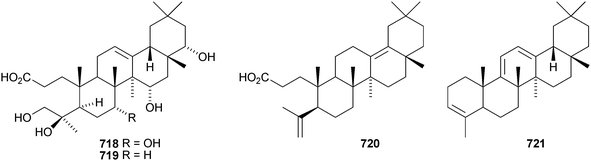
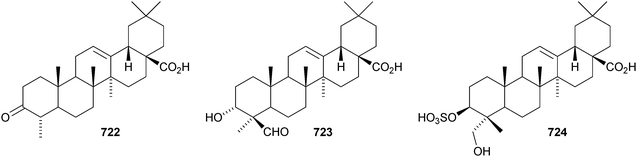
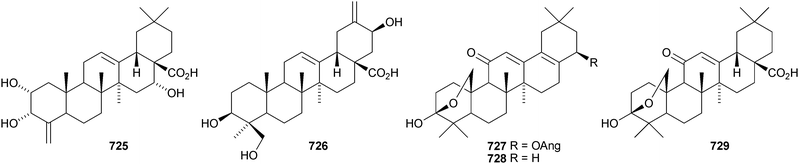
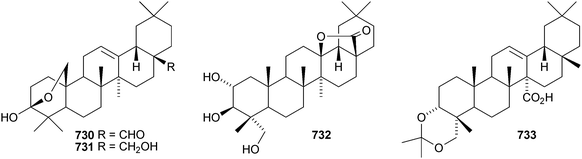
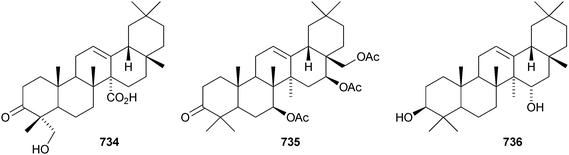
Two rearranged oleanane derivatives, dysoxyhainanins A 701 and B 702, have been identified from Dysoxylum hainanense.330 Dysoxyhainanin A 701 has a 3(2→1)-abeo rearranged skeleton with a formamide at C-2 and shows interesting antibacterial activity. Several 19(18→17)-abeo-28-noroleanenes have been isolated from rhizomes of Phlomis umbrosa including phlomisone 703, phlomistetraols A–C 704–706, phlomispentaol 707, phlomishexaols A 708 and B 709, phlomisin 710 and the related derivatives 711–714.331–333 The 3,4-secooleanane derivatives 715 from Christiana africana334 and 716–719 from the mangrove plant Hibiscus tiliaceus335 have been identified. The secooleananes 718 and 719 are shown in the reference with the 18α-configuration but there is no evidence for this stereochemistry. The ring-A cleaved oleanane 720 has been obtained from the floral spikes of Betula platyphylla var. japonica.216 24-Noroleana-3,9(11),12-triene 721 has been identified in olibanum from Boswellia serrata.336 It is possible that this nortriterpene is a pyrolysis product. 24-Nor-3-oxoolean-12-en-28-oic acid 722 has been named hederagonic acid, a name used previously for the 3-ketone of hederagenin.337 The 24-noroleanane derivative 722 occurs in Gypsohila oldhamiana, together with 3,4-di-epi-gypsogenin 723 and hederagenin 3-sulfate 724.Other noroleananes include 2α,3α,16α-trihydroxy-24-noroleana-4(23),12-dien-28-oic acid 725 from Salvia palaestina,338 the 30-nor-derivative 726 from Stauntonia obovatifoliola ssp. intermedia,322 and the 3,25-epoxy-28-noroleananes lantadienone 727 and camaradienone 728 from Lantana camara. The 3,25-epoxy derivative lantanoic acid 729 has also been found in Lantana camara,339whereas the related lantanolal 730 and lantanalol 731 are constituents of Rhus javanica var. roxburghiana.312 Dryobalanolide 732 has been isolated from Eucalyptus camaldulensis.340 Dryobalanolide 732 had previously been isolated as the 3,23-acetonide. The 3,23-acetonide of aceriphyllic acid 733 has been found in Aceriphyllum rossii together with the 3-ketone 734.341 The triacetate 735 has been isolated from the roots of Ligularia sagitta after acetylation.342 It is not clear whether the natural product is the corresponding triol or an acetylated compound. Foliasalacin C 736 is olean-12-ene-3β,15α-diol from the leaves of Salacia chinensis.213
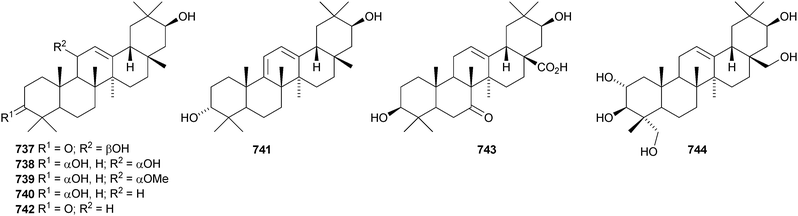
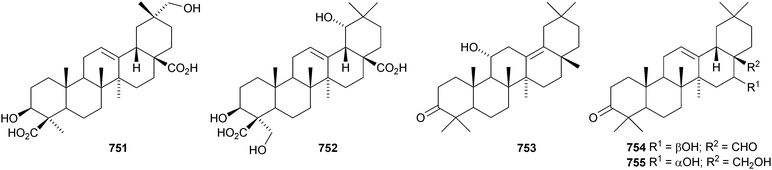
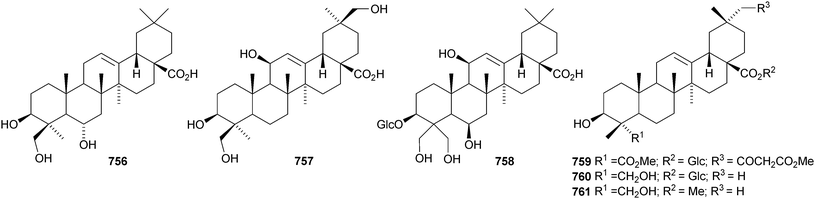
The 21β-hydroxyoleanane derivatives 737–741343 and 21β-hydroxyolean-12-en-3-one 742344 have been isolated from Hippocratea excelsa. Further 21-oxygenated oleananes include silymin B 743 from Silybum marianum,345 olean-12-ene-2α,3β,21β,23,28-pentol 744 from Laportea crenulata,346 olean-12-ene-3β,6β,21β-triol 745 from the trunk bark of Tabebuia heptaphylla,347 the 28-carboxylic acid 746 from neonauclea sessilifolia348 and the 11,13(18)-dienes 747–750 from Tetrapanax papyriferus.349 Ilexhainanins B 751 and D 752 are olean-12-ene-24,28-dioic acid derivatives from Ilex hainanensis.350 Other simple oleanane derivatives include wilforone 753 from Triperygium wilfordii,351 the 28-aldehyde 754 from Ligularia odontomanes,352 aegicornin 755 from Aegiceras corniculatum353 and salsolic acid 756354 and salsolins A 757 and B 758355 from Salsola baryosma. Kalidiumosides C 759 and D 760 and kalidiunin 761 have been found in aerial parts of Kalidium foliatum.356
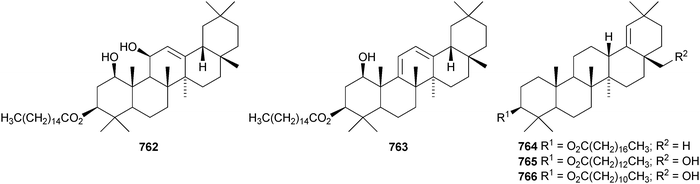
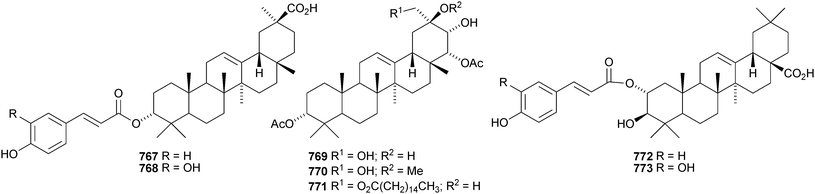
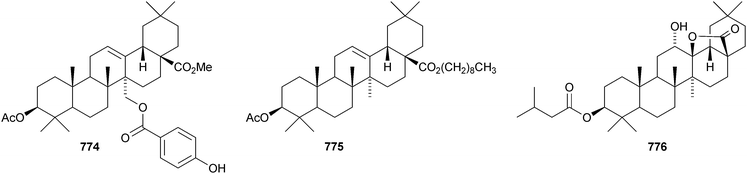
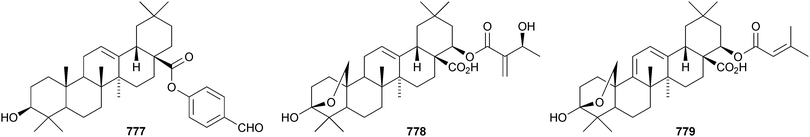
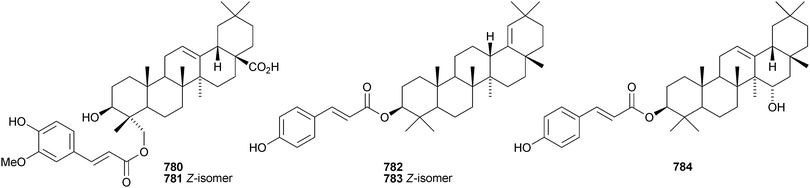
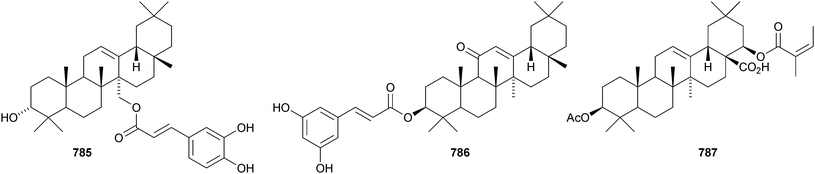
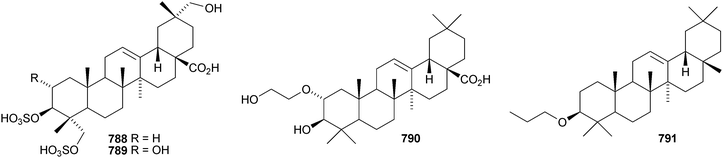
Celastrus rosthornianus is the source of the palmitoyl esters 762357 and 763.358 The ester 763 has also been isolated from Saussurea ussuriensis and named ussuriensin B.359 The fatty acid esters 764, from Maytenus salicifolia,360 and 765 and 766, from Scorzonera mongolica,361 have also been identified. Several esters have been isolated from Saussurea muliensis including the p-coumaroyl 767 and caffeoyl 768 oleanane derivatives and the 30-noroleanane esters 769–771.362 Other new oleanane esters include the maslinic esters 772 and 773 from Hippophae rhamnoides,363 the 27-(4-hydroxybenzoyl) derivative 774 from Heliceres angustifolia,314 lippiacin 775 from Lippia nodiflora,364 patrirupin A 776 from Patrinia rupestris,365 basilol 777 from Ocimum basilicum,313 camarolic acid 778 and lantrigloylic acid 779 from Lantana camara,366 the ferruloyl esters 780 and 781 from Ludwigia octovalvis,367 the p-coumaroyl esters 782 and 783 from Barringtonia racemosa368 and 784 from Rhizophora stylosa,369 the 27-caffeoyl ester 785 from Peganum nigellastrum,370 the 3,5-dihydroxycinnamoyl ester 786 from Drypetes tessmanniana299 and the angeloyl ester 787 from Lantana hispida.371 The disulfate esters 788 and 789 are constituents of Melissa officinalis.372
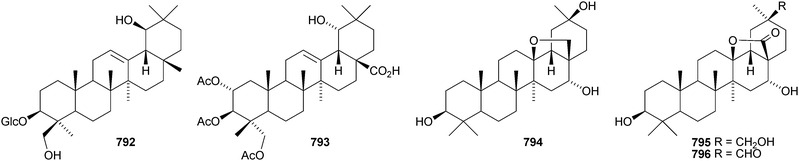
Psidiumoic acid 790, from Psidium guajava, is an unusual 2-hydroxyethyl ether.373 β-Amyrin propyl ether 791 has been found in Erythrina sigmoidea together with sigmoiside F 792, which has a new genin.374 Pteleopsoside is an oleanane saponin with a known genin from Pteleopsis hylodendron, where it occurs with the triacetate 793.375 Ardisicrenosides K and L are oleanane saponins from Ardisia crenata.376 Ardisicrenoside L has a new noroleanane genin 794. Some of the ardisianosides A–K, from Ardisia japonica, have new genins.377 Ardisianoside G has the same genin 794 as ardisicrenoside L whereas ardisianosides I, J and K have the genins 795–797, respectively.
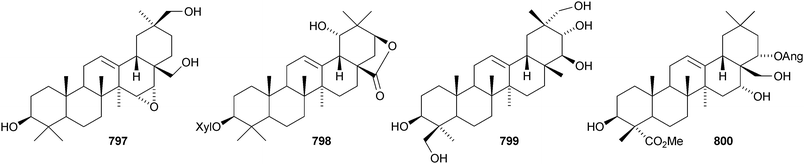
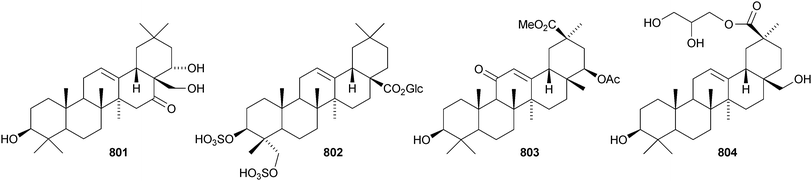
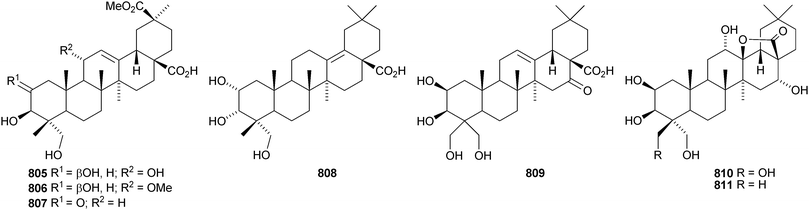
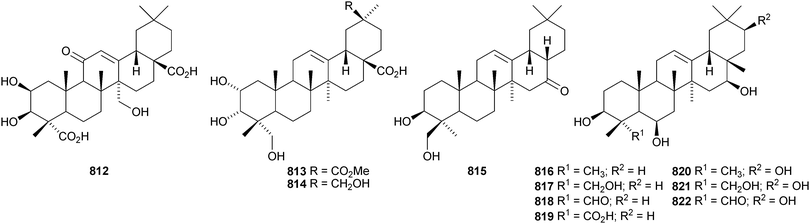
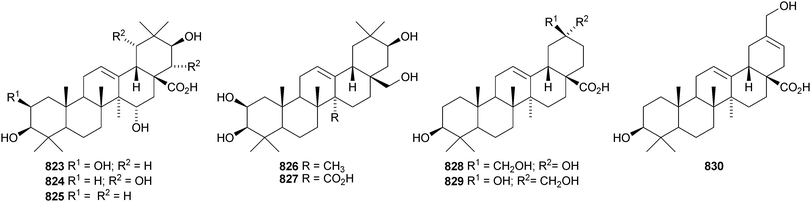
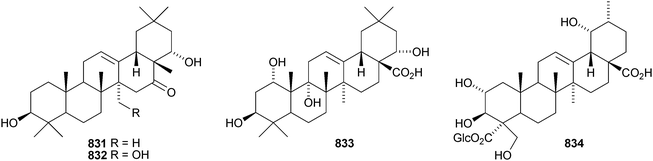
Other new oleanane saponins with new genins include the xyloside 798 from Astragalus corniculatus,378 six saponins from Astragalus flavescens with the genin 21-epi-kudzusapogenol A 799,379 theasaponins A4, A5, C1, E8, E9, G1 and H1 from Camellia sinensis including the genin 800,380 eryngiosides A–L from Eryngium yuccifolium including the genin 801,381 saponins from Fagonia arabica including the disulfate 802,382 saponins from Glycyrrhiza uralensis including 22β-acetoxyglycyrrhizin 803,383 davuricoside N from Lysimachia davurica with the genin 804,384 phytolaccasaponins N-1–N-5 from Phytolacca americana including the genins 805–807,385 three saponins from Pimenta dioica with the genin 808,386 saponins from Platycodon grandiflorum with the genins 16-oxoplatycodigenin 809387and the lactones 810 and 811,388 polygalasaponins XLVII–XL from Polygala japonica including the genin 812,389 saponins from Portulaca oleracea with the genins 813 and 814,390 saponins from Prunella vulgaris including the 28-noroleanane genin 815,391 seven saponins from Silphium radula with genins 816–822,392 stachyssaponins A–C from Stachys parviflora with the genins 823–825,393,394 brauhenosides A and B from Stocksia brauhica with genins 826 and 827,395 yemuosides YM17–YM20 from Stauntonia chinensis with genins 828–830,396 saponins from Stylosanthes erecta including genins 831 and 832,397 saponins from Terminalia arjuna with the genin 833398 and trachelosperoside F 834 from Trachelospermum jasminoides with a new 30-nor genin.399 New oleanane saponins with known genins that have been assigned trivial names are listed in Table 1.
| Trivial name | Plant species | Reference |
|---|---|---|
| Acanthopanaxoside E | Acanthopanax senticosus | 400 |
| Achyranthosides G and H | Achyranthes fauriei | 401 |
| Aesculiosides IIe–IIk and IIIa–IIIf | Aesculus pavia | 402 |
| Akebosides La and Lb | Akebia quinata | 403 |
| Ardisiacrenoside I | Ardisia crenata | 404 |
| Arganins L and O–R | Argania spinosa | 405 |
| Assamicins VI–VIII | Aesculus assamica | 406 |
| Asteratoidesoside A | Aster souliei | 407 |
| Bodinierin C | Elsholtzia bodinieri | 408 |
| Brevifoliasaponin | Calliandra brevifolia | 409 |
| Cauloside H | Caulophyllum thalictroides | 410 |
| Cernuasides A–D | Pulsatilla cernua | 411,412 |
| Chakasaponins V and VI | Camellia sinensis | 413 |
| Chionaeosides A–D | Paronychia chionaea | 414 |
| Clematiganoside A | Clematis ganpiniana | 415 |
| Codonolasides I–III | Codonopsis lanceolata | 416,417 |
| Congmuyanoside I | Aralia elata | 418 |
| Dexylosyltubeimoside III | Bolbostemma paniculatum | 419 |
| Dipterosides A–E | Dipteronia dyeriana | 420 |
| Floratheasaponins D–I | Camellia sinensis | 421 |
| Foliatheasaponins I–V | Camellia sinensis | 422 |
| Giganteasaponins 5 and 6 | Solidago gigantea | 423 |
| Giganteosides L–N | Cephalaria gigantea | 424 |
| Gleditzsioside Z | Gleditisia sinensis | 425 |
| Gummiferaosides A–C | Albizia gummifera | 426 |
| Gymnemosides W1 and W2 | Gymnema sylvestre | 427 |
| Gypsosaponins A (Gypoldoside A) –C | Gypsophila oldhamiana | 428,429 |
| Hehuanoside A | Albizia julibrissin | 430 |
| Helianthosides 4 and 5 | Helianthus annuus | 431 |
| Hydrocosisaponins A–F | Hydrocotyle sibthorpioides | 432 |
| Ilexpernoside F | Ilex pernyi | 433 |
| Ilexsaponin C | Ilex pubescens | 434 |
| Impatiprins A–C | Impatiens pritzellii var. hupehensis | 435 |
| Isoescins VIa–VIIa | Aesculus turbinata | 436 |
| Lancemasides B–G | Codonopsis lanceolata | 437,438 |
| Lonicerosides D and E | Lonicera japonica | 439 |
| Lysichrisides A and B | Lysimachia christinae | 440 |
| Montanosides 1 and 2 | Clematis montana | 441 |
| Nigellosides A–D | Nigella damascena | 442 |
| Oblonganosides L–M | Ilex oblonga | 443 |
| Onjisaponins | Polygala tenuifolia | 444,445 |
| Perennisaponins A–F | Bellis perennis | 446 |
| Perennisosides I–VII | Bellis perennis | 447 |
| Pharbitosides A and B | Pharbitis nil | 448 |
| Pithelucosides A–C | Pithecellobium lucidum | 449 |
| Puberosides A and B | Glochidion puberum | 450 |
| Raddeanoside R19 | Anemone raddeana | 451 |
| Repensosides A–F | Gypsophila repens | 452 |
| Sapinmusaponins K–N | Sapindus mukorossi | 217 |
| Serrulatins A–E | Photinia serrulata | 453 |
| Sigmoiside E | Erythrina sigmoidea | 454 |
| Stauntoside A | Suantonia chinensis | 455 |
| Stryphnosides A–F | Stryphnodendron fissuratum | 456 |
| Theasaponins A6, A7 and B5 | Camellia sinensis | 457 |
| Tenuifoside A | Polygala tenuifolia | 458 |
| Vaccaroside I | Vaccaria segetalis | 459 |
| Xanifolias Y0, Y2, Y3 and Y7 | Xanthoceras sorbifolia | 460 |
| Yemuosides YM21–YM25 | Stauntonia chinensis | 461 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2.
| Plant species | Reference |
|---|---|
| Achras sapota | 462 |
| Akebia quinata | 463,464 |
| Albizia lebbeck | 465 |
| Albizia procera | 466 |
| Androsace umbellata | 467 |
| Anemone flaccida | 468 |
| Ardisia gigantifolia | 469 |
| Arenaria juncea | 470 |
| Aronia melanocarpa | 471 |
| Artemisia sphaerocephala | 472 |
| Aster novi | 473 |
| Carthamus tinctorius | 474 |
| Chenopodium quinoa | 475 |
| Combretum laxum | 476 |
| Combretum molle | 477 |
| Cordia piauhiensis | 478 |
| Garcinia hanburyi | 309 |
| Gueldenstaedtia multiflora | 479 |
| Lactuca scariola | 480 |
| Lactuca scariola | 481 |
| Lonicera japonica | 482,483 |
| Lonicera macranthoides | 484 |
| Lysimchia davurica | 485 |
| Meryta denhamii | 486 |
| Myrsine africana | 487 |
| Nylandtia spinosa | 488 |
| Paronychia argentea | 489 |
| Phaseolus vulgaris | 490 |
| Polygala tenuifolia | 491 |
| Polyscias guilfoylei | 492 |
| Pulsatilla chinensis | 493 |
| Schima noronhae | 494 |
| Xanthium strumarium | 495 |
| Xanthoceras sorbifolia | 316,496,497 |
| Zygophyllum fabago | 498 |
A survey of the occurrence and biological activities of 13,28-epoxyoleanane saponins has been published.499
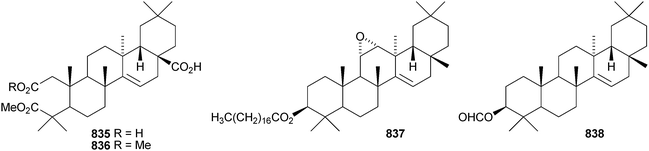
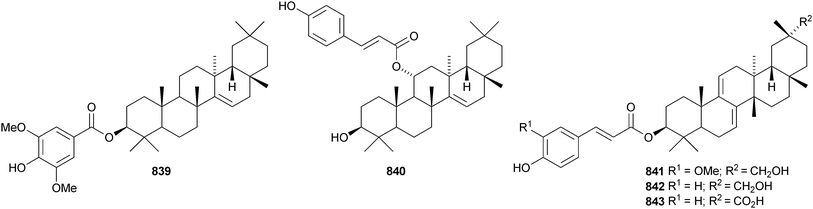
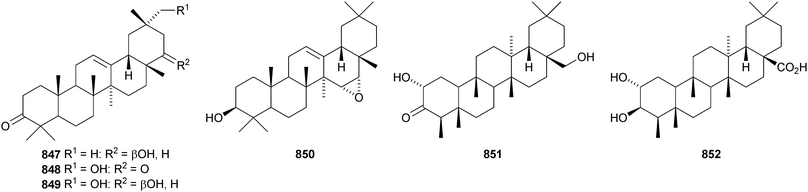
Two 2,3-secotaraxerane esters 835 and 836 have been found in Elateriospermum tapos.500 Crassifoate 837 is an epoxytaraxerane ester from Nepeta crassifolia.501 Other taraxerane esters include the formyl ester of taraxerol 838 from Rhizophora stylosa,369 fibrarecisin 839 from Fibraurea recisa502 and the p-coumaroyl ester 840 from Craibiodendron henryi.503 Four multiflorane esters 841–844 have been identified from Lagenaria siceraria.504 Spirowallichiione 845 is a rearranged multiflorane from Euphorbia wallichii.505Derris laxiflora contains trans-cinnamoylglutinol 846 and the oleanane derivatives 847–850.139
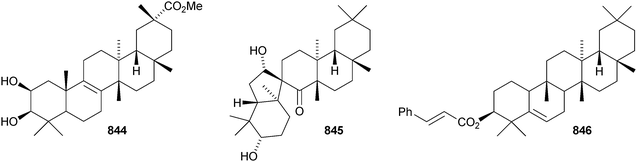
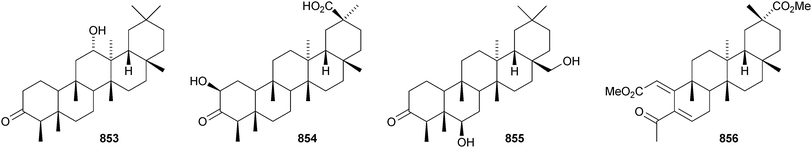
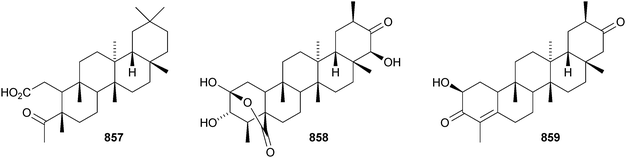
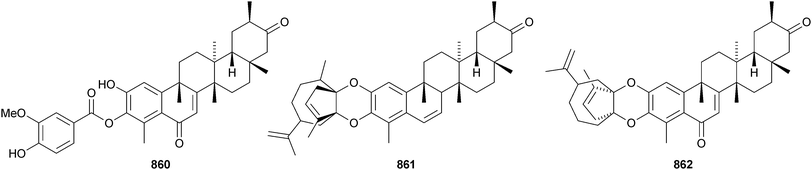
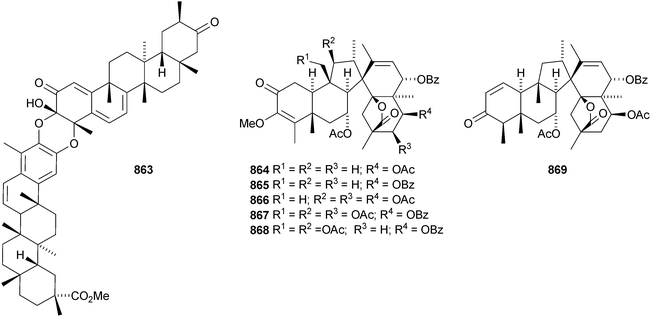
The structure of the friedelane triterpenoid endodesmiadiol 851, from Endodesmia calophylloides, was confirmed by X-ray analysis.506 Pluricostatic acid 852, from leaves of Marila pluricostata, is 2α,3β-dihydroxyfriedelan-28-oic acid.507 12α-Hydroxyfriedelan-3-one 853 is a constituent of Maytenus gonoclada,508 and 2β-hydroxy-3-oxofriedelan-30-oic acid 854 is found in Dichapetalum barteri.509 28-Hydroxyzeylanol 855 is a new friedelane from Dichapetalum gelonoides.235 Dzununcanone 856, from Hippocratea excelsa, is a 3,24-dinor-2,3-seco-friedelane derivative.344 The structure of dzununcanone 856 is drawn incorrectly in the reference. The related 3-nor-2,3-seco derivative 857 has been isolated from Passiflora wilsonii.510 Other norfriedelane derivatives include trifloralactone 858 and triptocalline B 859 from Microtropis triflora511 and milicifolines A–D 860–863 from Maytenus ilicifolia.512 Milicifolines B 861 and C 862 are related to the cheiloclines reported by the same group in 2005 (see Nat. Prod. Rep., 2008, 25, 794). Spirocarcolitones G–L 864–869, from the bark of Ruptiliocarpon caracolito, are rearranged friedelane derivatives.513
7 The ursane group
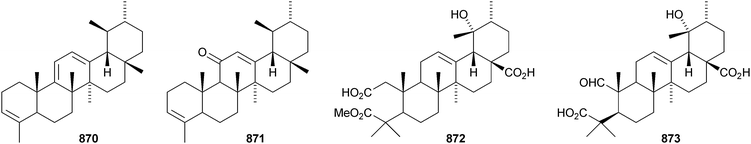
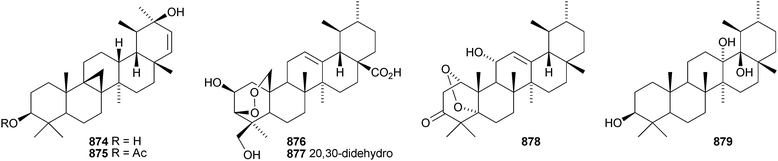
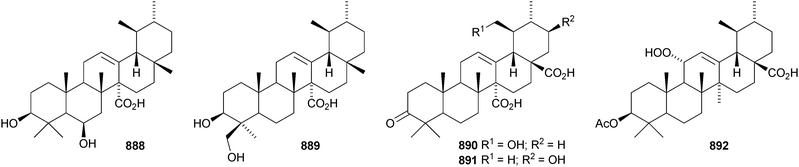
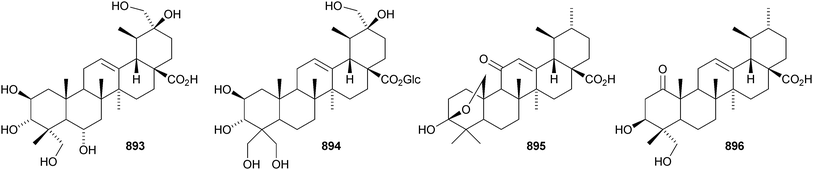
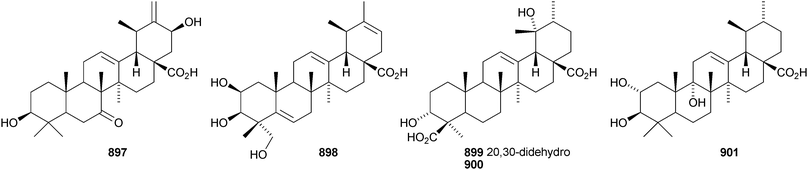
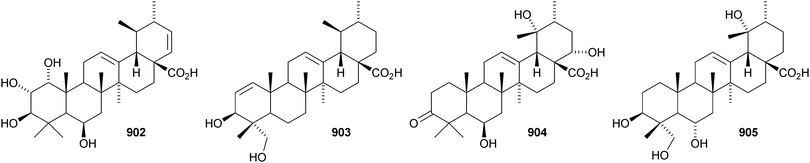
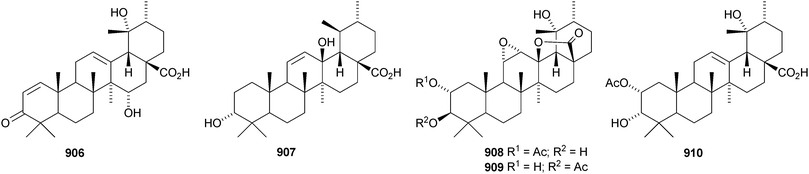
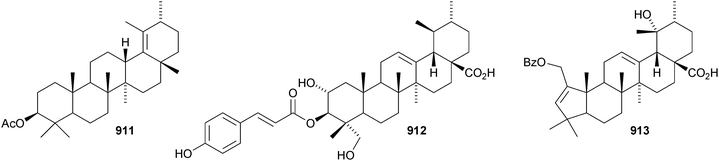
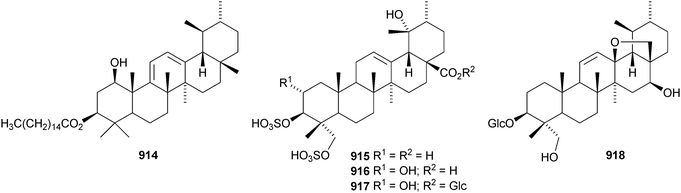
24-Norursa-3,9(11),12-triene 870 and 24-norursa-3,12-dien-11-one 871 have been identified in olibanum from Boswellia serrata.336 It is not clear whether these norursanes are natural or decomposition products. The 3-methyl ester of cecropracic acid 872 as been isolated together with the related 2-nor-aldehyde 873 from Potentilla multicaulis.514 9,26-Cyclours-21-ene-3β,20β-diol 874 and the corresponding 3-acetate 875 are constituents of the stem bark of Ficus cordata.515 The unusual 3,25-epidioxy derivatives 876 and 877 have been claimed from Gentiana aristata.516 The 1,5-epidioxy derivative 878 is found in Vladimiria muliensis together with ursane-3β,13α,18β-triol 879.517 Other simple ursane alcohol derivatives include urs-12-ene-1β,3β,11α-triol 880 from Plumbago zeylanica,518 sorbinol A 881 from Sorbus cashmariana,301 and 11α-methoxyurs-12-ene-3α,21β-diol 882 from Hippocratea excelsa.343 Ilexhainanins A 883 and C 884, from Ilex hainanensis, are urs-12-ene-24,28-dioic acids350 and nummularic acid 885, from Evolvulus nummularius, is 3β-urs-12-en-29-oic acid.519 3β-Hydroxy-12-oxoursan-28,13β-olide 886 has been reported as a natural product from Plumeria obtusa.520 This compound has previously been identified as an oxidation product of ursolic acid acetate.521 The ring-A-cleaved ursane 887 has been obtained from the floral spikes of Betula platyphylla var. japonica.216Astilbotriterpenic acid 888 is 3β,6β-dihydroxyurs-12-en-27-oic acid from Astilbe chinensis.522 The structure of 3β,24- dihydroxyurs-12-en-27-oic acid 889, also from Astilbe chinensis, has been confirmed by X-ray analysis.523 The urs-12-ene-27,28-dioic acids metatrichosins A 890 and B 891 have been found in Metadina trichotoma.524 Speciosaperoxide 892 is a hydroperoxide from Chaenomeles speciosa.525 2β,3α,6α,20β,23,30-Hexahydroxyurs-12-en-28-oic acid 893, together with the related 28-glucosyl ester 894, have been identified in Actinidia valvata.526 Further new urs-12-en-28-oic acid derivatives include camaranoic acid 895 from Lantana camara,339 cheiranthic acid 896 from Oenothera cheiranthifolia,527 silymin A 897 from Silybum marianum,345 madhunolic acid 898 from Madhuca latifolia,528 diospyric acids A 899 and B 900 from Diospyros kaki,529 santolinic acid 901 from Salvia santolinifolia,530 eleganenes A 902 and B 903 from Myricaria elegans,531 the 19α-alcohols 904 and 905 from Dischidia esquirolii532 and 906 from Canthium multiflorum.533 3α,13β-Dihydroxyurs-11-en-28-oic acid 907 has been claimed from Hedyotis crassifolia.534 The corresponding 28,13-lactone structure may be more likely. The 11α,12α-epoxy-28,13β-lactones 908 and 909 together with the acetate 910 are constituents of Cecropia catharinensis.535 Other ursane esters include 3β-acetoxyurs-18-ene 911 from Asteracantha longifolia,536 3-p-coumaroyl actinidic acid 912 from Actinidia arguta,537 hyptadienic acid 2-benzoate 913 from Hyptis verticillata538 and the palmitoyl ester ussuriensin A 914 from Saussurea ussuriensis.359 The disulfate esters 915–917 have been isolated from Melissa officinalis together with the glucoside 918, which has a new genin.372
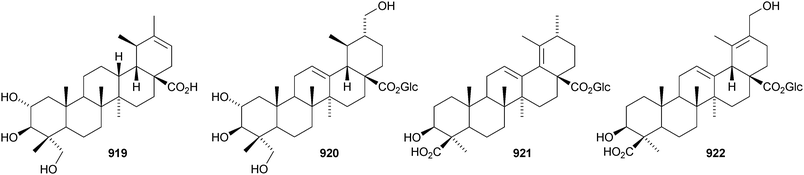
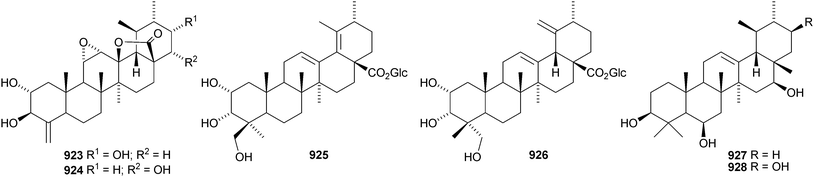
A new ursane saponin has been found in Centella asiatica together with its genin 919.539 Other ursane saponins with new genins include callianthaside A 920 from Pyrola calliantha,540 ilexhainosides A 921 and B 922 from Ilex hainanensis,541 ilexpernosides A and B with the 24-nor-genins 923 and 924 from Ilex pernyi,542 glucosyl esters 925 and 926 from Rosa laevigata543 and two saponins with the genins 927 and 928 from Silphium radula.392 New ursane saponins with known genins include ilexpernosides C–D and G–J from Ilex pernyi,433 ilexsaponin B4 from Ilex pubescens,434 kakisaponin A from Diospyros kaki,544 oblonganosides A–F545 and H–J443 from Ilex oblonga and zygophylosides O and P from Zygophyllum fabago.546 New ursane saponins with known genins that have not been assigned trivial names have been isolated from Combretum laxum,476Cordia piauhiensis,478 and Zygophyllum geslini.547
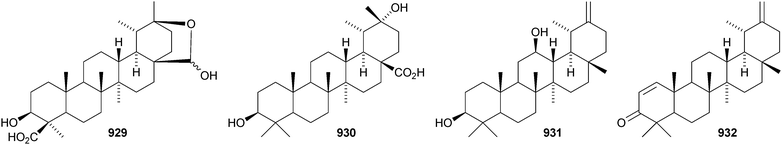
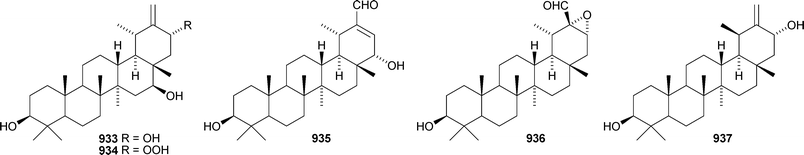
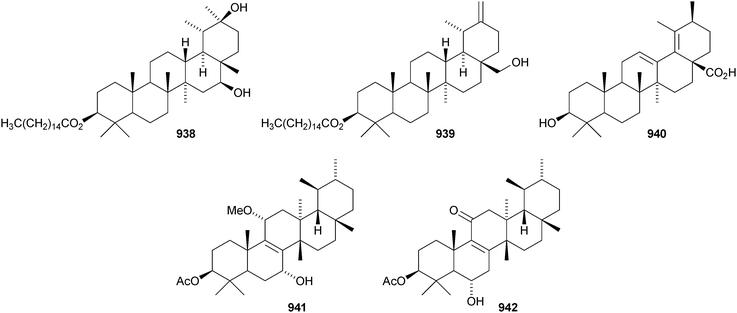
The 20,28-epoxytaraxastane 929 derivative is a constituent of Potentilla multicaulis.514 Punicanolic acid 930 is 3β,20α-dihydroxytaraxastan-28-oic acid from flowers of Punica granatum.548 Other new taraxastane triterpenoids include taraxast-20(30)ene-3β,12β-diol 931 from Craibiodendron yunnanense,549,550 taraxasta-1,20(30)-dien-3-one 932 from Sida acuta,551 taraxast-20(30)-ene-3β,16β,21α-triol 933 from Centipeda minima,552 the corresponding 21-hydroperoxide 934 from Arnica montana553 and 3β,22α-dihydroxytaraxast-20-en-30-al 935 from Polyalthia nemoralis.554 Oliganthas B 936 is a new taraxastane derivative from Saussurea oligantha.207 19β-Taraxast-20(30)-ene-3β,21α-diol 937 was isolated from Ixeris chinensis in 2006 and has now been identified in Cichorium intybus and named cichoridiol.263 The palmitoyl esters 938 and ussuriensin C 939 have been discovered in Conyza candanesis555 and Celastrus rosthornianus, respectively.359 Pubescenosides C and D, from Ilex pubescens, have the new genin 3β-hydroxytaraxasta-12,18-dien-28-oic acid 940.556 The structure of the baurene derivative 941, from Vladimiria muliensis, was confirmed by X-ray analysis.517 The related dinklagenonoate 942 is a constituent of Dorstenia dinklagei.557
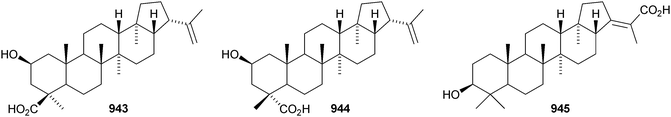
8 The hopane group
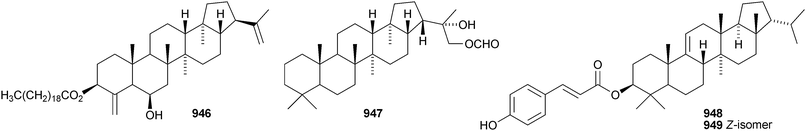
Dryopteric acids A 943 and B 944 are hopane derivatives from rhizomes of Dryopteris crassirhizoma.558 3β-Hydroxyhop-21E-en-29-oic acid 945 has been found in Dipentodon sinicus.559 The 21αH-24-norhopane ester 946 is a constituent of Harpullia arborea560 and the 29-formyl ester of hopane-22S,29-diol 947 is from Saxiglossum angustissimum.561 Two 21βH-arborinane esters 948 and 949 have been identified from Anoectochilus roxburghii as the E- and Z-isomers of p-coumaroyl sorghumol.5629 Miscellaneous compounds
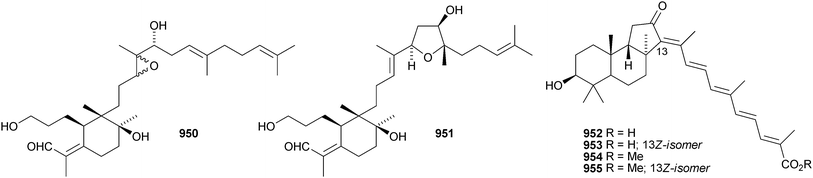
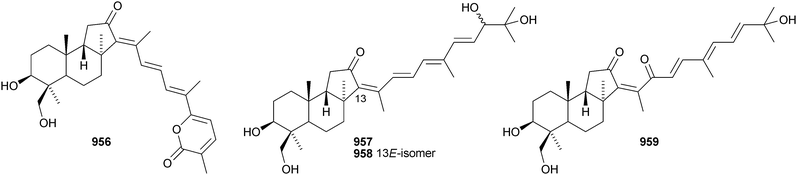
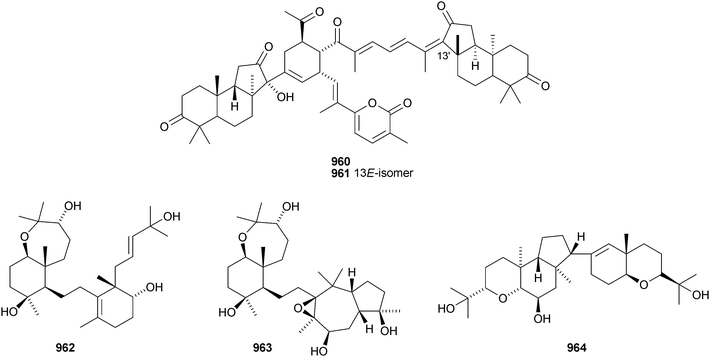
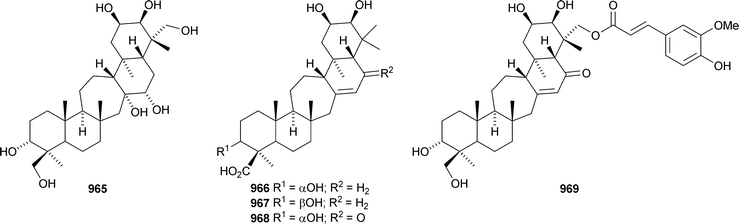
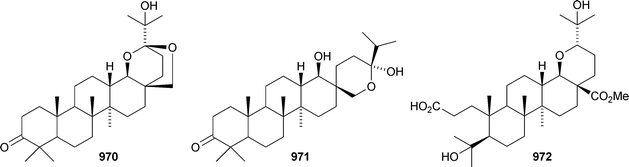
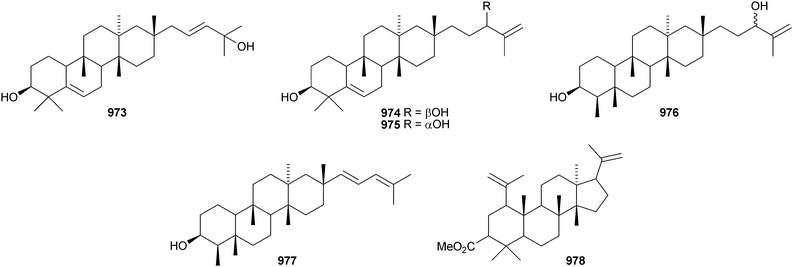
Iris tectorum is the source of iritectols A 950 and B 951.563 Stellettins L 952 and M 953 are isomalabaricane metabolites of the marine sponge Stelletta tenuis.564 The corresponding methyl esters, rhabdastrellins E 954 and F 955, are found in Rhabdastrella aff. distincta together with rhabdastrellins A–D 956–959.565 The bisisomalabaricanes jaspolides G 960 and H 961 are Diels–Alder-type adducts from a Jaspis species.566 The Red Sea sponge Siphonochalina siphonella produces siphonellinol C 962 and sipholenol I 963.567 Malbrancheosides A–D, metabolites of the fungus Malbranchea filamentosa, are glycosides of malbrancheogenin 964.568Five highly oxygenated serratane triterpenoids 965–969 have been isolated from Diphasiastrum complanatum.569 Ekeberin A 970 is a baccharane derivative from the stem bark of Ekebergia capensis12 and the spiro-hemiketal 971 is a constituent of the liverwort Lepidozia chordulifera.570 A 3,4-seco-baccharane derivative 972 has been found in the leaves and stem bark of Aglaia foveolata.571 Foliasalacins D1–D3973–975 are D:B-friedobaccharanes from leaves of Salacia chinensis.572 Two shionane derivatives 976 and 977 have been identified in Aster ageratoides var. oophyllus.573 The unusual elaeophorbate 978 has been claimed from leaves of Elaeophorbia drupifera.574
10 References
- T. Akihisa, Oreo Saiensu, 2007, 7, 445–453 ( Chem. Abstr. , 2007 , 147 , 419127 ) Search PubMed.
- A. Sharma, A. S. Mann, V. Gajbhiye and M. D. Kharya, Pharmacogn. Rev., 2007, 1, 137–142 Search PubMed.
- O. P. Sharma, S. Sharma, V. Pattabhi, S. B. Mahato and P. D. Sharma, Crit. Rev. Toxicol., 2007, 37, 313–352 CrossRef CAS.
- X. Huang and R. Yang, Redai Yaredai Zhiwu Xuebao, 2007, 15, 175–182 ( Chem. Abstr. , 2008 , 149 , 443673 ) Search PubMed.
- X. Zhang, J. Feng and X. Zhang, Xibei Zhiwu Xuebao, 2007, 27, 1484–1490 ( Chem. Abstr. , 2009 , 150 , 93532 ) Search PubMed.
- J.-P. Vincken, L. Heng, A. De Groot and H. Gruppen, Phytochemistry, 2007, 68, 275–297 CrossRef CAS.
- O. Guclu-Ustundag and G. Mazza, Crit. Rev. Food Sci. Nutr., 2007, 47, 231–258 CrossRef CAS.
- X. Cheng and Y. Xiong, Zhongcaoyao, 2007, 38, 792–795 ( Chem. Abstr. , 2009 , 150 , 297337 ) Search PubMed.
- J. P. Parente and B. Pereira da Silva, Curr. Topics Phytochem., 2007, 8, 33–45 Search PubMed.
- F.-C. Wang and R.-L. Zhang, Yaoxue Shijian Zazhi, 2007, 25, 140–141 ( Chem. Abstr. , 2008 , 148 , 134585 ) Search PubMed.
- J. C. Noro, L. R. Barrows, O. G. Gideon, C. M. Ireland, M. Koch, T. Matainaho, P. Piskaut, C. D. Pond and T. S. Bugni, J. Nat. Prod., 2008, 71, 1623–1624 CrossRef CAS.
- T. Murata, T. Miyase, F. W. Muregi, Y. Naoshima-Ishibashi, K. Umehara, T. Warashina, S. Kanou, G. M. Mkoji, M. Terada and A. Ishih, J. Nat. Prod., 2008, 71, 167–174 CrossRef CAS.
- Y. Morimoto, T. Okita, M. Takaishi and T. Tanaka, Angew. Chem. Int. Ed., 2007, 46, 1132–1135 CrossRef CAS.
- E. Manzo, M. Gavagnin, G. Bifulco, P. Cimino, S. Di Micco, N. L. Ciavatta, Y. W. Guo and G. Cimino, Tetrahedron, 2007, 63, 9970–9978 CrossRef CAS.
- N.-Y. Ji, X.-M. Li, H. Xie, J. Ding, K. Li, L.-P. Ding and B.-G. Wang, Helv. Chim. Acta, 2008, 91, 1940–1946 CrossRef CAS.
- Y. Matsuo, M. Suzuki, M. Masuda, T. Iwai and Y. Morimoto, Helv. Chim. Acta, 2008, 91, 1261–1266 CrossRef CAS.
- P. Metzger, M.-N. Rager and C. Fosse, Phytochemistry, 2008, 69, 2380–2386 CrossRef CAS.
- J. F. Arteaga, V. Domingo, J. F. Q. del Moral and A. F. Barrero, Org. Lett., 2008, 10, 1723–1726 CrossRef CAS.
- M. D. Kolesnikova, W. K. Wilson, D. A. Lynch, A. C. Obermeyer and S. P. T. Matsuda, Org. Lett., 2007, 9, 5223–5226 CrossRef CAS.
- M. Shibuya, T. Xiang, Y. Katsube, M. Otsuka, H. Zhang and Y. Ebizuka, J. Am. Chem. Soc., 2007, 129, 1450–1455 CrossRef CAS.
- M. D. Kolesnikova, A. C. Obermeyer, W. K. Wilson, D. A. Lynch, Q. Xiong and S. P. T. Matsuda, Org. Lett., 2007, 9, 2183–2186 CrossRef CAS.
- T.-K. Wu, H.-Y. Wen, C.-H. Chang and Y.-T. Liu, Org. Lett., 2008, 10, 2529–2532 CrossRef CAS.
- L. Kürti, R.-J. Chein and E. J. Corey, J. Am. Chem. Soc., 2008, 130, 9031–9036 CrossRef CAS.
- T.-K. Wu, C.-H. Chang, Y.-T. Liu and T.-T. Wang, Chem. Rec., 2008, 8, 302–325 CrossRef CAS.
- I. Abe, Yakugaku Zasshi, 2008, 128, 1109–1118 ( Chem. Abstr. , 2008 , 149 , 442543 ) CrossRef CAS.
- I. Abe, Nat. Prod. Rep., 2007, 24, 1311–1331 RSC.
- T. Kushiro and Y. Ebizuka, Tanpakushitsu Kakusan Koso, 2007, 52, 1730–1735 ( Chem. Abstr. , 2007 , 147 , 1117344 ) Search PubMed.
- M. Zhang, W.-L. Wang, Y.-C. Fang, T.-J. Zhu, Q.-Q. Gu and W.-M. Zhu, J. Nat. Prod., 2008, 71, 985–989 CrossRef CAS.
- S.-Y. Lee, H. Kinoshita, F. Ihara, Y. Igarashi and T. Nihira, J. Biosci. Bioeng., 2008, 105, 476–480 CrossRef CAS.
- M. Zhao, L.-J. Xu and C.-T. Che, Phytochemistry, 2008, 69, 527–532 CrossRef CAS.
- X.-Y. Hu, Y.-Q. Guo, W.-Y. Gao, T.-J. Zhang and H.-X. Chen, J. Asian Nat. Prod. Res., 2008, 10, 487–490 CAS.
- X. Y. Hu, Y. Q. Guo, W. Y. Gao, H. X. Chen and T. J. Zhang, Chin. Chem. Lett., 2008, 19, 438–440 CrossRef CAS.
- A.-C. Zhou, C.-F. Zhang and M. Zhang, Zhongguo Tianran Yaowu, 2008, 6, 109–111 ( Chem. Abstr. , 2008 , 149 , 432273 ) Search PubMed.
- H. Y. Gao, L. J. Wu, T. Nakane, O. Shirota and M. Kuroyanagi, Chem. Pharm. Bull., 2008, 56, 554–558 CrossRef CAS.
- H. Y. Gao, L. J. Wu, T. Nakane, O. Shirota and M. Kuroyanagi, Chem. Pharm. Bull., 2008, 56, 1352–1354 CrossRef CAS.
- B. Sun, B. Hou, J. Huang, L. Wu, M. Kuroyanagi and H. Gao, Arch. Pharmacal Res., 2008, 31, 1530–1533 Search PubMed.
- E. Ahmed, A. Malik, S. Ferheen, N. Afza, Azhar-ul-Haq, M. A. Lodhi and M. I. Choudhary, Chem. Pharm. Bull., 2006, 54, 103–106 CrossRef CAS.
- R.-W. Jiang, A. L. Lane, L. Mylacraine, K. I. Hardcastle, C. R. Fairchild, W. Aalbersberg, M. E. Hay and J. Kubanek, J. Nat. Prod., 2008, 71, 1616–1619 CrossRef CAS.
- C. Lei, S.-X. Huang, J.-X. Pu, J.-P. Liu, G.-Y. Yang, L.-B. Yang, Y. Lu, J.-J. Chen, Y. Lu, W.-L. Xiao and H.-D. Sun, Planta Med., 2008, 74, 292–295 CrossRef CAS.
- C. Feng, X.-M. Li, N.-Y. Ji and B.-G. Wang, Helv. Chim. Acta, 2008, 91, 850–855 CrossRef CAS.
- Z.-Q. Lu, G.-T. Chen, J.-Q. Zhang, H.-L. Huang, S.-H. Guan and D.-A. Guo, Helv. Chim. Acta, 2007, 90, 2245–2250 CrossRef CAS.
- C.-R. Chen, C.-W. Cheng, M.-H. Pan, Y.-W. Liao, C.-Y. Tzeng and C.-I. Chang, Chem. Pharm. Bull., 2007, 55, 908–911 CrossRef CAS.
- Y.-H. Lan, H.-Y. Wang, C.-C. Wu, S.-L. Chen, C.-L. Chang, F.-R. Chang and Y.-C. Wu, Chem. Pharm. Bull., 2007, 55, 1597–1599 CrossRef CAS.
- A. Petrova, S. Popov, M. Gjosheva and V. Bankova, Nat. Prod. Res., 2007, 21, 401–405 CrossRef CAS.
- V. Aeri, M. M. Rajkumari and M. Ali, Indian J. Nat. Prod., 2005, 21, 40–42 Search PubMed.
- R. S. El Dine, A. M. El Halawany, N. Nakamura, C.-M. Ma and M. Hattori, Chem. Pharm. Bull., 2008, 56, 642–646 CrossRef CAS.
- R. S. El Dine, A. M. El Halawany, C.-M. Ma and M. Hattori, J. Nat. Prod., 2008, 71, 1022–1026 CrossRef CAS.
- X.-M. Niu, S.-H. Li, W.-L. Xiao, H.-D. Sun and C.-T. Che, J. Asian Nat. Prod. Res., 2007, 9, 659–664 CrossRef CAS.
- F. Wang and J.-K. Liu, Chem. Pharm. Bull., 2008, 56, 1035–1037 CrossRef CAS.
- S.-H. Guan, J.-M. Xia, M. Yang, X.-M. Wang, X. Liu and D.-A. Guo, J. Asian Nat. Prod. Res., 2008, 10, 695–700 CrossRef CAS.
- Y. Qiao, X.-M. Zhang and M.-H. Qiu, Molecules, 2007, 12, 2038–2046 Search PubMed.
- S.-H. Guan, M. Yang, X.-M. Wang, J.-M. Xia, Z.-M. Zhang, X. Liu and D.-A. Guo, Magn. Reson. Chem., 2007, 45, 789–791 CrossRef CAS.
- E. de F. A. Smania, F. Delle Monache, R. A. Yunes, R. Paulert and A. Smania Junior, Rev. Bras. Farm., 2007, 17, 14–16 ( Chem. Abstr. , 2007 , 147 , 360408h ) Search PubMed.
- S. Zhou, Rec. Prog. Med. Plants, 2007, 17, 139–171 Search PubMed.
- G. Liu, C. Ding and X. Wang, Junwu Xuebao, 2007, 26, 470–476 ( Chem. Abstr. , 2008 , 149 , 569824 ) Search PubMed.
- X. Zhou, J. Lin, Y. Yin, J. Zhao, X. Sun and K. Tang, Am. J. Chin. Med., 2007, 35, 559–574 Search PubMed.
- Y. Liang, T. Chu and P. Ding, Zhongyao Xinyao Yu Linchuang Yaoli, 2007, 18, 168–172 ( Chem. Abstr. , 2007 , 147 , 124714 ) Search PubMed.
- B. Boh, M. Berovic, J. Zhang and L. Zhi-Bin, Biotechnol. Annu. Rev., 2007, 13, 265–301 Search PubMed.
- Y. Zheng and X.-W. Yang, J. Asian Nat. Prod. Res., 2008, 10, 289–292 CrossRef CAS.
- L. Zhou, Y. Zhang, L. A. Gapter, H. Ling, R. Agarwal and K.-Y. Ng, Chem. Pharm. Bull., 2008, 56, 1459–1562 CrossRef CAS.
- Y. Zheng and X.-W. Yang, J. Asian Nat. Prod. Res., 2008, 10, 640–646 CrossRef CAS.
- T. Akihisa, Y. Nakamura, H. Tokuda, E. Uchiyama, T. Suzuki, Y. Kimura, K. Uchikura and H. Nishino, J. Nat. Prod., 2007, 70, 948–953 CrossRef CAS.
- Y. Shiono, H. Sugawara, M. Nazarova, T. Murayama, K. Takahashi and M. Ikeda, J. Asian Nat. Prod. Res., 2007, 9, 531–535 CrossRef CAS.
- R. Stanikunaite, M. M. Radwan, J. M. Trappe, F. Fronczek and S. A. Ross, J. Nat. Prod., 2008, 71, 2077–2079 CrossRef CAS.
- S. Taji, T. Yamada, Y. In, S. Wada, Y. Usami, K. Sakuma and R. Tanaka, Helv. Chim. Acta, 2007, 90, 2047–2057 CrossRef CAS.
- T. Nakata, T. Yamada, S. Taji, H. Ohishi, S. Wada, H. Tokuda, K. Sakuma and R. Tanaka, Bioorg. Med. Chem, 2007, 15, 257–264 CrossRef CAS.
- S. Taji, T. Yamada, S. Wada, H. Tokuda, K. Sakuma and R. Tanaka, Eur. J. Med. Chem., 2008, 43, 2373–2379 CrossRef CAS.
- S. Taji, T. Yamada and R. Tanaka, Helv. Chim. Acta, 2008, 91, 1513–1524 CrossRef CAS.
- H. Li, L. Wang, S. Miyata and S. Kitanaka, J. Nat. Prod., 2008, 71, 739–741 CrossRef CAS.
- N. Wang, Z. Li, D. Song, W. Li, H. Fu, K. Koike, Y. Pei, Y. Jing and H. Hua, J. Nat. Prod., 2008, 71, 990–994 CrossRef CAS.
- X.-M. Gao, J.-X. Pu, W.-L. Xiao, S.-X. Huang, L.-G. Lou and H.-D. Sun, Tetrahedron, 2008, 64, 11673–11679 CrossRef CAS.
- J.-X. Pu, W.-L. Xiao, H.-M. Li, S.-X. Huang, S.-H. Li and H.-D. Sun, Helv. Chim. Acta, 2007, 90, 723–729 CrossRef CAS.
- I.-K. Lee, Y.-W. Jang, S. H. Yu and B.-S. Yun, Bioorg. Med. Chem. Lett, 2007, 17, 4906–4909 CrossRef CAS.
- I.-M. Chung, M. Ali, S.-C. Chun, O.-K. Lee and A. Ahmad, Asian J. Chem., 2007, 19, 1535–1543 CAS.
- I.-M. Chung, M. Ali and A. Ahmad, Indian J. Chem., Sect. B, 2007, 46, 516–522.
- I. M. Chung, M. Ali, Y.-M. Yang, C. A. M. Peebles, S.-C. Chun, S.-J. Lee, K.-Y. San and A. Ahmad, Bull. Korean Chem. Soc., 2007, 28, 1294 CAS.
- A. S. Antonov, A. I. Kalinovsky, V. A. Stonik, S. Sh. Afiyatullov, D. L. Aminin, P. S. Dmitrenok, E. Mollo and G. Cimino, J. Nat. Prod., 2007, 70, 169–178 CrossRef CAS.
- S. Sh. Afiyatullov, A. I. Kalinovsky, A. S. Antonov, L. P. Ponomarenko, P. S. Dmitrenok, D. L. Aminin, V. B. Krasokhin, V. M. Nosova and A. V. Kisin, J. Nat. Prod., 2007, 70, 1871–1877 CrossRef CAS.
- H. Han, Y. H. Yi, X. H. Wang, B. S. Liu, P. Sun and M. X. Pan, Chin. Chem. Lett., 2007, 18, 161–164 CrossRef CAS.
- N. H. Dang, N. V. Thanh, P. V. Kiem, L. M. Huong, C. V. Minh and Y. H. Kim, Arch. Pharmacal Res., 2007, 30, 1387–1391 Search PubMed.
- W. Yuan, Y. Yi, H. Tang, M. Xue, Z. Wang, G. Sun, W. Zhang, B. Liu, L. Li and P. Sun, Chem. Pharm. Bull., 2008, 56, 1207–1211 CrossRef CAS.
- J. Wu, Y.-H. Yi, H.-F. Tang, H.-M. Wu and Z.-R. Zhou, J. Asian Nat. Prod. Res., 2007, 9, 609–615 CrossRef CAS.
- W.-H. Yuan, Y.-H. Yi, M. Xue, H.-W. Zhang and M.-P. La, Zhongguo Tianran Yaowu, 2008, 6, 105–108 ( Chem. Abstr. , 2008 , 149 , 242492z ) Search PubMed.
- B.-S. Liu, Y.-H. Yi, L. Li, S.-L. Zhang, H. Han, Y.-Y. Weng and M.-X. Pan, Chem. Biodiversity, 2007, 4, 2845–2851 CrossRef CAS.
- S. A. Avilov, A. S. Silchenko, A. S. Antonov, V. I. Kalinin, A. I. Kalinovsky, A. V. Smirnov, P. S. Dmitrenok, E. V. Evtushenko, S. N. Federov, A. S. Savina, L. K. Shubina and V. A. Stonik, J. Nat. Prod., 2008, 71, 525–531 CrossRef CAS.
- A. S. Silchenko, S. A. Avilov, A. I. Kalinovsky, P. S. Dmitrenok, V. I. Kalinin, J. Morre, M. L. Deinzer, C. Woodward and P. D. Collin, Can. J. Chem., 2007, 85, 626–636 CrossRef CAS.
- B.-S. Liu, Y.-H. Yi, L. Li, P. Sun, W.-H. Yuan, G.-Q. Sun, H. Han and M. Xue, Chem. Biodiversity, 2008, 5, 1288–1297 CrossRef CAS.
- B.-S. Liu, Y.-H. Yi, P. Sun, H. Han, G.-Q. Sun, X.-H. Wang and Z.-L. Wang, Chem. Biodiversity, 2008, 5, 1425–1433 CrossRef CAS.
- J. Wu, Y.-H. Yi, H.-F. Tang, Z.-R. Zou and H.-M. Wu, Chem. Biodiversity, 2006, 3, 1249–1254 CrossRef CAS.
- P. Sun, B.-S. Liu, Y.-H. Yi, L. Li, M. Gui, H.-F. Tang, D.-Z. Zhang and S.-L. Zhang, Chem. Biodiversity, 2007, 4, 450–457 CrossRef CAS.
- S.-L. Zhang, L. Li, P. Sun and Y.-H. Yi, J. Asian Nat. Prod. Res., 2008, 10, 1097–1103 CrossRef CAS.
- H. Han, Y. Yi, L. Li, B. Yan, B. Liu and P. Sun, Zhongguo Haiyang Yaowu, 2007, 26, 21–24 ( Chem. Abstr. , 2008 , 149 , 327859z ) Search PubMed.
- H. Han, Y. H. Yi, L. Li, X. H. Wang, B. S. Lium, P. Sun and M. X. Pan, Chin. Chem. Lett., 2007, 18, 161–164 CrossRef CAS.
- H. Han, Y. H. Yi, B. S. Liu, X. H. Wang and M. X. Pan, Chin. Chem. Lett., 2008, 19, 1462–1464 CrossRef CAS.
- A. S. Antonov, S. A. Avilov, A. I. Kalinovsky, S. D. Anastyuk, P. S. Dmitrenok, E. V. Evushenko, V. I. Kalanin, A. V. Smirnov, S. Taboada, M. Ballerteros, C. Avila and V. A. Stonik, J. Nat. Prod., 2008, 71, 1677–1685 CrossRef CAS.
- W. H. Yuan, Y. H. Yi, L. Li, B. S. Liu, H. W. Zhang and P. Sun, Chin. Chem. Lett., 2008, 19, 457–460 CrossRef CAS.
- A. S. Silchenko, S. A. Avilov, V. I. Kalinin, V. A. Stonik, A. I. Kalinovsky, P. S. Dmitrenok and V. G. Stepanov, Russ. J. Bioorg. Chem., 2007, 33, 73–82 Search PubMed.
- A. S. Silchenko, S. A. Avilov, V. I. Kalinin, A. I. Kalinovsky, P. S. Dmitrenok, S. N. Fedorov, V. G. Stepanov, Z. Dong and V. A. Stonik, J. Nat. Prod., 2008, 71, 351–356 CrossRef CAS.
- R. Kumar, A. K. Chaturvedi, P. V. Kumar and V. Lakshmi, Bioorg. Med. Chem. Lett, 2007, 17, 4387–4391 CrossRef CAS.
- S.-Y. Zhang, H.-F. Tang and Y.-H. Yi, Fitoterapia, 2007, 78, 283–287 CrossRef CAS.
- V. I. Kalinin, D. L. Aminin, S. A. Avilov, A. S. Silchenko and V. A. Stonik, Stud. Nat. Prod. Chem., 2008, 35, 135–196 Search PubMed.
- P. Sun, Y.-H. Yi, L. Li and H.-F. Tang, Zhongguo Tianran Yaowu, 2008, 6, 241–250 Search PubMed.
- W.-L. Xiao, R. T. Li, S.-X. Huang, J.-X. Pu and H.-D. Sun, Nat. Prod. Rep., 2008, 25, 871–891 RSC.
- S.-X. Huang, R.-T. Li, J.-P. Liu, Y. Lu, Y. Chang, C. Lei, W.-L. Xiao, L.-B. Yang, Q.-T. Zheng and H.-D. Sun, Org. Lett., 2007, 9, 2079–2082 CrossRef CAS.
- S.-X. Huang, L.-B. Yang, W.-L. Xiao, C. Lei, J.-P. Liu, Y. Lu, Z.-Y. Weng, L.-M. Li, R.-T. Li, J.-L. Yu, Q.-T. Zheng and H.-D. Sun, Chem. Eur. J., 2007, 13, 4816–4822 CrossRef CAS.
- S.-X. Huang, Q.-B. Han, C. Lei, J.-X. Pu, W.-L. Xiao, J.-L. Yu, L.-M. Yang, H.-X. Xu, Y.-T. Zheng and H.-D. Sun, Tetrahedron, 2008, 64, 4260–4267 CrossRef CAS.
- S.-X. Huang, J. Yang, H. Huang, L.-M. Li, W.-L. Xiao, R.-T. Li and H.-D. Sun, Org. Lett., 2007, 9, 4175–4178 CrossRef CAS.
- W.-L. Xiao, S.-X. Hunag, L. Zhang, R.-R. Tian, L. Wu, X.-L. Li, J.-X. Pu, Y.-T. Zheng, Y. Lu, R.-T. Li, Q.-T. Zheng and H.-D. Sun, J. Nat. Prod., 2006, 69, 650–653 CrossRef CAS.
- R.-T. Li, W.-L. Xiao, Y.-H. Shen, Q.-S. Zhao and H.-D. Sun, Chem. Eur. J., 2005, 11, 2989–2996 CrossRef CAS 6763–6765.
- R.-T. Li, Q.-B. Han, Y.-T. Zheng, R.-R. Wang, L.-M. Yang, Y. Lu, S.-Q. Sang, Q.-T. Zheng, Q.-S. Zhao and H.-D. Sun, Chem. Commun., 2005, 2936–2938 RSC.
- C. Lei, S.-X. Huang, J.-J. Chen, J.-X. Pu, L.-M. Li, W.-L. Xiao, J.-P. Liu, L.-B. Yang and H.-D. Sun, Helv. Chim. Acta, 2007, 90, 1399–1405 CrossRef CAS.
- C. Lei, S.-X. Huang, J.-J. Chen, L.-B. Yang, W.-L. Xaio, Y. Chang, Y. Lu, H. Huang, J.-X. Pu and H.-D. Sun, J. Nat. Prod., 2008, 71, 1228–1232 CrossRef CAS.
- W.-L. Xiao, X.-L. Li, R.-R. Wang, L.-M. Yang, L.-M. Li, S.-X. Huang, J.-J. Pu, Y.-T. Zheng, R.-T. Li and H.-D. Sun, J. Nat. Prod., 2007, 70, 1056–1059 CrossRef CAS.
- W.-L. Xiao, J.-X. Pu, R.-R. wang, L.-M. Yang, X.-L. Li, S.-H. Li, R.-T. Li, S.-X. Huang, Y.-T. Zheng and H.-D. Sun, Helv. Chim. Acta, 2007, 90, 1505–1513 CrossRef CAS.
- W.-L. Xiao, C. Lei, J. Ren, T.-G. Liao, J.-X. Pu, C. U. Pittman Jr., Y. Lu, Y.-T. Zheng, H.-J. Zhu and H.-D. Sun, Chem. Eur. J., 2008, 14, 11584–11592 CrossRef CAS.
- W.-L. Xiao, L.-M. Yang, L.-M. Li, J.-X. Pu, S.-X. Huang, Z.-Y. Weng, C. Lei, J.-P. Liu, R.-R. Wang, Y.-T. Zheng, R.-T. Li and H.-D. Sun, Tetrahedron Lett., 2007, 48, 5543–5546 CrossRef CAS.
- W.-L. Xiao, S.-X. Huang, R.-J.-X. Pu, Y. Lu and Y.-T. Zheng, Phytochemistry, 2008, 69, 2862–2866 CrossRef CAS.
- G.-Y. Yang, W.-L. Xiao, Y. Chang, R.-R. Wang, J.-X. Pu, X.-M. Gao, C. Lei, Y. Lu, Y.-T. Zheng and H.-D. Sun, Helv. Chim. Acta, 2008, 91, 1871–1878 CrossRef CAS.
- D. M. Chenoweth, K. Chenoweth and W. A. Goddard III, J. Org. Chem., 2008, 73, 6853–6856 CrossRef CAS.
- X.-M. Gao, J.-X. Pu, S.-X. Huang, Y. Lu, L.-G. Lou, R.-T. Li, W.-L. Xiao, Y. Chang and H.-D. Sun, J. Nat. Prod., 2008, 71, 1182–1188 CrossRef CAS.
- W. Wang, Z. Xu, M. Yang, R. Liu, W. Wang, P. Liu and D. Guo, Magn. Reson. Chem., 2007, 45, 522–526 CrossRef CAS.
- J.-X. Pu, L.-M. Yang, W.-L. Xiao, R.-T. Li, C. Lei, X.-M. Gao, S.-X. Huang, S.-H. Li, Y.-T. Zheng, H. Huang and H.-D. Sun, Phytochemistry, 2008, 69, 1266–1272 CrossRef CAS.
- J.-X. Pu, S.-X. Huang, J. Ren, W.-L. Xiao, L.-M. Li, R.-T. Li, L.-B. Li, T.-G. Liao, L.-G. Lou, H.-J. Zhu and H.-D. Sun, J. Nat. Prod., 2007, 70, 1706–1711 CrossRef CAS.
- Z. Jia, Y. Lu, Z. Liao and D. Chen, Helv. Chim. Acta, 2007, 90, 1236–1243 CrossRef CAS.
- M. Chen and D.-F. Chen, Nat. Prod. Res., 2008, 22, 203–207 CrossRef CAS.
- L.-R. Sun, J. Yan, L. Lu, S.-J. Pei, Z.-R. Li, L. Zhou, X.-M. Zhang and M.-H. Qiu, Helv. Chim. Acta, 2007, 90, 1313–1318 CrossRef CAS.
- C. Dan, Y. Zhou, D. Ye, S. Peng, L. Ding, M. L. Gross and S. X. Qiu, Org. Lett., 2007, 9, 1813–1816 CrossRef CAS.
- H. Yoshimoto, M. Nishida and T. Nohara, Chem. Pharm. Bull., 2007, 55, 789–792 CrossRef.
- L.-R. Sun, C. Qing, Y.-L. Zhang, S.-Y. Jia, Z.-R. Li, S.-J. Pei, M.-H. Qiu, M. L. Gross and S. X. Qiu, Beilstein J. Org. Chem., 2007, 3 Search PubMed No. 3.
- S.-N. Chen, D. C. Lankin, D. Nikolic, D. S. Fabricant, Z.-Z. Lu, B. Ramirez, R. B. van Breemen, H. H. S. Fong, N. R. Farnsworth and G. F. Pauli, J. Nat. Prod., 2007, 70, 1016–1023 CrossRef CAS.
- S.-H. Lim, K. Mahmood, K. Komiyama and T.-S. Kam, J. Nat. Prod., 2008, 71, 1104–1106 CrossRef CAS.
- C.-R. Zhang, S.-P. Yang, Q. Zhu, S.-G. Liao, Y. Wu and J.-M. Yue, J. Nat. Prod., 2007, 70, 1616–1619 CrossRef CAS.
- T. Shen, W. Wan, H. Yuan, F. Kong, H. Guo, P. Fan and H. Lou, Phytochemistry, 2007, 68, 1331–1337 CrossRef CAS.
- T. Shen, H.-Q. Yuan, W.-Z. Wan, X.-L. Wang, X.-N. Wang, M. Ji and H.-X. Looou, J. Nat. Prod., 2008, 71, 81–86 CrossRef CAS.
- D. J. Canelon, A. I. Suarez, J. de Sanctis, M. Mijares and R. S. Compagnone, Nat. Prod. Commun., 2008, 3, 895–897 CAS.
- B. Rojano, E. Pérez, B. Figadère, M. T. Martin, M. C. Recio, R. Giner, J. L. Ríos, G. Schinella and J. Sáez, J. Nat. Prod., 2007, 70, 835–838 CrossRef CAS.
- N. Joycharat, H. Greger, O. Hofer and E. Saifah, Phytochemistry, 2008, 69, 206–211 CrossRef CAS.
- W.-S. Xiang, J.-D. Wang, X.-J. Wang and J. Zhang, J. Asian Nat. Prod. Res., 2008, 10, 1071–1085.
- H.-L. Chiu, J.-H. Wu, Y.-T. Tung, T.-H. Lee, S.-C. Chien and Y.-H. Kuo, J. Nat. Prod., 2008, 71, 1829–1832 CrossRef CAS.
- N. Sultana, Atta-ur-Rahman and A. Khalid, Nat. Prod. Res., 2008, 22, 37–47 CrossRef CAS.
- X. Huang, X. Zhu, L. Deng, Z. Deng and W. Lin, Chin. J. Ocean. Limn., 2006, 24, 443–448 Search PubMed.
- H. Haba, C. Lavaud, H. Harkat, A. A. Magid, L. Marcourt and M. Benkhaled, Phytochemistry, 2007, 68, 1255–1260 CrossRef CAS.
- Y.-G. Pei, Q.-X. Wu and Y.-P. Shi, J. Chin. Chem. Soc., 2007, 54, 1565–1572 CAS.
- S. Zahid, A. Ata and R. Samarasekera, Z. Naturforsch., B, 2007, 62, 280–284 CAS.
- F. Escalante-Erosa, G. E. Arvízu-Méndez and L. M. Peña-Rodríguez, Phytochem. Anal., 2007, 18, 188–192 CrossRef CAS.
- B. K. Mehta, U. Sharma, S. Agrawal, V. Pandit, N. Joshi and M. Gupta, Med. Chem. Res., 2008, 17, 462–473 Search PubMed.
- H.-F. Pi, P. Zhang, T. Zhu, H.-L. Ruan, Y.-H. Zhang, H.-D. Sun and J.-Z. Wu, Chin. Chem. Lett., 2007, 18, 418–420 CrossRef CAS.
- K. Toyoda, Y. Yaoita and M. Kikuchi, J. Tohoku Pharm. Univ., 2006, 53, 51–55 ( Chem. Abstr. , 2008 , 148 , 234027r ) Search PubMed.
- S. Takahashi, H. Satoh, Y. Hongo and H. Koshino, J. Org. Chem., 2007, 72, 4578–4581 CrossRef CAS.
- N. Li, X. Li, X.-Z. Li, P. Zhang and D.-L. Meng, J. Asian Nat. Prod. Res., 2008, 10, 119–124 CrossRef CAS.
- H. Yoshimitsu, M. Nishida and T. Nohara, Chem. Pharm. Bull., 2008, 56, 1625–1627 CrossRef CAS.
- X. Fu, X.-C. Li, T. J. Smillie, P. Carvalho, W. Mabusela, J. Syce, Q. Johnson, W. Folk, M. A. Avery and I. A. Khan, J. Nat. Prod., 2008, 71, 1749–1753 CrossRef CAS.
- Z. Zhao, K. Matsunami, H. Otsuka, T. Shinzato and Y. Takeda, Chem. Pharm. Bull., 2008, 56, 1153–1158 CrossRef CAS.
- Z. Ali, S. I. Khan, F. R. Fronczek and I. A. Khan, Phytochemistry, 2007, 68, 373–382 CrossRef CAS.
- Z. Ali, S. I. Khan and I. A. Khan, Planta Med., 2007, 73, 699–703 CrossRef CAS.
- Z. Ali, I. A. Khan and F. R. Fronczek, Acta Crystallogr., Sect. E, 2007, 63, o2101–o2103 CrossRef.
- Z. Ali, S. I. Khan, R. S. Pawar, D. Ferreira and I. A. Khan, J. Nat. Prod., 2007, 70, 107–110 CrossRef CAS.
- D. A. Iskenderov and M. I. Isaev, Chem. Nat. Compd., 2008, 44, 621–624 CrossRef CAS.
- M. I. Choudhary, S. Jan, A. Abbaskhan, S. G. Musharraf, Samreen, S. A. Sattar and Atta-ur-Rahman, J. Nat. Prod., 2008, 71, 1557–1560 CrossRef CAS.
- Q. T. Yu, P. Li, Z. M. Bi, J. Luo and X. D. Gao, Chin. Chem. Lett., 2007, 18, 554–556 CrossRef CAS.
- A. Perrone, M. Masullo, C. Bassarello, E. Bloise, A. Hamed, P. Nigro, C. Pizza and S. Piacente, Tetrahedron, 2008, 64, 5061–5071 CrossRef CAS.
- İ. Çaliş, A. A. Dönmez, A. Perrone, C. Pizza and S. Piacente, Phytochemistry, 2008, 69, 2634–2638 CrossRef CAS.
- J. S. Kim, M.-H. Yean, E.-J. Lee, H. S. Jung, J. Y. Lee, Y. J. Kim and S. S. Kang, Chem. Pharm. Bull., 2008, 56, 105–108 CrossRef CAS.
- L. Sun, Y. Yan, Y. Nian, L. Zhou, H. Zhang and M. Qiu, Molecules, 2008, 13, 1712–1721 Search PubMed.
- R.-L. Pan, D.-H. Chen, J.-Y. Si and X.-H. Zhao, J. Asian Nat. Prod. Res., 2007, 9, 97–102 CrossRef CAS.
- M. D. Alaniya, N. Sh. Kavtaradze, R. Faure and L. Debrauwer, Chem. Nat. Compd., 2008, 44, 324–326 CrossRef CAS.
- T. Kh. Naubeev, K. K. Uteniyazov, V. V. Kachala and A. S. Shashkov, Chem. Nat. Compd., 2007, 43, 166–169 CrossRef CAS.
- T. Kh. Naubeev and K. K. Uteniyazov, Chem. Nat. Compd., 2007, 43, 561–562.
- T. Kh. Naubeev, K. K. Uteniyazov, R. T. Tlegenov and K. U. Uteniyazov, O'zbekiston Kimyo Jurnali, 2008, 29–33 ( Chem. Abstr. , 2008 , 149 , 508855r ) Search PubMed.
- Y. Zhao, J.-P. Liu and P.-Y. Li, Nat. Prod. Res., 2007, 21, 494–499 CrossRef CAS.
- I. A. Sukhina, R. P. Mamedova, M. A. Agzamova and M. I. Isaev, Chem. Nat. Compd., 2007, 43, 159–161 CrossRef CAS.
- N. Li, X. Li, B.-L. Hou, P. Zhang and S.-L. Yang, Fitoterapia, 2008, 79, 314–316 CrossRef CAS.
- P. Zhang, Y. Cheng and Z. Ma, Nat. Prod. Commun., 2008, 3, 899–902 CAS.
- P. Zhang, Y.-Y. Cheng and Z.-J. Ma, J. Asian Nat. Prod. Res., 2008, 10, 1069–1074 CrossRef CAS.
- H. Sun, X.-T. Zhang, L. Wang, X.-Q. Zhang, Y. Wang, S.-B. Chen, P.-G. Xiao and W.-C. Ye, Helv. Chim. Acta, 2008, 91, 1961–1966 CrossRef CAS.
- M.-T. Liu, S. Lin, Y.-H. Wang, W.-Y. He, S. Li, S.-J. Wang, Y.-C. Yang and J.-G. Shi, Org. Lett., 2007, 9, 129–132 CrossRef CAS.
- D.-C. Wang, H. Xiang, D. Li, H.-Y. Gao, H. Cai, L.-J. Wu and X.-M. Deng, Phytochemistry, 2008, 69, 1434–1438 CrossRef CAS.
- C.-I. Chang, C.-R. Chen, Y.-W. Liao, H.-L. Cheng, Y.-C. Chen and C.-H. Chou, J. Nat. Prod., 2008, 71, 1327–1330 CrossRef CAS.
- D. B. Mekuria, T. Kashiwagi, S. Tebayashi and C.-S. Kim, Biosci. Biotechnol. Biochem., 2005, 69, 1706–1710 CrossRef CAS.
- S. Nakamura, T. Murakami, J. Nakamura, H. Kobayashi, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2006, 54, 1545–1550 CrossRef CAS.
- H. Matsuda, S. Nakamura, T. Murakami and M. Yoshikawa, Heterocycles, 2007, 71, 331–341 CrossRef CAS.
- L. Harinantenaina, M. Tanaka, S. Takaoka, M. Oda, O. Mogami, M. Uchida and Y. Asakawa, Chem. Pharm. Bull., 2006, 54, 1017–1021 CrossRef CAS.
- J. Chen, R. Tian, M. Qiu, L. Lu, Y. Zheng and Z. Zhang, Phytochemistry, 2008, 69, 1043 CrossRef CAS.
- T. Akihisa, N. Higo, H. Tokuda, M. Ukiya, H. Akazawa, Y. Tochigi, Y. Kimura, T. Suzuki and H. Nishino, J. Nat. Prod., 2008, 71, 1233–1239 CrossRef.
- J. Guan, H. Pan and Y. Zhao, Zhongcaoyao, 2007, 38, 1133–1135 ( Chem. Abstr. , 2009 , 150 , 442055s ) Search PubMed.
- J.-C. Chen, L. Lu, X.-M. Zhang, L. Zhou, Z.-R. Li and M.-H. Qiu, Helv. Chim. Acta, 2008, 91, 920–929 CrossRef CAS.
- Y. Liu, A. Ali and I. A. Khan, Planta Med., 2008, 74, 1291–1294 CrossRef CAS.
- Q.-Y. Li, H.-B. Chen, Z.-M. Liu, B. Wang and Y.-Y. Zhao, Magn. Reson. Chem., 2007, 45, 451–456 CrossRef CAS.
- Q. Y. Li, H. Liang, H. B. Chen, B. Wang and Y. Y. Zhao, Chin. Chem. Lett., 2007, 18, 843–845 CrossRef CAS.
- T. Kashiwagi, M. Takehiro, D. B. Mekuria, A. Dekebo, K. Sato, S. Tebayashi and C.-S. Kim, Z. Naturforsch., C, 2007, 62, 603–607 CAS.
- D. Kurnia, K. Akiyama and H. Hayashi, Biosci. Biotechnol. Biochem., 2008, 72, 618–620 CrossRef CAS.
- J.-C. Chen, G.-H. Zhang, Z.-Q. Zhang, M.-H. Qiu, Y.-T. Zheng, L.-M. Yang and K.-B. Yu, J. Nat. Prod., 2008, 71, 153–155 CrossRef CAS.
- D. C. Chaves, J. C. C. Assunção, R. Braz-Filho, T. L. G. Lemos and F. J. Q. Monte, Magn. Reson. Chem., 2007, 45, 389–392 CrossRef CAS.
- K. N. Maloney, M. Fujita, U. S. Eggert, F. C. Schroeder, C. M. Field, T. J. Mitchison and J. Clardy, J. Nat. Prod., 2008, 71, 1927–1929 CrossRef CAS.
- D.-C. Wang, H.-Y. Pan, X.-M. Deng, H. Xiang, H.-Y. Gao, H. Cai and L.-J. Wu, J. Asian Nat. Prod. Res., 2007, 9, 525–529 CrossRef CAS.
- M. Yoshikawa, T. Morikawa, H. Kobayashi, A. Nakamura, K. Matsuhira, S. Nakamura and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 428–434 CrossRef CAS.
- P. Bhandari, N. Kumar, B. Singh and V. K. Kaul, Phytochemistry, 2007, 68, 1248–1254 CrossRef CAS.
- T. Akihisa, Y. Hayakawa, H. Tokuda, N. Banno, N. Shimizu, T. Suzuki and Y. Kimura, J. Nat. Prod., 2007, 70, 783–788 CrossRef CAS.
- D. Li, T. Ikeda, T. Nohara, J. Liu, Y. Wen, T. Sakamoto and G. Nonaka, Chem. Pharm. Bull., 2007, 55, 1082–1086 CrossRef CAS.
- J. Guerrero-Analco, O. Medina-Campos, F. Brindis, R. Bye, J. Pedraza-Chaverri, A. Navarrete and R. Mata, Phytochemistry, 2007, 68, 2087–2095 CrossRef CAS.
- J.-C. Chen, Z.-Q. Zhang and M.-H. Qiu.
- S. Pointinger, S. Promdang, S. Vajrodaya, C. M. Pannell, O. Hofer, K. Mereiter and H. Greger, Phytochemistry, 2008, 69, 2696–2703 CrossRef CAS.
- C. Seger, S. Pointinger, H. Greger and O. Hofer, Tetrahedron Lett., 2008, 49, 4313–4315 CrossRef CAS.
- L. Tu, Y. Zhao, Z.-Y. Yu, Y.-W. Cong, G. Xu, L.-Y. Peng, P.-T. Zhang, X. Cheng and Q.-S. Zhao, Helv. Chim. Acta, 2008, 91, 1578–1587 CrossRef CAS.
- S.-M. Yang, C.-H. Tan, H.-F. Luo, D.-X. Wang and D.-Y. Zhu, Helv. Chim. Acta, 2008, 91, 333–337 CrossRef CAS.
- Z. Ahmad, I. Fatima, S. Mehmood, R. Ifzal, A. Malik and N. Afza, Helv. Chim. Acta, 2008, 91, 73–78 CrossRef CAS.
- X.-H. Li, H.-Y. Qi and Y.-P. Shi, J. Asian Nat. Prod. Res., 2008, 10, 397–402 CrossRef CAS.
- M. Xu, D. Wang, Y.-J. Zhang and C.-R. Yang, J. Nat. Prod., 2007, 70, 880–883 CrossRef CAS.
- P. A. de C. Braga, M. S. Soares, M. F. das, G. F. da Silva, P. C. Vieira, J. B. Fernandes and A. L. Pinheiro, Biochem. Syst. Ecol., 2006, 34, 282–290 CrossRef.
- X.-H. Xu, N.-Y. Yang, S.-H. Qian, N. Xie and J.-A. Duan, J. Asian Nat. Prod. Res., 2008, 10, 33–37 CrossRef CAS.
- V. Torpocco, H. Chávez, A. Estévez-Braun and A. G. Ravelo, Chem. Pharm. Bull., 2007, 55, 812–814 CrossRef CAS.
- Y. Zhao, J.-L. Ruan, J.-H. Wang, Y. Cong, S. Song, Y.-L. Cai, W. Fang and D.-N. Zhou, Nat. Prod. Res., 2008, 22, 233–240 CrossRef CAS.
- M. Yoshikawa, Y. Zhang, T. Wang, S. Nakamura and H. Matsuda, Chem. Pharm. Bull., 2008, 56, 915–920 CrossRef CAS.
- V. J. Ndlebe, N. R. Crouch and D. A. Mulholland, Phytochemistry, 2008, 69, 11–17.
- P. V. Srinivas, R. R. Rao and J. M. Rao, Chem. Biodiversity, 2006, 3, 930–934 CrossRef CAS.
- Y. Kashiwada, M. Sekiya, K. Yamazaki, Y. Ikeshiro, T. Fujioka, T. Yamagishi, S. Kitagawa and Y. Takaishi, J. Nat. Prod., 2007, 70, 623–627 CrossRef CAS.
- H.-C. Huang, M.-D. Wu, W.-J. Tsai, S.-C. Liao, C.-C. Liaw, L.-C. Hsu, Y.-C. Wu and Y.-H. Kuo, Phytochemistry, 2008, 69, 1609–1616 CrossRef CAS.
- R. S. Pawar, S. I. Khan and I. A. Khan, Planta Med., 2007, 73, 380–383 CrossRef CAS.
- L.-J. Wu, L.-B. Wang, H.-Y. Gao, B. Wu, X.-M. Song and Z.-S. Tang, Fitoterapia, 2007, 78, 556–560 CrossRef CAS.
- Y. Yuan, H. Gao, B. Wu, Z. Tang, D. Guo, X. Song and L. Wu, Asian J. Tradit. Med., 2006, 1, 91–93 Search PubMed.
- P.-Y. Liao, D. Wang, Y.-J. Zhang and C.-R. Yang, J. Agric. Food Chem., 2008, 56, 1751–1756 CrossRef CAS.
- F. Yin, Y.-N. Zhang, Z.-Y. Yang and L.-H. Hu, Chem. Biodiversity, 2006, 3, 771–782 CrossRef CAS.
- Z. Yang, Q. Chen and L. Hu, Phytochemistry, 2007, 68, 1752–1761 CrossRef CAS.
- Y. Zhou, Y.-H. Shen, C. Zhang, J. Su, R.-H. Liu and W.-D. Zhang, J. Nat. Prod., 2007, 70, 652–655 CrossRef CAS.
- M. Yoshikawa, S. Sugimoto, S. Nakamura and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 571–576 CrossRef CAS.
- S. Nakamura, S. Sugimoto, H. Matsuda and M. Yoshikawa, Heterocycles, 2007, 71, 577–588 CrossRef CAS.
- M. Yoshikawa, S. Sugimoto, S. Nakamura, H. Saumae and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 1034–1038 CrossRef CAS.
- J.-Z. Wang and J.-S. Yang, Youji Huaxue, 2006, 28, 69–72 ( Chem. Abstr. , 2008 , 148 , 466898d ).
- X.-M. Cui, Z.-Y. Jiang, J. Zeng, J.-M. Zhou, J.-J. Chen, X.-M. Zhang, L.-S. Xu and Q. Wang, J. Asian Nat. Prod. Res., 2008, 10, 845–849 CrossRef CAS.
- X.-Y. Wang, D. Wang, X.-X. Ma, Y.-J. Zhang and C.-R. Yang, Helv. Chim. Acta, 2008, 91, 60–66 CrossRef CAS.
- H.-P. Jiang, Y.-K. Qiu, D.-R. Cheng, T.-G. Kang and D. Qiang Dou, Magn. Reson. Chem., 2008, 46, 786–790 CrossRef CAS.
- L. B. Wang, Z. H. Wu, H. Y. Gao, J. Huang, B. H. Sun and L. J. Wu, Chin. Chem. Lett., 2008, 19, 837–840 CrossRef CAS.
- J. M. Jia, Z. Q. Wang and L. J. Wu, Chin. Chem. Lett., 2008, 19, 1099–1102 CrossRef CAS.
- Y. Liang and S. Zhao, Plant Biol., 2008, 10, 415–421 CrossRef CAS.
- L. Fang, A. Ito, H.-B. Chai, Q. Mi, W. P. Jones, D. R. Madulid, M. B. Oliveros, Q. Gao, J. Orjala, N. R. Farnsworth, D. D. Soejarto, G. A. Cordell, S. M. Swanson, J. M. Pezzuto and A. D. Kinghorn, J. Nat. Prod., 2006, 69, 332–337 CrossRef CAS.
- P. Tuchinda, J. Kornsakulkarn, M. Pohmakotr, P. Kongsaeree, S. Prabpai, C. Yoosook, J. Kasisit, C. Napaswad, S. Sophasan and V. Reutrakul, J. Nat. Prod., 2008, 71, 655–663 CrossRef CAS.
- B.-N. Su, H. Chai, Q. Mi, S. Riswan, L. B. S. Kardono, J. J. Afriastini, B. D. Santarsiero, A. D. Mesecar, N. R. Farnsworth, G. A. Cordell, S. M. Swanson and A. D. Kinghorn, Bioorg. Med. Chem, 2006, 14, 960–972 CrossRef CAS.
- R. S. Compagnone, A. C. Suarez, S. G. Leitao and F. Delle Monache, Rev. Bras. Farm., 2008, 18, 6–10 ( Chem. Abstr. , 2008 , 149 , 443711u ) Search PubMed.
- G.-H. Xu, J.-A. Kim, S.-Y. Kim, J.-C. Ryu, Y.-S. Kim, S.-H. Jung, M.-K. Kim and S.-H. Lee, Chem. Pharm. Bull., 2008, 56, 839–842 CrossRef CAS.
- C. Yan, Y. Wang and X.-J. Hao, Chin. Chem. Lett., 2008, 19, 937–939 CrossRef CAS.
- C. Yan, Y. Wang, H. Du and X.-J. Hao, Chin. J. Chem., 2008, 26, 1343–1345 CrossRef CAS.
- K. Mitsui, H. Saito, R. Yamamura, H. Fukaya, Y. Hitotsuyanagi and K. Takeya, Chem. Pharm. Bull., 2007, 55, 1442–1447 CrossRef CAS.
- B.-J. Xie, S.-P. Yang, H.-D. Chen and J.-M. Yue, J. Nat. Prod., 2007, 70, 1532–1535 CrossRef CAS.
- X.-N. Wang, C.-Q. Fan, S. Yin, L.-P. Lin, J. Ding and J.-M. Yue, Helv. Chim. Acta, 2008, 91, 510–519 CrossRef CAS.
- X.-H. Cai, Z.-Z. Du and X.-D. Luo, Helv. Chim. Acta, 2007, 90, 1980–1986 CrossRef CAS.
- H.-C. Huang, W.-J. Tsai, C.-C. Liaw, S.-H. Wu, Y.-C. Wu and Y.-H. Kuo, Chem. Pharm. Bull., 2007, 55, 1412–1415 CrossRef CAS.
- S. Yin, X.-N. Wang, C.-Q. Fan, S.-G. Liao and J.-M. Yue, Org. Lett., 2007, 9, 2353–2356 CrossRef CAS.
- B. T. Murphy, P. Brodie, C. Slebodnick, J. S. Miller, C. Birkinshaw, L. M. Randrianjanaka, R. Andriantsiferana, V. E. Rasamison, K. TenDyke, E. M. Suh and D. G. I. Kingston, J. Nat. Prod., 2008, 71, 325–329 CrossRef CAS.
- X.-H. Cai and X.-D. Luo, Chin. J. Chem., 2007, 25, 986–988 CrossRef CAS.
- O. J. H. Odera, M. Ndung'u and A. Hassanali, J. Kenya Chem. Soc., 2008, 5, 43–49 ( Chem. Abstr. , 2009 , 150 , 490420d ) Search PubMed.
- J. L. Chen, M. R. Kernan, S. D. Jolad, C. A. Stoddart, M. Bogan and R. Cooper, J. Nat. Prod., 2007, 70, 312–315 CrossRef CAS.
- S. Laphookhieo, W. Maneerat, S. Koysomboon, R. Kiattansakul, K. Chantrapromma and J. K. Syers, Can. J. Chem., 2008, 86, 205–208 CrossRef CAS.
- R. Malathi, S. S. Rajan, R. M. Kumar, S. Narasimhan and K. Ravikumar, Acta Crystallogr., Sect. E, 2007, 63, o2483–o2485 CrossRef.
- J. Kotoky, M. Kalita, G. K. Sarmah and B. Das, Indian J. Chem., Sect. B, 2007, 46, 1879–1882.
- G. D. Manners, J. Agric. Food Chem., 2007, 55, 8285–8294 CrossRef CAS.
- A. P. Breksa III, K. Dragull and R. Y. Wong, J. Agric. Food Chem., 2008, 56, 5595–5598 CrossRef.
- X.-W. Yang, H. Zhang and J. Hu, Fenxi Huaxue, 2008, 36, 219–222 ( Chem. Abstr. , 2008 , 148 , 580515b ) CAS.
- P. K. Cheplogoi, D. A. Mulholland, P. H. Coombes and M. Randrianarivelojosia, Phytochemistry, 2008, 69, 1384–1388 CrossRef CAS.
- N. T. Kipassa, T. Iwagawa, H. Okamura, M. Doe, Y. Morimoto and M. Nakatani, Phytochemistry, 2008, 69, 1782–1787 CrossRef CAS.
- S.-G. Liao, S.-P. Yang, T. Yuan, C.-R. Zhang, H.-D. Chen, Y. Wu, Y.-K. Xu and J.-M. Yue, J. Nat. Prod., 2007, 70, 1268–1273 CrossRef CAS.
- X.-N. Wang, S. Yin, C.-Q. Fan, L.-P. Lin, J. Ding and J.-M. Yue, Tetrahedron, 2007, 63, 8234–8241 CrossRef CAS.
- H.-D. Chen, S.-P. Yang, S.-G. Liao, B. Zhang, Y. Wu and J.-M. Yue, J. Nat. Prod., 2008, 71, 93–97 CrossRef CAS.
- Atta-ur-Rahman, S. Zareen, M. I. Choudhary, M. N. Akhtar and S. N. Khan, J. Nat. Prod., 2008, 71, 910–913 CrossRef.
- Y.-L. Shi and M.-F. Li, Prog. Neurobiol., 2007, 82, 1–10 CrossRef CAS.
- Q. Zhang, Y. Shi, X.-T. Liu, J.-Y. Liang, N. Y. Ip and Z.-D. Min, Planta Med., 2007, 73, 1298–1303 CrossRef CAS.
- F. Xie, C. F. Zhang, M. Zhang, Z. T. Wang and B. Y. Yu, Chin. Chem. Lett., 2008, 19, 183–186 CrossRef CAS.
- K. Mohamad, Y. Hirasawa, C. S. Lim, K. Awang, A. H. A. Hadi, K. Takeya and H. Morita, Tetrahedron Lett., 2008, 49, 4276–4278 CrossRef CAS.
- S. N. Ayyad, T. H. Hoye, L. Sreerama, A. M. Dawidar, W. M. Alarif and M. Abdel-Mogib, Rev. Latinoam. Quim., 2008, 36, 7–15 ( Chem. Abstr. , 2009 , 150 , 534470b ) Search PubMed.
- H. Zhang, O. A. Odeku, X.-N. Wang and J.-M. Yue, Phytochemistry, 2008, 69, 271–275 CrossRef CAS.
- N. T. Kipassa, H. Okamura, M. Doe, Y. Morimoto, T. Iwagawa and M. Nakatani, Heterocycles, 2008, 75, 157–164 CrossRef CAS.
- A.-E. Hay, J.-R. Ioset, K. M. Ahua, D. Diallo, R. Brun and K. Hostettmann, J. Nat. Prod., 2007, 70, 9–13 CrossRef CAS.
- Y.-Y. Chen, X.-N. Wang, C.-Q. Fan, S. Yin and J.-M. Yue, Tetrahedron Lett., 2007, 48, 7480–7484 CrossRef CAS.
- M. N. da Silva, M. S. P. Arruda, K. C. F. Castro, M. F. das and G. F. da Silva, J. Nat. Prod., 2008, 71, 1983–1987 CrossRef.
- C.-Q. Fan, X.-N. Wang, S. Yin, C.-R. Zhang, F.-D. Wang and J.-M. Yue, Tetrahedron, 2007, 63, 6741–6747 CrossRef CAS.
- R. C,-Zhang, S.-P. Yang, X.-Q. Chen, Y. Wu, X.-C. Zhen and J.-M. Yue, Helv. Chim. Acta, 2008, 91, 2338–2350 CrossRef.
- C.-R. Zhang, C.-Q. Fan, L. Zhang, S.-P. Yang, Y. Wu, Y. Lu and J.-M. Yue, Org. Lett., 2008, 10, 3183–3186 CrossRef CAS.
- C.-R. Zhang, S.-P. Yang, S.-G. Liao, C.-Q. Fan, Y. Wu and J.-M. Yue, Org. Lett., 2007, 9, 3383–3386 CrossRef CAS.
- K. Awang, C. S. Lim, K. Mohamad, H. Morita, Y. Hirasawa, K. Takeya, O. Thoison and A. H. A. Hadi, Bioorg. Med. Chem, 2007, 15, 5997–6002 CrossRef CAS.
- J. Wu, Q. Xiao, S. Zhang, X. Li, Z. Xiao, H. Ding and Q. Li, Tetrahedron, 2005, 61, 8382–8389 CrossRef CAS.
- J. Cui, J. Wu, Z. Deng, P. Proksch and W. Lin, J. Nat. Prod., 2007, 70, 772–778 CrossRef CAS.
- J. Cui, Z. Deng, J. Li, H. Fu, P. Proksch and W. Lin, Phytochemistry, 2005, 66, 2334–2339 CrossRef CAS.
- J. Wu, H. Ding, M. Li and S. Zhang, Z. Naturforsch., C, 2007, 62, 569–572 CAS.
- M. Li, J. Wu, S. Zhang, Q. Xiao and Q. Li, Magn. Reson. Chem., 2007, 45, 705–709 CrossRef CAS.
- S. Yin, X.-N. Wang, C.-Q. Fan, L.-P. Lin, J. Ding and J.-M. Yue, J. Nat. Prod., 2007, 70, 682–685 CrossRef CAS.
- H.-K. Fun, S. Chantrapromma, N. Boonnak, K. Chaiyadej and X.-L. Yu, Acta Crystallogr., Sect. E, 2006, 62, o3725–o3727 CrossRef.
- X.-N. Wang, C.-Q. Fan, S. Yin, L.-S. Gan and J.-M. Yue, Phytochemistry, 2008, 69, 1319–1327 CrossRef CAS.
- B.-J. Xie, S.-P. Yang and J.-M. Yue, Phytochemistry, 2008, 69, 2993–2997 CrossRef CAS.
- J. S. Yoon, S. H. Sung and Y. C. Kim, J. Nat. Prod., 2008, 71, 208–211 CrossRef CAS.
- P.-H. Zhao, L.-M. Sun, M.-A. Cao and C.-S. Yuan, Chem. Lett., 2007, 36, 1200 CrossRef CAS.
- P. H. Zhao, L. M. Sun, X. J. Liu, M. A. Cao and C. S. Yuan, Chem. Pharm. Bull., 2008, 56, 102–104 CrossRef CAS.
- L.-G. Lin, C.-P. Tang, C.-Q. Ke, Y. Zhang and Y. Ye, J. Nat. Prod., 2008, 71, 628–632 CrossRef CAS.
- Y.-L. Ren, Q.-R. Tang, Y.-T. Di, H.-P. He, Z. Zhang, S.-L. Li and X.-J. Hao, Helv. Chim. Acta, 2007, 90, 764–768 CrossRef CAS.
- X.-H. Yuan, B.-G. Li, C.-X. Xu, M. Zhou, H.-Y. Qi and G.-L. Zhang, Chem. Pharm. Bull., 2007, 55, 902–904 CrossRef CAS.
- Y.-T. Di, H.-P. He, H.-Y. Liu, P. Yi, Z. Zhang, Y.-L. Ren, J.-S. Wang, Q.-Y. Sun, F.-M. Yang, X. Fang, S.-L. Li, H.-J. Zhu and X.-J. Hao, J. Nat. Prod., 2005, 68, 1352–1355.
- L.-S. Gan, X.-N. Wang, Y. Wu and J.-M. Yue, J. Nat. Prod., 2007, 70, 1344–1347 CrossRef CAS.
- J. Wu, S. Zhang, T. Bruhn, Q. Xiao, H. Ding and G. Bringmann, Chem. Eur. J., 2008, 14, 1129–1144 CrossRef CAS.
- J. Cui, J. Ouyang, A. Deng and W. Lin, Magn. Reson. Chem., 2008, 46, 894–897 CrossRef CAS.
- T. Narender, T. Khaliq, Shweta, K. P. Reddy and R. K. Sharma, Nat. Prod. Commun., 2007, 2, 203–221 CAS.
- M. D. J. Dongfack, H. T. Van-Dufat, M.-C. Lallemand, J.-D. Wansi, E. Seguin, F. Tillequin and J. Wandji, Chem. Pharm. Bull., 2008, 56, 1321–1323 CrossRef CAS.
- G. Mustafa, E. Anis, S. Ahmed, I. Anis, H. Ahmed, A. Malik, S. Shahzad-ul-Hassan and M. I. Choudhary, J. Nat. Prod., 2000, 63, 881–883 CrossRef CAS.
- M. H. Kazmi, E. Ahmed, S. Hameed, A. Malik and M. Ashraf, Magn. Reson. Chem., 2007, 45, 416–419 CrossRef CAS.
- J. P. David, J. Ferrari, J. M. David, A. G. Guimarães, F. W. de M. Lima and G. L. S. de Souza, J. Braz. Chem. Soc., 2007, 18, 1585–1589 CAS.
- A. M. Rauter, I. Branco, R. G. Lopes, J. Justino, F. V. M. Silva, J. P. Noronha, E. J. Cabrita, I. Brouard and J. Bermejo, Fitoterapia, 2007, 78, 474–481 CrossRef CAS.
- G. Topçu, A. Ertaş, U. Kolak, M. Öztürk and A. Ulubelen, ARKIVOC, 2007, vii, 195–208.
- C. Mutai, D. Abatis, C. Vagias, D. Moreau, C. Roussakis and V. Roussis, Molecules, 2007, 12, 1035–1044 Search PubMed.
- L. M. Mbaze, H. M. P. Poumale, J. P. Wansi, J. A. Lado, S. N. Khan, M. C. Iqbal, B. T. Ngadjui and H. Laatsch, Phytochemistry, 2007, 68, 591 CrossRef CAS.
- M. Elbandy, T. Miyamoto and M.-A. Lacaille-Dubois, Chem. Pharm. Bull., 2007, 55, 808–811 CrossRef CAS.
- F. He, Q. Pan and Z. Min, Zhongcaoyao, 2006, 37, 168–171 ( Chem. Abstr. , 2008 , 148 , 187191c ) Search PubMed.
- L.-L. Wang, Z.-L. Li, D.-D. Song, L. Sun, Y.-H. Pei, Y.-K. Jing and H.-M. Hua, Planta Med., 2008, 74, 1735–1740 CrossRef CAS.
- K. P. Manoharan, F. J. Song, T. K. H. Benny and D. Yang, Magn. Reson. Chem., 2007, 45, 279–281 CrossRef CAS.
- J. Yang, X.-Q. Chen, X.-X. Liu, Y. Cao, M.-X. Lai and Q. Wang, Magn. Reson. Chem., 2008, 46, 794–797 CrossRef CAS.
- C.-L. Chiu, T.-H. Lee, Y.-Y. Shao and Y.-H. Kuo, J. Asian Nat. Prod. Res., 2008, 10, 684–688 CrossRef CAS.
- B. S. Siddiqui, H. Aslam, S. T. Ali, S. Begum and N. Khatoon, Chem. Pharm. Bull., 2007, 55, 516–519 CrossRef CAS.
- M.-H. Pan, C.-M. Chen, S.-W. Lee and Z.-T. Chen, Chem. Biodiversity, 2008, 5, 565–574 CrossRef CAS.
- L. He, Y.-S. Wang, Q.-J. Wang and Z.-P. Lou, Pharmazie, 2005, 60, 716–718.
- W. Li, X. Li, D.-L. Meng, P. Zhang and Z.-L. Li, J. Asian Nat. Prod. Res., 2007, 9, 7–11 CrossRef CAS.
- Z. Ma, Y. Hano, F. Qiu, G. Shao, Y. Chen and T. Nomura, Nat. Prod. Commun., 2008, 3, 149–154 CAS.
- I.-H. Chen, Y.-C. Du, A.-S. Lin, P.-W. Hsieh, C.-C. Wu, S.-L. Chen, H.-F. Yen, F.-R. Chang and Y.-C. Wu, J. Nat. Prod., 2008, 71, 1352–1357 CrossRef CAS.
- H. Shan, W. K. Wilson, D. R. Philips, B. Bartel and S. P. T. Matsuda, Org. Lett., 2008, 10, 1897–1900 CrossRef CAS.
- F. M. Abdel Bar, A. M. Zaghloul, S. V. Bachawal, P. W. Sylvester, K. F. Ahmad and K. A. El Sayed, J. Nat. Prod., 2008, 71, 1787–1790 CrossRef.
- A. A. El-Gamal, Nat. Prod. Res., 2008, 22, 191–197 CrossRef CAS.
- Y. Wei, C.-M. Ma, D.-Y. Chen and M. Hattori, Phytochemistry, 2008, 69, 1875–1879 CrossRef CAS.
- Z. Yang, S. Qian and M. Qin, Yaoxue Xuebao, 2008, 43, 388–391 ( Chem. Abstr. , 2009 , 150 , 163610d ) CAS.
- H.-S. Choi, H. J. Kim, S.-G. Nam, I.-S. Kim, K.-T. Lee, C.-S. Yook and Y. S. Lee, Chem. Pharm. Bull., 2008, 56, 1613–1616 CrossRef CAS.
- S. Okazaki, K. Kinoshita, K. Koyama, K. Takahashi and H. Yuasa, J. Nat. Med., 2007, 61, 24–29 Search PubMed.
- R. N. Yadava and N. Chakravarti, J. Enzym. Inhibition Med. Chem., 2008, 23, 543–548 Search PubMed.
- S. R. Giacomelli, G. Maldaner, C. Stücker, C. Marasciulo, J. Schmidt, L. Wessjohann, I. I. Dalcol and A. F. Morel, Planta Med., 2007, 73, 499–501 CrossRef CAS.
- W.-H. Li, X.-M. Zhang, R.-R. Tian, Y.-T. Zheng, W.-M. Zhao and M.-H. Qiu, J. Asian Nat. Prod. Res., 2007, 9, 551–555 CrossRef CAS.
- T. K. Tabapda, J. Liu, B. T. Ngadjui and B. Luu, Planta Med., 2007, 73, 376–380 CrossRef CAS.
- X.-F. He, X.-N. Wang, L.-S. Gan, S. Yin, L. Dong and J.-M. Yue, Org. Lett., 2008, 10, 4327–4330 CrossRef CAS.
- P. Liu, L. Y. Li, R. Q. Niu, W. P. Yin and T. Z. Zhao, Chin. Chem. Lett., 2008, 19, 1228–1230 CrossRef CAS.
- P. Liu, Z. Yao, W. Zhang, Y. Takaishi and H.-Q. Duan, Chem. Pharm. Bull., 2008, 56, 951–955 CrossRef CAS.
- P. Liu, Z. Yao, H.-Q. Li and H.-Q. Duan, Helv. Chim. Acta, 2007, 90, 601–606 CrossRef CAS.
- S. Michalet, L. Payen-Fattaccioli, C. Beney, P. Cégiéla, C. Bayet, G. Cartier, D. Noungoué-Tchamo, E. Tsamo, A.-M. Mariotte and M.-G. Dijoux-Franca, Helv. Chim. Acta, 2008, 91, 1106–1117 CrossRef CAS.
- L. Li, I. Sattler, Z. Deng, I. Groth, G. Walther, K.-D. Menzel, G. Peschel, S. Grabley and W. Lin, Phytochemistry, 2008, 69, 511–517 CrossRef CAS.
- L. O. Hanus, A. Moussaieff, T. Rezanka, S. Abu-Lafi and V. M. Dembitsky, Nat. Prod. Commun., 2007, 2, 139–142 CAS.
- J.-G. Luo, J. Liu and L.-Y. Kong, Chem. Biodiversity, 2008, 5, 751–757 CrossRef CAS.
- G. Cioffi, A. Bader, A. Malafronte, F. Dal Piaz and N. De Tommasi, Phytochemistry, 2008, 69, 1005–1012 CrossRef CAS.
- S. Begum, S. Q. Zehra and B. S. Siddiqui, Chem. Pharm. Bull., 2008, 56, 1317–1320 CrossRef CAS.
- D. Tsiri, N. Aligiannis, K. Graikou, C. Spyropoulos and I. Chinou, Helv. Chim. Acta, 2008, 91, 2110–2114 CrossRef CAS.
- I. S. Lee, J. K. Yoo, M. K. Na, B. S. Min, J. P. Lee, B. S. Yun, W. Y. Jin, H. J. Kim, U. J. Youn, Q. C. Chen, K. S. Song, Y. H. Seong and K. H. Bae, Chem. Pharm. Bull., 2007, 55, 1376–1378 CrossRef CAS.
- P.-L. Li and Z.-J. Jia, Helv. Chim. Acta, 2008, 91, 1717–1727 CrossRef CAS.
- D. Cáceres-Castillo, G. J. Mena-Rejón, R. Cedillo-Rivera and L. Quijano, Phytochemistry, 2008, 69, 1057–1064 CrossRef CAS.
- G. J. Mena-Rejón, A. R. Pérez-Espadas, R. E. Moo-Puc, R. Cedillo-Rivera, I. L. Bazzocchi, I. A. Jiménez-Diaz and L. Quijano, J. Nat. Prod., 2007, 70, 863–865 CrossRef CAS.
- E. Ahmed, A. Noor, A. Malik, S. Ferheen and N. Afza, Magn. Reson. Chem., 2007, 45, 79–81 CrossRef CAS.
- A. Khan, E. Haque, M. M M. Rahman, A. Mosaddik, M. Rahman and N. Sultana, Nat. Prod. Res., 2007, 21, 959–966 CrossRef CAS.
- F. R. Garcez, W. S. Garcez, T. S. Mahmoud, P. de O. Figueirdo and U. M. Resende, Quim. Nova, 2007, 30, 1887–1891 CAS.
- W.-Y. Kang, B.-R. Zhang and X.-J. Hao, Gaodeng Xuexiao Huaxue Xuebao, 2007, 28, 2096–2098 ( Chem. Abstr. , 2008 , 148 , 326782m ) CAS.
- J.-C. Ho, C.-M. Chen and L.-C. Row, Phytochemistry, 2007, 68, 631–635 CrossRef CAS.
- S.-X. Zhou, J.-S. Yang, F.-C. Liu and P.-F. Tu, Helv. Chim. Acta, 2007, 90, 121–127 CrossRef CAS.
- Q. Shen, Z. Yao, Y. Takaishi, Y. W. Zhang and H. Q. Duan, Chin. Chem. Lett., 2008, 19, 453–456 CrossRef CAS.
- D. Q. Fei, G. Wu, C. M. Liu and K. Gao, Chem. Pharm. Bull., 2007, 55, 577–579 CrossRef CAS.
- D. Zhang, S. Zhang and J. Wu, Zhongcaoyao, 2007, 38, 1601–1603 ( Chem. Abstr. , 2009 , 151 , 27969w ) Search PubMed.
- Z. Ahmad, S. Mehmood, R. Ifzal, A. Malik, N. Afza, F. Rashid, A. Mahmood and L. Iqbal, Polish J. Chem., 2007, 81, 1427–1432 Search PubMed.
- Z. Ahmad, S. Mehmood, I. Fatima, A. Malik, R. Ifzal, N. Afza, L. Iqbal, M. Latif and T. A. Nizami, Magn. Reson. Chem., 2008, 46, 94–98 CrossRef CAS.
- B. S. Siddiqui and Z. Z. Karzhaubekova, Chem. Pharm. Bull., 2007, 55, 1356–1360 CrossRef CAS.
- K.-W. Wang, Fitoterapia, 2008, 79, 311–313 CrossRef CAS.
- K.-W. Wang, Nat. Prod. Res., 2007, 21, 669–674 CrossRef CAS.
- X.-H. Li, J.-T. Feng and Y.-P. Shi, Can. J. Chem., 2008, 86, 281–284 CrossRef CAS.
- R. R. Silva de Miranda, G. D. de F. Silva, L. P. Duarte and S. A. Vierira Filho, Helv. Chim. Acta, 2007, 90, 652–658 CrossRef CAS.
- B. Wang, G. Q. Li, P. J. Qiu and H. S. Guan, Chin. Chem. Lett., 2007, 18, 708–710 CrossRef CAS.
- C.-M. Liu, H.-X. Wang, S.-L. Wei and K. Gao, J. Nat. Prod., 2008, 71, 789–792 CrossRef CAS.
- Z.-G. Yang, H.-R. Li, L.-Y. Wang, Y.-H. Li, S.-G. Lu, X.-F. Wen, J. Wang, A. Daikonya and S. Kitanaka, Chem. Pharm. Bull., 2007, 55, 15–18 CrossRef CAS.
- B. S. Siddiqui, F. Ahmad, F. A. Sattar and S. Begum, Arch. Pharmacal Res., 2007, 30, 1507–1510 Search PubMed.
- X.-P. Yang, C.-S. Yuan and Z.-J. Jia, J. Chin. Chem. Soc., 2007, 54, 459–463 CAS.
- S. Begum, S. Q. Zehra, B. S. Siddiqui, S. Fayyaz and M. Ramzan, Chem. Biodiversity, 2008, 5, 1856–1866 CrossRef CAS.
- C.-I. Chang and Y.-H. Kuo, J. Asian Nat. Prod. Res., 2007, 9, 67–72 CrossRef CAS.
- Y. Yang, Z. Deng, P. Proksch and W. Lin, Pharmazie, 2006, 61, 365–366 CAS.
- D.-L. Li, X.-M. Li and B.-G. Wang, Nat. Prod. Res., 2008, 22, 808–813 CrossRef CAS.
- Z.-Z. Ma, Y. Hano, F. Qiu, G. Shao, Y.-J. Chen and T. Nomura, J. Asian Nat. Prod. Res., 2007, 9, 575–578 CrossRef CAS.
- A. Jimenez-Arellanes, M. Meches, J. Torres and J. Luna-Herrera, J. Ethnopharmacol., 2007, 111, 202–205 CrossRef CAS.
- T. Mencherini, P. Picerno, C. Scesa and R. Aquino, J. Nat. Prod., 2007, 70, 1889–1894 CrossRef CAS.
- S. Begum, S. N. Ali, S. I. Hassan and B. S. Siddiqui, Nat. Prod. Res., 2007, 21, 742–748 CrossRef CAS.
- J. Kouam, A. L. Meli, M. I. Choudhary and Z. T. Fomum, Z. Naturforsch., B, 2008, 63, 101–104 CAS.
- Atta-ur-Rahman, S. Zareen, M. I. Chaudhary, M. N. Akhtar and F. N. Ngounou, Phytochemistry, 2008, 69, 2400–2405 CrossRef.
- D.-L. Liu, N.-L. Wang, X. Zhang, H. Gao and X.-S. Yao, J. Asian Nat. Prod. Res., 2007, 9, 119–127 CrossRef CAS.
- X. Chang, W. Li, Z. Jia, T. Satou, S. Fushiya and K. Koike, J. Nat. Prod., 2007, 70, 179–187 CrossRef CAS.
- I. Krasteva, S. Nikolov, M. Kaloga and G. Mayer, Nat. Prod. Res., 2007, 21, 941–945 CrossRef CAS.
- S. Avunduk, A.-C. Mitaine-Offer, Ö. Alankuş-Çalişkan, T. Miyamoto, S. G. Şenol and M.-A. Lacaille-Dubois, J. Nat. Prod., 2008, 71, 141–145 CrossRef CAS.
- M. Yoshikawa, T. Morikawa, S. Nakamura, N. Li, X. Li and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 57–63 CrossRef CAS.
- Z. Zhang, S. Li, S. Ownby, P. Wang, W. Yuan, W. Zhang and R. S. Beasley, Phytochemistry, 2008, 69, 2070–2080 CrossRef CAS.
- A. Perrone, M. Masullo, C. Bassarello, A. I. Hamed, M. A. Belisario, C. Pizza and S. Piacente, J. Nat. Prod., 2007, 70, 584–588 CrossRef CAS.
- L.-Y. Jinwei, J.-I. Nakajima, N. Kimura, K. Saito and S. Seo, Nat. Prod. Commun., 2007, 2, 243–248 CAS.
- L. Zhang, J. Tian, L. Xu, Z. Zou and S. Yang, Nat. Prod. Res., 2007, 21, 106–110 CAS.
- L. Wang, L. Bai, T. Nagasawa, T. Hasegawa, X. Yang, J.-I. Sakai, Y. Bai, T. Kataoka, S. Oka, K. Hirose, A. Tomida, T. Tsuruo and M. Ando, J. Nat. Prod., 2008, 71, 35–40 CrossRef CAS.
- F. A. Moharram, M. A. Mohamed, M. S. A. Marzouk and E. A. Aboutabi, Nat. Prod. Commun., 2007, 2, 895–900 CAS.
- W. Li, L. Xiang, J. Zhang, Y. N. Zheng, L. K. Han and M. Saito, Chin. Chem. Lett., 2007, 18, 306–308 CrossRef CAS.
- L. Zhang, Z.-H. Liu and J.-K. Tian, Molecules, 2007, 12, 832–841 Search PubMed.
- J. Fu, L. Zuo, J. Yang, R. Chen and D. Zhang, Phytochemistry, 2008, 69, 1617–1624 CrossRef CAS.
- H.-L. Xin, Y.-F. Xu, Y.-H. Hou, Y.-N. Zhang, X.-Q. Yue, J.-C. Lu and C.-Q. Ling, Helv. Chim. Acta, 2008, 91, 2075–2080 CrossRef CAS.
- X.-J. Gu, Y.-B. Li, P. Li, S.-H. Qian and J.-F. Zhang, Helv. Chim. Acta, 2007, 90, 72–78 CrossRef CAS.
- L. M. Calabria, S. Piacete, I. Kapusta, S. F. Dharmawardhane, F. M. Segarra, P. J. Pessiki and T. J. Mabry, Phytochemistry, 2008, 69, 961–972 CrossRef CAS.
- V. U. Ahmad, S. Arshad, S. Bader, S. Iqbal, A. Khan, S. S. Khan, J. Hussain, R. B. Tareen and A. Ahmed, Magn. Reson. Chem., 2008, 46, 986–989 CrossRef CAS.
- V. U. Ahmad, S. Arshad, S. Bader, A. Ahmed, S. Iqbal, A. Khan, S. S. Khan and R. B. Tareen, Nat. Prod. Commun., 2007, 2, 889–894 CAS.
- V. U. Ahmad, S. Bader, S. Arshad, F. V. Mohammad, A. Ahmed, S. Iqbal and S. S. Khan, Nat. Prod. Commun., 2008, 3, 159–162 CAS.
- H. Gao, Z. Wang, Z.-H. Yao, N. Wu, H.-J. Dong, J. Li, N.-L. Wang, W.-C. Ye and X.-S. Yao, Helv. Chim. Acta, 2008, 91, 451–459 CrossRef CAS.
- M. De Leo, R. Sanogo, N. De Tommasi and A. Braca, J. Nat. Prod., 2007, 70, 979–983 CrossRef CAS.
- M. S. Alam, G. Kaur, A. Ali, H. Hamid, M. Ali and M. Athar, Nat. Prod. Res., 2008, 22, 1279–1288 CrossRef CAS.
- X. Tan, H. Chen, M. Zhou and Y. Zhang, Zhongcaoyao, 2006, 37, 171–174 ( Chem. Abstr. , 2008 , 148 , 187192d ) Search PubMed.
- F. Li, W. Li, H. Fu, Q. Zhang and K. Koike, Chem. Pharm. Bull., 2007, 55, 1087–1089 CrossRef CAS.
- H. Ando, M. Fukumura, Y. Hori, Y. Hirai, K. Toriizuka, Y. Kuchino and Y. Ida, J. Nat. Med., 2008, 62, 57–62 Search PubMed.
- Z. Zhang and S. Li, Phytochemistry, 2007, 68, 2075–2086 CrossRef CAS.
- G.-Y. Liu, S.-C. Ma, J. Zheng, J. Zhang and R.-C. Lin, J. Integr. Plant Biol., 2007, 49, 196–201 CrossRef CAS.
- Z.-F. Zheng, J.-F. Xu, Z.-M. Feng and P. C. Zhang, J. Asian Nat. Prod. Res., 2008, 10, 833–839 CrossRef CAS.
- N. El Fakhar, Z. Charrouf, B. Coddeville, Y. Leroy, J. C. Michalski and D. Guillaume, J. Nat. Med., 2007, 61, 375–380 Search PubMed.
- H.-W. Liu, M.-Y. Wang, X.-Y. Song, Y. Xia, Y.-S. Zhao, X.-H. Song, M.-M. Jiang, X. Zhang, H. Gao, N.-L. Wang and X.-S. Yao, Helv. Chim. Acta, 2008, 91, 1704–1711 CrossRef CAS.
- S. Guo, Y. Yang, R. Ma, Q. Xu and P. Lao, Xibei Zhiwu Xuebao, 2007, 27, 2314–2319 ( Chem. Abstr. , 2009 , 151 , 27974u ) Search PubMed.
- H.-B. Hu, X.-D. Zheng, H.-S. Hu and Y.-F. Jian, Bull. Korean Chem. Soc., 2007, 28, 1519–1522 CAS.
- A. P. Barbosa, B. P. da Silva and J. P. Parente, Z. Naturforsch., B, 2008, 63, 894–902 CAS.
- A. Ali and I. A. Khan, Phytochemistry, 2008, 69, 1037–1042 CrossRef.
- T.-H. Xu, Y.-J. Xu, D. Han, H.-F. Zhao, S.-X. Xie and D.-M. Xu, J. Integr. Plant Biol., 2007, 49, 202–206 CrossRef CAS.
- T.-H. Xu, Y.-H. Xu, H.-X. Li, D. Han, H.-F. Zhao, S.-X. Xiem, Y. Li, J.-Z. Niu, Y.-S. Si and D.-M. Xu, J. Asian Nat. Prod. Res., 2007, 9, 705–711 CrossRef CAS.
- M. Yoshikawa, S. Sugimoto, S. Nakamura and H. Matsuda, Chem. Pharm. Bull., 2008, 56, 1297–1303 CrossRef CAS.
- S. Avunduk, M.-A. Lacaille-Dubois, T. Miyamoto, E. Bedir, S. G. Şenol and Ö. A. Çalişkan, J. Nat. Prod., 2007, 70, 1830–1833 CrossRef CAS.
- F. Sun, Q. He, P. G. Xiao, Y. Y. Cheng, F. Sun, Q. He, P. G. Xiao and Y. Y. Cheng, Chin. Chem. Lett., 2007, 18, 1078–1080 CrossRef CAS.
- J.-P. Li, Z.-M. Liang and Z. Yuan, Pharmazie, 2007, 62, 463–466 CAS.
- J.-P. Xu, H. Wang and Z. Yuan, Planta Med., 2008, 74, 1412–1415 CrossRef.
- L. Li, S. Song, Z. Liang and S. Xu, Asian J. Trad. Med., 2007, 2, 1–4 Search PubMed.
- T. Ma, J. Li, P. Tu and F. Lu, Zhongcaoyao, 2007, 38, 327–329 ( Chem. Abstr. , 2008 , 148 , 291767f ) Search PubMed.
- R. Guo, M. Luo, C.-L. Long, M.-L. Li, Z.-Q. Ouyang, Y.-P. Zhou, Y.-H. Wang, X.-Y. Li and Y.-N. Shi, Helv. Chim. Acta, 2008, 91, 1728–1735 CrossRef CAS.
- M. Yoshikawa, S. -Nakamura, Y. Kato, K. Matsuhira and H. Matsuda, Chem. Pharm. Bull., 2007, 55, 598–605 CrossRef CAS.
- T. Morikawa, S. Nakamura, S. Nakamura, Y. Kato, O. Muraoka, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2007, 55, 293–298 CrossRef CAS.
- A. Lendl and G. Reznicek, Sci. Pharm., 2007, 75, 111–120 CrossRef CAS.
- N. Tabatadze, R. Elias, R. Faure, P. Gerkens, M. C. de Pauw-Gillet, E. Kemertelidze, A. Chea and E. Ollivier, Chem. Pharm. Bull., 2007, 55, 102–105 CrossRef CAS.
- Z.-Z. Gao, Y.-F. Xia, X.-J. Yao, Y. Dai and Q. Wang, Nat. Prod. Res., 2008, 22, 320–332 CrossRef CAS.
- S. Gao, A. Norris, J. S. Miller, F. Ratovoson, J. Razafitsalama, R. Andriantsiferana, V. E. Rasamison, K. TenDyke and D. G. I. Kingston, J. Nat. Prod., 2007, 70, 361–366 CrossRef.
- X.-M. Zhu, P. Xie, Y.-T. Di, S.-L. Peng, L.-S. Ding and M.-K. Wang, J. Integr. Plant Biol., 2008, 50, 589–592 CrossRef CAS.
- Q. Zheng, W. Li, L. Han and K. Koike, Chem. Pharm. Bull., 2007, 55, 646–650 CrossRef CAS.
- H. Bai, Y. Zhong, Y.-Y. Xie, Y.-S. Wang, L. Liu, L. Zhou, J. Wang, Y.-L. Mu and C.-X. Zuo, Chem. Biodiversity, 2007, 4, 955–960 CrossRef CAS.
- T.-H. Xu, H.-T. Li, Y.-J. Xu, H.-F. Zhao, S.-X. Xie, D. Han, Y.-S. Si, Y. Li, J.-Z. Niu and D.-M. Xu, J. Asian Nat. Prod. Res., 2008, 10, 771–775 CrossRef CAS.
- M. Ukiya, T. Akihisa, K. Yasukawa, K. Koike, A. Takahashi, T. Suzuki and Y. Kimura, J. Nat. Prod., 2007, 70, 813–816 CrossRef CAS.
- H.-C. Huang, C.-C. Liaw, L.-J. Zhang, H.-U. Ho, L.-M. Y. Kuo, Y.-C. Shen and Y. -H. Kuo, Phytochemistry, 2008, 69, 1597–1603 CrossRef CAS.
- G.-B. Xie, J. Zheng, P.-F. Tu and G.-Q. Liu, Helv. Chim. Acta, 2008, 91, 1630–1639 CrossRef CAS.
- F. Feng, M.-X. Zhu, N. Xie, W.-Y. Liu, D.-J. Chen and Q.-D. You, J. Asian Nat. Prod. Res., 2008, 10, 71–75 CrossRef CAS.
- X.-F. Zhou, X.-Y. Zhao, L. Tang, H.-L. Ruan, Y.-H. Zhang, H.-F. Pi, W.-L. Xiao, H.-D. Sun and J.-Z. Wu, J. Asian Nat. Prod. Res., 2007, 9, 379–385 CrossRef CAS.
- X.-W. Yang, J. Zhao and M. Hattori, J. Asian Nat. Prod. Res., 2008, 10, 243–247 CrossRef CAS.
- M. Ichikawa, S. Ohta, N. Komoto, M. Ushijima, Y. Kodera, M. Hayama, O. Shirota, S. Sekita and M. Kuroyanagi, J. Nat. Med., 2008, 62, 423–429 Search PubMed.
- M. Ushijima, N. Komoto, Y. Sugizono, I. Mizuno, M. Sumihiro, M. Ichikawa, M. Hayama, N. Kawahara, T. Nakane, O. Shirota, S. Sekita and M. Kuroyanagi, Chem. Pharm. Bull., 2008, 56, 308–314 CrossRef CAS.
- L.-M. Lin, X.-G. Zhang, J.-J. Zhu, H.-M. Gao, Z.-M. Wang and W.-H. Wang, J. Asian Nat. Prod. Res., 2008, 10, 925–929 CrossRef CAS.
- L. J. Tian, N.-Y. Yang and W.-Q. Chen, J. Asian Nat. Prod. Res., 2008, 10, 265–269 CrossRef CAS.
- J. S. Jangwan and M. Dobhal, J. Indian Chem. Soc., 2008, 85, 313–316 CAS.
- H. Yoshimitsu, M. Nishida, M. Okawa and T. Nohara, Chem. Pharm. Bull., 2007, 55, 488–491 CrossRef CAS.
- Z.-J. Wu, M.-A. Ouyang, C.-Z. Wang, Z.-K. Zhang and J.-G. Shen, J. Agric. Food Chem., 2007, 55, 1712–1717 CrossRef CAS.
- J. Liu, X. Yang, J. He, M. Xia, L. Xu and S. Yang, J. Mass Spectrom., 2007, 42, 861–873 CrossRef CAS.
- C. Li, J. Yang, S. Yu, N. Chen, W. Xue, J. Hu and D. Zhang, Planta Med., 2008, 74, 133–144 CrossRef CAS.
- M. Yoshikawa, X. Li, E. Nishida, S. Nakamura, H. Matsuda, O. Muraoka and T. Morikawa, Chem. Pharm. Bull., 2008, 56, 559–568 CrossRef CAS.
- T. Morikawa, X. Li, E. Nishida, Y. Ito, H. Matsuda, S. Nakamura, O. Muraoka and M. Yoshikawa, J. Nat. Prod., 2008, 71, 828–835 CrossRef CAS.
- D. Y. Jung, H. Ha, H. Y. Lee, C. Kim, J.-H. Lee, K. Bae, J. S. Kim and S. S. Kang, Chem. Pharm. Bull., 2008, 56, 203–206 CrossRef CAS.
- S. G. Ma, Y. C. Hu, S. S. Yu, Y. Zhang, X. G. Chen, J. Liu and Y. X. Liu, J. Nat. Prod., 2008, 71, 41–46 CrossRef CAS.
- Z. Zhang, Z.-L. Gao, X. Fang, Y.-H. Wang, H. Xiao, X.-J. Hao, G.-M. Liu and H.-P. He, J. Asian Nat. Prod. Res., 2008, 10, 1029–1034 CrossRef CAS.
- F. Li, L. S. Ding and M. K. Wang, Chin. Chem. Lett., 2008, 19, 305–307 CrossRef CAS.
- M. Elbandy, T. Miyamoto and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2007, 90, 260–270 CrossRef CAS.
- Y.-L. Song, Y.-H. Wang, Q. Lu, H.-J. Qiao and Y.-X. Cheng, Helv. Chim. Acta, 2008, 91, 665–672 CrossRef CAS.
- J. Keuam, X. S. Noundou and L. B. K. Mabeku, J. Chem. Soc. Ethiopia, 2007, 21, 373–378 Search PubMed.
- H. Gao, X. Zhang, N.-L. Wang, H.-W. Liu, Q.-H. Zhang, S.-S. Song, Y. Yu and X.-S. Yao, J. Asian Nat. Prod. Res., 2007, 9, 175–182 CrossRef CAS.
- A. Yokosuka, S. Kawakami, M. Haraguchi and Y. Mimaki, Tetrahedron, 2008, 64, 1474–1481 CrossRef CAS.
- T. Morikawa, H. Matsuda, N. Li, X. Li and M. Yoshikawa, Helv. Chim. Acta, 2007, 90, 2342–2348 CrossRef CAS.
- T.-H. Xu, G. Lv, Y.-J. Xu, S.-X. Xie, H.-F. Zhao, D. Han, Y.-S. Si, Y. Li, J.-Z. Niu and D.-M. Xu, J. Asian Nat. Prod. Res., 2008, 10, 803–806 CrossRef CAS.
- C.-H. Ma, M.-S. Fan, L.-P. Lin, W.-D. Tang, L.-G. Lou, J. Ding and C.-G. Huang, J. Asian Nat. Prod. Res., 2008, 10, 177–184 CrossRef CAS.
- P.-K. Chan, M. Zhao, C.-T. Che and E. Mak, J. Nat. Prod., 2008, 71, 1247–1250 CrossRef CAS.
- H. Gao, Z. Wang, L. Yang, Y. Yu, Z.-H. Yao, N.-L. wang, G.-X. Zhou, W.-C. Ye and X.-S. Yao, Magn. Reson. Chem., 2008, 46, 630–637 CrossRef CAS.
- R. Ahmed, F. Rashid, V. U. Ahmed, F. V. Mohammad, M. Noorwala, N. Bibi and S. U. Kazmi, J. Asian Nat. Prod. Res., 2008, 10, 7–16 CrossRef CAS.
- Y. Mimaki, S. Doi, M. Kuroda and A. Yokosuka, Chem. Pharm. Bull., 2007, 55, 1319–1324 CrossRef CAS.
- Y. Mimaki and S. Doi, Nat. Prod. Commun., 2008, 3, 903–910 CAS.
- J. P. Scott, W. F. Tinto and W. F. Reynolds, Nat. Prod. Commun., 2008, 3, 1763–1770 CAS.
- F. R. Melek, T. Miyase, N. S. Ghaly and M. Nabil, Phytochemistry, 2007, 68, 1261–1266 CrossRef CAS.
- Y. Wang, D. Zhang, W. Ye, Z. Yin, K.-P. Fung, S. Zhao and X. Yao, Planta Med., 2008, 74, 1280–1284 CrossRef CAS.
- L. T. Zhang, Y. W. Zhang, Y. Takaishi and H. Q. Duan, Chin. Chem. Lett., 2008, 19, 190–192 CrossRef CAS.
- P. Wen, X.-M. Zhang, N.-L. Wang and X.-S. Yao, J. Asian Nat. Prod. Res., 2008, 10, 873–880 CrossRef CAS.
- G. Gaidi, T. Miyamoto and M.-A. Lacaille-Bubois, Pharmazie, 2005, 60, 635–637 CAS.
- M. Yu, X. Li, C.-C. Zhao, J. Xu and P. Zhang, J. Asian Nat. Prod. Res., 2007, 9, 365–372 CrossRef CAS.
- L. Li, M. Li, D. Zhao and X. Liu, Nat. Prod. Res., 2008, 22, 1633–1636 CrossRef CAS.
- S. M. A. Khalik, Bull. Fac. Pharm. (Cairo Univ.), 2006, 44, 85–89 ( Chem. Abstr. , 2008 , 149 , 466264q ) Search PubMed.
- R. N. Yadava and C. Navneeta, Int. J. Chem. Sci., 2007, 5, 903–910 ( Chem. Abstr. , 2008 , 149 , 99362z ) Search PubMed.
- T. Kuljanabhagavad, P. Thongphasuk, W. Chamulitrat and M. Wink, Phytochemistry, 2008, 69, 1919–1926 CrossRef CAS.
- E. Bisoli, W. S. Garcez, L. Hamerski, C. Tieppo and F. R. Garcez, Molecules, 2008, 13, 2717–2728 Search PubMed.
- B. K. Ponou, L. Barboni, R. B. Teponno, M. Mbiantcha, T. B. Nguelefack, H.-J. Park, K.-T. Lee and L. A. Tapondjou, Phytochem. Lett., 2008, 1, 183–187 Search PubMed.
- R. P. Santos, E. R. Silveira, D. E. de A. Uchôa, O. D. L. Pessoa, F. A. Viana and R. Braz-Filho, Magn. Reson. Chem., 2007, 45, 692–694 CrossRef CAS.
- Y. Tsunoda, M. Okawa, J. Kinjo, T. Ikeda and T. Nohara, Chem. Pharm. Bull., 2008, 56, 1138–1142 CrossRef CAS.
- R. N. Yadava and J. Jharbade, Fitoterapia, 2008, 79, 245–249 CrossRef CAS.
- R. N. Yadava and J. Jharbade, Nat. Prod. Res., 2007, 21, 500–506 CrossRef CAS.
- N. Kumar, P. Bhandari, B. Singh and V. K. Kaul, Nat. Prod. Commun., 2007, 2, 633–636 CAS.
- C.-W. Choi, H. A. Jung, S. S. Kang and J. S. Choi, Arch. Pharm. Res., 2007, 30, 1–7 Search PubMed.
- Y. Chen, X. Feng, X. Jia, M. Wang, J. Liang and Y. Dong, Chem. Nat. Compd., 2008, 44, 39–43 CrossRef CAS.
- L. Zhang, B. Li, J. Tian, L. Xu and S. Yang, Chem. Nat. Compd., 2007, 43, 567–570 CrossRef CAS.
- G. Cioffi, F. Dal Piaz, A. Vassallo, F. Venturella, P. De Capraris, F. De Simone and N. De Tommasi, J. Nat. Prod., 2008, 71, 100–1004.
- Y.-P. Zou, C.-H. Tan, B.-D. Wang, D.-Y. Wang and S.-K. Kim, Helv. Chim. Acta, 2008, 91, 2168–2173 CrossRef CAS.
- C. Diome, A.-C. Mitaine-Offer, T. Miyamoto, C. Delaude, J.-F. Mirjolet, O. Duchamp and M.-A. Lacaille-Dubois, J. Nat. Prod., 2007, 70, 1680–1682 CrossRef CAS.
- A. Braca, A. Bader, T. Siciliano and N. De Tommasi, Magn. Reson. Chem., 2008, 46, 88–93 CrossRef CAS.
- M. Dong, X. He and R. H. Liu, J. Agric. Food Chem., 2007, 55, 6044–6051 CrossRef CAS.
- T. H. Xu, G. Lv, T. H. Liu, Y. J. Xu, Y. S. Si, S. X. Xie, H. F. Zhao, D. Han and D. M. Xu, Chin. Chem. Lett., 2008, 19, 817–820 CrossRef CAS.
- G. Cioffi, A. Vassallo, L. Lepore, F. Venturella, F. Dal Piaz and N. De Tommasi, Nat. Prod. Commun., 2008, 3, 1667–1670 CAS.
- Q. Li, X. Zhang, Y. Wang, W. Ye and X. Yao, Yaoxue Xuebao, 2007, 42, 862–866 ( Chem. Abstr. , 2009 , 150 , 480308j ).
- T. Ohtsuki, T. Miyagawa, T. Koyano, T. Kowithayakorn, N. Kawahara, Y. Goda and M. Ishibashi, J. Nat. Prod., 2008, 71, 918–921 CrossRef CAS.
- R. N. Yadava and J. Jharbade, Asian J. Chem., 2007, 19, 1224–1230 CAS.
- W. Li, X. Li, J. Yang, D.-L. Meng and N. Li, J. Asian Nat. Prod. Res., 2008, 10, 260–264 CrossRef.
- W. Li, X. Li, J. Yang, L.-H. Li, N. Li and D.-L. Meng, Pharmazie, 2006, 61, 810–811 CAS.
- Y.-L. Feng, H.-R. Li, L.-Z. Xu and S.-L. Yang, J. Asian Nat. Prod. Res., 2007, 9, 505–510 CrossRef CAS.
- K. Foubert, M. Theunis, S. Apers, A. J. Vlietinck and L. Pieters, Curr. Org. Chem., 2008, 12, 629–642 CrossRef CAS.
- D. Pattamdilok and R. Suttisri, J. Nat. Prod., 2008, 71, 292–294 CrossRef.
- S. A. Ibrahim and M. S. Ali, Turkish J. Chem., 2007, 31, 463–470 ( Chem. Abstr. , 2008 , 148 , 163615t ) Search PubMed.
- J.-L. Fu, C.-H. Tan, L.-P. Lin, J. Huang and D.-Y. Zhu, Nat. Prod. Res., 2007, 21, 351–353 CrossRef CAS.
- X.-Z. Huang, Y. Liu, S.-S. Yu, Y.-C. Hu and J. Qu, J. Integr. Plant Biol., 2007, 49, 1615–1618 CrossRef CAS.
- C.-R. Chen, H.-W. Chen and C.-I. Chang, Chem. Pharm. Bull., 2008, 56, 385–388 CrossRef CAS.
- M. S. Ali, S. Ahmed and M. Saleem, Molecules, 2008, 13, 405–411 Search PubMed.
- E. T. Ngouamegne, R. S. Fongang, S. Ngouela, F. F. Boyom, M. Rohmer, E. Tsamo, J. Gut and P. J. Rosenthal, Chem. Pharm. Bull., 2008, 56, 374–377 CrossRef CAS.
- D. A. Olmedo, J. L. López-Pérez, E. del Olmo, Y. Vásquez, A. San Feliciano and M. P. Gupta, Molecules, 2008, 13, 2915–2924 Search PubMed.
- M. L. Oliveira, L. P. Duarte, G. D. F. Silva, S. A. Vieira Filho, V. F. Knupp and F. G. P. Alves, Magn. Reson. Chem., 2007, 45, 895–898 CrossRef CAS.
- I. Addae-Mensah, S. Adu-Kumi, R. Waibel and I. V. Oppong, ARKIVOC, 2007, ix, 71–79.
- G.-P. Li, L.-J. Yang, J.-F. Zhao, X.-D. Yang and L. Li, Linchan Huaxue Yu Gongye, 2007, 27, 27–30 ( Chem. Abstr. , 2008 , 148 , 210244h ) Search PubMed.
- K.-W. Wang, H. Zhang and Y.-J. Pan, Helv. Chim. Acta, 2007, 90, 277–281 CrossRef CAS.
- F. Gutiérrez, A. Estévez-Braun, A. G. Ravelo, L. Astudillo and R. Zárate, J. Nat. Prod., 2007, 70, 1049–1052 CrossRef CAS.
- M. Asim, H. Hussien, J. T. Arnason, L. Poveda and T. Durst, J. Nat. Prod., 2007, 70, 1228–1232 CrossRef CAS.
- P.-L. Li, C.-J. Lin, Z.-X. Zhang and Z.-J. Jia, Chem. Biodiversity, 2007, 4, 17–24 CrossRef CAS.
- H. M. P. Poumale, R. T. Kengap, J. C. Tchouankeu, F. Keumedjio, H. Laatsch and B. T. Ngadjui, Z. Naturforsch., B, 2008, 63, 1335–1338 CAS.
- Q.-X. Wu, X. Liu and Y.-P. Shi, Chem. Biodiversity, 2007, 4, 175–182 CrossRef CAS.
- J.-J. Chen, D.-Q. Fei, S.-G. Chen and K. Gao, J. Nat. Prod., 2008, 71, 547–550 CrossRef CAS.
- X.-Y. Huang, M.-T. Tan, Q. Wu, Y. Chen and H.-S. Wang, J. Chin. Pharm. Sci., 2008, 17, 144–147 Search PubMed.
- B. Dinda, B. Ghosh, S. Arima, N. Sato and Y. Harigaya, Indian J. Chem., Sect. B, 2007, 46, 492–498.
- F. I. Ali, I. A. Hashmi and B. S. Siddiqui, Nat. Prod. Commun., 2008, 3, 125–128 CAS.
- P. L. Majumder and M. Chakraborty, Tetrahedron, 1979, 35, 2397–2403 CrossRef CAS.
- Y.-B. Zhang, X.-Y. Peng and H.-X. Sun, Chem. Biodiversity, 2008, 5, 189–196 CrossRef CAS.
- J.-Y. Hu, Z. Yao, Y.-Q. Xu, P. Liu and H.-Q. Duan, Acta Crystallogr., Sect. E, 2007, 63, o2375–o2377 CrossRef.
- Y. Zhang and N. Tan, Yunnan Zhiwu Yanjiu, 2006, 28, 673–675 ( Chem. Abstr. , 2008 , 148 , 444838g ) Search PubMed.
- Y.-L. Song, L. Zhang, J.-M. Gao, G.-H. Du and Y.-X. Cheng, J. Asian Nat. Prod. Res., 2008, 10, 214–217 CrossRef CAS.
- H.-L. Xin, X.-Q. Yue, Y.-F. Xu, Y.-C. Wu, Y.-N. Zhang, Y.-Z. Wang and C.-Q. Ling, Helv. Chim. Acta, 2008, 91, 575–580 CrossRef CAS.
- T. Nakanishi, Y. Inatomi, H. Mutata, S.-S. Ishida, Y. Fujino, K. Miura, Y. Yasuno, A. Inada, F. A. Lang and J. Murata, Chem. Pharm. Bull., 2007, 55, 334–336 CrossRef CAS.
- B. S. Siddiqui, S. Khan, M. N. Karder and S. Perwaiz, J. Asian Nat. Prod. Res., 2007, 9, 293–297 CrossRef CAS.
- P. T. Thuong, C. H. Lee, T. T. Dao, P. H. Nguyen, W. G. Kim, S. J. Lee and W. K. Oh, J. Nat. Prod., 2008, 71, 1775–1778 CrossRef CAS.
- Z. Ahmad, S. Mehmood, R. Ifzal, A. Malik, N. Afza, M. Ashraf and E. Jahan, Turkish J. Chem., 2007, 31, 495–501 ( Chem. Abstr. , 2008 , 148 , 163616u ) Search PubMed.
- M. Ahmad, W. Ahmad, S. Khan, M. Zeeshan, M. Nisar, F. Shaheen and M. Ahmad, J. Enzym. Inhibition Med. Chem., 2008, 23, 1023–1027 Search PubMed.
- X. Ma, C. Yang and Y. Zhang, Magn. Reson. Chem., 2008, 46, 571–575 CrossRef CAS.
- M. Traoré, J. W. Jaroszewski, C. E. Olsen, J. B. Ouédraogo, G. I. Peirre, O. G. Nacoulma, T. R. Guiguemdé and S. B. Christensen, Planta Med., 2008, 74, 560–562 CrossRef CAS.
- C. H. Bui and K. P. Nguyen, Tap Chi Hoa Hoc, 2007, 45, 363–367 ( Chem. Abstr. , 2008 , 148 , 303435m ) Search PubMed.
- E. C. Machado, R. A. Yunes, A. Malheiros, E. C. Gomez and F. Delle Monache, Nat. Prod. Res., 2008, 22, 1310–1316 CrossRef CAS.
- A. Ali and S. K. Tripathi, Asian J. Chem., 2007, 19, 3765–3769 CAS.
- D. S. Jang, G. Y. Lee, J. Kim, Y. M. Lee, J. M. Kim, Y. S. Kim and J. S. Kim, Arch. Pharm. Res., 2008, 31, 666–670 Search PubMed.
- D. A. C. Biggs, R. B. R. Porter, W. F. Reynolds and L. A. Williams, Nat. Prod. Commun., 2008, 3, 1759–1762 CAS.
- Q. L. Yu, H. Q. Duan, W. Y. Gao and Y. Takaishi, Chin. Chem. Lett., 2007, 18, 62–64 CrossRef CAS.
- Y.-L. Chen, C.-H. Tan, J.-J. Tan, S.-J. Qu, H.-B. Wang, Q. Zhang, S.-H. Jiang and D.-Y. Zhu, Helv. Chim. Acta, 2007, 90, 2421–2426 CrossRef CAS.
- S. Zhou, J. Yang, F. Liu and P. Tu, Magn. Reson. Chem., 2007, 45, 179–181 CrossRef CAS.
- G.-B. Xie, L.-D. Lei and P.-F. Tu, Magn. Reson. Chem., 2007, 45, 997–1000 CrossRef CAS.
- J.-Q. Yuan, X.-Z. Yang, J.-H. Miao, C.-P. Tang, C.-Q. Ke, J.-B. Zhang, X.-J. Ma and Y. Ye, Molecules, 2008, 13, 2229–2237 Search PubMed.
- G. Chen, J. Xue, S.-X. Xu and R.-Q. Zhang, J. Asian Nat. Prod. Res., 2007, 9, 347–353 CrossRef CAS.
- Z.-J. Wu, M.-A. Ouyang, C.-Z. Wang and Z.-K. Zhang, Chem. Pharm. Bull., 2007, 55, 422–427 CrossRef CAS.
- Y.-L. Feng, B. Wu, H.-R. Li, Y.-Q. Li, L.-Z. Xu, S.-L. Yang and S. Kitanaka, Chem. Pharm. Bull., 2008, 56, 858–860 CrossRef CAS.
- D. Smati, A.-C. Mitaine-Offer, T. Miyamoto, V. Hammiche and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2007, 90, 712–719 CrossRef CAS.
- Y. Xie, T. Morikawa, K. Ninomiya, K. Imura, O. Muraoka, D. Yuan and M. Yoshikawa, Chem. Pharm. Bull., 2008, 56, 1628–1631 CrossRef CAS.
- S.-X. Yang, J.-M. Gao, J.-C. Qin, L. Zhou, M.-H. Chiu and L. Wang, Helv. Chim. Acta, 2007, 90, 1477–1481 CrossRef CAS.
- H.-L. Ma, J.-Y. Qin, L. Wang, S.-X. Yang, M.-H. Chiu, X.-M. Zhang and J.-M. Gao, Chem. Nat. Compd., 2008, 44, 44–47 CrossRef CAS.
- C.-R. Chen, L.-H. Chao, M.-H. Pan, Y.-W. Liao and C.-I. Chang, J. Chin. Chem. Soc., 2007, 54, 41–45 CAS.
- H. Liang, F. Bao, X. Dong, Q. Lu and Y. Cheng, Yunnan Zhiwu Yanjiu, 2007, 29, 479–482 ( Chem. Abstr. , 2009 , 150 , 163652u ) Search PubMed.
- K. Maeda, T. Naitou, K. Umishio, T. Fukuhara and A. Motoyama, Biol. Pharm. Bull., 2007, 30, 873–879 CrossRef CAS.
- X.-F. He, X.-N. Wang, C.-Q. Fan, L.-S. Gan, S. Yin and J.-M. Yue, Helv. Chim. Acta, 2007, 90, 783–791 CrossRef CAS.
- W. D. Xie, X. Gao and Z. J. Jia, Arch. Pharm. Res., 2007, 30, 547–551 Search PubMed.
- J.-R. Wang, H. Zhou, Z.-H. Jiang and L. Liu, Chem. Biodiversity, 2008, 5, 1369–1376 CrossRef CAS.
- B. Vouffo, K. Krohn, S. F. Kouam, H. Hussain, E. Dongo, K. Meier and B. Schulz, Biochem. Syst. Ecol., 2008, 36, 655–658 CrossRef CAS.
- J. S. Lee, H. Miyashiro, N. Nakamura and M. Hattori, Chem. Pharm. Bull., 2008, 56, 711–714 CrossRef CAS.
- G. Ye, H. Peng, M. Fan and C. Huang, Biochem. Syst. Ecol., 2007, 35, 905–908 CrossRef CAS.
- R. Poovapatthanachart and W. Thanakijcharoenpath, Fitoterapia, 2008, 79, 498–500 CrossRef CAS.
- H. F. Pi, Z. H. Zhong, H. L. Ruan, P. Zhang, Y. H. Zhang and J. Z. Wu, Chin. Chem. Lett., 2008, 19, 835–836 CrossRef CAS.
- M.-H. Han, X.-W. Yang and Y.-P. Jin, Phytochem. Anal., 2008, 19, 438–443 CrossRef CAS.
- R. Fang, P. J. Houghton, C. Luo and P. J. Hylands, Phytochemistry, 2007, 68, 1242–1247 CrossRef CAS.
- H.-W. Lin, Z.-L. Wang, J.-H. Wu, N. Shi, H.-J. Zhang, W.-S. Chen, S. L. Morris-Natschke and A.-S. Lin, J. Nat. Prod., 2007, 70, 1114–1117 CrossRef CAS.
- F. Lv, M. Xu, Z. Deng, N. J. de Voogd, R. V. M. van Soest, P. Proksch and W. Lin, J. Nat. Prod., 2008, 71, 1738–1741 CrossRef CAS.
- S. Tang, Z. Deng, P. Proksch and W. Lin, Tetrahedron Lett., 2007, 48, 5443–5447 CrossRef CAS.
- S. Jain, S. Laphookhieo, Z. Shi, L.-W. Fu, S.-I. Akiyama, Z.-S. Chen, D. T. A. Youssef, R. W. M. van Soest and K. A. El Sayed, J. Nat. Prod., 2007, 70, 928–931 CrossRef CAS.
- D. Wakana, T. Hosoe, T. Itabashi, H. Okada, K. Fukushima and K.-I. Kawai, Heterocycles, 2008, 75, 1109–1122 CrossRef CAS.
- J. Jian, P. Yi, B. Chen, L. Lu, Z. Li, X. Zhang, L. Zhou and M. Qiu, Phytochemistry, 2008, 69, 506–510 CrossRef.
- H. Zapp, K. Orth, J. Zapp, J. D. Connolly and H. Becker, Nat. Prod. Commun., 2008, 3, 1755–1758 CAS.
- A. A. Salim, H.-B. Chai, I. Rachman, S. Riswan, L. B. S. Kardono, N. R. Farnsworth, E. J. Carcache-Blanco and A. D. Kinghorn, Tetrahedron, 2007, 63, 7926–7934 CrossRef CAS.
- Y. Zhang, S. Nakamura, T. Wang, H. Matsuda and M. Yoshikawa, Tetrahedron, 2008, 64, 7347–7352 CrossRef CAS.
- F.-L. Yan, S.-M. Yao and Y. Zhou, J. Chin. Chem. Soc., 2007, 54, 1321–1324 CAS.
- P. W. K. Ahiahonu and D. B. Goodenowe, Fitoterapia, 2007, 78, 337–341 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |