DNA photocleavage in anaerobic conditions by a Ru(II) polypyridyl complex with long wavelength MLCT absorption†
Qian-Xiong
Zhou
ab,
Wan-Hua
Lei
a,
Chao
Li
a,
Yuan-Jun
Hou
a,
Xue-Song
Wang
*a and
Bao-Wen
Zhang
*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: xswang@mail.ipc.ac.cn; g203@mail.ipc.ac.cn
bGraduate School of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 2nd November 2009
Abstract
Ru(II) polypyridyl complexes possessing long wavelength absorption and an efficient DNA photocleavage activity exhibit a potential application in photodynamic therapy (PDT). In this article, we reported a Ru(II) polypyridyl complex, [Ru(bpy)2(dpb)]2+ (bpy = 2,2′-bipyridine, dpb = 2,3-bis(2-pyridyl)benzoquinoxaline), that exhibits a very long wavelength 1MLCT absorption, with a maximum at 550 nm, and DNA photocleavage activity in anaerobic conditions in the presence of suitable oxidative quenchers, showing a promising potential application in the PDT of hypoxic tumors.
Introduction
Photodynamic therapy (PDT), a promising treatment modality for malignant tumors, relies on the generation of cytotoxic agents upon the irradiation of a photosensitizer.1 Cytotoxic agents include radicals or radical ions generated via electron transfer or hydrogen abstraction processes, i.e. type I mechanisms, and singlet oxygen (1O2) generated via energy transfer processes, i.e. type II mechanisms. In contrast to type II mechanisms, a highly oxygen-dependent process, a type I mechanism, may still apply at low O2 concentrations, and therefore may extend PDT applications into hypoxic cellular areas of solid tumor tissue that are resistant to conventional therapies, including radiotherapy.Transition metal complexes with DNA photocleavage activity have received significant attention for their potential use as DNA structural probes and as anticancer agents.2 Among them, Ru(II) polypyridyl complexes have been extensively studied owing to their rich photophysical, photochemical and redox properties.3 So far, a large number of Ru(II) complexes have provided good 1O2 quantum yields and high DNA photocleavage activities.4 Since DNA is one of the possible targets for PDT,5 the PDT potential of Ru(II) polypyridyl complexes has been proposed recently.4g However, most DNA-photocleaving Ru(II) polypyridyl complexes suffer from short wavelength absorption, with the absorption maxima of the longest wavelength absorption band (generally a metal-to-ligand charge transfer (MLCT) transition) shorter than 500 nm, limiting their use in PDT. An ideal PDT photosensitizer needs a strong absorptivity within the phototherapeutic window of 600–900 nm, where the tissue penetration of light is optimal. Moreover, the DNA photocleavage activities of these Ru(II) complexes are highly oxygen dependent and not suitable for applications in hypoxic tumors.
In addition to relying on 1O2, some Ru(II) complexes based on bpz (2,2′-bipyrazine), tap (1,4,5,8-tetraazaphenanthrene) or hat (1,4,5,8,9,12-hexaazatriphenylene) ligands can photocleave DNA, even in anaerobic conditions, by virtue of their strongly oxidizing ability.4a,6 However, the MLCT absorption maxima of these Ru complexes are all shorter than 500 nm. Alternatively, Ru(II) complexes can also exhibit DNA photocleavage activities through their oxidized Ru(III) species if they possess strong enough oxidizing abilities to oxidize DNA bases (e.g. guanine).7 Ru(III) species can be in situ-generated via electron transfer from excited Ru(II) complexes to oxidative quenchers, the so called flash quench approach,7 and can damage DNA in anaerobic conditions,7e offering opportunities to develop oxygen-independent PDT sensitizers. To develop long wavelength-absorbing Ru polypyridyl complexes, we recently focused our attention on the dpb ligand (dpb = 2,3-bis(2-pyridyl)benzoquinoxaline; Scheme 1). The highly delocalized π-system of the dpb ligand renders its corresponding Ru complexes a much longer wavelength MLCT absorption.8 In this article, we investigate the Ru(II) complex [Ru(bpy)2(dpb)]2+ (bpy = 2,2′-bipyridine; Scheme 1), which shows a very long wavelength 1MLCT absorption, with a maximum at 550 nm, and an efficient DNA photocleavage ability in anaerobic conditions in the presence of suitable oxidative quenchers.
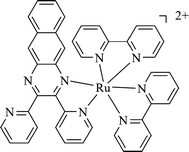 | ||
| Scheme 1 The chemical structure of the investigated complex. | ||
Results and discussion
Fig. 1 shows the normalized absorption spectra of [Ru(bpy)2(dpb)]2+, [Ru(bpy)3]2+ and the dpb ligand. Comparisons among them lead to following assignments for the absorption transitions of [Ru(bpy)2(dpb)]2+: bpy- and dpb-based transitions at 286 and 315 nm, a dpb-based transition at 365 nm, and an MLCT transition for Ru → dpb centered at 550 nm. The presence of the dpb ligand makes [Ru(bpy)2(dpb)]2+ undergo a 100 nm red shift in its MLCT absorption maximum with respect to [Ru(bpy)3]2+. Upon 1 h of visible irradiation (≥470 nm), the absorption and 1H NMR spectrum of a [Ru(bpy)2(dpb)]2+ solution (in PBS buffer or d6-DMSO/D2O) remained unchanged, reflecting its photochemical stability (ESI, Fig. S1 and S2†).![The normalized absorption spectra of [Ru(bpy)2(dpb)]2+, [Ru(bpy)3]2+ and dpb in acetonitrile (top), and the emission spectrum of [Ru(bpy)2(dpb)]2+ in acetonitrile at room temperature (bottom).](/image/article/2010/NJ/b9nj00465c/b9nj00465c-f1.gif) | ||
| Fig. 1 The normalized absorption spectra of [Ru(bpy)2(dpb)]2+, [Ru(bpy)3]2+ and dpb in acetonitrile (top), and the emission spectrum of [Ru(bpy)2(dpb)]2+ in acetonitrile at room temperature (bottom). | ||
The emission of [Ru(bpy)2(dpb)]2+ extends into the NIR region, meaning that an NIR fiber optic spectrometer with a wavelength range of 880–1684 nm was used to examine it; an emission with a maximum at 927 nm was observed (Fig. 1). [Ru(bpy)3]2+, bpy and dpb have no signals under similar conditions, excluding the artifact origin of the observed emission signal. Based on the emission spectrum, a 0–0 transition energy of 1.4 eV was roughly estimated for the 3MLCT state of [Ru(bpy)2(dpb)]2+ (see ESI†).
[Ru(bpy)2(dpb)]2+ displayed a half-wave oxidation potential at +1.64 V vs. NHE in acetonitrile (Fig. S3†), assignable to the Ru(III/II) couple and implying that [Ru(III)(bpy)2(dpb)]3+ has a reduction potential of +1.64 V, a potential high enough to oxidize guanine (E1/2 = 1.29 V vs. NHE). This was corroborated by the observed catalytic anodic current in the cyclic voltammograms of [Ru(bpy)2(dpb)]2+ in PBS buffer upon the addition of pBR322 plasmid DNA (Fig. 2). In the absence of pBR322 plasmid DNA, the cyclic voltammogram of [Ru(bpy)2(dpb)]2+ was quasi-reversible. However, the anodic current of [Ru(bpy)2(dpb)]2+ increased greatly with increasing pBR322 plasmid DNA concentration. The catalytic enhancement of the anodic current can be attributed to the following EC mechanism (eqn (1) and eqn (2)):9
| [Ru(II)(bpy)2(dpb)]2+ → [Ru(III)(bpy)2(dpb)]3+ (E) | (1) |
| [Ru(III)(bpy)2(dpb)]3+ + DNA → [Ru(II)(bpy)2(dpb)]2+ + DNAox (C) | (2) |
| ΔG = ED+/D − EA/A−1 − ED* − C | (3) |
![Cyclic voltammograms of [Ru(bpy)2(dpb)]2+ (100 μM) in 10 mM NaCl/220 mM sodium phosphate buffer (pH = 7.4) in the presence of various concentrations of pBR322 plasmid DNA. Scan rate: 150 mV s−1.](/image/article/2010/NJ/b9nj00465c/b9nj00465c-f2.gif) | ||
| Fig. 2 Cyclic voltammograms of [Ru(bpy)2(dpb)]2+ (100 μM) in 10 mM NaCl/220 mM sodium phosphate buffer (pH = 7.4) in the presence of various concentrations of pBR322 plasmid DNA. Scan rate: 150 mV s−1. | ||
![The agarose gel electrophoresis pattern of supercoiled pBR322 plasmid DNA (31 μM base pair) upon visible light irradiation (≥470 nm) for 1 h in Tris-CH3COOH/EDTA buffer (pH = 7.4) under an N2 atmosphere (lane 1–4) or in air (lane 5–6). Lane 1: DNA + R + K3Fe(CN)6, lane 2: DNA + R + [Co(NH3)5Cl]Cl2, lane 3: DNA + R + MV2+, lane 4: DNA + R, lane 5: DNA + R, lane 6: DNA alone. R represents [Ru(bpy)2(dpb)]2+. SC and NC denote supercoiled circular and nicked circular forms, respectively. The concentration of [Ru(bpy)2(dpb)]2+ was 70 μM and that of the quencher was 210 μM.](/image/article/2010/NJ/b9nj00465c/b9nj00465c-f3.gif) | ||
| Fig. 3 The agarose gel electrophoresis pattern of supercoiled pBR322 plasmid DNA (31 μM base pair) upon visible light irradiation (≥470 nm) for 1 h in Tris-CH3COOH/EDTA buffer (pH = 7.4) under an N2 atmosphere (lane 1–4) or in air (lane 5–6). Lane 1: DNA + R + K3Fe(CN)6, lane 2: DNA + R + [Co(NH3)5Cl]Cl2, lane 3: DNA + R + MV2+, lane 4: DNA + R, lane 5: DNA + R, lane 6: DNA alone. R represents [Ru(bpy)2(dpb)]2+. SC and NC denote supercoiled circular and nicked circular forms, respectively. The concentration of [Ru(bpy)2(dpb)]2+ was 70 μM and that of the quencher was 210 μM. | ||
The DNA photocleavage abilities of [Ru(bpy)2(dpb)]2+ under various conditions were examined by the agarose gel electrophoresis pattern of supercoiled pBR322 plasmid DNA under visible light irradiation (≥ 470 nm) (Fig. 3). Under aerobic conditions, [Ru(bpy)2(dpb)]2+ displays a weak photocleavage activity (lane 5) due to its moderate 1O2 quantum yield (Φ = 0.22; measured in acetonitrile using [Ru(bpy)3]2+ as the standard (Φ = 0.57 in CH3CN)13). The photocleavage was not inhibited by superoxide dismutase, catalase and mannitol, a scavenger of O2−˙, H2O2 and ˙OH,14 but restricted markedly by NaN3, a well known scavenger of 1O2,15 confirming the 1O2 mechanism further. Thus, it is not surprising that, under an N2 atmosphere, the photocleavage activity of [Ru(bpy)2(dpb)]2+ diminished greatly (lane 4; a trace amount of the NC form observed in this case may be attributed to residual O2). In the presence of K3FeCN6 or [Co(NH3)5Cl]2+ (lane 1 and 2), [Ru(bpy)2(dpb)]2+ exhibited an efficient DNA photocleavage activity under an N2 atmosphere, while the sample containing MV2+ (lane 3) did not show DNA cleavage. Control experiments indicate that the DNA cleavage in anaerobic conditions only took place with a combination of light, photosensitizer and quencher (K3FeCN6 or [Co(NH3)5Cl]2+). All of these findings are consistent with the flash quench mechanism, in which the in situ-generated Ru(III) species accounts for the single strand scission that leads to the transformation of plasmid DNA from its SC form to its NC form.7e We also compared the DNA cleavage activities of [Ru(bpy)2(dpb)]2+ and [Ru(bpy)3]2+ under anaerobic conditions in the presence of K3FeCN6 (Fig. 4). The observed much higher DNA cleavage activity of [Ru(bpy)2(dpb)]2+ is at least partly the result of its greatly red shifted absorption spectrum compared to that of [Ru(bpy)3]2+.
![The agarose gel electrophoresis pattern of supercoiled pBR322 plasmid DNA (31 μM base pair) upon visible light irradiation (≥470 nm) for 1 h in Tris-CH3COOH/EDTA buffer (pH = 7.4) in air (lane 1) or under an N2 atmosphere (lanes 2–5). Lane 1: DNA alone, lane 2: DNA + R1, lane 3: DNA + R1 + K3Fe(CN)6, lane 4: DNA + R2 + K3Fe(CN)6, lane 5: DNA + R2. R1 and R2 represent [Ru(bpy)2(dpb)]2+ and [Ru(bpy)3]2+, respectively. %NC represents the percentage of the NC form. The concentration of [Ru(bpy)2(dpb)]2+ and [Ru(bpy)3]2+ was 70 μM, and that of the quencher was 210 μM.](/image/article/2010/NJ/b9nj00465c/b9nj00465c-f4.gif) | ||
| Fig. 4 The agarose gel electrophoresis pattern of supercoiled pBR322 plasmid DNA (31 μM base pair) upon visible light irradiation (≥470 nm) for 1 h in Tris-CH3COOH/EDTA buffer (pH = 7.4) in air (lane 1) or under an N2 atmosphere (lanes 2–5). Lane 1: DNA alone, lane 2: DNA + R1, lane 3: DNA + R1 + K3Fe(CN)6, lane 4: DNA + R2 + K3Fe(CN)6, lane 5: DNA + R2. R1 and R2 represent [Ru(bpy)2(dpb)]2+ and [Ru(bpy)3]2+, respectively. %NC represents the percentage of the NC form. The concentration of [Ru(bpy)2(dpb)]2+ and [Ru(bpy)3]2+ was 70 μM, and that of the quencher was 210 μM. | ||
Conclusions
In summary, [Ru(bpy)2(dpb)]2+, with a long wavelength absorption, exhibits an efficient DNA photocleavage activity under anaerobic conditions in the presence of a suitable oxidative quencher. We are currently pursuing biocompatible electron acceptors (e.g., redox enzyme systems) to replace K3FeCN6 and [Co(NH3)5Cl]2+. This work demonstrates that dyads comprising of a long wavelength-absorbing Ru(II) complex and a biocompatible electron acceptor are significant for developing novel PDT agents applicable to hypoxic conditions.Experimental
Materials
2,2,6,6-Tetramethyl-4-piperidone (TEMP), sodium azide (NaN3), RuCl3·3H2O, 2,3-diaminonaphthalene, 2,2′-bipyridine, 1,3-diphenylisobenzofuran (DPBF), tetra-n-butylammonium hexafluorophosphate ([N(C4H9)4]PF6), gel loading buffer, tris-hydroxymethyl-aminomethane (Tris base), superoxide dismutase (SOD), and catalase were purchased from Sigma-Aldrich. The supercoiled pBR322 plasmid DNA was purchased from TaKaRa Biotechology.Spectroscopic measurements
1H NMR spectra were obtained on a Bruker DMX-400 MHz spectrophotometer. ESI and MALDI-TOF mass spectra were determined on Q-Tof (Waters) and Biflex III (Bruker) mass spectrometers, respectively. Elemental analyses were performed on an Elementar Vario EL instrument.UV-vis absorption spectra were recorded on a Shimadzu UV-1601 spectrophotometer. NIR luminescence spectra were recorded on an NIR fiber optic spectrometer (NIR-512L-1.7T1), while light excitation was from a 532 nm laser obtained from a Tsunami-Spitfire-OPA-800C system (USA, Spectra-Physics) using Millennia (532 nm, CW) as a laser source.
Redox potentials in CH3CN were measured on an EG&G Model 283 potentiostat/galvanostat in a three-electrode cell with a microdisc Pt working electrode, a Pt wire counterelectrode and a saturated calomel electrode (SCE) as a reference. Cyclic voltammetry was conducted at a scan rate of 150 mV s−1 in N2-saturated, anhydrous CH3CN containing 0.1 M [N(C4H9)4]PF6 as the supporting electrolyte.
Synthesis of [Ru(bpy)2(dpb)](PF6)2
0.30 g Ru(bpy)2Cl2 and 0.22 g dpb were refluxed in 25 mL ethanol–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 3 h under an N2 atmosphere. The solution was filtered after cooling. After removal of the solvent, the solid was purified on silica gel using CH3CN–H2O/KNO3 (40
1) for 3 h under an N2 atmosphere. The solution was filtered after cooling. After removal of the solvent, the solid was purified on silica gel using CH3CN–H2O/KNO3 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. The compound was dissolved in water and precipitated with NH4PF6. The red solid was filtered, washed with water and vacuum dried. Yield 76%. 1H NMR (400 MHz, in d6-acetone): δ = 7.45–7.48 (m, 2H), 7.56–7.61 (m, 4H), 7.68–7.74 (m, 3H), 7.85–7.93 (m, 2H), 8.06–8.11 (m, 3H), 8.23–8.35 (m, 6H), 8.42–8.48 (m, 3H), 8.56–8.64 (m, 3H), 8.75–8.76 (d, 1H, J = 4.3 Hz), 8.85 (s, 1H) and 8.99–9.03 (m, 2H). MALDI-TOF-MS: 893.17 (M − PF6), 748.18 (M − 2PF6). ESI-MS: m/z = 373.6 (M − 2PF6)2+. Anal. calc. for C42H30F12N8P2Ru·H2O: C, 47.78; H, 3.06; N, 10.61. Found: C, 47.75; H, 3.05; N, 10.63%.
1) as the eluent. The compound was dissolved in water and precipitated with NH4PF6. The red solid was filtered, washed with water and vacuum dried. Yield 76%. 1H NMR (400 MHz, in d6-acetone): δ = 7.45–7.48 (m, 2H), 7.56–7.61 (m, 4H), 7.68–7.74 (m, 3H), 7.85–7.93 (m, 2H), 8.06–8.11 (m, 3H), 8.23–8.35 (m, 6H), 8.42–8.48 (m, 3H), 8.56–8.64 (m, 3H), 8.75–8.76 (d, 1H, J = 4.3 Hz), 8.85 (s, 1H) and 8.99–9.03 (m, 2H). MALDI-TOF-MS: 893.17 (M − PF6), 748.18 (M − 2PF6). ESI-MS: m/z = 373.6 (M − 2PF6)2+. Anal. calc. for C42H30F12N8P2Ru·H2O: C, 47.78; H, 3.06; N, 10.61. Found: C, 47.75; H, 3.05; N, 10.63%.
The DNA photocleavage abilities of the complexes were evaluated using supercoiled pBR322 plasmid DNA as the target. The mixture of examined complex and supercoiled pBR322 DNA in Tris-CH3COOH/EDTA buffer (pH 7.4) was irradiated on a “merry-go-round” apparatus for 1 h by a medium pressure sodium lamp (500 W, with glass filters; λ ≥ 470 nm). After irradiation, gel loading buffer was added. The sample was then subjected to agarose gel electrophoresis and analyzed using the Gel Doc XR system (Bio-Rad).
1O2 quantum yields were measured by the chemical trapping method using DPBF as the chemical trap for 1O2.16 A series of 2 mL air-saturated acetonitrile solutions containing 1,3-diphenylisobenzofuran (DPBF) and the examined photosensitizer, of which the absorbance at 480 nm originating from the photosensitizer was adjusted to be the same, were separately charged into open 1 cm path length fluorescence cuvettes and illuminated with 480 nm light (obtained from a Hitachi F-4500 fluorescence spectrophotometer). The consumption of DPBF was followed by monitoring the fluorescence intensity decrease at the emission maximum (λex = 405 nm) after different irradiation times. [Ru(bpy)3]2+ was used as a standard, whose 1O2 quantum yield was determined to be 0.57 in air-saturated acetonitrile.13
Acknowledgements
The authors are grateful for financial support from NNSFC (20772133, 20873170) and CAS (KJCX2.YW.H08).References
- (a) T. J. Dougherty, Photochem. Photobiol., 1993, 58, 895 CrossRef CAS; (b) M. R. Detty, S. L. Gibson and S. J. Wagner, J. Med. Chem., 2004, 47, 3897 CrossRef CAS; (c) T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, J. Natl. Cancer Inst., 1998, 90, 889 CrossRef CAS; (d) D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380 CrossRef CAS.
- B. Armitage, Chem. Rev., 1998, 98, 1171 CrossRef CAS.
- (a) K. E. Erkkila, D. T. Odom and J. K. Barton, Chem. Rev., 1999, 99, 2777 CrossRef CAS; (b) B. M. Zeglis, V. C. Pierre and J. K. Barton, Chem. Commun., 2007, 4565 RSC.
- (a) A. B. Tossi and J. M. Kelly, Photochem. Photobiol., 1989, 49, 545 CrossRef CAS; (b) H. Y. Mei and J. K. Barton, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 1339 CAS; (c) A. Hergueta-Bravo, M. E. Jiménez-Hernández, F. Montero, E. Oliveros and G. Orellana, J. Phys. Chem. B, 2002, 106, 4010 CrossRef CAS; (d) H.-Y. Ding, X.-S. Wang, L.-Q. Song, J.-R. Chen, J.-H. Yu, L. Chao and B.-W. Zhang, J. Photochem. Photobiol., A, 2006, 177, 286 CrossRef CAS; (e) Y. Liu, R. Hammitt, D. A. Lutterman, R. P. Thummel and C. Turro, Inorg. Chem., 2007, 46, 6011 CrossRef CAS; (f) G. J. Ryan, S. Quinn and T. Gunnlaugsson, Inorg. Chem., 2008, 47, 401 CrossRef CAS; (g) Y. Liu, R. Hammitt, D. A. Lutterman, R. P. Thummel and C. Turro, Inorg. Chem., 2009, 48, 375 CrossRef CAS; (h) A. Chouai, S. E. Wicke, C. Turro, J. Bacsa, K. R. Dunbar, D. Wang and R. P. Thummel, Inorg. Chem., 2005, 44, 5996 CrossRef CAS.
- (a) K. Kawai, Y. Osakada, M. Fujitsuka and T. Majima, J. Phys. Chem. B, 2007, 111, 2322 CrossRef CAS; (b) A. Ikeda, Y. Doi, M. Hashizume, J. Kikuchi and T. Konishi, J. Am. Chem. Soc., 2007, 129, 4140 CrossRef CAS; (c) J. W. Hofman, V. Zeeland F, S. Turker, H. Talsma, S. A. G. Lambrechts, D. V. Sakharov, W. E. Hennink and C. F. van Nostrum, J. Med. Chem., 2007, 50, 1485 CrossRef CAS.
- (a) C. Sentagne, J. C. Chambron, J. P. Sauvage and N. Paillous, J. Photochem. Photobiol., B, 1994, 26, 165 CrossRef CAS; (b) J. P. Lecomte, A. Kirsch-De Mesmaeker, M. M. Feeney and J. M. Kelly, Inorg. Chem., 1995, 34, 6481 CrossRef CAS; (c) J. P. Lecomte, A. Kirsch-De Mesmaeker, J. M. Kelly, A. B. Tossi and H. Görner, Photochem. Photobiol., 1992, 55, 681 CAS.
- (a) E. D. A. Stemp, M. R. Arkin and J. K. Barton, J. Am. Chem. Soc., 1997, 119, 2921 CrossRef CAS; (b) M. R. Arkin, E. D. A. Stemp, S. C. Pulver and J. K. Barton, Chem. Biol., 1997, 4, 389 CrossRef CAS; (c) K. L. Nguyen, M. Steryo, K. Kurbanyan, K. M. Nowitzki, S. M. Butterfield, S. R. Ward and E. D. A. Stemp, J. Am. Chem. Soc., 2000, 122, 3585 CrossRef CAS; (d) S. Delaney, M. Pascaly, P. K. Bhattacharya, K. Han and J. K. Barton, Inorg. Chem., 2002, 41, 1966 CrossRef CAS; (e) P. K.-L. Fu, P. M. Bradley, D. V. Loyen, H. Durr, S. H. Bossmann and C. Turro, Inorg. Chem., 2002, 41, 3808 CrossRef CAS; (f) K. E. Augustyn, E. D. A. Stemp and J. K. Barton, Inorg. Chem., 2007, 46, 9337 CrossRef CAS; (g) S. E. Evans, A. Grigoryan and V. A. Szalai, Inorg. Chem., 2007, 46, 8349 CrossRef CAS.
- (a) Y. Y. Ng, C. M. Che and S. M. Peng, New J. Chem., 1996, 20, 781 Search PubMed; (b) M. Milkevitch, H. Storrie, E. Brauns, K. J. Brewer and B. W. Shirley, Inorg. Chem., 1997, 36, 4534 CrossRef CAS; (c) M. Milkevitch, B. W. Shirley and K. J. Brewer, Inorg. Chim. Acta, 1997, 264, 249 CrossRef CAS; (d) R. L. Williams, H. N. Toft, B. Winkel and K. J. Brewer, Inorg. Chem., 2003, 42, 4394 CrossRef CAS.
- (a) D. H. Johnston, K. C. Glasgow and H. H. Thorp, J. Am. Chem. Soc., 1995, 117, 8933 CrossRef CAS; (b) F. Boussicault and M. Robert, Chem. Rev., 2008, 108, 2622 CrossRef CAS.
- M. Guo, F. Sulc, M. W. Ribbe, P. J. Farmer and B. K. Burgess, J. Am. Chem. Soc., 2002, 124, 12100 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, Boca Raton, FL, USA, 75th edn, 1994, pp. 8–27 Search PubMed.
- D. Rehm and A. Weller, Isr. J. Chem., 1970, 8, 259 CAS.
- A. A. Abdel-Shafi, P. D. Beer and R. J. Mortimer, J. Phys. Chem. A, 2000, 104, 192–202 CrossRef CAS.
- (a) A. E. Friedman, J.-C. Chambron, J.-P. Sauvage, N. J. Turro and J. K. Barton, J. Am. Chem. Soc., 1990, 112, 4960 CrossRef CAS; (b) O. Baudoin, M.-P. Teulade-Fichou, J.-P. Vigneron and J. M. Lehn, Chem. Commun., 1998, 2349 RSC.
- Y. Li and M. A. Trush, Cancer Res., 1994, 54, 1895.
- R. H. Young, K. Wehrly and R. L. Martin, J. Am. Chem. Soc., 1971, 93, 5774 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Further experimental data and spectra. See DOI: 10.1039/b9nj00465c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2010 |
