A cucurbit[8]uril inclusion complex with 1,7-dimethyl-1,4,7,10-tetraazacyclododecane tetrachloride†
Xiao-jun
Wu
a,
Kai
Hu
a,
Xiang-gao
Meng
b and
Gong-zhen
Cheng
*a
aCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan, P. R. China. E-mail: gzhcheng@whu.edu.cn
bCollege of Chemistry, Central China Normal University, Wuhan, P. R. China
First published on 27th November 2009
Abstract
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex between cucurbit[8]uril and protonated 1,7-dimethyl-1,4,7,10-tetraazacyclododecane tetrahydrochloride was prepared. Its structure was characterized using NMR and single crystal X-ray diffraction. This new macrocycle complex shows a beautiful sexfoil packing structure, very different from that of the free cucurbit[8]uril, which is perfectly aligned. A higher inclination angle of about 78° 15′ is observed between the two macrocycle planes.
1 inclusion complex between cucurbit[8]uril and protonated 1,7-dimethyl-1,4,7,10-tetraazacyclododecane tetrahydrochloride was prepared. Its structure was characterized using NMR and single crystal X-ray diffraction. This new macrocycle complex shows a beautiful sexfoil packing structure, very different from that of the free cucurbit[8]uril, which is perfectly aligned. A higher inclination angle of about 78° 15′ is observed between the two macrocycle planes.
Cucurbit[n]uril (CB[n], n = 5–10), featuring a hydrophobic cavity and polar carbonyl groups surrounding the portals, has the remarkable property of encapsulating hydrophobic or/and positively charged guest molecules through supramolecular host–guest interactions.1–6 As macrocycles, CB[n]s can form stable host–guest complexes with ions, aliphatic and aromatic amines, toluene, ferrocene, etc. The cucurbituril homologue CB[8], a member of the CB[n] family comprising eight glycoluril units, has recently attracted much attention. The larger cavity of CB[8], compared to that of extensively studied CB[6], enables it to form different types of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest inclusion complexes,5 1
1 host–guest inclusion complexes,5 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complexes7 and even 1
2 host–guest complexes7 and even 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complexes.8 Of special interest is the ability of CB[8] to form macrocycles within macrocycle complexes.9 The discovery of novel macrocycles in macrocycle complexes of CB[8] and their transition metal complexes have opened up new opportunities in the realms of CB[n] chemistry. The encapsulation of cyclen and cyclam macrocycles in CB[8] has been examined, and their conformational details revealed by means of NMR and XRD.8 Moreover, the inclusion complex of the cyclen guest in the γ-cyclodextrin (γ-CD) host, which has a similar cavity size to that of CB[8], has also been reported.10
1 ternary complexes.8 Of special interest is the ability of CB[8] to form macrocycles within macrocycle complexes.9 The discovery of novel macrocycles in macrocycle complexes of CB[8] and their transition metal complexes have opened up new opportunities in the realms of CB[n] chemistry. The encapsulation of cyclen and cyclam macrocycles in CB[8] has been examined, and their conformational details revealed by means of NMR and XRD.8 Moreover, the inclusion complex of the cyclen guest in the γ-cyclodextrin (γ-CD) host, which has a similar cavity size to that of CB[8], has also been reported.10
1,7-Dimethyl-1,4,7,10-tetraazacyclododecane tetrachloride (DMC) is a macrocyclic polyamine. It can not only hydrolyze double-stranded DNA under physiological conditions,11 but also induce HeLa cell apoptosis by showing antitumor activity.12 By considering the bio-character of this cyclen derivative and its capacity to coordinate metal ions, the structural characteristics of the DMC guest included in the CB[8] host (Scheme 1) have been examined in the present work. This macrocycle complex is expected to have practical importance as a drug carrier13 in the field of medicine.
![The molecular structures of (a) host CB[8] and (b) guest DMC.](/image/article/2010/NJ/b9nj00218a/b9nj00218a-s1.gif) | ||
| Scheme 1 The molecular structures of (a) host CB[8] and (b) guest DMC. | ||
The crystal structure indicates that one DMC guest is encapsulated in the cavity of the CB[8] host, forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Fig. 1(a)). It can be seen that the inner macrocycle is significantly tilted with respect to the outer macrocycle. The average plane of the DMC is almost perpendicular to that of the CB[8]; the angle between the two ring planes is about 78° 15′ (Fig. 1(b)). To our knowledge, this is the largest relative inclination angle that has been reported with respect to macrocycles in similar macrocycle complexes. The angles between the two average planes of cyclen@CB[8] and cyclam@CB[8] are about 38° and 59°,9 respectively; and that for CB[5]@CB[10] is about 64°.14 It is assumed that the larger ring size of the inner guest is responsible for the larger inclination angle, which is in agreement with the presumption proposed by Kim et al.9 Apart from the relatively large inclusion angle between the two macrocycle planes, the inclusion also results in the deformation of the host rings, which has not previously been reported. It can be seen that after inclusion (Fig. 1(a)), the CB[8] ring changes from a round cycle with a cavity entrance diameter of 9.9 Å, defined as the distance between the two opposite carbonyl oxygens of one rim in the free state, to an ellipse with major and minor axes of 9.3 and 10.7 Å, respectively. This variation in the skeleton of CB[8] has been reported when unsaturated viologen analogues complex with CB[8].15 The DMC guest molecule is located in the center of the host cavity, with the ring plane being parallel to that of major axis plane (Fig. 1(b)). Meanwhile, the two methyl groups of the guest reside each side of the ring plane like two “arms” propping tightly against the inside wall of the host. Actually, it is the accommodation of the two “arms” of the host inside the CB[8] cavity that results in the deformation of the outer ring.
1 complex (Fig. 1(a)). It can be seen that the inner macrocycle is significantly tilted with respect to the outer macrocycle. The average plane of the DMC is almost perpendicular to that of the CB[8]; the angle between the two ring planes is about 78° 15′ (Fig. 1(b)). To our knowledge, this is the largest relative inclination angle that has been reported with respect to macrocycles in similar macrocycle complexes. The angles between the two average planes of cyclen@CB[8] and cyclam@CB[8] are about 38° and 59°,9 respectively; and that for CB[5]@CB[10] is about 64°.14 It is assumed that the larger ring size of the inner guest is responsible for the larger inclination angle, which is in agreement with the presumption proposed by Kim et al.9 Apart from the relatively large inclusion angle between the two macrocycle planes, the inclusion also results in the deformation of the host rings, which has not previously been reported. It can be seen that after inclusion (Fig. 1(a)), the CB[8] ring changes from a round cycle with a cavity entrance diameter of 9.9 Å, defined as the distance between the two opposite carbonyl oxygens of one rim in the free state, to an ellipse with major and minor axes of 9.3 and 10.7 Å, respectively. This variation in the skeleton of CB[8] has been reported when unsaturated viologen analogues complex with CB[8].15 The DMC guest molecule is located in the center of the host cavity, with the ring plane being parallel to that of major axis plane (Fig. 1(b)). Meanwhile, the two methyl groups of the guest reside each side of the ring plane like two “arms” propping tightly against the inside wall of the host. Actually, it is the accommodation of the two “arms” of the host inside the CB[8] cavity that results in the deformation of the outer ring.
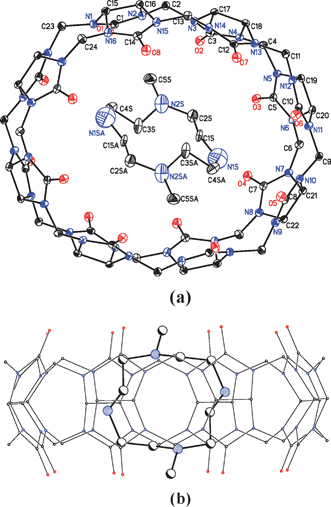 | ||
| Fig. 1 The X-ray crystal structure of the inclusion complex: (a) top view and (b) side view. Colour code: oxygen: red, nitrogen: blue and carbon: white. Hydrogen atoms, counterions (Cl−) and solvent (H2O) molecules are omitted for clarity. | ||
When the 2D packing structure of the inclusion complex is examined, it can be seen that the structure of the complex is significantly different from that of free CB[8]. The packing structure changes from the perfectly aligned packing in three dimensions of free CB[8]16 to a special sexfoil arrangement of the complex in the ab plane (Fig. 2(a)). Nine coordination molecules compose a three-dimensional sexfoil. Six complex molecules among this assembly, due to their parallel planes along the c axis, are presented in a ball and stick-type view (Fig. 2(b)). It is also observed that these parallel molecules show an angle of about 120° with the next plane. Moreover, the double-membered pillars are shared with the next sexfoil structure. It can also be seen in Fig. 2(c) that the guest molecules have an interesting arrangement; a sandwich-like configuration is observed, with the upper and lower panels parallel to each other, while the middle one is perpendicular.
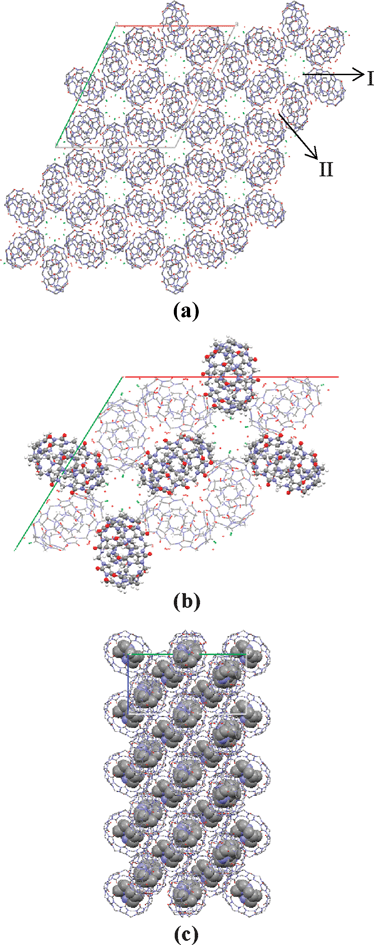 | ||
| Fig. 2 Packing diagram of the inclusion complexes in the (a) and (b) ab plane, and (c) bc plane. Colour code: oxygen: red, nitrogen: blue, carbon: gray and chlorine: green. In (b), the ball-stack style notation presents two overlapped molecules, while the stick notation presents only one molecule for clarity. | ||
Moreover, this interesting disposition of complexes does not preclude the forming of channels in the c dimension, as can be seen in Fig. 2(a). Two kinds of channels are formed: one (I) is surrounded by the hydrophobic walls of complexes in a hexagonal form and the other (II) is derived from three hydrophilic complex surfaces forming a sharp triangle. Since the guest DMC is fully protonated, small species, including four Cl− counterions and a total of 22 water molecules for each complex, are observed in these channels. As shown in the packing structure in the ab plane, water molecules, as well as chlorine ions, reside in channels I, with a total of 12 species located next to each other. For channel II, only water molecules are observed. Water molecules are also observed inside the macrocycles.
To summarize, in the ab plane, the complexes are like perfectly arranged six-petalled flowers, with the CB[8] complexes acting as petals, and the water molecules and chlorine ions acting as pistils. The crystal structure indicates that the van der Waals forces between the inner guest and the inside wall of the outer ring are responsible for the stability of the inclusive complex, while the hydrogen bonds between the water and the oxygen atoms of CB[8] play a critical role in stabilizing the beautiful arrangement of these crystals. Moreover, chlorine anions may also play an important role by reinforcing the water network in a manner similar to that assigned to its stabilizing CB[7] hydrate fibers.17
An X-ray crystal analysis revealed how the complex was arranged in the solid state, while NMR measurements showed how the molecules interacted in solution. The 1H and 13C NMR spectra of the free host, guest and the product complex in D2O without DCl were obtained. Three peaks at 4.160, 5.453 and 5.643 ppm in the low-field region of the 1H NMR spectrum of DMC@CB[8] could be attributed to CB[8]; no obvious chemical shift differences with free CB[8] being seen. However, for the DMC guest, the 1H NMR chemical shifts of the complex were significantly affected by inclusion. All of the resonances of the DMC protons are shifted to a higher field relative to that of free DMC. Meanwhile, the resonance pattern changed from three groups of broad peaks at 2.547 (2H), 2.916 (4H) and 3.051 (2H) ppm in free DMC to four triplet peaks at 1.941 (2H), 2.112 (2H), 2.325 (2H) and 2.804 (2H) ppm in its complexed form (Fig. 3). These variations probably result from complex formation between CB[8] and DMC, as could been seen in the crystal structure, confirming that the DMC in the cavity of CB[8] is fixed by its two methyl groups “propping” themselves against the wall of the host like two arms (Fig. 1). This conformational constraint results in the protons of DMC suffering from different chemical environments. The shielding of protons in the cavity is enhanced, causing the magnetic resonances to shift to higher fields. Therefore, the resolution of the protons is improved. In addition, this resonance shifting was also observed in the 13C NMR spectrum of the DMC in the complex, with its resonances moving to a slightly higher field (by 0.2–0.7 ppm). This helped confirm that an inclusion complex was formed between CB[8] and DMC. Indeed, only one set of NMR signals for product was observed, suggesting that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex of DMC@CB[8] was formed, in agreement with the conclusion drawn from the X-ray study.
1 host–guest complex of DMC@CB[8] was formed, in agreement with the conclusion drawn from the X-ray study.
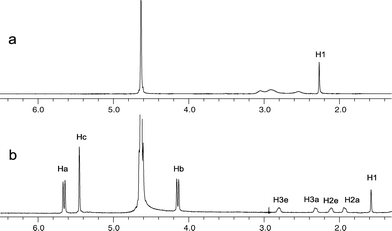 | ||
| Fig. 3 The 1H NMR spectra of DMC in its (a) free state and (b) included state. | ||
Besides the crystal structure, further conformational details of guest molecule DMC in the cavity of CB[8] could also be extracted by 1H NMR spectroscopy. Except for the relative up-field shift of the bound DMC proton resonances, the appearance of the peaks also showed some differences between its free and bound forms. Before inclusion, the resonances of the methylene protons in the guest overlapped into humps (Fig. 3a), while after inclusion, four well baseline-separated peaks were observed with same integral value (Fig. 3b). From left to right, these four resonances could be assigned as 3e, 3a, 2e and 2a; the axial and equatorial ethylene protons could therefore be distinguished. This assignment was confirmed by combining the results of COSY, HSQC and HMBC studies (see the ESI†). It is undoubted that the resolution enhancement of the spectrum is due to the inclusion of DMC. The substituted methyl groups of the guest play a vital role in resolution simplification. In its free form, rotation of the a–e bond of DMC is faster than the NMR time scale, so only one averaged resonance is observed. When DMC is encapsulated in CB[8], the relatively small space in the host’s inner ring restricts the motion of the guest. A more rigid conformation of the DMC is therefore present, which results in relatively slow guest tumbling. This is reflected in its spectrum, where the a and e methylene proton resonances are distinguishable. This sustained conformation of DMC in CB[8] also increases the stability of the inclusion complex.
In conclusion, the DMC@[CB[8] macrocycle complex was examined by X-ray crystallography and solution NMR spectroscopy. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex showed different conformational characteristics compared to previous work. Firstly, the average planes of the inner and outer macrocycles were almost perpendicular to each other, with the angle between the average plane being 78° 15′. Secondly, a unique packing structure was observed: a sexfoil-type arrangement of complexes present in the ab plane and two kinds of channels filled with small species. Thirdly, guest molecules in the cavity were restricted by the host, resulting in distinguishable a–e methylene protons.
1 host–guest complex showed different conformational characteristics compared to previous work. Firstly, the average planes of the inner and outer macrocycles were almost perpendicular to each other, with the angle between the average plane being 78° 15′. Secondly, a unique packing structure was observed: a sexfoil-type arrangement of complexes present in the ab plane and two kinds of channels filled with small species. Thirdly, guest molecules in the cavity were restricted by the host, resulting in distinguishable a–e methylene protons.
This work was financially supported by the National Natural Science Foundation of China (no. 20572083).
Experimental
The synthesis and purification of CB[8] was completed by the modification of a literature procedure.18 Mass spectra were recorded on a Voyager-DE STR MALDI-TOF instrument.An aqueous solution of CB[8] and DMC in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 was heated in a 100 °C water bath for 1.5 h then slowly cooled to room temperature. After 4 d, colorless crystals of CB[8]@DMC were produced (19.6 mg, yield 56.6%).
1.7 was heated in a 100 °C water bath for 1.5 h then slowly cooled to room temperature. After 4 d, colorless crystals of CB[8]@DMC were produced (19.6 mg, yield 56.6%).
NMR spectra were recorded in D2O at 25 °C using a Varian Inova 600 spectrometer. 1H NMR (ppm, D2O): 1.58 (s, 6H), 1.94 (m, 2H), 2.11 (m, 2H), 2.33 (m, 2H), 2.80 (m, 2H), 4.16 (d, 8H), 5.45 (s, 16H) and 5.64 (d, 8H). 13C NMR (ppm, D2O): 41.32, 42.34, 51.39, 54.05, 72.22 and 157.03. MS (MALDI-TOF): m/z (%) = 1529.40 (100) [M + H]+, M = [(C48H48N32O16)(C10H24N4)].
Crystal data for DMC@[CB[8]: [(C48H48N32O16)(C10H24N4)·4HCl·16H2O, Mf = 2071.70, rhombohedral, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (no. 148), a = b = 37.08856(10), c = 16.3396(9) Å, V = 20
(no. 148), a = b = 37.08856(10), c = 16.3396(9) Å, V = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310.5(14) Å3, Z = 9, ρc = 1.524 g cm−3. In the range of 1.29 ≤ θ ≤ 25.00, a total of 66
310.5(14) Å3, Z = 9, ρc = 1.524 g cm−3. In the range of 1.29 ≤ θ ≤ 25.00, a total of 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 reflections were collected, of which 7946 were independent (Rint = 0.0878) and 591 were observed with I > 2σ(I). The unit cell parameters were determined by 7946 observed reflections. The structure was solved by the Patterson method (SHELXS-97). All non-hydrogen atoms were refined anisotropically (SHELXL-97). Hydrogen atoms were determined by theoretical calculations and refined isotropically. The final full-matrix least-squares refinement gave R1 [I > 2σ(I)] = 0.0854, wR2 = 0.2489, R1 (all data) = 0.1385, wR2 = 0.2255 and GOF = 0.898.†
104 reflections were collected, of which 7946 were independent (Rint = 0.0878) and 591 were observed with I > 2σ(I). The unit cell parameters were determined by 7946 observed reflections. The structure was solved by the Patterson method (SHELXS-97). All non-hydrogen atoms were refined anisotropically (SHELXL-97). Hydrogen atoms were determined by theoretical calculations and refined isotropically. The final full-matrix least-squares refinement gave R1 [I > 2σ(I)] = 0.0854, wR2 = 0.2489, R1 (all data) = 0.1385, wR2 = 0.2255 and GOF = 0.898.†
References
- W. L. Mock, in Comprehensive Supramolecular Chemistry, ed. F. Vögtle, Pergamon, Oxford, 1996, vol. 2, pp. 477–493 Search PubMed.
- J. W. Lee, S. Samal, N. Selvapalam, H.-J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621–630 CrossRef CAS.
- K. Kim and H.-J. Kim, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, Marcel Dekker, New York, 2004, pp. 390–397 Search PubMed.
- K. Kim, Chem. Soc. Rev., 2002, 31, 96–107 RSC.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS.
- K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267–279 RSC.
- J. Kim, I.-S. Jung, S.-Y. Kim, E. Lee, J.-K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS.
- H.-J. Kim, J. Heo, W. S. Jeon, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 1526–1528 CrossRef CAS.
- S.-Y. Kim, I.-S. Jung, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 2119–2121 CrossRef CAS.
- A. Gafni and Y. Cohen, J. Org. Chem., 1997, 62, 120–125 CrossRef CAS.
- S.-H. Wan, F. Liang, X.-Q. Xiong, L. Yang, X.-J. Wu, P. Wang, X. Zhou and C.-T. Wu, Bioorg. Med. Chem. Lett., 2006, 16, 2804–2806 CrossRef CAS.
- L. Yang, F. Liang, M. Liu, C. Zheng, S. Wan, X. Xiong, X. Zhang, C. Sheng and X. Zhou, Bioorg. Med. Chem. Lett., 2007, 17, 1818–1822 CrossRef CAS.
- N. J. Wheate, D. P. Buck, A. I. Day and J. G. Collins, Dalton Trans., 2006, 3, 451–458 RSC.
- A. Day, R. J. Blanch, A. P. Arnold, S. Lorenzo, G. R. Lewis and I. Dance, Angew. Chem., Int. Ed., 2002, 41, 275–277 CrossRef CAS.
- L. G. Kuz’mina, A. I. Vedernikov, N. A. Lovova, J. A. K. Howard, Y. A. Strelenko, V. P. Fedin, M. V. Alfimov and S. P. Gromov, New J. Chem., 2006, 30, 458–466 RSC.
- D. Bardelang, K. A. Udachin, D. M. Leek and J. A. Ripmeester, CrystEngComm, 2007, 9, 973–975 RSC.
- I. Hwang, W. S. Jeon, H.-J. Kim, D. Kim, H. Kim, N. Selvapalam, N. Fujita, S. Shinkai and K. Kim, Angew. Chem., Int. Ed., 2007, 46, 210–213 CrossRef CAS.
- S. M. Liu, X. J. Wu, Z. X. Huang, J. H. Yao, F. Liang and C. T. Wu, J. Inclusion Phenom. Macrocyclic Chem., 2004, 50, 203–207 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR correlation data. CCDC reference number 689822. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b9nj00218a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2010 |
