Metallo–allixinate complexes with anti-diabetic and anti-metabolic syndrome activities
Hiromu
Sakurai
*a,
Akira
Katoh
b,
Tamas
Kiss
c,
Tamas
Jakusch
c and
Masakazu
Hattori
d
aDepartment of Pharmaco-analytical and Biocoordination Chemistry, Faculty of Pharmaceutical Sciences, Suzuka University of Medical Science, 3500-3 Minami-Tamagaki-cho, Suzuka, Mie 513-8670, Japan. E-mail: sakuirai@suzuka-u.ac.jp; Fax: +81-59-368-1271
bDepartment of Materials and Life Science, Faculty of Science and Technology, Seikei University, 3-3-1 Kitamachi, Kichijoji, Musashino, Tokyo 180-8633, Japan
cDepartment of Inorganic and Analytical Chemistry, Biocoordination Research Group of Hungarian Academy of Science, University of Szeged, H-6701 Szeged, Hungary
dDivision of Diabetes, Clinical Research Institute for Endocrine and Metabolic Diseases, National Hospital Organization, Kyoto Medical Center, 1-1 Mukouhata-cho, Fushimi-ku, Kyoto 612-8555, Japan
First published on 13th September 2010
Abstract
Metabolic syndrome and the accompanied diabetes mellitus are both important diseases worldwide due to changes of lifestyle and eating habits. The number of patients with diabetes worldwide is estimated to increase to 300 million by 2025 from 150–220 million in 2010. There are two main types of diabetes. In type 1 diabetes, caused by destruction of pancreatic β-cells resulting in absolute deficiency of intrinsic insulin secretion, the patients require exogenous insulin injections several times a day. In type 2 diabetes, characterized by insulin resistance and abnormal insulin secretion, the patients need exercise, diet control and/or several types of hypoglycemics. The idea of using metal ions for the treatment of diabetes originates from the report in 1899. The research on the role of metal ions that may contribute to the improvement of diabetes began. The orally active metal complexes containing vanadyl (oxidovanadium(IV)) ion and cysteine or other ligands were first proposed in 1990, and a wide class of vanadium, copper and zinc complexes was found to be effective for treating diabetes in experimental animals. We noticed a characteristic compound, allixin, which is a non-sulfur component in dry garlic. Its vanadyl and zinc complexes improved both types of diabetes following oral administration in diabetic animals. We then developed a new zinc complex with thioxoallixin-N-methyl (tanm), which is both a sulfur and N-methyl derivative of allixin, and found that this complex improves not only diabetes but also metabolic syndrome. Furthermore, new zinc complexes inspired from the zinc-tanm were prepared; one of them exceeded the activity of zinc-tanm. The mechanism of such complexes was studied in adipocytes. We describe here the usefulness of the development of metal-based complexes in the context of potential therapeutic application for diabetes and metabolic syndrome.
 Hiromu Sakurai | Hiromu Sakurai received his PhD in Pharmaceutical Sciences from Kyoto University. He was appointed a lecturer at Kyoto Pharmaceutical University and an associate professor at University of Tokushima. He was promoted to a professor at Kyoto Pharmaceutical University. Currently he is a professor of pharmacoanalytical and biocoordination chemistry at Suzuka University of Medical Science. His work includes development of metallopharmaceutics, particularly anti-diabetic metal complexes, biocoordination chemistry, pharmacometallomics, development of in vivo real time EPR and CL measurement methods, and new designs of the periodic table of the elements. |
 Akira Katoh | Akira Katoh was born in 1953. He received the degree of Doctor of Science in 1982 from University of Tsukuba. In 1988–1989, he performed postdoctoral research at University of Miami under the supervision of Prof. G. W. Gokel. He was appointed as professor of the Department of Industrial Chemistry, Faculty of Engineering, Seikei University, in 1995 and then as professor of Department of Materials and Life Science, Faculty of Science and Technology in 2005. His current research interest focuses on synthesis of various types of heterocyclic compounds and their application to functional molecules and chemotherapeutic agents. |
 Tamas Kiss | Tamas Kiss was born in Debrecen, Hungary in 1950. He received his PhD in chemistry from the Kossuth Lajos University, Debrecen in 1976, in 1995 became Professor of Inorganic Chemistry at the same University. In 1996 he moved to the University of Szeged and was elected to the Chair of the Department of Inorganic and Analytical Chemistry. His research interests include solution equilibria and biospeciation of essential and toxic metal ions in biological fluids and tissues. More recently, his research interest has turned to structural modeling of metalloenzymes and metalloproteins. |
 Tamas Jakusch | Tamas Jakusch was born in Mosonmagyaróvár, Hungary in 1973. He received his PhD in chemistry from the University of Szeged, in 2005. He is currently working as an assistant professor at the same University. He spent two years (2005–2007) at University of Michigan as a research fellow. His research interests include metal ion especially vanadium(IV and V) speciation studies with different methods (pH-metry, UV-VIS, EPR, NMR), functional and structural mimicking of the vanadium dependent haloperoxidase enzyme and preparation of anticancer ruthenium complexes. |
 Masakazu Hattori | Masakazu Hattori received a PhD in Experimental Pathology from the John Curtin School of Medical Research at the Australian National University and a DMSc in diabetes from Kyoto University. He was principal investigator in the Section of Immunology and Immunogenetics at Joslin Diabetes Center, Harvard Medical School in 1984–2004. Currently, he is chief of diabetes research at National Hospital Organization Kyoto Medical Center and visiting professor of medicine (diabetes) at Tokyo Women's Medical University Medical Center East. His research interests are to identify the MHC-linked type 1 diabetes genes and susceptibility genes to diabetic nephropathy in man and mouse. |
1. Introduction: background of the research
Brief history of chemotherapy
The bioavailability of metal ions originates from the birth of life in the oceans of the prehistoric age. This fact enables us not only to control our homeostasis but also to treat some diseases using metal ions and their complexes with biomolecules and synthetic organic compounds.Chemotherapy is a method for treatment of diseases by chemical compounds. The first modern concept of chemotherapy was created by Paul Ehrlich (1854–1915) in 1909, when Ehrlich and his co-worker Sahachirho Hata (1873–1938) discovered an inorganic arsenic (As)-containing compound, Arsphenamin or Salvarsan 606 to treat syphilis, which was an obstinate disease in those days.1 This great finding in chemotherapy was followed by the discovery of a peptide hormone, insulin, by Frederic Banting (1891–1941), Charles Best (1899–1978) and John Macleod (1876–1935) in 1921,2 an antibiotic, penicillin by Alexander Fleming (1881–1955), Howard Florey (1898–1968), Ernst Chain (1906–1979) and Norman Heatley (1911–2004) in 1928,3 and synthesized chemicals, sulfonamides, by Gerhard Domagk (1895–1964) in 1939.4
Chemotherapy using metallopharmaceutics is yet limited,5 although several new candidates such as metal-containing anticancer agents are now proposed.5 Creation of metallopharmaceutics is an important research field of Metallomics. Many chemists and bioinorganic chemists have made an endeavour to develop new metallopharmaceutics over the past century.
Diabetes mellitus and properties of insulin
Insulin is the most important hormone for the chemotherapy of diabetes mellitus. This hormone, which is composed of 51 amino acid residues and has a molecular weight of 5808 Da, is secreted from the islets of Langerhans in the pancreas and regulates the energy and glucose metabolism in the body. Insulin induces both the uptake of glucose in the cells of the liver, muscle and fat tissues and the storage of glucose as glucagon in the liver and muscle.6 If the secretion and activity level of insulin deteriorates, diabetes will occur, and then exogenous insulin is required to treat the diabetic state. The first trial of insulin administration was performed on a 14-year-old youth in Toronto. The finding of insulin and the following research revealed that there are several types of diabetes in humans.7–9Therapeutics for diabetes mellitus
Currently, diabetes mellitus is classified into two major types.7–9 Patients with type 1 diabetes lack intrinsic secretion of insulin by β-cell destruction and are dependent on an exogenous insulin supply by subcutaneous injection for survival. However, most patients with type 2 diabetes are insulin resistant, in which the insulin sensitivity of the target cells (muscles, liver, adipose tissues) is lowered and the relative insulin level in the blood is deficient. Patients with type 2 diabetes thus rely on chemotherapy involving several types of synthetic anti-diabetic pharmaceutics, such as insulin secretion promoters, insulin resistance improvers, and glucose absorption suppressors (α-glucosidase inhibitors) under the diet and exercise control.7–9Such pharmaceutics, however, have limited effects and sometimes induce side effects. To conquer the weakness of the current pharmaceutics available for type 2 diabetes, a new class of hypoglycemics such as glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors has recently been developed and clinically used. The proposed mechanism for the compounds is thought to involve suppression of GLP-1 degradation.10,11
Anti-diabetic vanadyl complexes
During research into developing synthetic therapeutics for diabetes, several metal ions and their complexes with organic ligands have been found to exhibit hypoglycemic effects in animals and humans. For example, before the discovery of insulin by Banting and Best, French physicians reported the improved glucose-lowering effect of sodium metavanadate (NaVO3) in human diabetes.12 Oral administration of either vanadyl sulfate (VOSO4) or NaVO3 has been shown to clinically improve human diabetes.13 These observations encouraged inorganic and bioinorganic chemists to promptly create the anti-diabetic metal complexes.5,13–20Since 1990, a wide class of vanadyl (oxidovanadium(IV)) complexes involving bis(methylcysteinato) [VO(cysm)2]- (1990),21 bis(L-tartrato) [(V2O4)(L-tart)2]- (1990),21 bis(maltolato) [VO(ma)2]- (1992),22 bis(pyrrolidine-N-dithiocarbamato) [VO(pdc)2]- (1994),23 bis(picolinato) [VO(pa)2]- (1995),24 and bis(1-oxy-2-pyridinethiolato) [VO(opt)2]- (1999)25 have been found to improve the hyperglycemic state in streptozotocin-induced type 1-like diabetes in rats (STZ-rats). In particular, studies on VO(pa)2 with a VO(N2O2) coordination environment13,15,17,26 and bis(3-hydroxy-4-pyronato) [VO(3hp)2]-,15,17,27 bis(1,4-dihydro-2-methyl-4-oxo-3-pyridinolato)- and bis(1,2-dihydro-2-oxo-1-pyrimidinolato)oxidovanadium(IV) complexes28 with a VO(O4) coordination environment have been intensively performed to find more potent analogues than the parent complexes, leading to the discovery of the linear relationship between in vitro insulin-mimetic activity and the partition coefficient of these complexes.
Anti-diabetic zinc complexes
There has been research into the production of more potent complexes than those mentioned above, and since 2000 oral administration of a wide variety of zinc(II) (Zn) complexes involving bis(6-methylpicolinato) [Zn(6mpa)2]-,29 bis(maltolato) [Zn(ma)2],30 bis(1-oxy-2-pyridonato) [Zn(opd)2]-,30,31 and bis(1-oxy-2-pyridinethiolato) [Zn(opt)2]31 has been shown to exhibit anti-diabetic activity and ameliorate hyperinsulinemia and massive hereditary obesity in mice. In addition, structure–activity relationships on zinc complexes with dithiocarbamates32 and pyridine-2-sulfonates33 were also examined with regard to the in vitro insulin-mimetic activity and partition coefficient of the complexes, leading to the discovery of new potential complexes such as bis(pyrrolidine-N-dithiocarbamato)Zn [Zn(pdc)2]32 and bis(3-methylpyridine-2-sulfonato)Zn,33 respectively. The former complex, with a Zn(S4) coordination environment, improved hyperglycemia and insulin resistance in type 2 diabetic mice on daily oral administration.32 Oral administration of Zn(3hp)2-related complexes with a Zn(O4) coordination environment induced high quality anti-diabetic properties.27 Bis(β-thujaplicinato)Zn or bis(hinokitiolato)Zn [Zn(hkt)2]34 with the same Zn(O4) coordination environment exhibited not only anti-diabetic activity but also anti-metabolic syndrome activity in respect to hypoglycemic effect and adiponectin secretion enhancing effect, when it was given to STZ-rats by daily intraperitoneal injections. Adiponectin is a protein hormone produced and secreted exclusively by adipocytes that regulates the metabolism of lipids and glucose.35 The hormone is known to influence the body's response to insulin and its levels are generally reduced under type 2 diabetes. The monitoring of adiponectin is thus essential for evaluating compounds with anti-metabolic syndrome effects. The Zn(hkt)2 complex actually triggered the development of new zinc complexes with anti-metabolic syndrome activity.Allixin as a phytoalexin
During the development of synthetic metal complexes with anti-diabetic effects, the search for ligands for metal ions such as vanadyl and zinc has been performed to include those not only forming a M(O4) (M = metal ions) coordination environment but also those originating from nature. After many trials, we have found that allixin, a phytoalexin of garlic, is a good candidate for this purpose.36–38Allixin (3-hydroxy-5-methoxy-6-methyl-2-pentyl-4H-pyran-4-one), a non-sulfur compound with a γ-pyrone structure (Fig. 1), was the first compound isolated from garlic as a phytoalexin, a product induced in plants by continuous stress. Among several induction methods, Kodera et al. have found that allixin crystals can be induced at an extremely high concentration (1400 ng mg−1 wet garlic weight) on the surface of garlic (Allium satiuum L.) after approximately 2 years of storage at room temperature in a dark room.
 | ||
| Fig. 1 Fresh garlic and structures of allixin and its vanadyl and zinc complexes. | ||
Allixin is an interesting compound with several unique biological properties such as anticancer activity, inhibition of aflatoxin B1 DNA binding activity, neurotropic activity, and inhibitory activity for the growth of Helicobacter pylori.36–40 By using this unique allixin, the physicochemical and biological studies on metallo–allixinate complexes have been performed with the great help of Dr Kodera and the Wakunaga Pharmaceutical Co. Ltd.
This review describes the frontier of research for the development of vanadyl- and zinc-allixinate complexes focusing on anti-diabetic and anti-metabolic syndrome activities, with respect to hyperglycemia, glucose intolerance, insulin resistance, hyperleptinemia, obesity, hypertension and low plasma adiponectin level.
2. Preparation of metallo–allixinate and related complexes
Vanadyl- and zinc-allixinate complexes
The bis(allixinato)oxidovanadium(IV) complex, VO(alx)2, was prepared by adding an aqueous solution of VOSO4·2.8H2O (1 mmol) to a suspension of allixin (2 mmol) in H2O, adjusting the pH of the mixture to 8.0 with 2 mol L−1 KOH, and heating it at 80 °C for 10 h.27 The bis(allixinato)Zn complex, Zn(alx)2, was also prepared by adding the aqueous solution of LiOH·H2O (3.0 mmol) to the aqueous mixture of allixin (3.0 mmol) and ZnSO4·7H2O (1.5 mmol), followed by stirring at pH 7–8 at room temperature41 (Fig. 1).Sulfur and nitrogen substitutes of zinc-allixinate complex
In order to enhance the anti-diabetic and anti-metabolic syndrome effects in animals, sulfur and/or nitrogen substitutes of allixin were prepared.42 Allixin was treated with phosphorus pentasulfide (P2S5) to give thioxoallixin. Methylamine underwent Michael-type addition to thioxoallixin and subsequent ring opening–ring closure produced the desired 1,6-dimethyl-3-hydroxy-5-methoxy-2-pentyl-4(1H)-pyrdinethione (tanm). Two equimolar amounts of tanm were treated with ZnSO4 in the presence of KOH to produce Zn(tanm)242 (Fig. 2).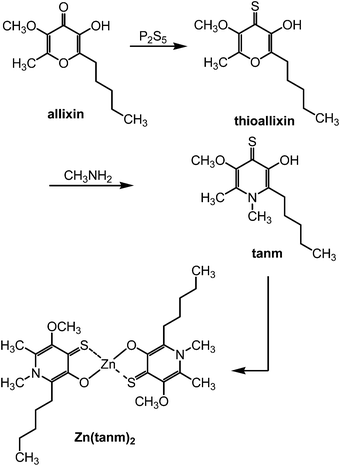 | ||
| Fig. 2 Synthesis of Zn(tanm)2 complex inspired from allixin. | ||
New zinc complexes inspired from Zn(tanm)2 complex
With the objective of providing a new family of insulin-mimetic complexes with VO(S2O2) and Zn(S2O2) coordination environments and searching for a new lead compound in place of tanm, vanadyl and zinc complexes with three types of heterocyclic compound, such as 3,2-hopsR, 5,4-hopsR1R2, and hoqltR, with various substituents were synthesized (Fig. 3(A)).43–49 The structures of allixin and thioxoallixin related compounds are also depicted in Fig. 3(B). | ||
| Fig. 3 (A) Structures and characteristics of tanm and its related compounds: R = alkyl and (CH2)nPh for 3,2-hopsR; R1 and R2 = alkyl for 5,4-hopsR1R2; R = OCH3, CH3, H, Br, NO2 for hoqltR. (B) Structural formulae of maltol(ma) and its analogues discussed in this paper. | ||
1-Substituted 3-hydroxy-2(1H)-pyridinethione (3,2-hopsR) is regarded as a structural isomer of tanm, and there are the following characteristics between 2-alkoxymethyl-1-alkyl-5-hydroxy-4(1H)-pyridinethione (5,4-hopsR1R2) and tanm; (a) 5,4-hopsR1R2 possesses the same 4(1H)-pyridinethione skeleton, which is illustrated in red in Fig. 3, as tanm, (b) 5,4-hopsR1R2 has the same S,O binding donors as tanm on complexation, (c) the zinc complex of 5,4-hopsR1R2 has the same molecular formula (C26H40N2O4S2Zn) as that of tanm. In addition, 2-aryl-3-hydroxy-1-methyl-4(1H)-quinolinethione (hoqltR) is regarded as the benzene-fused tanm.
3,2-hopsR was prepared from 3-methoxy-2(1H)-pyridinone (commercially available) via 3 steps;43–45 (1) N-alkylation of 3-methoxy-2(1H)-pyridinone with alkyl halides in the presence of NaOH, (2) conversion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O into C
O into C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S with Lawesson's reagent, and (3) deprotection with 1 mol L−1 BBr3 in CH2Cl2. Treatment of two equimolar amounts of 3,2-hopsR with VOSO4 and ZnSO4 afforded the corresponding metal complexes (Fig. 4). The structural assignment of vanadyl and zinc complexes was carried out by 1H-NMR (for Zn complex), IR (υV
S with Lawesson's reagent, and (3) deprotection with 1 mol L−1 BBr3 in CH2Cl2. Treatment of two equimolar amounts of 3,2-hopsR with VOSO4 and ZnSO4 afforded the corresponding metal complexes (Fig. 4). The structural assignment of vanadyl and zinc complexes was carried out by 1H-NMR (for Zn complex), IR (υV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and MALDI-TOF MS spectroscopy, and combustion analysis.
O) and MALDI-TOF MS spectroscopy, and combustion analysis.
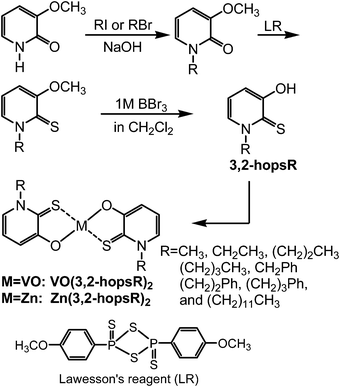 | ||
| Fig. 4 Synthesis of vanadyl and zinc complexes of 3,2-hopsR. | ||
5,4-hopsR1R2 was synthesized from kojic acid (commercially available) via 5 steps as shown in Fig. 5; (1) protection of the OH group at the C-5 position with a benzyl group by reaction of kojic acid with benzyl chloride in the presence of NaOH,45,46 (2) O-benzyl kojic acid was treated with alkyl iodides in the presence of NaH to give the O-alkylated products, (3) alkylamines underwent Michael-type addition to O,O′-disubstituted kojic acids to give the corresponding 4(1H)-pyridinones, (4) debenzylation with 10% Pd–C as a catalyst under a hydrogen atmosphere, and (5) conversion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O into C
O into C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S by treatment of the resulting 5-hydroxy-4(1H)-pyridinones with P2S5 in the presence of hexamethyldisiloxane (HMDSO).47 Treatment of two equimolar amounts of 5,4-hopsR1R2 with ZnSO4 afforded the corresponding zinc complexes, Zn(5,4-hopsR1R2)2.
S by treatment of the resulting 5-hydroxy-4(1H)-pyridinones with P2S5 in the presence of hexamethyldisiloxane (HMDSO).47 Treatment of two equimolar amounts of 5,4-hopsR1R2 with ZnSO4 afforded the corresponding zinc complexes, Zn(5,4-hopsR1R2)2.
 | ||
| Fig. 5 Synthesis of zinc complexes of 5,4-hopsR1R2. | ||
The synthetic procedure for hoqltR is depicted in Fig. 6. N-Methylanthranilic acid was allowed to react with (p-substituted)phenacyl bromide in the presence of K2CO3 to yield the corresponding O-phenacyl anthranilates.48 They were subjected to the ring closure reaction in polyphosphoric acid48,49 at 100 °C and subsequent conversion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O into C
O into C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S with Lawesson's reagent to give the desired hoqltR. Treatment of two equimolar amounts of hoqltR with ZnSO4 afforded zinc complexes, Zn(hoqltR)2 (Fig. 6).
S with Lawesson's reagent to give the desired hoqltR. Treatment of two equimolar amounts of hoqltR with ZnSO4 afforded zinc complexes, Zn(hoqltR)2 (Fig. 6).
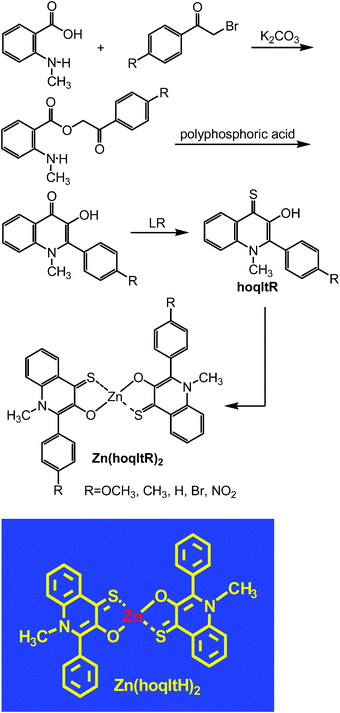 | ||
| Fig. 6 Synthesis of zinc complexes of hoqltR. | ||
3. Biospeciation of metallo–allixinates
The fate of any metal compounds after oral administration appears to be rather complex, as it includes processes such as crossing hydrophobic membranes, transportation by body fluids, binding to cell receptors, etc. Accordingly, different characteristics of the compounds may be important in each partial process, which will finally determine the efficacy of delivering the effective substance, the metal ion, into the cell. However, the actual solution state of the metal–ligand systems in the biological fluids (biospeciation) remains to be clarified, and their lipophilicity/hydrophilicity needs to be characterized by partition measurements. Furthermore, their interactions with important endogenous plasma and cell constituents should also be clarified in order to assess their affinity to relevant biomolecules.Stability constants for metal complexes of allixin and some ligands inspired from thioxoallixin-N-methyl
Allixin is a natural pyrone derivative and its vanadyl and zinc complexes were found to be very efficient in lowering high blood glucose levels in animal models in both type 1 and 2 diabetes.27,41,43,50 Pyrone derivatives form O,O coordinated complexes with metal ions. Recently, it was also found that the zinc complexes of the S,O analogues of pyrone derivatives (such as thioxomaltol (tma) or 1,2-dimethyl-3-hydroxypyridine-4-thione (3,4-hops) (Fig. 3) can be considered as more efficient potential drug candidates for the treatment of diabetes.45 Based on our inorganic chemistry background knowledge the affinity of vanadium(IV,V) and zinc(II) to O,O and S,O binding donors is not expected to be the same.51 Accordingly, it is worth comparing the metal binding ability of the basic ring donors together with that of allixin and the new synthetic 3,4-hops derivatives. Although there were some previous results for the basic ring compounds in the literature,50,52–55 because of solubility problems, all proton- and metal–ligand systems had to be re-measured in the same solvent (1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 65
water 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 mixture) for the sake of comparison. The detailed results and their discussion can be read elsewhere.56
35 mixture) for the sake of comparison. The detailed results and their discussion can be read elsewhere.56
In order to compare the vanadium(IV,V) and zinc(II) binding ability of the various ligands with O,O or S,O binding donors, the stability constants of the neutral bis complexes and the pM values (at pH = 8.35, neutral condition in the solvent mixture, cM = 1 mmol L−1, and cL/cM = 2) are listed in Table 1. The data indicate that zinc is bound more strongly to the softer S,O donor ligands (tma, 3,4-hops, hoqltH and 5,4-hopsHexMe) (Fig. 3–6). The pyridinone derivatives are stronger metal ion binders than the pyrone ones due to the electronic stabilization through the ring-N (Δlog K ∼ 4–5), while the change from O,O to S,O binding donor results again in a similar increase in the stability of the corresponding complexes. The pZn values follow practically the same order as the stability of the mono and bis complexes of the ligands.56 This is in agreement with the data obtained in the more polar solvent 15% CH3OH–H2O mixture.51 If we compare the metal binding ability of the ligands with S,O binding donors based on the stability constants of the bis complexes, the order is as follows: 3,4-hops ≥ hoqltH > 5,4-hopsHexMe > tma. However, because of the different protonation constants of these ligands (pKa of tma: 10.73, 3,4-hops: 12.25, hoqltH: 12.99, 5,4-hopsHexMe: 10.97), the conditional stability at pH = 8.35 is different (hoqltH > 5,4-hopsHexMe = 3,4-hops > tma) to that shown by the pZn values. The ligand 5,4-hopsHexMe shows remarkable Zn binding ability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 65
water 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 mixed solvent. The values are expressed as the means and standard deviations. Conditional stability constants for [VO2A(OH)]− and pM values (at pH = 8.35, cM = 1 mmol L−1, cL/cM = 2) for vanadyl and zinc are also given
35 mixed solvent. The values are expressed as the means and standard deviations. Conditional stability constants for [VO2A(OH)]− and pM values (at pH = 8.35, cM = 1 mmol L−1, cL/cM = 2) for vanadyl and zinc are also given
| ma | 3,4-hopo | allixin | tma | 3,4-hopos | hoqltH | 5,4-hopsHexMe | ||
|---|---|---|---|---|---|---|---|---|
| a Conditional stability constant at pH = 8.35 (neutral conditions). | ||||||||
| log βMA2 | VIVO | 20.0 ± 0.2 | 27.3 ± 0.1 | 22.1 ± 0.2 | 21.0 ± 0.3 | 24.4 ± 0.2 | 24.7 ± 0.1 | 23.0 ± 0.3 |
| Zn(II) | 11.9 ± 0.1 | 15.7 ± 0.1 | 12.5 ± 0.2 | 16.4 ± 0.2 | 21.3 ± 0.2 | 20.9 ± 0.1 | 19.5 ± 0.1 | |
| VVO2a | 5.6 ± 0.1 | 6.8 ± 0.2 | 4.8 ± 0.2 | — | — | — | — | |
| log βVO2A(OH)a | VVO2 | 2.2 ± 0.2 | 4.1 ± 0.3 | 2.4 ± 0.1 | 1.8 ± 0.1 | 2.9 ± 0.1 | 1.8 ± 0.2 | 2.4 ± 0.1 |
| pM | VIVO | 8.4 | 10.7 | 8.8 | 8.7 | 7.9 | 7.7 | 10.0 |
| Zn | 4.0 | 4.7 | 3.8 | 5.7 | 6.0 | 6.0 | 6.9 |
Species distribution curves for metal complexes with allixin and some ligands inspired from thioxoallixin-N-methyl
The species distribution curves for the Zn-allixin, Zn-3,4-hoqltH and Zn-5,4-hopsHexMe systems shown in Fig. 7(A–C) also indicate that at neutral pH the percentage of the uncharged bis complex ZnA2 (A = ligand) is extremely high in the Zn-5,4-hopsHexMe system (it is the predominant species in a wide pH range, pH 6–12), providing better membrane permeability for the metal ion. At the same time, due to the lower stability, the uncharged bis complex with allixin is present at only about 60% at neutral pH.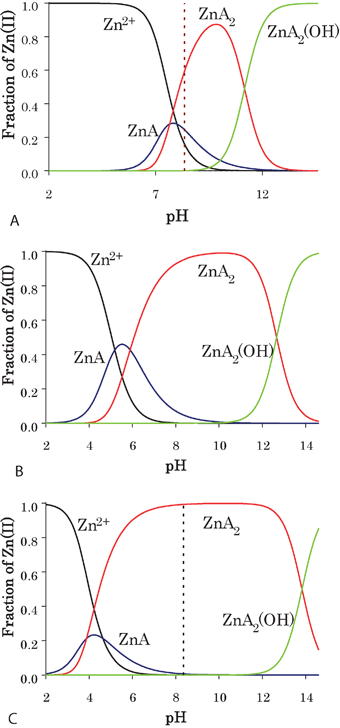 | ||
Fig. 7 Concentration distribution diagrams for the zinc systems (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal to ligand ratio, CZn(II) = 1 mmol L−1, t = 25 °C and I = 0.1 mol L−1 KCl in 1,4-dioxane 2 metal to ligand ratio, CZn(II) = 1 mmol L−1, t = 25 °C and I = 0.1 mol L−1 KCl in 1,4-dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 65 water 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 mixed solvent). (A) allixin; (B) hoqltH; (C) 5,4-hopsHexMe (The dashed line represents the neutral pH = 8.35 in the solvent mixture). 35 mixed solvent). (A) allixin; (B) hoqltH; (C) 5,4-hopsHexMe (The dashed line represents the neutral pH = 8.35 in the solvent mixture). | ||
Interestingly, the situation is not so clear with the vanadium complexes. With the vanadyl ion, significantly lower overall stability constants were obtained in water for the thioxo derivatives compared with the corresponding oxo analogues.52 This was explained by the hard character of vanadyl and the soft character of sulfur. As is seen in Table 1, in 65% dioxane–water mixture, only 3,4-hopo and 3,4-hops follow this trend. In other cases the thioxo derivative forms more stable complexes, but it is probably only the effect of the solvent. This observation is supported by the fact that the stability increase is even larger with the synthetic 3,4-hops derivatives (3,4-hoqltH and 5,4-hopsHexMe) in comparison with allixin, which have large hydrophobic side chains making the molecules even less polar (see Fig. 3 and 5).
The binding ability of the vanadate ion is significantly weaker than those of the previous two metal ions. The extent of complex formation could hardly be observed by pH-potentiometry. Thus, interactions between the metal ion and the ligands were studied at neutral pH in the same solvent mixture by 51V-NMR spectrometry; the determined conditional stability constants can be found in Table 1. Ligands with O,O binding donors form mono ([(VO2)L(OH)]−) and bis complexes ([(VO2)L2]−), while the thioxo derivatives, under the given conditions, form only one type of complex, most probably the mono complex. The conditional stability of the ([(VO2)L(OH)]− does not vary too much, only 3,4-hopo, and somewhat 3,4-hops, has a little higher affinity to vanadate. The lack of the formation of bis complexes results in a lower amount of vanadate binding to the thioxo derivatives.
Due to the low extent of complex formation (<20% at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal ion to ligand ratio at cM = 1 mmol L−1), the complexes of allixin and synthetic thioxo derivatives with vanadate, differently from those of vanadyl and zinc, are considered to have lower potential as candidates for the improvement of diabetes.
2 metal ion to ligand ratio at cM = 1 mmol L−1), the complexes of allixin and synthetic thioxo derivatives with vanadate, differently from those of vanadyl and zinc, are considered to have lower potential as candidates for the improvement of diabetes.
4. Insulin-mimetic activity and anti-diabetic and anti-metabolic syndrome effects of metallo–allixinate complexes
In vitro evaluation of insulin-mimetic effect of metallo-complexes
It is essential to establish a reliable and simple in vitro appraisal system without using radioisotopes to evaluate metal ions and metal complexes for their potential insulin-mimetic and anti-diabetic effects. Such an appraisal system, demonstrating both increase of lipolysis and stimulation of glucose transport, was developed in 199557 and 200458 with respect to the interaction of chemical compounds with isolated rat adipocytes. In this system, the adipose cells are prepared from epididymal fat tissue and treated with adrenaline (epinephrine).When insulin receptors on the cells are activated by inhibiting protein tyrosine phosphatase (PTB1B), which is related to the activation of cytosolic nonreceptor tyrosine kinase, the following reactions involving direct phosphorylation of insulin receptor substrate 1 (IRS-1), activation of phosphatidylionsitol 3 kinase (PI3K), glucose transporter 4 (GLUT4) translocation to the cell membrane and activation of phosphodiesterase (PDE) are induced in the cells.59 In fact, vanadyl and zinc ions, in place of insulin, induce insulin-mimetic effects with regard to both incorporation of glucose in the rat adipocytes and inhibition of free fatty acids (FFA) release from the adipocytes.13,60–62 Both vanadyl and zinc ions are found to simultaneously act on multiple sites in the adipocytes, we thus named it as “the ensemble mechanism”.17 By using simple determination kits (Fuji Dry Chem (Fuji Medical Co.) for the determination of glucose concentration and FFA kit (NEFA C-test Wako, Wako Pure Chemicals) for the determination of FFA concentration,57,58 the insulin-mimetic effect of vanadyl-3-hydroxy-4-pyronate (VO(3hp)2) and the related complexes were examined (Fig. 8). Among 5 complexes, VO(alx)2 exhibited the strongest effect in terms of FFA release inhibition (IC50 (50% inhibitory concentration of FAA release from the cells) = 553 μmol L−1) and glucose uptake (EC50 (50% enhancing concentration of glucose uptake) = 24 μmol L−1).27
 | ||
| Fig. 8 Structures of bis(3-hydroxypyronato)oxidovanadium(IV) (VO(3hp)2) and its related complexes. Abbreviations are as follows. VO(ma)2 = bis(maltolato)oxidovanadium(IV), VO(ema)2 = bis(ethylmaltolato)oxidovanadium(IV), VO(ka)2 = bis(kojato)oxidovanadium(IV), and VO(alx)2 = bis(allixinato)oxidovanadium(IV). | ||
In addition, a good linear correlation (r = 0.99) was observed between the inhibitory effect of FFA release (reciprocal IC50 value) and the partition coefficient (log P) of the complexes, indicating that the insulin-mimetic effect correlates positively with the permeability of the complexes through the cell membrane.27 An increase in the cell membrane penetration of the complexes will enhance the insulin-mimetic effect.
Likewise, in evaluation of in vitro insulin-mimetic effect of a series of zinc-3-hydroxy-4-pyronate (Zn(3hp)2) complexes, the Zn(alx)2 complex exhibited the highest insulin-mimetic effect (reciprocal IC50), where it correlated linearly with the partition coefficient (log P) of the ligand (r = 0.99), indicating that the effect of Zn(3hp)2-related complexes is dependent on the membrane permeability of the ligand.42
Improvement of diabetes mellitus and metabolic syndrome by metallo–allixinate complexes in animals
Based on these findings, the effect of oral administration of VO(alx)2 was examined in the obesity-linked type 2 diabetic KK-Ay mice, which is characterized by hyperphagia due to leptin resistance, followed by obesity and development of hyperleptinemia, insulin resistance, hyperinsulinemia, diabetes, dyslipidemia and hypertension after approximately 8 weeks of age.63 The hyperglycemic state of KK-Ay mice was ameliorated within 10 days after VO(alx)2 was administered daily at the doses of 3–7 mg V kg−1 of body weight for 4 weeks. Similarly, a significant reduction or improvement was observed in the levels of HbA1C, plasma insulin and leptin, glucose tolerance test (OGTT), epididymal fat pad weight, food intake and systolic blood pressure.63 VO(alx)2, however, failed in achieving a lowered level of adiponectin, which can enhance insulin sensitivity.
 | ||
| Fig. 9 Structures of bis(allixinato)zinc (Zn(alx)2) and its related complexes. Abbreviations are as follows. Zn(talx)2 = bis(thioxoallixinato)zinc, Zn(anm)2 = bis(allixin-N-methyl)zinc, and Zn(tanm)2 = bis(thioxoallixin-N-methyl)zinc. | ||
The Zn(tanm)2 exhibited an extremely high in vitro insulin-mimetic effect (IC50 = 11 ± 1 μmol L−1) compared with others (IC50 = 31–220 μmol L−1).42,45 Daily oral administrations of Zn(tanm)2 to KK-Ay mice at the dose of 15 mg Zn kg−1 of body weight for 4 weeks significantly improved hyperglycemia, OGTT, insulin resistance, hyperleptinemia, obesity and hypertension. Interestingly, this complex increased the depressed plasma adiponectin levels in the mice.66 On the basis of these results, Zn(tanm)2 is proposed to be an active therapeutic for treating obesity-linked type 2 diabetes and metabolic syndrome.
Adiponectin is an adipocytokine, which is synthesized and released by adipocytes, abundantly present in plasma and leads to enhanced insulin action, indicating that this adipocytokine maintains insulin sensitivity and glucose homeostasis. However, adiponectin levels are reduced in patients with increased insulin resistance due to conditions such as obesity, type 2 diabetes and hypertension. Thus, adipocytokine is considered as a good biomarker to assess efficacy for developing therapeutics for type 2 diabetes.67–70 It is already known that thiazolidinedione derivatives (TDZs) increase the adiponectin level as potential insulin-sensitizing therapeutics.71 However, TZDs are associated with edema, body weight gain, congestive heart failure, osteoporosis and liver dysfunction in type 2 diabetic patients.71 In contrast, Zn(tanm)2 neither induces such symptoms nor exerts appreciable toxic effects in the liver of animals.66 Zn(tanm)2 is thus the first example which improves not only type 2 diabetes but also metabolic syndrome with respect to the adipocytokine level.
In vitro and in vivo activities of new zinc complexes inspired from Zn(tanm)2
In terms of IC50 values, all zinc complexes, Zn(3,2-hopsR)2 (Fig. 4), showed high insulin-mimetic activities 7 to 15 times higher than ZnSO4 at the μmol L−1 level; ZnSO4: 292 ± 31, R = CH3: 18.4 ± 0.9, R = CH2CH3: 18.4 ± 1.5, R = (CH2)2CH3: 17.6 ± 1,2, R = (CH2)3CH3: 21.3 ± 1.5, R = CH2Ph: 21.5 ± 0.7, R = (CH2)2Ph: 36.1 ± 2.0, R = (CH2)3Ph: 24.1 ± 2.7, R = (CH2)11CH3: 292 ± 31.44 In contrast, no relationship between IC50 values and the balance of the hydrophilicity/hydrophobicity was observed. In addition, VO(3,2-hopsR)2 afforded essentially no activity, probably due to the insolubility of the complexes in the KRB buffer.IC50 values of all zinc complexes, Zn(5,4-hopsR1R2)2 (Fig. 5), were larger than that of Zn(tanm)2 (IC50 = 11 ± 1 μmol L−1),42 R1 = (CH2)5CH3, R2 = CH3: 131 ± 16, R1 = (CH2)4CH3, R2 = CH2CH3: 184 ± 11, R1 = (CH2)3CH3, R2 = (CH2)2CH3: 52 ± 12, R1 = (CH2)2CH3, R2 = (CH2)3CH3: 101 ± 18 μmol L−1. It is likely that zinc complexes bearing R1 and R2 with similar methylene chain lengths show high insulin-mimetic activities (A. Katoh, unpublished data).
In terms of IC50 values, some zinc complexes, Zn(hoqltR)2 (Fig. 6), showed high insulin-mimetic activities in the μmol L−1 level; R = OCH3: 131 ± 66, R = CH3: 51 ± 1, R = H: 6.7 ± 1.8 μmol L−1, R = Br: none, R = NO2: none. It is worthy of note that Zn(hoqltH)2 exhibits the highest insulin-mimetic activity among the complexes reported previously. The hypoglycemic effect of Zn(hoqltH)2 was then examined in diabetic KK-Ay mice. When this complex was given by oral administration at the dose of 15 mg Zn kg−1 body weight for 14 days, the normoglycemic effect was observed one week after the complex administration began (Fig. 10). The results of OGTT and HbA1C level in diabetic KK-Ay mice showed that the complex improves the type 2 diabetic state (A. Katoh et al., to be published elsewhere).
 | ||
| Fig. 10 Changes in blood glucose levels in (●) control KK-Ay mice and (□) KK-Ay mice treated with Zn(hoqltH)2 at the dose of 15 mg Zn kg−1 of body weight by daily oral administration for 14 days. *Significance at p < 0.001 versus control. | ||
The observations described above strongly indicate that the development of new zinc complexes inspired by Zn(tanm)2 may provide highly potent candidates for treating not only type 2 diabetes but also metabolic syndrome.
5. Mechanism of action of metallo–allixinate complexes
Mode of action of vanadyl complexes
For many years possible mechanisms of action of insulin-mimetic vanadyl and zinc complexes have been examined in isolated rat adipocytes or cultured cell systems.17,57,59,62,72 Both vanadyl and zinc complexes enhanced glucose uptake into the adipocytes without adding hormones and inhibited epinephrine-induced FFA release,15,17,59,60,62,64 similar to the action of insulin. These results show that vanadyl and zinc complexes have common “insulin-mimetic” activities. In examining the mechanism of action of these complexes, several inhibitors, such as the selective insulin receptor β-subunit (IRβ) inhibitor (HNMPA-(AM)3), PI3K inhibitor (wortmannin), glucose transporter inhibitor (cytochalasin-B), and PDE inhibitor (cilostamide) have been used.57,62 The inhibition of FFA release by vanadyl and zinc complexes was reversed by these inhibitors, indicating that the complexes simultaneously act on several target sites such as IRβ, PI3K, PDE3B, and GLUT4 (“ensemble mechanism”).17 However, the critical mechanism of action of the vanadyl and zinc complexes with respect to the regulation of the insulin and lipolysis signaling pathways remained unclear. We thus tried to discriminate the mode of action of vanadyl and zinc complexes using high potent metallo–allixinate complexes.The action site of VO(alx)2 was examined in terms of the levels of phosphoproteins in the insulin signaling pathway in the 3T3-L1 adipocytes. VO(alx)2 enhanced not only the tyrosine phosphorylation of IRβ and IRS but also of Akt (akt8) and mitogen-activated protein kinase (MAPK).73 In addition, VO(alx)2 regulated the downstream effects of Akt, such as the stimulation of GLUT4 translocation to the plasma membrane and the activation of forkhead transcription factor class O1 (FOXO1).73,74
As described before, VO(alx)2 could not rescue the adiponectin level in the plasma,63 which suggested that VO(alx)2 affects the organs such as muscle and liver rather than the adipose tissues in both STZ and KK-Ay diabetic mice. However, the reason why VO(alx)2 is unable to rescue the level of adiponectin in diabetic animals remains unresolved.
Anti-metabolic syndrome activity of zinc complexes
The problem of low levels of adiponectin in diabetes and metabolic syndrome has been overcome by the zinc complexes as described. Surprisingly, the continued administration of both Zn(alx)2 and Zn(tanm)2 not only improved hyperglycemia and the levels of insulin and leptin but also increased the adiponectin level, which is reduced in diabetic KK-Ay mice.66 These findings indicate that Zn(alx)2 and Zn(tanm)2 may affect the adipose tissues rather than the liver and muscle tissues.Zn(alx)2 and Zn(tanm)2 induced Akt/PKB activation, leading to the translocation of GLUT4 to the plasma membrane and glucose uptake. In addition, zinc was incorporated into the adipocytes in a dose- and time-dependent manner when each complex was added to the cells; thus, it was concluded that the activation of Akt/PKB-GLUT4 signaling was dependent on the intracellular zinc concentration.74 The imbalance in the intracellular zinc concentration has been reported in diabetes and other disorders.75,76 From the previous findings, it will be suggested that Zn(alx)2 and Zn(tanm)2 improve the imbalance in the intracellular zinc concentration and consequently exhibit insulin-mimetic activity.
Differential action sites of vanadyl and zinc complexes
Critical action sites were examined on the Zn(alx)2 and Zn(tanm)2 complexes. The PI3K inhibitor wortmannin inhibited the activation of Akt/PKB induced by both complexes, and both complexes did not induce the tyrosine phosphorylation of IRβ and IRS. Previously, it has been reported that zinc induces the degradation of the phosphatase and tensin homologue deleted on chromosome 10 (PTEN).77 Similarly, it has been observed that zinc complexes inhibit the enzymatic activity of PTEN (W. Basuki, unpublished data). In addition, the overexpression of PTEN in mice impaired the insulin signaling pathway and resulted in insulin resistance.78 Based on these observations, it is proposed that Zn(alx)2 and Zn(tanm)2 affect PTEN and activate PI3K-Akt/PKB signaling.Furthermore, it was analyzed whether Zn(alx)2 and Zn(tanm)2 inhibit the secretion of FFA from adipocytes (the inhibition of lipolysis signaling). Adipocytes play a role in regulating the level of endogenous FFA in the body.79,80 The degradation of triglycerides is controlled by hormone-sensitive lipase (HSL). This lipase is phosphorylated by cAMP-dependent kinase (PKA) that is activated by the increase in the intracellular cAMP concentration.79,81 On the other hand, PDE3B, that is activated by Akt/PKB, catalyzes the conversion of cAMP to 5′-AMP,82–84 indicating that this enzyme is a negative regulator of PKA signaling. Adipocytes, treated with Zn(alx)2 and Zn(tanm)2, attenuated PKA-dependent HSL phosphorylation and consequently inhibited FFA secretion from the adipocytes.74 Alternatively, the PI3K inhibitor reversed this effect. Therefore, the suppression of FFA release by Zn(alx)2 and Zn(tanm)2 indicates the activation of PI3K-Akt/PKB-PDE3B signaling (Fig. 11).
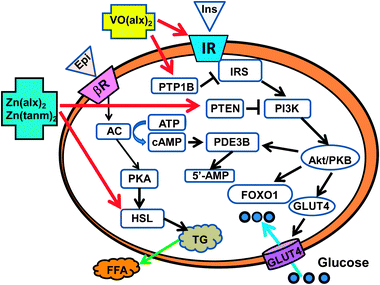 | ||
| Fig. 11 Differential action sites of vanadyl and zinc complexes in adipocytes. VO(alx)2 acts on IRβ and/or PTP1B, and activates the downstream insulin receptor proteins. The activation of Akt/PKB by VO(alx)2 finally induces the translocation of GLUT4 to the plasma membrane and regulates the transcription factor FOXO1. Zn(alx)2 and Zn(tanm)2 suppress the activity of PTEN and subsequently activate the downstream proteins of PI3K. Zn complexes thus activate Akt/PKB and stimulate the translocation of GLUT4 to the plasma membrane. The activation of Akt by Zn(alx)2 and Zn(tanm)2 induces the enzymatic activity of PDE3B, which uses c-AMP as a substrate, and thus follows the suppression of PKA and HSL activities. Then, FFA release is suppressed. Abbreviations used are as follows. IR, insulin receptor; IRS, insulin receptor substrate; PI3K, phosphatidylinositol 3-kinase; PTP1B, protein tyrosine phosphatase-1B; PTEN, phosphatase and tensin homologue deleted on chromosome 10; Akt/PKB, Akt/protein kinase B; GLUT4, glucose transporter 4; FOXO1, forkhead transcription factor class O1; PDE3B, phosphodiestarase 3B; βR, β-receptor; AC, adenylate cyclase; PKA, cAMP-dependent phosphokinase; HSL, hormone-sensitive lipase; FFA, free fatty acid. This figure is summarized from the results of ref. 13–16, 50, 57–62, 73 and 74. | ||
In addition, VO(alx)2, Zn(alx)2, and Zn(tanm)2 also play unique roles in cells; VO(alx)2 regulates the activation of the FOXO1,73 while Zn(alx)2 and Zn(tanm)2 regulate the activation of HSL, resulting in the suppression of FFA release.74 Recently, the participation of FOXO1 in the insulin-mimetic signal transduction by some zinc complexes has also been confirmed.85
On the basis of the results, it is concluded that a common mechanism of action of VO(alx)2, Zn(alx)2, and Zn(tanm)2 is the induction of Akt/PKB activity that results in the translocation of GLUT4 to the plasma membrane. However, their critical action sites are slightly different from each other. VO(alx)2 targets IRβ and/or PTPase,73 while Zn(alx)2 and Zn(tanm)2 target PTEN74 (Fig. 11). Such different action sites of the metal complexes may depend on the chemical characteristics of the vanadyl and zinc metal ions. The characteristics of the ligand are important for passage through the plasma membrane.
Gene expression profiling
Furthermore, since oral administration of VO(alx)2 was found to enhance the phosphorylation of Akt and glycogen synthase kinase-3β (GSK3β), located downstream of the insulin receptor cascade in the skeletal muscles of STZ-mice,50 the gene expression in the muscles of the mice before and after insulin- or VO(alx)2-treatment was analyzed.50 The treatment with insulin or VO(alx)2 normalized the gene expression levels of 152 up-regulated and 11 down-regulated genes, especially in the case of up-regulation of Cyp2E1 and FOXO1 in the muscles of the diabetic mice. The restorations by VO(alx)2 of these genes are considered to be due to the enhancement of protein phosphorylation leading to the activation or inactivation of the transcriptional machinery, suggesting that the insulin-mimetic effect of VO(alx)2 in diabetes is due to changes in the protein phosphorylations and their gene expression levels.50These results will be important for developing novel molecular targeting strategies for insulin-mimetic complexes.
6. Conclusion and future aspects
Nature sometimes gives us valuable compounds. Garlic, a species in the onion family Alliaceae, is a gift from nature and has been used globally for thousands of years for culinary and medicinal purposes in most races. The garlic bulk is the most commonly used part of the plant, where plenty of low molecular weight sulfur-containing compounds as allicin, dialkylsulfides, dithiis, alliin and S-alkylcysteine have been found for many years.In 1999, an unusual non-sulfur compound, allixin, was found from the bulb and unique biological properties of this compound have been revealed. Allixin, with a hydroxy-γ-pyrone structure, is also a good ligand for several metal ions. Noticing the characteristics of the compound, we tested the prepared metal complexes in the treatment of diabetes and metabolic syndrome in experimental animals.
As expected, we found that both vanadyl- and zinc-allixin complexes ameliorate diabetes and metabolic syndrome. Throughout our research endeavour on discovering new metal therapeutics, we attempted valuable methods and found that suitable drug designs of allixin enhanced the biological and pharmaceutical activities of the original vanadyl and zinc complexes. Consequently, we established the usefulness of Zn(tanm)2 with high anti-diabetic and anti-metabolic syndrome effects in animals. Furthermore, this complex inspired us to design more potent complexes, and a desired Zn(hoqltH)2 complex was found. The detailed biological and pharmaceutical properties of the complex need further research before aiming for clinical use in diabetic patients in the future.
Metallo–allixinate complexes are now evolving in a new Metallomics world, and the fruitful outcome may offer a novel medicine with high quality of anti-diabetic and anti-metabolic syndrome activity for humans in the future.
Acknowledgements
The authors gratefully acknowledge the financial support from the grants-in-aid for scientific research (B), scientific research on priority areas and specially promoted research provided by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of the Japanese Government. The authors would also like to thank all the researchers and students who were involved in the research and are cited in the references for their excellent contributions.References
- N. C. Lloyd, H. W. Morgan, B. K. Nicholson and R. S. Ronimis, Angew. Chem., Int. Ed., 2005, 44, 941–944 CrossRef CAS.
- G. S. Eisenbarth, Diabetes, 2010, 59, 759–774 CrossRef CAS.
- J. W. Bonnett and K. T. Chung, Adv. Appl. Microbiol., 2001, 49, 163–184 Search PubMed.
- W. Bocker, J. Vet. Pharmacol. Ther., 1994, 17, 309–316 CrossRef.
- H. Sakurai, J. Health Sci., 2010, 56, 129–143 CrossRef CAS.
- K. Chang, A. M. Jorgensen, P. Bardrum and J. J. Led, Biochemistry, 1997, 36, 9409–9422 CrossRef CAS.
- Textbook of Medical Physiology, ed. A. C. Guyton and J. E. Hall, Elsevier Sanders, Philadelphia, 11 edn, 2006 Search PubMed.
- American Diabetes Association, Diabetes Care, 2007, 298, suppl. (1), s43–48 Search PubMed.
- B. B. Turkoski, Orthop. Nurs., 2006, 25, 227–231 Search PubMed.
- M. Kirby, D. M. Yu, S. O'Connor and M. D. Gorrell, Clin. Sci., 2009, 118, 31–41 Search PubMed.
- P. L. Brubaker, Endocrinology, 2010, 151, 1984–1989 CrossRef CAS.
- B. Lyonnet, X. Martz and E. Martin, Presse Med., 1899, 1, 191–192 Search PubMed.
- H. Sakurai, H. Yasui and Y. Yoshikawa, Expert Opin. Invest. Drugs, 2003, 12, 1189–1203 Search PubMed.
- H. Sakurai, Y. Yoshikawa and H. Yasui, Chem. Soc. Rev., 2008, 37, 2383–2392 RSC.
- H. Sakurai, Expert Opin. Drug Discovery, 2007, 2, 873–887 Search PubMed.
- H. Sakurai, Biomed. Res. Trace Elements, 2007, 18, 241–248 CAS.
- H. Sakurai, A. Katoh and Y. Yoshikawa, Bull. Chem. Soc. Jpn., 2006, 79, 1645–1664 CrossRef CAS.
- K. H. Thompson and C. Orvig, J. Inorg. Biochem., 2006, 100, 1925–1935 CrossRef CAS.
- D. C. Crans, J. J. Smee, E. Gaidamauskas and L. Yang, Chem. Rev., 2004, 104, 849–902 CrossRef CAS.
- D. Rehder, Inorg. Chem. Commun., 2003, 6, 604–617 CrossRef CAS.
- H. Sakurai, K. Tshuchiya, M. Nukatsuka, J. Kawada, S. Ishikawa, H. Yoshida and M. Komatsu, J. Clin. Biochem. Nutr., 1990, 8, 193–200 Search PubMed.
- J. H. McNeill, V. G. Yuen, H. R. Hoveyda and C. Orvig, J. Med. Chem., 1992, 35, 1489–1491 CrossRef CAS.
- H. Watanabe, M. Nakai, K. Komazawa and H. Sakurai, J. Med. Chem., 1994, 37, 876–877 CrossRef CAS.
- H. Sakurai, K. Fujii, H. Watanabe and H. Tamura, Biochem. Biophys. Res. Commun., 1995, 214, 1095–1101 CrossRef CAS.
- H. Sakurai, H. Sano, T. Takino and H. Yasui, Chem. Lett., 1999, 913–914 CrossRef CAS.
- H. Yasui, A. Tamura, T. Takino and H. Sakurai, J. Inorg. Biochem., 2002, 91, 327–338 CrossRef CAS.
- Y. Adachi, J. Yoshida, Y. Kodera, A. Katoh, J. Takada and H. Sakurai, J. Med. Chem., 2006, 49, 3251–3256 CrossRef CAS.
- A. Katoh, M. Yamaguchi, K. Taguchi, R. Saito, Y. Adachi, Y. Yoshikawa and H. Sakurai, Biomed. Res. Trace Elements, 2006, 17, 1–10 CAS.
- Y. Yoshikawa, E. Ueda, K. Kawabe, H. Miyake, T. Takino, H. Sakurai and Y. Kojima, J. Biol. Inorg. Chem., 2002, 7, 68–73 CrossRef CAS.
- Y. Yoshikawa, E. Ueda, K. Kawabe, H. Miyake, H. Sakurai and Y. Kojima, Chem. Lett., 2000, 874–875 CrossRef.
- Y. Kojima and H. Sakurai, European patent, EP 1256342AT Search PubMed.
- Y. Yoshikawa, Y. Adachi and H. Sakurai, Life Sci., 2007, 80, 759–766 CrossRef CAS.
- Y. Yoshikawa, M. Morishita, M. Nishide, E. Ueda, I. Kinoshita, K. Okada, N. Kajiwara, H. Sakurai and Y. Kojima, Bull. Chem. Soc. Jpn., 2007, 80, 530–532 CrossRef CAS.
- M. Yamane, Y. Adachi, Y. Yoshikawa and H. Sakurai, Chem. Lett., 2005, 34, 1694–1695 CrossRef CAS.
- Y. Matsuzawa, FEBS Lett., 2006, 580, 2917–2921 CrossRef CAS.
- Y. Kodera, M. Ayabe, K. Ogasawara, S. Yoshida, N. Hayashi and K. Ono, Chem. Pharm. Bull., 2002, 50, 405–407 CrossRef CAS.
- Y. Kodera, M. Ichikawa, J. Yoshida, N. Kashimoto, N. Ueda, I. Sumioka, N. Ida and K. Ono, Chem. Pharm. Bull., 2002, 50, 354–363 CrossRef CAS.
- Y. Kodera, M. Ayabe, K. Ogasawara and K. Ono, Chem. Pharm. Bull., 2001, 49, 1636–1637 CrossRef CAS.
- T. Moriguchi, H. Matsuura, Y. Itakura, H. Katsuki, H. Saito and N. Nishiyama, Life Sci., 1997, 61, 1413–1420 CrossRef CAS.
- G. B. Mahady, H. Matsuura and S. L. Pendland, Am. J. Gastroenterol., 2001, 96, 3454–3455 CrossRef CAS.
- Y. Adachi, J. Yoshida, Y. Kodera, A. Katoh, Y. Yoshikawa, Y. Kojima and H. Sakurai, J. Biol. Inorg. Chem., 2004, 9, 885–893 CrossRef CAS.
- Y. Adachi, J. Yoshida, Y. Kodera and H. Sakurai, Chem. Lett., 2005, 34, 656–657 CrossRef CAS.
- B. L. Ellis, A. K. Duhme, R. C. Hider, M. B. Hossain, S. Rizvi and D. van der Helm, J. Med. Chem., 1996, 39, 3659–3670 CrossRef CAS.
- A. Katoh, H. Yokoyama, Y. Matsumura, Y. Yoshikawa, H. Yasui and H. Sakurai, Heterocycles, 2010, 81, 585–600 CrossRef CAS.
- A. Katoh, Y. Matsumura, Y. Yoshikawa, H. Yasui and H. Sakurai, J. Inorg. Biochem., 2009, 103, 567–574 CrossRef CAS.
- Y. Ma, W. Luo, P. J. Quinn, Z. Liu and R. C. Hider, J. Med. Chem., 2004, 47, 6349–6362 CrossRef CAS.
- B. L. Tran and S. M. Cohen, Chem. Commun., 2006, 203–205 RSC.
- P. Hradil, J. Hlavac and K. Lemr, J. Heterocycl. Chem., 1999, 36, 141–144 CrossRef CAS.
- D. Y. Yushchenko, M. D. Bilokin, O. V. Pyvovarenko, G. Duportail, Y. Mely and V. G. Pivovarenko, Tetrahedron Lett., 2006, 47, 905–908 CrossRef CAS.
- M. Hiromura, Y. Adachi, M. Machida, M. Hattori and H. Sakurai, Metallomics, 2009, 1, 92–100 RSC.
- S. Chaves, R. Jelic, C. Mendonca, M. Carrasco, Y. Yoshikawa, H. Sakurai and M. A. Santos, Metallomics, 2010, 2, 220–227 RSC.
- Y. Monga, K. H. Thompson, V. G. Yuen, V. Sharma, B. O. Patrick, J. H. McNeill and C. Orvig, Inorg. Chem., 2005, 44, 2678–2688 CrossRef CAS.
- T. Jakusch, K. Gajda-Schrantz, Y. Adachi, H. Sakurai, T. Kiss and L. Horváth, J. Inorg. Biochem., 2006, 100, 1521–1526 CrossRef CAS.
- P. Buglyó, T. Kiss, E. Kiss, D. Sanna, E. Garribba and G. Micera, Dalton. Trans., 2002, 2275–2282 RSC.
- T. Kiss, E. Kiss, G. Micera and D. Sanna, Inorg. Chim. Acta, 1998, 283, 202–210 CrossRef CAS.
- É. Sija, T. Jakusch, T. Kiss and H. Sakurai, in preparation.
- M. Nakai, H. Watanabe, C. Fujiwara, H. Kakegawa, T. Satoh, J. Takada, R. Matsushita and H. Sakurai, Biol. Pharm. Bull., 1995, 18, 719–725 CAS.
- Y. Adachi and H. Sakurai, Chem. Pharm. Bull., 2004, 52, 428–433 CrossRef CAS.
- H. Sakurai, Y. Kojima, Y. Yoshikawa, K. Kawabe and H. Yasui, Coord. Chem. Rev., 2002, 226, 187–198 CrossRef CAS.
- H. Sakurai, Chem. Rec., 2002, 2, 237–248 CrossRef CAS.
- I. Goldwaser, S. Qian, E. Gershonov, M. Fridkin and Y. Schechter, Mol. Pharmacol., 2000, 56, 736–746.
- K. Kawabe, Y. Yoshikawa, H. Yasui and H. Sakurai, Life Sci., 2006, 78, 2860–2866 CrossRef CAS.
- Y. Adachi, Y. Yoshikawa, J. Yoshida, Y. Kodera, A. Katoh, J. Takada and H. Sakurai, Biochem. Biophys. Res. Commun., 2006, 345, 945–950 CrossRef CAS.
- H. Sakurai, A. Tamura, J. Fugono, H. Yasui and T. Kiss, Coord. Chem. Rev., 2003, 245, 31–37 CrossRef CAS.
- A. Katoh, T. Tsukahara, R. Saito, K. K. Ghosh, Y. Yoshikawa, Y. Kojima, A. Tamura and H. Sakurai, Chem. Lett., 2002, 114–115 CrossRef CAS.
- Y. Adachi, J. Yoshida, Y. Kodera, T. Kiss, T. Jakusch, E. A. Enyedy, Y. Yoshikawa and H. Sakurai, Biochem. Biophys. Res. Commun., 2006, 351, 165–170 CrossRef CAS.
- Y. Matsuzawa, T. Funahashi and T. Nakamura, Ann. N. Y. Acad. Sci., 1999, 892, 146–154 CrossRef CAS.
- R. B. Ceddia, H. A. Keistinen, J. R. Zierath and G. Sweeney, FASEB J., 2002, 16, 1163–1176 CrossRef CAS.
- E. W. Shek and M. W. Brands, Hypertensions, 1998, 31, 409–414 Search PubMed.
- M. Aizawa-Abe, Y. Ogawa, H. Masutaki, K. Ebihara, N. Satoh, H. Iwai, N. Matsuoka, T. Hayashi, K. Hosoda, G. Inoue, Y. Yoshimasa and K. Nakao, J. Clin. Invest., 2000, 105, 1243–1252 CrossRef CAS.
- D. E. Moller, Nature, 2001, 414, 821–827 CrossRef.
- Y. Yoshikawa, E. Ueda, Y. Kojima and H. Sakurai, Life Sci., 2004, 75, 741–751 CrossRef CAS.
- M. Hiromura, A. Nakayama, Y. Adachi, M. Doi and H. Sakurai, J. Biol. Inorg. Chem., 2007, 12, 1275–1287 CrossRef CAS.
- A. Nakayama, M. Hiromura, Y. Adachi and H. Sakurai, J. Biol. Inorg. Chem., 2008, 13, 675–684 CrossRef CAS.
- M. Ghayour-Mobarhan, A. Taylor, S. A. New, D. J. Lamb and G. A. Ferns, Ann. Clin. Biochem., 2005, 42, 364–375 CrossRef CAS.
- N. Cohen and A. Golik, Heart Failure Rev., 2006, 11, 19–24 Search PubMed.
- W. Wu, X. Wang, W. Zhang, W. Reed, J. M. Samet, Y. E. Whang and A. J. Ghio, J. Biol. Chem., 2003, 278, 28258–28263 CrossRef CAS.
- D. F. Lazar and A. R. Saltiel, Nat. Rev. Drug Discovery, 2006, 5, 333–342 CrossRef CAS.
- K. N. Frayn, Diabetologia, 2002, 45, 1201–1210 CrossRef CAS.
- G. Boden and G. I. Shulman, Eur. J. Clin. Invest., 2002, 32, 14–23 CrossRef CAS.
- G. Y. Carmen and S. M. Victor, Cell. Signalling, 2006, 18, 401–408 CrossRef CAS.
- T. Kitamura, Y. Kitamura, S. Kuroda, Y. Hino, M. Ando, K. Kotani, H. Konishi, H. Matsuzaki, U. Kikkawa, W. Ogawa and M. Kasuga, Mol. Cell. Biol., 1999, 19, 6286–6296 CAS.
- E. Degerman, C. J. Smith, H. Tornqvist, V. Vasta, P. Belfrage and V. C. Manganiello, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 533–537 CrossRef CAS.
- H. Eriksson, M. Ridderstrale, E. Degerman, D. Ekholm, C. J. Smith, V. C. Manganiello, P. Belfrage and H. Tornqvist, Biochim. Biophys. Acta, Mol. Cell Res., 1995, 1266, 101–107 CrossRef.
- A. R. Cameron, S. Anil, E. Sutherland, J. Harthill and G. Rena, Metallomics, 2010, 2, 195–203 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
