Using a dual-stable isotope tracer method to study the uptake, xylem transport and distribution of Fe and its chelating agent from stereoisomers of an Fe(III)-chelate used as fertilizer in Fe-deficient Strategy I plants
Irene
Orera
a,
José A.
Rodríguez-Castrillón
b,
Mariella
Moldovan
b,
José I.
García-Alonso
b,
Anunciación
Abadía
a,
Javier
Abadía
a and
Ana
Álvarez-Fernández
*a
aDepartment of Plant Nutrition, Aula Dei Experimental Station (CSIC), P.O. Box 13034, 50080 Zaragoza, Spain. E-mail: ana.alvarez@eead.csic.es; Fax: +34 976716145; Tel: +34 976716052
bDepartment of Physical and Analytical Chemistry, University of Oviedo, 33006 Oviedo, Spain
First published on 17th August 2010
Abstract
A dual-stable isotope tracer experiment was carried out with Fe-deficient sugar beet plants grown hydroponically and resupplied with differentially Fe labeled racemic and meso Fe(III)-chelates of the ethylendiamine di(o-hydroxyphenylacetic) acid (o,oEDDHA). No short-term Fe isotope exchange reactions occurred in the nutrient solution and plants did not discriminate between 54Fe and 57Fe. After 3–6 h, stable Fe isotopes, chelating agents and chelates were analyzed in roots, xylem sap and leaves by ICP-MS and HPLC-ESI/TOFMS. Ferric chelate reductase rates, xylem transport and total uptake were 2-fold higher with the meso isomer than with the racemic one. Both chelating agent isomers were incorporated and distributed by plants at similar rates, in amounts one order of magnitude lower than those of Fe. After 6 h of Fe resupply, most of the Fe acquired was localized in roots, whereas most of the chelating agent was in leaves. In a separate experiment, Fe-deficient sugar beet and tomato plants were treated with different concentrations of Fe(III)-o,oEDDHA (with a meso/racemic ratio of 1). The xylem sap Fe concentration at 24 h was unaffected by the chelate concentration, with xylem Fe(III)-o,oEDDHA accounting for 1–18% of total Fe and xylem meso/racemic ratio close to 1. Although most of the Fe coming from Fe(III)-o,oEDDHA was taken up through a reductive dissociative mechanism, a small part of the Fe may be taken up via non-dissociative mechanisms.
Introduction
Ethylendiamine di(o-hydroxyphenylacetic) acid (o,oEDDHA) is a xenobiotic water-soluble compound, known for its high Fe(III)-binding ability.1 In biomedical studies, Fe(III)-o,oEDDHA has been used in magnetic resonance imaging2 and positron emission tomography.3 In agricultural practice, the deficiency of Fe in crops (also called Fe-chlorosis) is commonly remedied by soil applications of Fe(III)-o,oEDDHA.4 Iron chlorosis is characterized by a marked decrease in leaf chlorophyll, that in turn results in losses in crop quality and yield;5 the most common cause of Fe deficiency is low soil Fe bioavailability, due to the occurrence of this metal in oxy-hydroxide insoluble forms. The efficiency of Fe(III)-o,oEDDHA as an Fe fertilizer is due to the remarkable stability over a wide range of pH values1 and the low reactivity in soils.6 In spite of the high cost of Fe(III)-o,oEDDHA, fertilization with this compound is one of the most widespread practices to control Fe-chlorosis in high-value crops and soil-less horticulture.4Iron uptake from this and other Fe(III)-chelates is carried out in the so-called Strategy I plant species by a root ferric chelate reductase (FCR) enzyme.7,8 Radioactivity assays using 59Fe(III)–14C-EDDHA in the nutrient solution confirmed that Fe-deficient Strategy I plants have a splitting Fe uptake mechanism, with final root 59Fe/14C ratios being very different in Fe-deficient and Fe-sufficient plants (approximately 25 and 6, respectively).9 In the case of Fe-deficient plants most of the EDDHA remains in the soil solution, possibly facilitating further solubilization and transport of native Fe to the rhizosphere, in what has been called ‘shuttle effect’.4,10
In spite of the existence of the splitting uptake mechanism, both synthetic Fe(III) chelating agents and Fe(III)-chelates have been found in plants after fertilization. Radioactivity assays with 14C labeled Fe(III)-EDDHA found significant amounts of 14C in Strategy I and II plants,9,11,12 indicating that either the whole chelating agent or one or more breakdown product(s) could enter the plant. Iron(III)-EDDHA was also found, using UV-Vis detection, in xylem exudates of zinnia, sunflower and soybean,13 as well as in tobacco,14 pepper, lettuce and tomato tissues.15 Recently, significant amounts of both Fe(III)-o,oEDDHA and o,oEDDHA have been found in sugar beet and tomato tissues using a very selective and sensitive technique, HPLC-ESI/TOFMS.16
Commercially available Fe(III)-o,oEDDHA fertilizers contain a mixture (approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of two groups of stereoisomers, the meso form [(R,S–Fe(III)-o,oEDDHA)] and the d,l racemic mixture [(R,R-Fe(III)-o,oEDDHA) + (S,S–Fe(III)-o,oEDDHA)]. Although most studies on Fe(III)-o,oEDDHA have not considered the existence of this heterogeneity, significant differences in the physico-chemical properties of the stereoisomers have been observed, including different stability constants of the complexes with Fe (log K values of 35.86 and 34.15 for the racemic and meso forms, respectively)1 and different chromatographic mobilities.17 Moreover, some animal and plant studies have reported differences in the in vivo behavior of the stereoisomers. Meso Fe(III)-o,oEDDHA was found to bind non-specifically to the bilirrubin binding site of human serum albumin, whereas the racemic isomer used a single high-affinity site.18 An in vivo study with rats reported a more rapid blood clearance and a higher Fe liver uptake in the case of the meso stereoisomer as compared to the racemic one.19 In Strategy I plant species grown in a nutrient solution containing both isomers (in a 1
1) of two groups of stereoisomers, the meso form [(R,S–Fe(III)-o,oEDDHA)] and the d,l racemic mixture [(R,R-Fe(III)-o,oEDDHA) + (S,S–Fe(III)-o,oEDDHA)]. Although most studies on Fe(III)-o,oEDDHA have not considered the existence of this heterogeneity, significant differences in the physico-chemical properties of the stereoisomers have been observed, including different stability constants of the complexes with Fe (log K values of 35.86 and 34.15 for the racemic and meso forms, respectively)1 and different chromatographic mobilities.17 Moreover, some animal and plant studies have reported differences in the in vivo behavior of the stereoisomers. Meso Fe(III)-o,oEDDHA was found to bind non-specifically to the bilirrubin binding site of human serum albumin, whereas the racemic isomer used a single high-affinity site.18 An in vivo study with rats reported a more rapid blood clearance and a higher Fe liver uptake in the case of the meso stereoisomer as compared to the racemic one.19 In Strategy I plant species grown in a nutrient solution containing both isomers (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), it was found that meso Fe(III)-o,oEDDHA was preferentially depleted from the solution.20,21 However, this effect did not occur with Strategy II species lacking FCR activity.20 Although bean plants treated separately with racemic and meso59Fe(III)-EDDHA did not show significant differences in Fe plant uptake,22 higher in vivo root FCR reduction rates were found for the meso than for the racemic isomer in cucumber plants.23
1 ratio), it was found that meso Fe(III)-o,oEDDHA was preferentially depleted from the solution.20,21 However, this effect did not occur with Strategy II species lacking FCR activity.20 Although bean plants treated separately with racemic and meso59Fe(III)-EDDHA did not show significant differences in Fe plant uptake,22 higher in vivo root FCR reduction rates were found for the meso than for the racemic isomer in cucumber plants.23
A new approach to study plant Fe uptake and translocation is the use of multiple-stable isotope tracer methodologies, which also provide the possibility to carry out long-term experiments (Fe has four stable isotopes: 54Fe, 56Fe, 57Fe and 58Fe; see review by Álvarez-Fernández).24 The main drawbacks of using stable isotopes are the high cost and the special instrumentation (e.g. inductively coupled plasma mass spectrometry (ICP-MS) or electrospray time-of-flight mass spectrometry (ESI/TOFMS)) needed to determine them in complex matrices. ICP-MS has been used successfully for Fe stable isotope studies in plants because of the high selectivity and sensitivity; Fe uptake from 57Fe(III)-o,oEDDHA and 57Fe(III)-o,pEDDHA has been recently studied in cucumber,25 tomato and peach26 plants. A different technique, HPLC-ESI/TOFMS, has been recently used for the determination of stable Fe isotope-labeled synthetic Fe(III)-chelates in agricultural matrices27 and plant tissues.16 Iron-57 Mössbauer spectroscopy has also been used to study the chemistry of Fe in plants,28 although this technique has sensitivity limitations.
No data are yet available, to the best of our knowledge, on the possible direct competition during plant uptake between the two Fe(III)-o,oEDDHA stereoisomers. The aim of this work was to study the uptake, movement and distribution of Fe and ligands from racemic and meso Fe(III)-o,oEDDHA chelates, when applied simultaneously to the roots of Fe-deficient Strategy I plants. Sugar beet plants grown in nutrient solution were treated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of racemic and meso-o,oEDDHA isomers, each one labeled with a different Fe stable isotope (54Fe or 57Fe). Then, the contents of the Fe stable isotopes in different plant parts were determined by ICP-MS, whereas those of the ligands (racemic and meso-o,oEDDHA) were determined by HPLC-ESI/TOFMS.
1 mixture of racemic and meso-o,oEDDHA isomers, each one labeled with a different Fe stable isotope (54Fe or 57Fe). Then, the contents of the Fe stable isotopes in different plant parts were determined by ICP-MS, whereas those of the ligands (racemic and meso-o,oEDDHA) were determined by HPLC-ESI/TOFMS.
Experimental
Chemicals and reagents
All solutions were prepared with analytical grade type I water (Milli-Q Synthesis, Millipore, Bedford, MA) with the exception of nutrient solutions, which were prepared with analytical grade type II water (Milli-RX20, Millipore). Reagent-grade glacial acetic acid, hydrochloric acid (35%) and ammonium hydroxide (25%) were purchased from Panreac Química S. A. (Barcelona, Spain). Ammonium acetate (99.99%, Sigma), Li hydroxide monohydrate (99.99%, Sigma), nitric acid (65% TraceSELECT Ultra, Fluka), formic acid (50%, Sigma), methanol and 2-propanol (both LC-MS grade, Riedel-de-Haën) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nitric acid 68% and H2O2 30% (Suprapur) were obtained from Merck (Darmstadt, Germany).Pure chelating agents used were ethylenediamine tetraacetic acid (EDTA; 99%, Merck), o,oEDDHA (98%, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of racemic and meso; LCG Promochem, Barcelona, Spain) and ethylenediamine-N-N′bis(2-hydroxy-4-methylphenylacetic) acid (o,oEDDHMA; 98%, LCG Promochem). A commercial Fe(III)-EDDHA (6% soluble Fe) was also used in some experiments (Syngenta Agro, Madrid, Spain). Natural abundance Fe (thereafter called Fenat, with isotopic abundances of 5.8% 54Fe, 91.7% 56Fe, 2.1% 57Fe and 0.2% 58Fe) was supplied as Fe standard Titrisol (1000 mg Fe in 15% HCl, Merck). Labeled 57Fe oxide (Fe2O3, 98% Fe, 95.06% 57Fe) and labeled 54Fe oxide (Fe2O3, 99.8% Fe, 99.8% 54Fe) were obtained from Cambridge Isotope Laboratories (Andover, MA). A certified Fe standard solution for ICP-MS analyses (Iron ICP standard, 1000 mg L−1) was purchased from Merck.
1 mixture of racemic and meso; LCG Promochem, Barcelona, Spain) and ethylenediamine-N-N′bis(2-hydroxy-4-methylphenylacetic) acid (o,oEDDHMA; 98%, LCG Promochem). A commercial Fe(III)-EDDHA (6% soluble Fe) was also used in some experiments (Syngenta Agro, Madrid, Spain). Natural abundance Fe (thereafter called Fenat, with isotopic abundances of 5.8% 54Fe, 91.7% 56Fe, 2.1% 57Fe and 0.2% 58Fe) was supplied as Fe standard Titrisol (1000 mg Fe in 15% HCl, Merck). Labeled 57Fe oxide (Fe2O3, 98% Fe, 95.06% 57Fe) and labeled 54Fe oxide (Fe2O3, 99.8% Fe, 99.8% 54Fe) were obtained from Cambridge Isotope Laboratories (Andover, MA). A certified Fe standard solution for ICP-MS analyses (Iron ICP standard, 1000 mg L−1) was purchased from Merck.
Preparation of Fe(III)-o,oEDDHA chelates
Racemic- and meso-o,oEDDHA were separated from 98% o,oEDDHA by selective precipitation of racemic Fe(III)-o,oEDDHA with Mg(II), using the method of Yunta et al.1 The racemic isomer has two possible configurations, the RR form (see structure in Fig. 1) and the SS form, the latter being less likely. In fact, the first X-ray structural analysis of the Mg-[racemic-Fe(III)-o,oEDDHA]2 did not show any evidence of the SS form, but this does not preclude its existence in solution.1 It has been estimated that 0.5% of the SS form could be present as the racemic mixture of the Fe(III)-o,oEDDHA.1 In contrast to the racemic, the meso Fe(III)-o,oEDDHA has two possible configurations, the RS and the SR forms, which are indistinguishable because of the symmetric structure of o,oEDDHA (see structure in Fig. 1). The SS and RR forms are enantiomers; the RS and the SR forms are diastereomers of the SS and RR forms. The purity of racemic- (99.7%) and meso-o,oEDDHA (95.0%) was determined by HPLC-ESI/TOFMS (see below; Orera et al.).16 Stock solutions (1 mM) of racemic Fenat(III)-o,oEDDHA, racemic54Fe(III)-o,oEDDHA, racemic57Fe(III)-o,oEDDHA, meso Fenat(III)-o,oEDDHA, meso54Fe(III)-o,oEDDHA, meso57Fe(III)-o,oEDDHA, Fenat(III)-o,oEDDHA, Fenat(III)-EDTA and Fenat(III)-o,oEDDHMA were prepared by slowly adding acidic Fe solutions (9.2 mM 54Fe, 8.2 mM 57Fe or 35.8 mM Fenat in 15-35% HCl, in 5% excess over the molar amount of each chelating agent) over high-pH pure chelating agent solutions.17 During the addition of Fe, pH was maintained in the range 6–8 by adding NH4OH. Solutions were set to pH 7.0 with NH4OH and HCl and made up to volume with Milli-Q water, equilibrated overnight in the dark at room temperature and filtered through a 0.45 μm PVDF membrane. Standard solutions of Fe(III)-chelates with concentrations lower than 100 mM were prepared daily from stocks. Solutions of a Fe(III)-EDDHA commercial fertilizer (6% of soluble Fe) were used where indicated. | ||
| Fig. 1 Protocol for the dual-stable Fe isotope tracer experiment. Iron deficient sugar beet plants were treated with differentially labeled (57Fe and 54Fe) racemic and meso Fe(III)-o,oEDDHA. | ||
Plant material
Sugar beet (Beta vulgaris L. cv. ‘Orbis’) and tomato (Lycopersicon esculentum L. cv. ‘Tres Cantos’) plants were grown in a growth chamber with a photosynthetic photon flux density (PPFD) of 350 μmol m−2 s−1 photosynthetically active radiation at leaf level, a photoperiod of 16 h light/8 h dark, a temperature of 23/18 °C day/night, and 80% relative humidity. Seeds were germinated and grown in vermiculite for two weeks. Seedlings were grown for two more weeks in half-strength Hoagland solution with 45 μM Fenat(III)-EDTA, pH 5.5, and then transplanted to plastic buckets (four plants per bucket) containing half-strength Hoagland solution with 0 μM Fe. pH was buffered at approximately 7.0 by adding 1 mM NaOH and 1 g l−1 CaCO3 for sugar beet plants and 0.5 mM KOH for tomato plants. The total amount of solution was held constant by refilling with distilled water. Sugar beet and tomato plants showing Fe chlorosis were used after growth in 0 μM Fe for approximately 14 and 10 d, respectively.Some Fe-deficient sugar beet and tomato plants were transferred to two L plastic pots (four plants per pot) containing nutrient solution supplemented with different concentrations of the Fe(III)-EDDHA commercial fertilizer solution (0, 35, 73, 107, 262, 427, 693 and 1470 μM Fe(III)-o,oEDDHA for sugar beet and 0, 31, 54, 82, 177, 383, 798 and 2038 μM Fe(III)-o,oEDDHA for tomato). Plants were treated with Fe for 24 h. Xylem sap was collected from sugar beet and tomato plants by centrifugation of petioles29 and plant de-topping,30 respectively, filtered through 0.45 μm PVDF membranes and stored at −20 °C until analysis. All fresh xylem sap samples were assessed for contamination using c-mdh (EC 1.1.1.37) as a cytosolic marker,29 and no contamination was found.
In a different experiment, Fe-deficient sugar beet plants (70 ± 19 g FW per plant) were transferred to two L plastic pots (four plants per pot) containing nutrient solution supplemented with two different Fe treatments: (A) 30 μM racemic54Fe-o,oEDDHA plus 30 μM meso57Fe-o,oEDDHA or (B) 30 μM racemic57Fe-o,oEDDHA plus 30 μM meso54Fe-o,oEDDHA (see Fig. 1 for a description of the protocol). Three and six h after Fe resupply, xylem sap, young and old leaves and main and fine roots were collected from Fe-resupplied and untreated Fe-deficient plants grown in the same conditions. Leaves and roots were washed with distilled water. Approximately one g of fresh material was frozen in liquid N2 and stored at −20 °C. The rest of leaves and roots were dried in an oven at 60 °C, ground in a ZrO2 ball mill (MM301, Retsch, Haan, Germany) and stored at room temperature until analysis.
Root Fe(III)-chelate reductase activity
The FCR activity of roots of intact, illuminated Fe-deficient sugar beet plants was followed by measuring spectrophotometrically the formation of the Fe(II) complex with 4,7-diphenyl-1,10-phenanthrolinedisulfonic acid (BPDS) at 535 nm.23,31 Individual plants were placed into 0.5 L beakers containing 1 mM MES (pH 6.0), 100 μM BPDS and 60 μM racemic or meso Fenat(III)-o,oEDDHA in MilliQ water. Beakers were fully covered with aluminium foil to exclude light, the solution was aerated continuously and aliquots (3 mL) were removed at 0.5, 1, 2 and 5 h. Since both Fe(III)-chelates used as substrates had a significant absorption at 535 nm, measurements were also made at 480 nm to calculate both the Fe(III)BPDS3 and Fe(III)-chelate concentrations in the FCR assay solutions.23 Beakers without plants were used to correct for non-enzymatic Fe(III) reduction.Extraction of o,oEDDHA from plant materials
Sugar beet tissue extraction was performed as described in Orera et al.16 Frozen leaves (1 g FW) or roots (2 g FW) were ground with 2 mL ammonium acetate (1 mM pH 6.0) containing the internal standard Fenat(III)-o,oEDDHMA in a ZrO2 ball mill at 30 rps for 1–2 min. The suspension was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 20 min at 4 °C and the supernatant was collected. The pellet was resuspended with 1 mL of ammonium acetate solution and centrifuged again. This step was repeated once more and supernatants were combined and made up to 5 g final weight with ammonium acetate solution. An aliquot of the extract (2 g) was treated with NH4OH until basic pH was attained, an excess of Fe(III) was added and pH was adjusted to 6–7 with HCl. Solutions were filtered and made up to 5 g final weight.
000 g for 20 min at 4 °C and the supernatant was collected. The pellet was resuspended with 1 mL of ammonium acetate solution and centrifuged again. This step was repeated once more and supernatants were combined and made up to 5 g final weight with ammonium acetate solution. An aliquot of the extract (2 g) was treated with NH4OH until basic pH was attained, an excess of Fe(III) was added and pH was adjusted to 6–7 with HCl. Solutions were filtered and made up to 5 g final weight.
Plant tissue digestion
Xylem sap was digested with 1% HNO3 (TraceSELECT Ultra). Tissues were digested in a closed vessel microwave oven (Milestone Model MLS 1200, Sorisole, Italy). Approximately 0.1 g or 0.05 g of sugar beet leaves or roots, respectively, were digested with 6 mL HNO3 (26%) and 2 mL H2O2 (30%). The microwave digestion program was 3 min at 95 °C, 10 min at 160 °C, 3 min at 185 °C and 15 min at 185 °C. At the end of the process, digests were diluted to 50 mL with Milli-Q water and stored at 4 °C until analysis.Iron determination
Total Fe was determined in sugar beet and tomato xylem sap by graphite furnace atomic absorption spectrometry (Varian 3000, Palo Alto, CA, USA). The isotope composition of Fe (54Fe, 56Fe, 57Fe and 58Fe) in sugar beet leaves, roots and xylem sap was determined by quadrupole ICP-MS. The device (model Agilent 7500ce; Agilent Technologies, Tokyo, Japan) was equipped with an octapole collision cell to remove polyatomic interferences and was operated with a RF power of 1500 W and cooling and carrier gas flows of 15 and 1.1 L min−1, respectively. The collision cell was operated with an He gas flow of 4.0 mL min−1 and a cell exit, octapole and quadrupole bias voltages of −72.0, −18.0 and −16.0 V, respectively. Torch position and ion lens voltage settings were optimized daily for optimum sensitivity with a solution of 1 ng g−1 Li, Co, Y, Tl and Ce mixture in 1% (w/w) HNO3. A solution of 1% (w/w) HNO3 was also used to check the background level caused by polyatomic Ar interferences. The possible contribution of spectral interferences of 40Ca16O, 40Ca16O1H, 54Cr and 58Ni was corrected mathematically by measuring ion signals at masses 43 for Ca, 52 for Cr and 60 for Ni. Mass bias correction was carried out internally by minimizing the sum of squared residuals.32 The ICP-MS instrument was controlled with ICP-MS ChemStation software version B.03.04 (Agilent Technologies).The isotope composition of the stable isotope-enriched Fe spikes was determined by direct ICP-MS nebulization. Measured isotope abundances (in % 54Fe, 56Fe, 57Fe and 58Fe) were 0.14, 4.74, 94.63 and 0.49 for the 57Fe-enriched solution and 96.32, 3.26, 0.41 and 0.01 for the 54Fe-enriched solution, respectively. Iron enriched solutions were mixed with the Fenat certified standard and Fe concentrations were calculated by reverse isotope dilution analysis.
The determination of the molar fractions of 54Fe, 57Fe and Fenat present in the different plant materials was carried out by direct ICP-MS nebulization of the plant digests after 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution and employing a modification of the mathematical isotope pattern deconvolution procedure described elsewhere.25 For the purpose of this study three different isotope patterns were fitted to the linear model as shown in eqn (1):
10 dilution and employing a modification of the mathematical isotope pattern deconvolution procedure described elsewhere.25 For the purpose of this study three different isotope patterns were fitted to the linear model as shown in eqn (1):
 | (1) |
Racemic and meso Fe(III)-o,oEDDHA determination in nutrient solution and xylem sap
Racemic and meso Fe(III)-o,oEDDHA were determined directly by HPLC-ESI/TOFMS in nutrient solutions and in sugar beet and tomato xylem sap.16,27Racemic- and meso-o,oEDDHA determination in plant tissues
In sugar beet tissue (leaf and root) extracts, racemic- and meso-o,oEDDHA were determined by HPLC-ESI/TOFMS as racemic and meso Fe(III)-o,oEDDHA, after addition of an excess of Fe(III) to ensure the chelation of all o,oEDDHA chemical forms with Fe(III).16 This is a necessary step, because the chelating agent o,oEDDHA could occur in tissue homogenates in chemical forms other than Fe(III)-o,oEDDHA.16Statistical analysis
ANOVA tests were carried out using the SPSS software v. 15.0 (SPSS Inc.). Means were compared using the Duncan′s LSD test.Results
Effect of Fe(III)-o,oEDDHA nutrient solution concentration on the xylem sap concentrations of total Fe and racemic and meso Fe(III)-o,oEDDHA
Sugar beet and tomato Fe-deficient plants were treated with different concentrations of commercial Fe(III)-o,oEDDHA, which contains a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of racemic and meso isomers. Xylem sap concentrations of total Fe and racemic and meso Fe(III)-o,oEDDHA were measured 24 h after treatment (Fig. 2). Iron(III)-o,oEDDHA treatments increased the xylem sap total Fe concentrations in sugar beet and tomato (Fig. 2a and b, respectively). However, all different Fe(III)-o,oEDDHA treatments led to similar (not significantly different at p < 0.05) Fe concentrations in the xylem sap of each species, with values being 4- to 5-fold larger in tomato than in sugar beet.
1 mixture of racemic and meso isomers. Xylem sap concentrations of total Fe and racemic and meso Fe(III)-o,oEDDHA were measured 24 h after treatment (Fig. 2). Iron(III)-o,oEDDHA treatments increased the xylem sap total Fe concentrations in sugar beet and tomato (Fig. 2a and b, respectively). However, all different Fe(III)-o,oEDDHA treatments led to similar (not significantly different at p < 0.05) Fe concentrations in the xylem sap of each species, with values being 4- to 5-fold larger in tomato than in sugar beet.
 | ||
| Fig. 2 Xylem sap concentrations of total Fe and racemic and meso Fe(III)-o,oEDDHA measured 24 h after treatment. | ||
Significant amounts of the Fe(III)-o,oEDDHA chelate were found in sugar beet and tomato xylem sap in all the Fe(III)-o,oEDDHA treatments (Fig. 2c and d). In the 30–450 μM Fe(III)-o,oEDDHA concentration treatment range, the xylem sap Fe(III)-o,oEDDHA concentration was approximately 0.3 and 1.5 μM in sugar beet and tomato, respectively. However, treatments with higher Fe(III)-o,oEDDHA concentrations (700–800 μM) led to 6- to 7-fold increases in Fe concentrations in both species. The highest Fe(III)-o,oEDDHA concentrations used did not change significantly the xylem sap Fe(III)-o,oEDDHA concentration in sugar beet (Fig. 2c), whereas in tomato plants a further increase was found (Fig. 2d).
The meso/racemic ratio in xylem sap was approximately 1.0 in all Fe(III)-o,oEDDHA treatments and in both plant species. Visual symptoms (red leaf spots) of Fe(III)-o,oEDDHA toxicity were observed only in tomato plants treated with the highest chelate concentration. Iron(III)-o,oEDDHA was 1–8% and 1–18% of the total Fe in the xylem sap of sugar beet and tomato plants, respectively, depending on the Fe(III)-o,oEDDHA concentrations applied; the highest values were found in plants treated with the highest Fe(III)-o,oEDDHA concentrations.
Plant uptake, long-distance transport and distribution of Fe and racemic- and meso-o,oEDDHA
We applied simultaneously two Fe(III)-o,oEDDHA stereoisomers, each one labeled with a different stable Fe isotope, to Fe-deficient sugar beet plants for 3 and 6 h. Treatments were: (A) 30 μM racemic54Fe(III)-o,oEDDHA plus 30 μM meso57Fe(III)-o,oEDDHA, and (B) 30 μM racemic57Fe(III)-o,oEDDHA plus 30 μM meso54Fe(III)-o,oEDDHA (see Fig. 1). First, to assess the possible existence of isotope exchange reactions, the concentrations of Fe(III)-o,oEDDHA isomers in the nutrient solutions were determined by HPLC-ESI/TOFMS at 0 and 6 h after preparation. Racemic and meso Fe(III)-o,oEDDHA were well separated (Fig. 3). Also, the high mass resolution of the detector allowed for the accurate determination of the racemic and meso Fe(III)-o,oEDDHA chelates with 54Fe (Fig. 3a and b), 56Fe (present in a very small amount, Fig. 3c and d) and 57Fe (Fig. 3e and f); chromatograms for the o,oEDDHA chelates with 54Fe, 56Fe and 57Fe were extracted at m/z ratios 410.0, 412.0 and 413.0, respectively, with a precision of ±0.02 m/z. In both treatments, the relative amounts of the different stable Fe isotope-labeled Fe(III)-o,oEDDHA chelates in nutrient solutions were similar 0 and 6 h after preparation: values found were (in %; 54Fe-o,oEDDHA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56Fe-o,oEDDHA
56Fe-o,oEDDHA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57Fe-o,oEDDHA) 92
57Fe-o,oEDDHA) 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for the racemic and 1
3 for the racemic and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 for the meso isomer in treatment A and 3
95 for the meso isomer in treatment A and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 for the racemic and 96
91 for the racemic and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for the meso in treatment B. These results indicate that metal isotope exchange reactions between isomers did not occur in the nutrient solution in the time period considered.
3 for the meso in treatment B. These results indicate that metal isotope exchange reactions between isomers did not occur in the nutrient solution in the time period considered.
 | ||
| Fig. 3 The concentrations of the racemic and meso Fe(III)-o,oEDDHA chelates with 54Fe, 56Fe and 57Fe in the nutrient solutions were determined by HPLC-ESI/TOFMS at 0 and 6 h after preparation. | ||
The amount of Fe taken up from the solution by whole sugar beet plants (Feex-meso + Feex-rac) was only a small fraction of the total Fe (Fenat + Feex-meso + Feex-rac), both at 3 and 6 h after Fe-resupply. The amounts of Fenat in the plant did not change significantly upon Fe resupply (Fig. 4a). The amounts of Feex-meso taken up from the nutrient solution were higher than those of Feex-rac at both sampling times (Fig. 4a). At 6 h, increases in Feex-meso and Feex-rac were found as compared to the values observed at 3 h (1.5- and 1.4-fold, respectively; Fig. 4a). The Feex-meso/Feex-rac ratios in whole sugar beet plants were approximately 2.3 and 2.2 at 3 and 6 h, respectively (Table 1), values significantly different (at p < 0.001) to the Femeso/Ferac ratio of 1.0–1.1 found in nutrient solutions. This indicates that plants took up Fe preferentially from the meso isomer as compared to the racemic one.
 | ||
| Fig. 4 The amount of Fe taken up by whole sugar beet plants (Feex-meso + Feex-rac) was one order of magnitude higher than those of racemic- and meso-o,oEDDHA. | ||
| 3 h | 6 h | |
|---|---|---|
| Material | Feex-meso/Feex-rac | |
| Nutrient solution | 1.1 ± 0.1a | 1.0 ± 0.0a |
| Whole plant | 2.3 ± 0.3bc | 2.2 ± 0.2b |
| Fine roots | 3.0 ± 0.6c | 2.4 ± 0.3b |
| Main root | 2.4 ± 0.4bc | 1.8 ± 0.2b |
| Xylem sap | 1.9 ± 0.2b | 2.1 ± 0.2b |
| Old leaves | 1.6 ± 0.1b | 2.1 ± 0.2b |
| Young leaves | 1.5 ± 0.1b | 1.9 ± 0.1b |
| meso/racemic Fe(III)-o,oEDDHA | ||
| Nutrient solution | 1.1 ± 0.1n.s. | 1.0 ± 0.1n.s. |
| Xylem sap | 0.9 ± 0.1n.s. | 1.3 ± 0.2n.s. |
| meso-/racemic-o,oEDDHA | ||
| Whole plant | 1.2 ± 0.1n.s. | 1.0 ± 0.1n.s. |
| Fine roots | 1.3 ± 0.3n.s. | 1.4 ± 0.2n.s. |
| Old leaves | 1.3 ± 0.1n.s. | 1.0 ± 0.1n.s. |
| Young leaves | 1.0 ± 0.1n.s. | 0.9 ± 0.1n.s. |
The contents of Feex-rac, Feex-meso and Fenat were determined in four different sugar beet tissues: fine and main roots, old leaves and young leaves (Fig. 5). Pre-existing Fe (Fenat) was the main component of total Fe in all plant parts, with contents being unaffected by Fe resupply (Fig. 5). Young leaves, old leaves, main root and fine roots accounted for 12–17, 22–25, 3–4 and 58–59% of the total Fe taken up from the nutrient solution at 3–6 h (Fig. 5); values increased significantly in young leaves (2-fold) and fine roots (1.4-fold) from 3 to 6 h after Fe resupply. The Feex-meso/Feex-rac ratios were in the range 1.5–3.0 in all tissues (Table 1), values significantly higher (at p < 0.01) than the Femeso/Ferac ratio of 1.0–1.1 found in nutrient solutions. The highest Feex-meso/Feex-rac ratios were found in fine roots, with values of 3.0 and 2.4 at 3 and 6 h, respectively (Table 1).
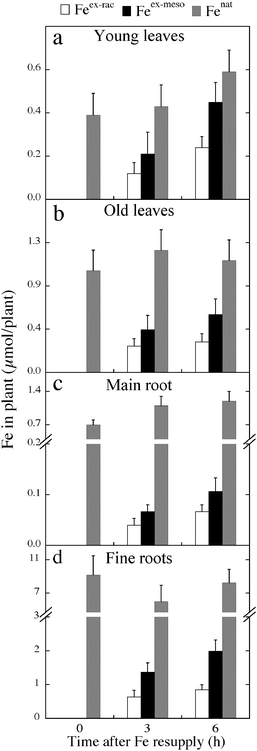 | ||
| Fig. 5 The contents of Feex-rac, Feex-meso and Fenat were determined in four different sugar beet tissues: young leaves, old leaves, main root and fine roots. | ||
When considering the total chelating agents (estimated after adding an excess of Fe(III), see Orera et al.),16 the contents of meso- and racemic-o,oEDDHA in the whole plant were similar, both at 3 and 6 h after Fe resupply (Fig. 4b). At 6 h, the contents of meso- and racemic-o,oEDDHA increased (1.5- and 1.7-fold, respectively) when compared to the values found at 3 h (Fig. 4b). Ratios of meso-o,oEDDHA/racemic-o,oEDDHA in sugar beet plants were approximately 1.2 and 1.0 at 3 and 6 h, respectively (Table 1), indicating that the racemic and meso isomers were incorporated at similar rates. Racemic- and meso-o,oEDDHA were similarly distributed in all tissues (Fig. 6), with meso/racemic ratios in the range 0.9–1.3 (Table 1); main roots were not analyzed because the analyte recoveries were not good enough for this tissue (data not shown). Most of the o,oEDDHA was located in leaves (78–84%), with values of approximately 98 ± 18 and 108 ± 12 nmol plant−1 in young and old leaves, respectively; although there was a tendency to increase with time, no significant differences between treatment times were found.
 | ||
| Fig. 6 Distribution of racemic- and meso-o,oEDDHA in different sugar beet tissues. | ||
The amounts of meso- and racemic-o,oEDDHA taken up by the plant were one order of magnitude lower than those of Fe, with plant Feex-meso/meso-o,oEDDHA, Feex-rac/racemic-o,oEDDHA and (Feex-meso + Feex-rac)/(racemic- + meso-o,oEDDHA) ratios in the ranges 17–18, 8–10 and 13–14, respectively. The total amount of o,oEDDHA (racemic plus meso) found would account, if totally chelated with Fe, for 8–7% of the total Feex-meso + Feex-rac, respectively, at 3–6 h after resupply. The contents of racemic- and meso-o,oEDDHA found in young and old leaves and fine roots were 1–2 orders of magnitude lower than those of Fe. The Feex-meso/meso-o,oEDDHA ratios were in the ranges of 8–6, 8–10 and 45–57 in young leaves, old leaves, and fine roots, respectively, whereas Feex-rac/racemic-o,oEDDHA ratios were in the ranges of 3–4, 5–6 and 23–35 in the same plant tissues. The total amount of o,oEDDHA (racemic plus meso) found would account, if totally chelated with Fe, for 22–25, 14–14 and 2–3% of the total Feex-meso + Feex-rac in young leaves, old leaves and fine roots, respectively, at 3–6 h after resupply.
The xylem concentrations of Fenat did not change significantly upon Fe resupply (Fig. 7a). The concentrations of Feex-rac and Feex-meso transported in xylem sap were in the ranges 0.4-0.9 and 0.7-1.9 μM, respectively, with values 2.1- and 2.7-fold higher at 6 than at 3 h for Feex-rac and Feex-meso, respectively (Fig. 7a). The xylem sap Feex-meso/Feex-rac ratios were 1.9 and 2.1 at 3 and 6 h, respectively (Table 1), values significantly different (at p < 0.01) from the 1.0–1.1 Femeso/Ferac ratio found in nutrient solutions. This indicates that the xylem was relatively enriched in Feex-meso when compared to the nutrient solution.
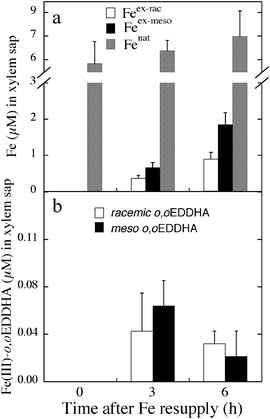 | ||
| Fig. 7 The concentrations of Fe (Feex-rac, Feex-meso and Fenat) and Fe(III)-o,oEDDHA (racemic and meso) transported in sugar beet xylem sap. | ||
When considering the chelates, the concentrations of racemic and meso Fe(III)-o,oEDDHA in xylem sap were measurable but low, with the two isomers being transported in similar amounts (meso/racemic ratios of 0.9 and 1.3 at 3 and 6 h, respectively (Table 1). Total Fe(III)-o,oEDDHA concentrations being transported at 3 and 6 h were 0.10 and 0.06 μM, respectively (values not significantly different at p < 0.05; Fig. 7b). The relative amounts of Fe(III)-chelate transported in the xylem were two orders of magnitude lower than those of Fe, with the concentration of meso+racemic Fe(III)-o,oEDDHA accounting for only 1 and <1% of the total Fe at 3 and 6 h, respectively. When looking only at the labeled Fe coming from the nutrient solution, the concentration of meso+racemic Fe(III)-o,oEDDHA accounted for 10 and 2% of the (Feex-meso + Feex-rac) at 3 and 6 h, respectively.
Iron reduction from racemic and meso Fe(III)-o,oEDDHA substrates
Root FCR activity was measured with intact Fe-deficient sugar beet plants at different times, using as reduction substrates racemic and meso Fenat(III)-o,oEDDHA separately (Fig. 8). In both cases the amount of Fe reduced was linear with time, confirming previous results with sugar beet.33 The root FCR activity was 2.7-fold higher with the meso isomer than with the racemic one. | ||
| Fig. 8 Root FCR activity was measured with intact Fe-deficient sugar beet plants at different times, using as reduction substrates racemic and meso Fenat(III)-o,oEDDHA separately. | ||
Discussion
When Fe-deficient Beta vulgaris plants (a Strategy I species) were treated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of racemic and meso Fe(III)-o,oEDDHA labeled differentially with 54Fe and 57Fe, Fe was preferentially taken up from the meso isomer when compared to the racemic one (ratios Feex-meso/Feex-rac of 2.2). This is the first time that a dual-stable Fe isotope tracer approach has been used in plant Fe nutrition. Plants did not discriminate between 54Fe and 57Fe and no short-term Fe isotope exchange reactions occurred with the Fe(III)-chelates used. The preferential plant uptake found for the Feex-meso is in agreement with the relative depletion of the meso Fe(III)-o,oEDDHA isomer observed previously in nutrient solutions with other Strategy I plant species.20,21 Previous studies comparing the efficiency of the racemic and meso Fe(III)-o,oEDDHA have always applied these isomers individually, not as a mixture; these studies gave contradictory results, since bean plants treated with racemic and meso59Fe-o,oEDDHA separately did not lead to significant differences in Fe uptake,22 whereas in Fe-deficient cucumber plants resupplied with Fe the final Fe shoot concentrations were 1.5-fold higher with meso than with racemic Fe-o,oEDDHA.23
1 mixture of racemic and meso Fe(III)-o,oEDDHA labeled differentially with 54Fe and 57Fe, Fe was preferentially taken up from the meso isomer when compared to the racemic one (ratios Feex-meso/Feex-rac of 2.2). This is the first time that a dual-stable Fe isotope tracer approach has been used in plant Fe nutrition. Plants did not discriminate between 54Fe and 57Fe and no short-term Fe isotope exchange reactions occurred with the Fe(III)-chelates used. The preferential plant uptake found for the Feex-meso is in agreement with the relative depletion of the meso Fe(III)-o,oEDDHA isomer observed previously in nutrient solutions with other Strategy I plant species.20,21 Previous studies comparing the efficiency of the racemic and meso Fe(III)-o,oEDDHA have always applied these isomers individually, not as a mixture; these studies gave contradictory results, since bean plants treated with racemic and meso59Fe-o,oEDDHA separately did not lead to significant differences in Fe uptake,22 whereas in Fe-deficient cucumber plants resupplied with Fe the final Fe shoot concentrations were 1.5-fold higher with meso than with racemic Fe-o,oEDDHA.23
The Fe tracers provided by both Fe(III)-o,oEDDHA diasteroisomers reached all plant tissues. Approximately 60% of the Fe tracers (Feex-meso + Feex-rac) taken up from Fe(III)-o,oEDDHA by Fe-deficient sugar beet were still localized in the fine roots at 3–6 h after Fe-resupply. At that time, leaves had incorporated only 12–17% (young leaves) and 22–25% (old leaves) of the total Fe tracers taken up. These results are in agreement with a previous study, where 80% of the Fe delivered to Fe-deficient cucumber plants by 100 μM of 57Fe(III)-o,oEDDHA was also located in the roots 1 h after the treatment.25 Average plant concentrations of Fe tracers (Feex-meso and Feex-rac) were 0.6 and 1.1 μmol Fe g DW−1 in sugar beet (this study) and cucumber25 plants, respectively. In leaves, the concentrations of Fe tracers were 2- to 4-fold higher in sugar beet (this study) than in tomato and peach treated for 18–28 d with 5 μM 57Fe(III)-o,oEDDHA26 (0.7-1.4 and 0.3 μmol g DW−1, respectively); this could be associated to dilution effects during long term plant growth and/or to the different Fe concentrations applied. The distribution inside the plant of the Fe tracers (Feex-meso and Feex-rac) was similar, with percentages of the Fe allocated in each tissue (fine roots![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) main roots
main roots![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) old leaves
old leaves![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) young leaves, at 3–6 h after resupply) of 62–60
young leaves, at 3–6 h after resupply) of 62–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3–3
3–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21–23
21–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–16 for the meso isomer and 55–56
11–16 for the meso isomer and 55–56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3–4
3–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23–27
23–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13–19 for the racemic one. This indicates that there were no major differences in the plant allocation of the Fe delivered by both Fe-carriers (Feex-meso/Feex-rac ratios in the range of 2.4–1.6), except for fine roots, where higher ratios (3.0–2.4) were found, indicating a preferential accumulation of Feex-meso.
13–19 for the racemic one. This indicates that there were no major differences in the plant allocation of the Fe delivered by both Fe-carriers (Feex-meso/Feex-rac ratios in the range of 2.4–1.6), except for fine roots, where higher ratios (3.0–2.4) were found, indicating a preferential accumulation of Feex-meso.
Iron-deficient sugar beet and tomato plants were found to incorporate the o,oEDDHA chelating agent in all tissues during Fe resupply, using a highly selective and sensitive HPLC-ESI/TOFMS method for the determination of o,oEDDHA.16 This is in agreement with previous findings using HPLC-UV/Vis and 14C-radioactive determinations.9,12–15 The concentrations of o,oEDDHA found in sugar beet tissues (0.1-0.2 μmol g DW−1) are within the range found in tomato (0.1–0.4 μmol g DW−1),15,16 pepper (0.1–0.4 μmol g DW−1)15 and tobacco plants (0.1–0.2 μmol g DW−1).14 These values are equivalent to approximately 30–70 μg o,oEDDHA g DW−1. Approximately 80% of the chelating agent taken up was in the leaves after 6 h, in marked contrast with the 40% value found for Fe. This may suggest that o,oEDDHA is transported in the transpiration stream, in agreement with the hypothesis that plant uptake of the chelating agents (with or without the metal) occurs by absorption through openings in the root endodermis and the Casparian strip.15,34 This type of absorption should be proportional to the time exposure of the plant to the chelate and the chelate concentration applied. In our study, the o,oEDDHA content in Fe-deficient sugar beet plants was only 1.5-fold higher on the average when the time of exposure doubled, with the highest increases being found in fine roots and young leaves, although the xylem concentrations did not change significantly. Also, whereas the Fe(III)-o,oEDDHA sugar beet and tomato xylem concentrations were similar at Fe(III)-o,oEDDHA concentrations below 500 μM, Fe(III)-o,oEDDHA concentrations above this limit caused significant increases (6–7 fold) in the Fe(III)-o,oEDDHA xylem concentrations in both species. These results may suggest that o,oEDDHA uptake is not fully explained by a simple passive mechanism, and that the occurrence of a rather complex process is more likely.
The racemic- and meso-o,oEDDHA isomers were in similar amounts (ratio of 1.0) in all tissues analyzed, irrespective of the resupply time (3-6 h) and chelate concentration (30–2000 μM Fe-o,oEDDHA). In a previous study using long-term applications to pepper and tomato plants, the meso/racemic ratios were slightly higher in leaves than in roots (approximately 1.0–1.4 and 1.0, respectively) and this was attributed to a preferential translocation of the meso isomer from roots to shoots and/or degradation of the racemic isomer in leaves.15 Other authors observed that the meso Fe(III)-o,oEDDHA isomer disappeared in higher amounts from the nutrient solution when compared with the racemic one when Strategy I plants were used20 suggesting that plants take up the meso isomer preferentially; however, in that study plant tissue o,oEDDHA was not quantified, and nutrient solution racemic and meso Fe(III)-o,oEDDHA was measured without considering that o,oEDDHA can be chelated with other metals after Fe release by the FCR.
The o,oEDDHA plant contents were one order of magnitude lower than those of Fe tracers (Feex-meso + Feex-rac) taken up from Fe(III)-o,oEDDHA. These results are in accordance with the values obtained with tomato and pepper plants,15 and support that the main mechanism of Fe acquisition from Fe(III)-o,oEDDHA by Fe-deficient Strategy I plants involves the release of Fe from the racemic and meso o,oEDDHA carriers. This dissociative Fe acquisition mechanism was first demonstrated by Tiffin and Brown,12 and Römheld and Marschner,9 labeling Fe and o,oEDDHA with 55Fe (or 59Fe) and 14C, respectively. The ratios (Feex-meso + Feex-rac)/o,oEDDHA differed among tissues (43–32, 10–50, 7–7 and 4–5 in fine roots, xylem, old leaves and young leaves, respectively, after 3–6 h of resupply), and point out to the preferential accumulation of Fe and chelating agent in roots and leaves, respectively. It is remarkable that the concentration of meso + racemic Fe(III)-o,oEDDHA accounted for 10 and 2% of the Fe tracers taken up (Feex-meso + Feex-rac) at 3 and 6 h, respectively. Also, ratios did not change much with resupply time, with the exception of the xylem, where the ratio (Feex-meso + Feex-rac)/(racemic + meso Fe(III)-o,oEDDHA) increased 5-fold between 3 to 6 h of resupply. This may suggest a differential uptake kinetics for Fe and chelating agent uptake, with a lag phase in the increase of xylem total Fe concentration when compared to that of Fe(III)-o,oEDDHA. The Feex-meso/meso-o,oEDDHA ratio in the plant was approximately 2-fold higher than that of the Feex-rac/racemic-o,oEDDHA, showing that the dissociative mechanism used by Fe-deficient sugar beet plants was more effective for the meso than for the racemic Fe(III)-o,oEDDHA isomer. The presence in the plants of significant amounts of chelating agents observed in this and other studies13–16 opens the possibility that a small part of the Fe delivered by the Fe(III)-o,oEDDHA may be taken up via non-dissociative mechanisms. This may occur at all times, but the relative contribution of these mechanisms would be specially important both in the short term after Fe fertilization and also when root FCR activity is down-regulated by the Fe status (e.g., after fertilization as in López-Millán et al.31 or in continuous low intensity Fe supply).
A 2.7-fold higher sugar beet root reduction rate was found for the meso isomer than for the racemic one, using the strong Fe(II) chelating agent BPDS to assess separately both stereoisomers; in a previous study a 7.5-fold increase was found with Fe-deficient cucumber roots.23 The mechanism of Fe uptake from Fe(III)-chelates by Fe-deficient Strategy I plant species involves a reduction step,35 followed by the spontaneous release or competitive sequestration of the reduced species.4 A study on the reduction kinetics of various synthetic weak hexadentate polyaminocarboxylate Fe(III)-chelates (lacking phenolic groups, e.g. Fe(III)-EDTA and Fe(III)-DTPA) by Fe-deficient peanut roots showed that the differences in enzyme affinity (Km) and maximal reduction rate (Vmax) could be attributed to differences in chemical characteristics such as formation constant (KFe(III)L; LogKFe(III)L in the range 18.2–31.2) and ionic charge (in the range 0 to −2).36 However, the differences in these parameters between the meso and racemic Fe(III)-o,oEDDHA stereo-isomers are relatively small (LogKFe(III)L of 34.2 and 35.9, respectively; charge of −1 in both cases), and therefore cannot explain the 3- to 8-fold differences in root reduction rate found here and in the Lucena and Chaney23 study.
The differences in FCR reduction rates between racemic and meso Fe(III)-o,oEDDHA could be related to the pH dependence of the corresponding redox potentials. The cytoplasmatic reductant acting as electron donor for the root FCR is thought to be cytoplasmic NAD(P)H (midpoint potentials (E0′) of approximately −320 mV),37 with the Fe(III)-chelate, not Fe(III), being the electron acceptor.38 The efficacy of the root FCR to release Fe from Fe(III)-chelates depends markedly on the redox potential in the cell surface environment.39,40 The reduction of the complex Fe(III)-o,oEDDHA itself by the FCR enzyme has been recently questioned, because the very negative reduction potential (−560 mV at the pH 7.4 typical of calcareous soils)41 and the high spin Fe42 of Fe(III)-o,oEDDHA make such reaction thermodynamically unfavorable. The same authors proposed that the electron acceptor of the FCR enzyme could be the Fe(III)-o,oEDDHA monoprotonated specie ([Fe(III)HL]) instead of the major [Fe(III)L]− species. In Fe-deficient Strategy I plants, the induction of a root plasma membrane H+-ATPase is a core component of the response to Fe deficiency, favoring the activity of the root FCR.43 At lower pH values, the concentration of the protonated species increase, while the Fe(III)-o,oEDDHA redox potential increases to −480 and −370 mV at pH 6.0 and 5.0, respectively,41 values closer to that of the electron donor. To the best of our knowledge, the redox properties of the individual racemic and meso Fe(III)-o,oEDDHA isomers have not been reported. However, the pH-dependence of the redox potential is determined by the protonation constants of the oxidized and reduced chelate forms,39,40 which are known for the racemic and meso Fe(III)-o,oEDDHA isomers. In the meso isomer the [Fe(III)HL] species was more stable than the [Fe(III)L]− species (LogK of 36.6 and 34.2, respectively), whereas in the racemic isomer the constants of the [Fe(III)HL] and [Fe(III)L]− species were similar (LogK of 35.1 and 35.9, respectively).1 The tendency of the meso isomer to form protonated species as the pH decrease when compared with the racemic one could be behind the higher relative efficiency of the meso isomer as an Fe source.
In the protonated species of racemic and meso Fe(III)-o,oEDDHA, o,oEDDHA coordinates with Fe as a pentadentate ligand. Iron(III)-chelate redox potentials depend on the first Fe coordination shell, which changes with ligand denticity (the number of atoms in a single ligand that bind to a central Fe atom in a coordination complex), among others.39 For instance, the redox potential of 12 hydroxymate siderophore Fe(III)-complexes, with homologous binding groups but with different denticity, followed the sequence hexadentate > tetradentate > bidentate.39 In the racemic and meso Fe(III)-o,oEDDHA [FeL]− species, the Fe(III) coordination arrangement is a six-coordinate, closed roughly octahedral field, and o,oEDDHA coordinates as a hexadentate ligand with 6 donor groups available for metal chelation (2 amino, 2 carboxylate and 2 phenolate groups). In the Fe(III)-o,oEDDHA [FeHL] species (that becomes more abundant when pH decreases), the arrangement is a six-coordinate, open octahedral field, and o,oEDDHA coordinates as a pentadentate ligand with a phenolic oxygen atom free and the vacant Fe coordination position occupied by a water molecule.1 The Fe coordination arrangement of the Fe(III)-chelate has been hypothesized to be the origin of the better efficiency as a root FCR enzyme substrate (using BPDS and pH 6) of the regioisomer Fe(III)-o,pEDDHA when compared of Fe(III)-o,oEDDHA; the accessibility of the Fe atom for the FCR enzyme would be facilitated by the octahedral open form of Fe(III)-o,pEDDHA when compared to the octahedral closed form of the Fe(III)-o,oEDDHA [FeL]− species predominant at pH 6.44 In fact, the Fe(III)-o,oEDDHA [FeHL] species has a first coordination shell similar to that of Fe(III)-o,pEDDHA, in both cases coordinating as pentadentate ligands.
The meso isomer appears to be the major contributor to the exceptional efficiency of Fe(III)-o,oEDDHA as a plant Fe source in nutrient solution (and therefore in similar conditions such as soil-less horticulture). However, both isomers behaved similarly as Fe sources in calcareous soil conditions,22 where soil related factors can limit the efficiency of the meso isomer (e.g. by adsorption onto soil surfaces6,45 or metal and ligand exchange reactions). This is similar to what occurs with the Fe(III)-chelate of the pentadentate ligand o,pEDDHA, which was capable of providing sufficient Fe to plants in nutrient solution but not in calcareous soil.26
A rough comparison of rates of reduction and uptake can be made in the sugar beet experiment with Fe tracers. The amount of Fe tracer taken up (Feex-meso + Feex-rac) in leaves and the whole plant was approximately 11–15 and 40–62% of the Fe reduced in the presence of BPDS. The total Fe xylem concentrations, considering transpiration rates of 20 g water h−1 plant−1,46 would correspond to Fe transport rates equivalent to approximately 10% of the Fe reduced by the FCR; however, considering xylem Fe tracer concentrations, values would be equivalent to approximately 2% of the Fe reduced by the FCR. In previous studies, Fe-deficient peanut plants showed similar rates of Fe(III)-DTPA-reduction and 59Fe-uptake,47 whereas in Fe-deficient cucumber plants the xylem Fe concentration accounted for only 1% of the Fe reduced in the BPDS assay.23 These results point out to the need for a system to assess physiological Fe reduction rates directly, in the absence of strong Fe(II) chelators such as BPDS, which can lead to overestimations of the reduction rates.48
Conclusion
The usefulness of dual-stable Fe isotope tracer experiments in plant Fe nutrition has been proven, since plants did not discriminate between 54Fe and 57Fe and no short-term Fe isotope exchange reactions occurred with the Fe(III)-chelates used. The meso isomer appears to be the major contributor to the exceptional efficiency of Fe(III)-o,oEDDHA to deliver Fe to plants in nutrient solution, with rates of FCR, xylem transport and total uptake (in both cases considering the Fe previously chelated) 2-fold higher than those found for the racemic isomer. Both isomers of the chelating agent were incorporated and distributed by plants at similar rates, in amounts one order of magnitude lower than those of Fe. After 6 h of Fe resupply, most of the Fe acquired was still localized in roots, whereas most of the chelating agent was localized in leaves. Although most of the Fe coming form the Fe(III)-o,oEDDHA was taken up by the plant through a dissociative reduction mechanism, a small part of the Fe delivered by the Fe(III)-o,oEDDHA may be taken up via non-dissociative mechanisms, probably using the transpiration stream as the driving force for entry, and this may be important in the short term after Fe fertilization and also when root FCR activity is down-regulated.Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation (MICINN) (projects AGL2007-61948 and AGL2009-09018, co-financed with FEDER), the Commission of European Communities (ISAFRUIT Project, Thematic Priority 5-Food Quality and Safety, of the 6th Framework Programme of RTD; Contract No. FP6-FOOD-CT-2006-016279), the trilateral Project Hot Iron (ERA-NET Plant Genome Research KKBE; MICINN EUI2008-03618) and the Aragón Government (Group A03). IO was supported by a CONAID-DGA predoctoral fellowship and a CAI-Europa grant. The authors thank Dr J. Orduna (ICMA, CSIC-University of Zaragoza, Spain) for drawing the molecular structures of racemic and meso Fe(III)-o,oEDDHA, J. Ascaso (Digital-Works, Huesca, Spain) for designing Fig. 1, A. Poc and A. Calviño for their assistance in growing plants, I. Bellosta for carrying out o,oEDDHA extractions from the plant materials and C. Sariego (Centro Científico-Tecnológico “Severo Ochoa”, University of Oviedo, Spain) for help with ICP-MS.References
- F. Yunta, S. García-Marco, J. J. Lucena, M. Gómez-Gallego, R. Alcázar and M. A. Sierra, Inorg. Chem., 2003, 42, 5412–5421 CrossRef CAS
.
- R. B. Lauffer, A. C. Vincent, S. Padmanabhan, A. Villringer, S. Saini, D. R. Elmaleh and T. J. Brady, Magn. Reson. Med., 1987, 4, 582–590 CrossRef CAS
.
- S. L. Madsen, C. J. Bannochie, M. J. Welch, C. J. Mathias and A. E. Martell, Int. J. Rad. Appl. Instrum. B, 1991, 18, 289–294 Search PubMed
.
-
J. J. Lucena, in Iron Nutrition in Plants and Rhizospheric Microorganisms, ed. L. L. Barton and J. Abadía, Springer, Dordrecht, The Netherlands, 2006, pp. 103–128 Search PubMed
.
-
A. Álvarez-Fernández, J. Abadía and A. Abadía, in Iron Nutrition in Plants and Rhizospheric Microorganisms, ed. L. L. Barton and J. Abadía, Springer, Dordrecht, The Netherlands, 2006, pp. 103–128, pp. 85–101 Search PubMed
.
- A. Álvarez-Fernández, M. A. Sierra and J. J. Lucena, Plant Soil, 2002, 241, 129–137 CrossRef CAS
.
- J. Jeong and E. L. Connolly, Plant Sci., 2009, 176, 709–714 CrossRef CAS
.
- N. J. Robinson, C. M. Procter, E. L. Connolly and M. L. Guerinot, Nature, 1999, 397, 694–697 CrossRef CAS
.
- V. Römheld and H. Marschner, J. Plant Nutr., 1981, 3, 551–560 CrossRef
.
- J. J. Lucena, J. Plant Nutr., 2003, 26, 1969–1984 CrossRef CAS
.
- R. A. Jeffreys, V. Q. Hale and A. Wallace, Soil Sci., 1961, 92, 268–273 CrossRef CAS
.
- L. O. Tiffin and J. C. Brown, Plant Physiol., 1961, 36, 710–714 CrossRef CAS
.
- L. O. Tiffin, J. C. Brown and R. W. Krauss, Plant Physiol., 1960, 35, 362–367 CrossRef CAS
.
- R. A. Jeffreys and A. Wallace, Agron. J., 1968, 60, 613–618 CrossRef CAS
.
- H. F. Bienfait, J. García-Mina and A. M. Zamarreño, Soil Sci. Plant Nutr., 2004, 50, 1103–1110 Search PubMed
.
- I. Orera, A. Abadía, J. Abadía and A. Álvarez-Fernández, Rapid Commun. Mass Spectrom., 2009, 23, 1694–1702 CrossRef CAS
.
- J. J. Lucena, P. Barak and L. Hernández-Apaolaza, J. Chromatogr., A, 1996, 727, 253–264 CrossRef CAS
.
- R. B. Lauffer, A. C. Vincent, S. Padmanabhan, A. Villringer, W. L. Greif, D. Elmaleh and T. J. Brady, Abstr. Pap. Am. Chem. Soc., 1986, 192, 87
.
- S. L. Madsen, C. J. Bannochie, A. E. Martell and M. J. Welch, Abstr. Pap. Am. Chem. Soc., 1990, 199, 12
.
- M. Cerdán, A. M. S. Juárez, J. D. Jordá and D. Bermúdez, J. Agric. Food Chem., 2006, 54, 1387–1391 CrossRef CAS
.
- D. G. Hill-Cottingham and C. P. Lloyd-Jones, J. Exp. Bot., 1965, 16, 233–242 CrossRef CAS
.
- D. P. Ryskiewich and G. Boka, Nature, 1962, 193, 472–473 CrossRef CAS
.
- J. J. Lucena and R. L. Chaney, J. Plant Nutr., 2006, 29, 423–439 CrossRef CAS
.
-
A. Álvarez-Fernández, in Iron Nutrition in Plants and Rhizospheric Microorganisms, ed. L. L. Barton and J. Abadía, Springer, Dordrecht, The Netherlands, 2006, pp. 437–448 Search PubMed
.
- J. A. Rodríguez-Castrillón, M. Moldovan, J. I. García-Alonso, J. J. Lucena, M. L. García-Tome and L. Hernández-Apaolaza, Anal. Bioanal. Chem., 2008, 390, 579–590 CrossRef CAS
.
- C. L. Rojas, F. J. Romera, E. Alcántara, R. Pérez-Vicente, C. Sariego, J. I. García-Alonso, J. Boned and G. Martí, J. Agric. Food Chem., 2008, 56, 10774–10778 CrossRef CAS
.
- A. Álvarez-Fernández, I. Orera, J. Abadía and A. Abadía, J. Am. Soc. Mass Spectrom., 2007, 18, 37–47 CrossRef CAS
.
- K. Kovács, E. Kuzmann, E. Tatar, A. Vertés and F. Fodor, Planta, 2009, 229, 271–278 CrossRef CAS
.
- A. F. López-Millán, F. Morales, A. Abadía and J. Abadía, Plant Physiol., 2000, 124, 873–884 CrossRef CAS
.
- A. F. López-Millán, F. Morales, Y. Gogorcena, A. Abadía and J. Abadía, J. Plant Physiol., 2009, 166, 375–384 CrossRef CAS
.
- A. F. López-Millán, F. Morales, Y. Gogorcena, A. Abadía and J. Abadía, Aust. J. Plant Physiol., 2001, 28, 171–180 Search PubMed
.
- J. A. Rodríguez-Castrillón, M. Moldovan, J. R. Encinar and J. I. García-Alonso, J. Anal. At. Spectrom., 2008, 23, 318–324 RSC
.
- S. Susín, A. Abadía, J. A. González-Reyes, J. J. Lucena and J. Abadía, Plant Physiol., 1996, 110, 111–123 CAS
.
- B. Nowack, R. Schulin and B. H. Robinson, Environ. Sci. Technol., 2006, 40, 5225–5232 CrossRef CAS
.
- R. L. Chaney, J. C. Brown and L. O. Tiffin, Plant Physiol., 1972, 50, 208–213 CrossRef CAS
.
- R. L. Chaney, Acta Bot. Neerl., 1989, 38, 155–163 Search PubMed
.
-
M. Vuletic, V. H. T. Sukalovic and Z. Vucinic, in Biophysics from molecules to brain in Memory of Radoslav K. Andjus, ed. Z. Vucinic, B. Djuricic, S. S. Stojilkovic and P. R. Andjus, New York Academic Sciences, New York, 2005, pp. 244–258 Search PubMed
.
- P. R. Moog and W. Bruggemann, Plant Soil, 1994, 165, 241–260 CrossRef CAS
.
- A. L. Crumbliss and J. M. Harrington, Adv. Inorg. Chem., 2009, 61, 179–250 CAS
.
- J. M. Harrington and A. L. Crumbliss, BioMetals, 2009, 22, 679–689 Search PubMed
.
- M. Gómez-Gallego, D. Pellico, P. Ramírez-López, M. J. Mancheño, S. Romano, M. C. de la Torre and M. A. Sierra, Chem.–Eur. J., 2005, 11, 5997–6005 CrossRef CAS
.
- M. Gómez-Gallego, I. Fernández, D. Pellico, A. Gutiérrez, M. A. Sierra and J. J Lucena, Inorg. Chem., 2006, 45, 5321–5327 CrossRef CAS
.
- S. Santi and W. Schmidt, New Phytol., 2009, 183, 1072–1084 Search PubMed
.
- S. García-Marco, N. Martínez, F. Yunta, L. Hernández-Apaolaza and J. J. Lucena, Plant Soil, 2006, 279, 31–40 CrossRef CAS
.
- L. Hernández-Apaolaza and J. J. Lucena, J. Agric. Food Chem., 2001, 49, 5258–5264 CrossRef CAS
.
- A. Larbi, A. Abadía, J. Abadía and F. Morales, Photosynth. Res., 2006, 89, 113–126 CrossRef CAS
.
- R. L. Chaney and P. F. Bell, J. Plant Nutr., 1987, 10, 963–994 CrossRef CAS
.
- J. L. Pierre, M. Fontecave and R. R. Crichton, BioMetals, 2002, 15, 341–346 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2010 |
