Metallomics study in CSF for putative biomarkers to predict cerebral vasospasm†
Yaofang
Zhang
a,
Joseph F.
Clark
b,
Gail
Pyne-Geithman
c and
Joseph
Caruso
*a
aDepartment of Chemistry, University of Cincinnati, OH 45221-0172, USA. E-mail: joseph.caruso@uc.edu
bDepartment of Neurology, University of Cincinnati, Cincinnati, OH 45267-0536, USA
cDepartment of Neurosurgery, University of Cincinnati, Cincinnati, OH 45267-0515, USA
First published on 17th August 2010
Abstract
Cerebral vasospasm (CV) refers to physical narrowing of brain cerebral arteries due to over-contraction of the arterial wall, which often arises following a subarachnoid hemorrhage (SAH). CV is frequently associated with poorer outcomes in those patients. Between the ictus of SAH and its CV complication, there is a 3–7 days delay, which provides a time window to predict and possibly prevent the onset CV. Since the precise pathomechanism of CV is still unclear and approaches for predicting it are inefficient, more effective ways of predicting CV need to be developed. As a protective nourishing fluid flows through the subarachnoid space, cerebrospinal fluid (CSF) closely relates to the health states of the central nervous system (CNS). Analysis of CSF can provide invaluable information to diagnose, treat and prevent diseases of the CNS because of its relatively direct representation of events in the brain. Therefore, we assume that the components in CSF and their alterations may reflect the state of aneurismal SAH and the development of vasospasm. In this study, three types of CSF from healthy control, and patients who suffered SAH and its complication, CV, were investigated via two-dimensional separations in combination with elemental and molecular mass spectrometry detection for the identification of elemental species. Size exclusion chromatography (SEC) was initially used with selective metal detection by inductively coupled plasma mass spectrometry (ICPMS) for characterizing size distribution of metal species. Various molecular distribution patterns were exhibited at different metal detection points (Fe, Ni, Cu, Zn and Pb). Further identification of possible metallopeptides and metalloprotein in tryptic digested fractions from the three sample types were made via reverse phase (RP)-Chip and electrospray mass spectrometry (MS) in combination with the Spectrum Mill data base search engine accessing appropriate data bases. Comparisons were generated to show suggested protein similarities or differences across the three CSF sample types. Six protein families with possible protein markers were further identified, and may be considered as possible focus areas for discovering valuable biomarkers to preclude the debilitating or deadly vasospasm.
Introduction
Cerebral vasospasm (CV), a complication of subarachnoid hemorrhagic stroke (SAH), is the prolonged, physical narrowing of brain arteries in the subarachnoid space, with the precise cause still uncertain.1–3 SAH usually results from a ruptured aneurysm, or may come from traumatic brain injury.4,5 CV is often more closely associated with ruptured aneurysm but can be seen with post traumatic brain injury. After treatment of bleeding (clipping the aneurysm for example), still around 40% of patients who survive SAH will develop CV.6–9 The most serious, with its interruption of blood supply, results in neurologic deficit, infarction and irreversible brain damage.Yearly, it is estimated that 1 million people worldwide die or are seriously debilitated by CV, and the cost for care is in the region of hundreds of millions of dollars or more annually. The standard treatment still involves aggressive therapy with a calcium channel blocker and triple H therapy (hemodilution, hypervolemia and hypertension) to improve blood flow to the brain.10,11 While overall mortality post SAH and CV has declined in the last 10 years, the role of these treatments is questionable. Despite intensive research efforts, promising results in animal studies did not translate into significant results in human clinical trials. Between the ictus of SAH and its CV complication, there is a 3–7 day delay, which provides a time window chance to predict and possibly prevent the onset of CV.12 The observation of vasospasm in the first three days following SAH is rare.10,12–14 Since the precise pathomechanism of CV is still unclear and approaches for predicting are inefficient,15–17 more effective ways of predicting CV need to be developed.
The study of diagnostic and prognostic biomarkers for human diseases is expanding rapidly with its increasingly important role in drug development and healthcare improvement. By characterizing specific diseases and treatment responses at the DNA, RNA, protein, or metabolite level, biomarkers play key roles in better prediction and preclusion of disease.18,19 Therapeutic strategies to preclude the debilitating event cannot be rationally designed without better identification and validation of novel biomarkers to allow tailoring appropriate intervention strategies.
Cerebrospinal fluid (CSF), flows through the subarachnoid space, bringing nutrition and rinsing away metabolic waste to maintain the homeostasis of the whole central nervous system (CNS).20,21 Thus many studies have been carried out on CSF with respect to predicting, diagnosing and treating neurological diseases. It is therefore, reasonable to assume that the components in CSF and their alterations may reflect the state of aneurismal SAH and the development of vasospasm. The comprehensive analysis to ascertain differences in composition, especially proteins and metabolites, between SAH and SAH-CV patient CSF samples may ultimately lead to the discovery of valuable biomarkers.
To date, little has been done in studying metal ions and metalloenzymes as possible biomarkers in CSF. Moreover, various metals are involved in many biological activities such as signaling, gene expression, regulation and catalysis.22–24 So far relevant studies in CSF have been performed in various CNS diseases, mainly focusing on metal homeostasis. In the investigation of metals in Parkinson's disease (PD), significantly lower levels of Co, Cr, Fe, Pb, Si and Sn were observed in the CSF of PD patients compared to controls.25,26 From studies on Alzheimer's disease (AD), abnormal interactions of β-amyloid with Zn, Cu and Fe were found for contributing an important factor in neuronal toxicity and increased oxidative stress. Cu, Zn, Fe, Mg and Cr exhibit significant inverse correlation of CSF β-amyloid.27,28 Observing dementia with Lewy bodies (DLB) suggested that elevations of CSF–Mg, CSF–Ca and CSF–Cu may be responsible for neurodegenerative process in DLB and the concentration of CSF–Mg and CSF–Ca could be diagnostic markers in distinguishing DLB from AD.29 Increased levels of Cu, Fe and Zn were also discovered in the CSF of demyelinating disease compared to controls with raised protein levels, which indicated these metal ions are transferred into the CSF through their protein chelation.30 Years of observations on the therapeutic process of Wilson's disease with cerebral manifestation, confirmed that monitoring CSF Cu concentration is a valuable quantitative parameter for reflecting the normalization of copper in the brain, as well as for adjusting therapy to the individual metabolic situation and evaluating new therapeutic strategies.31–33 These correlations between metal levels and neurodegenerative diseases suggest further investigations regarding the important roles metals in CSF play in disease progression, and in particular the elucidation of the appropriate metal species, not just the total metal amount. A rare and recent study by Michalke et al. monitored 13 manganese species in CSF using CZE–ICPMS (capillary zone electrophoresis inductively coupled plasma mass spectrometry). And other species in CSF containing Pb, Mg, Zn, Fe or Cu were also separated using different chromatographic techniques.34,35
To date, there are very few studies about metals in CSF with regard to cerebral vasospasm, except a report by Sato et al. that showed a significantly lower level of Mg in the CSF of patients with vasospasm36 and a more recent study in our group.37
As a biological fluid, CSF inherently contains a complex matrix that is further complicated by the hemorrhaging blood. Additionally, there are further difficulties in studying metalloproteins, in as much as the data base libraries are in their stages of infancy. This requires state-of-the-art analytical techniques including multidimensional chromatographic separations in conjunction with elemental and molecular mass spectrometries.38
Two technologies, ICPMS and HPLC-chip-MS (that is, elemental and molecular mass spectrometries), demonstrate significant advantages for specific elemental and molecular detection.39–41 ICPMS (inductively coupled plasma mass spectrometry) is a sensitive and powerful tool providing simultaneous elemental analysis at trace to ultra trace levels. With chromatographic techniques coupled with mass spectrometry, metal and metalloid containing components in biological samples can be speciated and will be utilized with low abundance proteins42–45 with the intent to generate data suggesting even more comprehensive studies. Of particular interest is the possibility of finding groups or families of metalloproteins that may have diagnostic or prognostic implications for the SAH and SAH-CV diseased states.
Experimental
Chemicals and standards
The reagents used in these experiments were analytical grade or higher and used without further purification: urea, ammonium bicarbonate and trifluoroacetic acid (Fisher, Fair Lawn, NJ); iodoacetamide, dithiothreitol and tris-hydroxymethyl-aminomethane (Tris) (Sigma-Aldrich, St. Louis, MO); hydrogen chloride (HCl) (J.T. Baker, Phillipsburg, NJ)The 30 mM Tris solution (pH = 7.4) used as SEC chromatographic buffer was prepared in 18.2 MΩ cm−1 doubly deionized water (DDW) (Sybron Barnstead, Boston, MA, USA) with HCl for pH adjustment. Acetonitrile and high purity water used for nanoHPLC were acquired from Burdick and Jackson (Muskegon, MI). Acetic acid was provided by Agilent (Santa Clara, CA).
The standard protein ladders powder with known molecular weight were from Biorad (Hercules, CA) and suspended in 30 mM Tris-HCl (pH = 7.5) buffer as stock standards and kept at −20 °C. Fresh standard protein solution was prepared from stock prior to analysis by appropriate dilution.
Sample preparation and treatment
CSF samples from three types of patients, normal healthy control, subarachnoid hemorrhage (SAH) and vasospasm (CV) after SAH, were obtained from Department of Neurology at the University of Cincinnati, OH, with all required approvals and documentation from the appropriate institutional review board. The health type set had two samples from two individual patients named as control 1 and control 2. The SAH type of CSF included four samples, identified as SAH-1, SAH-2, SAH-3 and SAH-4, respectively. The vasospastic type set also consisted of four CSF samples from four patients indicated as CV-1, CV-2, CV-3 and CV-4, respectively.All CSF samples were mixed gently to avoid possible gradient effects and divided as aliquots in plastic (polypropylene) tubes. The aliquots were immediately frozen at −80 °C and stored. Before analysis, the CSF samples were thawed slowly on ice, processed and analyzed immediately.
It is important to note that the occurrence of hemorrhage in the subarachnoid space inevitably introduces blood products into CSF resulting in a mixture characteristic of diseased sample matrix. Previous studies have partly revealed that the degeneration of blood products leads to the formation of oxidative substances such as oxyhemoglobin which might trigger the overcontraction of the arterial wall and brain ischemia.10,46 Therefore, instead of removing blood composition from CSF samples by ultrafiltration or other purifying procedures, keeping the intrinsic condition of the diseased CSF samples (mixed with some blood) and studying the detailed difference among the disease states and control can provide more comprehensive information for diagnostic and therapeutic purposes.
After thawing, CSF samples were passed through 0.22 μm filters (Agilent Technologies, Santa Clara, CA) prior to LC separation to eliminate various insoluble materials. A Sorvall RC-5B refrigerated superspeed centrifuge (Dupont Instruments, Wilmington, DE) was utilized at 2000 rpm for 10 min.
Based on the results from SEC-ICPMS screening, fractions were collected offline from the SEC separation and were then digested by trypsin as follows: a 500 μl aliquot of each CSF sample faction with large molecular weight was preconcentrated to 50 μl with a SpeedVac concentrator connected with refrigerated vapor trap (Thermo scientific, Waltham, MA). Then 20 μl of 8 M urea, 0.4 M ammonium bicarbonate solution (4.8 g of urea and 0.316 g of ammonium bicarbonate in 10 ml of DDW) and 5 μl of 45 mM of dithiothreitol (DTT) (6.9 mg of DTT in 1 ml of DDW) solution were added to the concentrated CSF fractions, mixed by gently vortexing and then incubated at 50 °C for 30 min to denature protein. After incubation, the sample was cooled to room temperature, and a further 5 μl of 100 mM iodoacetamide (IAA) solution (18.59 mg of IAA in 1 ml DDW) was added and incubated at room temperature in the dark for 15 min. Then, 5 μl of 0.1 μg μl−1 trypsin (re-suspending 20 μg of Promega sequence grade modified trypsin in 200 μl of 50 mM acetic acid re-suspension buffer associated with trypsin supply) was introduced and incubated at 37 °C for 20 h. Stopping the digestion was accomplished by acidifying the sample with 10 μl of 3% trifluoroacetic acid (TFA) solution. The digested samples were then filtered with 0.22 μm filters for further analysis on NanoLC-Chip-ESI-ITMS.
Instrumentation
The size distribution characterization of metal species from three types of CSF samples were performed on Superdex 200 HR 10/30 (Amersham Biosciences, Inc., Piscataway, NJ) size exclusion column (SEC) with linear separation range of 10–600 kDa for globular proteins and 1–100 kDa for dextrans.47 Calibration of the column was accomplished with a mixture of standard proteins from Biorad including thyroglobulin (670 kDa), bovine gamma–globulin (158 kDa), chicken ovalbumin (44 kDa), equine myoglobin (16.7 kDa) and vitamin B12 (1350 Da) using UV/VIS detection. Retention times (in min) plotted versus the logarithm of molecular mass (in kDa) showed a good linear response in this range (r2 = 0.993). Separations were carried out isocratically at 0.4 ml min−1 using 30 mM Tris as mobile phase. The pH of the Tris buffer was adjusted with HCl to 7.4, which is within the general pH range (7.3–7.4) of normal cerebrospinal fluid.48,49
An Agilent 7500ce inductively coupled plasma mass spectrometer (ICPMS) (Agilent Technologies, Santa Clara, CA) fitted with a MicroMist nebulizer (Glass Expansion, West Melbourne, Australia) was connected to Scott double channel spray chamber (2 °C), shield torch, and collision/reaction cell for the specific detection of metals. The performance of the system was optimized to give a stable signal with maximum intensity for 1 μg L−17Li+, 89Y+ and 205Tl+ in 2% HNO3. Metal screening was for 57Fe, 60Ni, 63Cu, 66Zn, 208Pb simultaneously under optimized conditions. However, polyatomic interferences such as 57KO, 63NaAr, 60CaO, 66PCl, affect the detection of target masses. Thus, He was chosen as collision gas for minimizing polyatomic interferences and providing enhanced sensitivity of which the mechanisms have been well discussed and widely used.50–52 The connection between LC and ICPMS was achieved via a commercially available 0.25 mm I.D. polyether ether ketone (PEEK) coated silica tubing by connecting the UV outlet to the ICP nebulizer. The following operating parameters of ICPMS are used: forward power 1500 W, sampling depth 7 mm, quadrupole bias −16 V, octopole bias −18 V, collision/reaction cell gas (He) flow rate 3.0 ml min−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 as the target number and 150 ms as the maximum accumulation number. The fragmentation procedure was performed in Auto MS(2) mode: the number of precursor ions per scan was 6, and the MS/MS fragmentation amplitude was 1.3.
000 as the target number and 150 ms as the maximum accumulation number. The fragmentation procedure was performed in Auto MS(2) mode: the number of precursor ions per scan was 6, and the MS/MS fragmentation amplitude was 1.3.
Results and discussion
Size characterization of metal species in CSF
Three types of CSF samples were first analyzed using size exclusion chromatography (SEC) coupled with ICPMS to gain information about size distribution characteristics of various metal species. Comprehensive comparisons were performed to determine the typical metal specific profiles and variation among individual patients for the same type of sample, and to establish molecular level differences among non-diseased samples and the two diseased sample types. The SEC column has a wide separation range of ca. 1–600 kDa for various molecules, which is suitable for evaluating the molecular mass range that the metal moieties fall within. The utilization of ICPMS provides virtually simultaneous scanning of metal species over the different molecular size ranges when coupled with SEC and it has inherently high sensitivity, large dynamic range and low detection limits.The SEC chromatographic profiles for metal containing species are shown in Fig. 1 (screening results for the same metal are arranged in the same row, while results belonging to the same type of CSF—control, SAH or CV—are demonstrated in the same column). Standard proteins with known molecular weights were run for column calibration prior to sample analysis, and the molecular mass range is labeled in the upper part of the graph. These ranges may not be representative for globular proteins. As depicted in these chromatograms, the diseased CSF samples exhibit more metal molecular weight ranges than the non-disease control.
 | ||
| Fig. 1 SEC-ICPMS of three types of CSF samples for iron (m/z 57), copper (m/z 63), nickel (m/z 60), zinc (m/z 66) and lead (m/z 208). In the upper part, the elution of MW standards is indicated. | ||
The peak areas vary among the four CSF samples from the different SAH patients, as they do for the CSF CV samples. It is possible that large intensity increases of metals in diseased samples relative to healthy control samples might be due to the hemorrhagic blood inherent to these samples and the different extent of hemorrhage may contribute to peak area differences. However, metalloproteins are common in both fluids and free metal ions can be easily transported from blood to CSF causing changes in metal–protein interactions and various metallo species in CSF. The metal level fluctuations within the same sample types or across different diseased states, still provides information that may have biomarker implications.
In the case of Fe, Fig. 1, two peaks were observed at retention times of 30 min and 42.5 min from healthy patient CSF, and four peaks appeared in all diseased patient samples with approximate retention times of 24 min, 30 min, 36 min and 42.5 min (the last not visible in the figure due to the increased range on y axis to allow the larger intensities to be shown) demonstrating different molecular weights (or different hydrodynamic radii, which more accurately describes the separation mechanism). The peak that eluted at ca. 24 min corresponds to the molecular weight of transferrin (80 kDa). Peaks with the wide shoulders shown at ca. 30 min likely indicate hemoglobin (68 kDa) with the largest peak starting at 32 min and ending around 40 min suggesting multiple Fe-containing species, but not identifiable from SEC-ICPMS alone.
SEC is limited in identifying molecules of similar size; hence multiple CSF proteins will co-elute under one visible peak envelope. These are likely high abundance metalloproteins; however, they also serve as internal indicators to estimate other molecular sizes.
The Cu-specific chromatogram, Fig. 1, revealed four peaks in diseased samples (two major peaks approximately at 30 min and 35 min, two minor peaks approximately at 46.5 min and 50 min) and three peaks in the controls (the peak at 46.5 min is not seen). Two Cu species, albumin (65 kDa) and superoxide dismutase 1 (SOD 1, 33 kDa) may be considered as related to the first two major peaks. There is no significant difference in the peak pattern between the two diseased sample types (SAH and CV), due in part to the relatively poor resolution with SEC and the sample complexity. Studies described below show that SEC is a useful screening technique for metal species when followed by molecular mass spectrometry studies. Albumin is a protein that is known to bind to proteins and metabolites in the blood stream and cerebrospinal fluid. Metals such as Pb binding to albumin can facilitate the safe excretion of “toxic” metals via the kidneys, whereas binding of metabolites such as thyroid hormone to albumin allows the hormone to travel safely in the blood with decrease risk of degradation. So binding to albumin has the function of albumin chaperoning the metal as opposed to the metal acting on the protein. Metals are common cofactors for proteins and enzymes as well. In this case the metal binding to the enzyme impacts the enzyme function. SOD is an enzyme that binds Cu and Zn to modulate the catalytic activity of SOD. Finally, muscle protein/enzymes such as myosin bind their substrate ATP only when the ATP is bound to magnesium. Therefore, the measure of metalloproteins can provide insight into the action of the metals on those proteins as well as the action of the proteins on those metals.
For Ni, Fig. 1, about four peaks were detected in diseased sample, two were baseline separated and the rest were not, but there are some higher MW species. The main Ni containing species eluted after 40 min, which shows most Ni existing as low molecular weight substances in the samples. To prevent corrosion, many medical and surgical instruments are nickel plated, including hypodermic syringes. Thus the possibility of Ni contamination during the sampling process should be carefully considered. However, Ni is an essential component of a metalloenzyme involved in the detoxification processes and in pathogenesis.57 A considerable increase of the arterial blood pressure was found with exogenous nickel ingestion.58 And human exposure to Ni is in various ways due to its popular and frequent use in industry as alloy and plating.59
The Zn-specific chromatogram, Fig. 1, revealed up to 7 peaks with distributions over a wide molecular weight range. The first peak eluted about 18 min that represents a high molecular weight >600 kDa might suggest a Zn metalloprotein, alpha-2-macroglobulin, with molecular weight of 720 kDa. The second peak at 24 min is coincident with the Fe SEC peak. Consistent with the Cu profile, the peaks ca. 30 min and 35 min might contain the Cu and Zn associated proteins, albumin and SOD 1. The main peak at 38 min is likely to correspond to several Zn bound proteins including carbonic anhydrase (29 kDa) and zinc finger proteins. The last peak at low molecular weight represents Zn peptides or possible inorganic zinc complexes.
Elevated lead levels in diseased samples are interesting observations, especially since lead is not an essential element. Only two peaks show in the control sample, while two more peaks appear in the higher molecular range (approximately at 18 min and 24 min) in diseased sample types. People can be exposed to lead through various sources, including drinking water, old lead paint and lead acid batteries.60,61 Studies have shown that lead raises superoxide and hydrogen peroxide in human vascular smooth muscle cells, as well as altering vascular reactivity, causing lead-associated hypertension and vascular lesions.62,63 Chronically elevated Pb in serum has also been found to inhibit the proliferation of vascular endothelial cells and repair of wounded vascular areas which may impact on recovery of the cerebral vasculature after vasospasm.6,64 Also Pb can strongly bind to glucose-regulated protein (GRP78) and Pb may contribute to protein conformational diseases, such as Alzheimer's (AD).65 Therefore, the presence of increased Pb in the diseased patient samples should be further studied with regard to its possible influence on CNS diseases. Also, as indicated above, there is the possibility that if lead is binding to albumin, it could be carried away as a means of detoxification.
As depicted in all of the SEC chromatograms, for a given sample type, the results from different patients demonstrate the same distribution pattern for all detected metals, which suggest a reasonable stability for the metalloproteins. These data show that SEC is useful for screening metals in different molecular weight regions, but at most is tentative for unambiguous identification. Thus, one or more additional chromatographic techniques are required to provide sufficient separation for mass spectrometric identification or quantification.
Additionally, the analysis of CSF samples with SEC provides a good opportunity for fraction collection over appropriate MW ranges; these fractions, then to be further analyzed via electrospray MS, as one possibility. A detailed summary from metal specific chromatograms is given in Table 1a. Also, protein analysis in various samples is shown in Table 1b. As shown in the table, eight specific effluent regions in decreasing order of molecular weight are indicated. For further investigation, individual SEC fractions were collected offline from control 2, SAH 4 and CV4 samples according to Table 1 in preparation for the molecular MS investigation.
| Time/min | Metals | |
|---|---|---|
| 17–19 | Zn, Pb | |
| 22–25 | Fe, Zn, Pb | |
| 26.5–30 | Ni, Cu, Pb | |
| 30–33 | Ni, Fe | |
| 33–38 | Fe, Ni, Cu, Zn | |
| 38–41 | Zn | |
| 42–45.5 | Ni, Zn, Pb | |
| 4.5–47.5 | Ni, Cu | |
| Sample name | [Protein], μg/ml | sd |
|---|---|---|
| Control 1 | 742.22 | 16.33 |
| Control 2 | 29.26 | 0.27 |
| SAH 1 | 236.66 | 5.14 |
| SAH 2 | 1647.03 | 31.28 |
| SAH 3 | 761.48 | 30.1 |
| SAH 4 | 1200.37 | 193.96 |
| CV 1 | 1075.55 | 78.08 |
| CV 2 | 1370 | 112.57 |
| CV 3 | 1333.33 | 245.26 |
| CV 4 | 1761.11 | 164.28 |
MS Analysis of SEC fractions
The identification of proteins (including metalloproteins) from biological samples, in general, can be implemented by two main strategies. The first is by using established biological approaches based on enzymatic activity or antibody immunoassay to qualitatively detect with relatively high specificity. It is possible, however, that alternative approaches need to be utilized for protein characterization. Mass spectrometry is a powerful approach for identifying proteins in conjunction with continually developing protein databases. With ICPMS, metal cohort fractions can be identified, providing an important target for molecular MS methods employing electrospray MS or MALDI MS.In this application, all CSF fractions collected from SEC were further analyzed on nanoLC-Chip-ESI-ITMS. In keeping with the performance and mass range of the ion trap mass spectrometer utilized, the fractions collected over wide molecular weight ranges were first tryptic digested, although considering higher charged species was an additional alternative.
The significant design of the on-chip enrichment column provides an online desalting and enrichment prior to separation on the on-chip capillary column. As illustrated,39,44 the injected sample is first introduced with 97% of water mobile phase while the analysis column is flushed with the same mobile phase. Ultimately proteins are transferred from the enrichment column into the capillary analytical column for gradient separation.
Fig. 2 and 3 illustrate the base peak chromatograms (BPC) of reverse phase separations on fractions collected on 22–25 min and 33–38 min, respectively, from three patient types. BPC represents the intensity of the most abundant peak in the analysis, which in these cases provides better S/N ratios compared to the total ion chromatograms. Peptide identification and protein prediction were attained through the Spectrum Mill search engine accessing the SwissProt database, with the peptide and protein information shown in the figures (the complete set of results is presented in ESI†).
 | ||
| Fig. 2 Base peak chromatograms of nanoLC-Chip-ESI-ITMS analysis on 22–25 min fractions via SEC-ICPMS (shown on the left). | ||
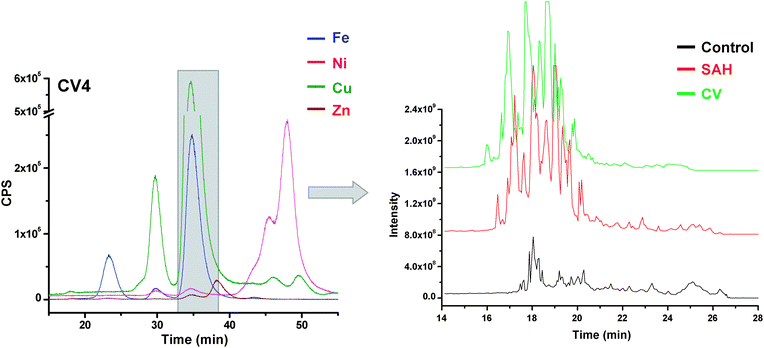 | ||
| Fig. 3 Base peak chromatograms of nanoLC-Chip-ESI-ITMS analysis on 33–38 min fractions via SEC-ICPMS (shown at the left). | ||
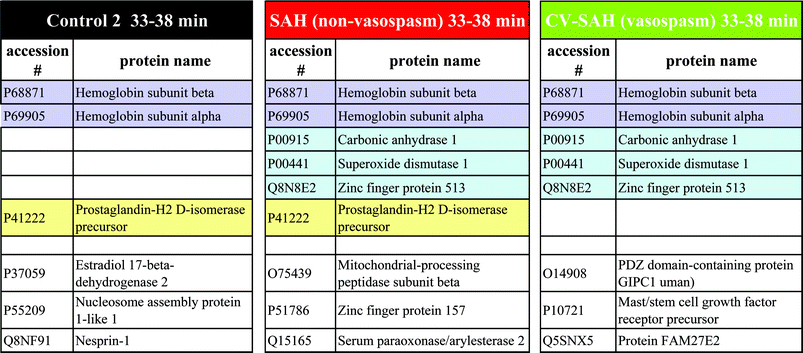
Comparison tables were generated to show suggested protein similarities or differences across the three CSF sample types. Tables 2 and 3 present the sample comparisons on fractions at 22–25 min and 33–38 min, respectively. In the blue–grey area at the top of the tables, similarities are shown among three CSF types, whereas with the green highlight, similarities are only between the two diseased states. The yellow highlight shows similarities between control and SAH sample, but importantly shows the sharp differences between the two diseased states. The bottom unhighlighted area demonstrates dissimilarities across the three sample types. All of these similarities or differences provide protein information that will inform the understanding of the molecular level events that either preclude or are directly inherent with hemorrhagic strokes and their vasospasm complications. It is important to note that it is likely that not all of the reports will be metalloproteins, for the SEC fractions, while collected based on a metal target or cohort will include non-metalloproteins as well when considering the relatively wide peaks. Unfortunately, at this time the major databases available do not specifically indicate the metal association(s).

|
In addition to observing relatively high abundance proteins such as albumin, transferrin, apolipoprotein and keratin, some low abundance proteins are found in diseased samples, which may have promise for disease biomarkers. For example, the proteins such as actin, actinin and fructose-bisphosphate aldolase from CV samples are involved in muscle motility and metabolism, which suggests vascular smooth muscle dysfunction consistent with damaged or pathologic arteries. It is possible, therefore, that profound vasospasm and vascular remodeling, which occurs during vasospasm, resulted in some cell death or dumping of cytosolic contents from vascular smooth muscle cells.
|
There is no report of Cu and Pb being present in the peptide or protein sequence. However, it is interesting to note that many peptides and proteins reported have multiple sulfur containing amino acids, cysteine and methionine, in their sequences, which provide metal ion binding sites, though not exclusively, since nitrogen and oxygen are also possibilities. Additionally, the formation (stability) constants Log Kf for Cu2+ and Pb2+ with methionine are 4.37, 4.38 at ML1 and 14.75, 8.62 at ML2 respectively. With cysteine, Log Kf for Cu and Pb are 7.0, 12.2 at ML1 and 15.72, 15.9 at ML2, respectively.66 The thermodynamic basis for binding or forming the metal complex is clearly strong. Also other studies showed that Cu, Zn and Pb can bind with some Ca proteins (actin and actinin) through multiple binding sites. Thus, it is possible that Cu and Pb interact with Ca proteins or other species when there are a relatively high number of sulfur sites.
With careful consideration of all peptide and protein reports, 6 families of proteins that are expressed uniquely or frequently in the SAH patients with vasospasm are identified:
(1) mitochondrial and metabolic proteins
(2) membrane, trans-membrane and membrane structural proteins
(3) muscle and motility proteins
(4) immune system and reactive oxygen species proteins
(5) signaling cascade proteins and
(6) protein processing and pathology proteins.
Each of the families can generate a cause and effect scenario concerning the hemorrhage, hemorrhage complications and resultant vasospasm as reflected in the CSF. Growing literature has suggested that the immune system, as well as reactive oxygen species is a contributory cause of CV post SAH.67 Thus, it is not a surprise that several proteins related to these pathways are seen from our current study in the CSF of vasospasm patients. For instance, proteins such as catalase and clusterin of the compliment system are increased in the vasospasm patients. However, attention on all families provides more comprehensive information than focusing on any one family, since incorporation of multiple analytes representing diagnostic families, increases fidelity for interpreting diagnostic information. Thus, in this study, the observation of these families that reflect mechanisms involved in the pathogenesis following subarachnoid hemorrhage can facilitate the exploitation of powerful tool for predicting, diagnosing and treating these disorders.
Conclusion
Three types of CSF from healthy control patients and patients who suffered subarachnoid hemorrhage and its complication, cerebral vasospasm, were separated using size exclusion chromatography with selective metal detection by ICPMS for characterizing size distribution of metal species. Hemorrhagic and vasospastic CSF mixed with blood, increased metal levels and more metal species eluted from diseased samples than from the control. Various molecular distribution patterns were exhibited at different metal detection points (Fe, Ni, Cu, Zn and Pb) and the metal ion presence in different MW portions are summarized. Further identification on possible metallopeptides and metalloprotein fractions from three types of sample were made via electrospray MS in combination with the Spectrum Mill data base search engine and SwissProt database. This resulted in peptide and protein comparisons across the three types of CSF. Six families with possible protein markers were further identified, and may be considered as possible focus areas for predicting, diagnosing and treating the hemorrhage to preclude the debilitating or deadly vasospasm.Acknowledgements
The authors are grateful to Agilent Technologies for their continuing support of our metallomics studies. We also thank CEM corporation for support via a microwave digestion system.References
- B. W. Robert Loch Macdonald, Cerebral vasospasm: advances in research and treatment, Thieme, 2005 Search PubMed.
- J. F. Clark, et al., Chemical and biochemical oxidations in spinal fluid after subarachnoid hemorrhage, Front. Biosci., 2008, 13(13), 1806–1812 CrossRef CAS.
- R. Loch Macdonald, Management of cerebral vasospasm, Neurosurg. Rev., 2006, 29(3), 179–193 CrossRef CAS.
- A. Pasqualin, C. Vivenza and L. Rosta, Cerebral vasospasm after head injury, Neurosurgery, 1984, 15(6), 855–858 CrossRef CAS.
- B. Aminmansour, et al., Cerebral vasospasm following traumatic subarachnoid hemorrhage, Journal of Research in Medical Sciences, 2009, 14(6), 343–348 Search PubMed.
- J. Ellis, et al., A preliminary study of metalloproteins in CSF by CapLC-ICPMS and nanoLC-CHIP/ITMS, J. Proteome Res., 2008, 7(9), 3747–3754 CrossRef CAS.
- J. I. Suarez, R. W. Tarr and W. R. Selman, Aneurysmal Subarachnoid Hemorrhage, N. Engl. J. Med., 2006, 354(4), 387–396 CrossRef CAS.
- R. R. Vindlacheruvu and A. D. Mendelow, Subarachnoid haemorrhage, Practitioner, 2002, 246(1638), 608–614 Search PubMed.
- M. C. Loftspring, et al., An in vitro model of aneurysmal subarachnoid hemorrhage: Oxidation of unconjugated bilirubin by cytochrome oxidase, J. Neurochem., 2007, 102(6), 1990–1995 CrossRef CAS.
- L. C. T. V. S. P. Murthy, M. P. Bhatia and B. T. Prabhakar, Cerebral vasospasm: Aetiopathogenesis and intensive care management, Indian Journal of Critical Care Medicine, 2005, 9(1), 42–46 Search PubMed.
- K. H. Lee, T. Lukovits and J. A. Friedman, “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage, Neurocritical Care, 2006, 4(1), 68–76 Search PubMed.
- X. Liu-DeRyke and D. H. Rhoney, Cerebral vasospasm after aneurysmal subarachnoid hemorrhage: An overview of pharmacologic management, Pharmacotherapy, 2006, 26(2), 182–203 CrossRef CAS.
- D. Daniele and G. B. Bradac, Aneurysm and subarachnoid haemorrhage: Diagnostic angiography, Rivista di Neuroradiologia, 2002, 15(5), 523–530 Search PubMed.
- K. F. Lindegaard, et al., Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements, Acta Neurochir., 1989, 100(1–2), 12–24 CrossRef CAS.
- M. E. Baldwin, et al., Early vasospasm on admission angiography in patients with aneurysmal subarachnoid hemorrhage is a predictor for in-hospital complications and poor outcome, Stroke, 2004, 35(11), 2506–2511 CrossRef.
- M. Clarke, Systematic review of reviews of risk factors for intracranial aneurysms, Neuroradiology, 2008, 50(8), 653–664 CrossRef.
- R. Moftakhar, et al., Utility of computed tomography perfusion in detection of cerebral vasospasm in patients with subarachnoid hemorrhage, Neurosurgical focus [electronic resource], 2006, 21(3) Search PubMed.
- S. Bogdanovic and B. Langlands, The Convergence of Biomarkers and Diagnostics: Therapy Area Analyses, Key Products and Future Trends, Business Insights Ltd, London, 2008 Search PubMed.
- K. A. Phillips, S. Van Bebber and A. M. Issa, Diagnostics and biomarker development: Priming the pipeline, Nat. Rev. Drug Discovery, 2006, 5(6), 463–469 CrossRef CAS.
- R. Jurado and H. K. Walker, in Clinical Methods: The History, Physical, and Laboratory Examinations, Part IV The Neurologic System, ed. H. K. Walker, W. D. Hall and J. W. Hurst, Butterworth Publishers, Stoneham (MA), Cerebrospinal Fluid, 3rd edn, 1990, ch. 74 Search PubMed.
- J. H. Wood, in Neurobiology of Cerebrospinal Fluid, ed. J. H. Wood, Plenum Press, New York, 1980, vol. 1 Search PubMed.
- W. Shi and M. R. Chance, Metallomics and metalloproteomics, Cell. Mol. Life Sci., 2008, 65(19), 3040–3048 CrossRef CAS.
- H. Haraguchi, Metallomics as integrated biometal science, J. Anal. At. Spectrom., 2004, 19(1), 5–14 RSC.
- J. S. Garcia, C. S. De Magalhaes and M. A. Z. Arruda, Trends in metal-binding and metalloprotein analysis, Talanta, 2006, 69(1), 1–15 CrossRef CAS.
- A. Alimonti, et al., Elemental profile of cerebrospinal fluid in patients with Parkinson's disease, J. Trace Elem. Med. Biol., 2007, 21(4), 234–241 CrossRef CAS.
- B. Bocca, et al., Metal changes in CSF and peripheral compartments of parkinsonian patients, J. Neurol. Sci., 2006, 248(1–2), 23–30 CrossRef CAS.
- L. Gerhardsson, et al., Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer's disease, Dementia Geriatr. Cognit. Disord., 2008, 25(6), 508–515 Search PubMed.
- D. Strozyk, et al., Zinc and copper modulate Alzheimer Aβ levels in human cerebrospinal fluid, Neurobiol. Aging, 2009, 30(7), 1069–1077 CrossRef CAS.
- F. Bostrom, et al., CSF Mg and Ca as diagnostic markers for dementia with Lewy bodies, Neurobiol. Aging, 2009, 30(8), 1265–1271 CrossRef.
- K. Gellein, et al., Trace elements in cerebrospinal fluid and blood from patients with a rare progressive central and peripheral demyelinating disease, J. Neurol. Sci., 2008, 266(1–2), 70–78 CrossRef CAS.
- C. Hartard, et al., Wilson's disease with cerebral manifestation: Monitoring therapy by CSF copper concentration, J. Neurol., 1993, 241(2), 101–107 CrossRef CAS.
- H. J. Stuerenburg, CSF copper concentrations, blood-brain barrier function, and coeruloplasmin synthesis during the treatment of Wilson's disease, J. Neural Transm., 2000, 107(3), 321–329 CrossRef CAS.
- B. Weisner, C. Hartard and C. Dieu, CSF copper concentration: A new parameter for diagnosis and monitoring therapy of Wilson's disease with cerebral manifestation, J. Neurol. Sci., 1987, 79(1–2), 229–237 CrossRef CAS.
- B. Michalke, et al., Size characterization of manganese species from human serum and cerebrospinal fluid using size exclusion chromatography coupled to inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2007, 22(3), 267–272 RSC.
- V. Nischwitz, A. Berthele and B. Michalke, Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: An approach to investigate the permeability of the human blood-cerebrospinal fluid-barrier, Anal. Chim. Acta, 2008, 627(2), 258–269 CrossRef CAS.
- N. Sato, et al., Changes in trace elements of cerebrospinal fluid after subarachnoid hemorrhage, and effects of trace elements on vasospasm, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 150(1–4), 214–217 CrossRef CAS.
- J. Ellis, et al., Studying protein phosphorylation in low MW CSF fractions with capLC-ICPMS and nanoLC-CHIP-ITMS for identification of phosphoproteins, J. Proteome Res., 2008, 7(11), 4736–4742 CrossRef CAS.
- B. Canas Montalvo, et al., Mass spectrometry technologies for proteomics, Briefings Funct. Genomics Proteomics, 2006, 4(4), 295–320 Search PubMed.
- M. Vollmer and T. van de Goor, HPLC-Chip/MS technology in proteomic profiling, Methods Mol. Biol., 2009, 544, 3–15 CAS.
- J. A. Caruso and M. Montes-Bayon, Elemental speciation studies—New directions for trace metal analysis, Ecotoxicol. Environ. Saf., 2003, 56(1), 148–163 CrossRef CAS.
- J. A. Caruso, et al., Modeling and separation-detection methods to evaluate the speciation of metals for toxicity assessment, J. Toxicol. Environ. Health, Part B, 2006, 9(1), 41–61 CAS.
- F. Brambilla, et al., A label-free internal standard method for the differential analysis of bioactive lupin proteins using nano HPLC-Chip coupled with Ion Trap mass spectrometry, Proteomics, 2009, 9(2), 272–286 CrossRef CAS.
- J. Hardouin, et al., Usefulness of an integrated microfluidic device (HPLC-Chip-MS) to enhance confidence in protein identification by proteomics, Rapid Commun. Mass Spectrom., 2006, 20(21), 3236–3244 CrossRef CAS.
- J. Hardouin, R. Joubert-Caron and M. Caron, HPLC-chip-mass spectrometry for protein signature identifications, J. Sep. Sci., 2007, 30(10), 1482–1487 CrossRef CAS.
- H. Yin and K. Killeen, The fundamental aspects and applications of Agilent HPLC-Chip, J. Sep. Sci., 2007, 30(10), 1427–1434 CrossRef CAS.
- A. G. Kolias, J. Sen and A. Belli, Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: Putative mechanisms and novel approaches, J. Neurosci. Res., 2009, 87(1), 1–11 CrossRef CAS.
- G. Healthcare and A. P. biotech, Superdex 200 HR 10/30 Instructions, GE healthcare Search PubMed.
- R. A. Mitchell, et al., Stability of cerebrospinal fluid pH in chronic acid–base disturbances in blood, Journal of applied physiology, 1965, 20(3), 443–452 Search PubMed.
- E. M. Enevoldsen, et al., Dynamic changes in regional CBF, intraventricular pressure, CSF pH and lactate levels during the acute phase of head injury, J. Neurosurg., 1976, 44(2), 191–214 CrossRef CAS.
- R. K. Marcus, Collisional dissociation in plasma source mass spectrometry: A potential alternative to chemical reactions for isobar removal, J. Anal. At. Spectrom., 2004, 19(5), 591–599 RSC.
- A. Sarmiento-Gonzalez, et al., ICP-MS multielemental determination of metals potentially released from dental implants and articular prostheses in human biological fluids, Anal. Bioanal. Chem., 2005, 382(4), 1001–1009 CrossRef CAS.
- A. P. Vonderheide, et al., Use of optional gas and collision cell for enhanced sensitivity of the organophosphorus pesticides by GC-ICP-MS, J. Anal. At. Spectrom., 2003, 18(9), 1097–1102 RSC.
- Y. Zhang, K. M. Kubachka and J. A. Caruso, Enhancing determination of organophosphate species in high inorganic phosphate matrices: application to nerve agent degradation products, Anal. Methods, 2010 10.1039/c0ay00230e.
- M. Durren, et al., Impaired plasma antioxidative defense and increased nontransferrin-bound iron during high-dose chemotherapy and radiochemotherapy preceding bone marrow transplantation, Free Radical Biol. Med., 2000, 28(6), 887–894 CrossRef.
- Y. Fujiwara and T. Kaji, Possible mechanism for lead inhibition of vascular endothelial cell proliferation: a lower response to basic fibroblast growth factor through inhibition of heparan sulfate synthesis, Toxicology, 1999, 133(2–3), 147–157 CrossRef CAS.
- S. Shull, et al., Differential regulation of antioxidant enzymes in response to oxidants, Journal of Biological Chemistry, 1991, 266(36), 24398–24403 CAS.
- E. Permyakov, in Metalloproteomics, Wiley Series on Protein and Peptide Science, ed. V. N. Uversky, Wiley, New Jersey, 2009 Search PubMed.
- V. Morvai, et al., The effects of simultaneous alcohol and nickel sulfate poisoning on the cardiovascular system of rats, Acta Physiologica Hungarica, 1993, 81(3), 239–251 Search PubMed.
- P. G., Human Exposure to Nickel, IARC Sci Publ., 1984, 53, 469–85 Search PubMed.
- P. Ragan and T. Turner, Working to prevent lead poisoning in children: getting the lead out, JAAPA: official journal of the American Academy of Physician Assistants, 2009, 22(7), 40–45 Search PubMed.
- M. D. Sanborn, et al., Identifying and managing adverse environmental health effects: 3. Lead exposure, Canadian Medical Association Journal, 2002, 166(10), 1287–1292 Search PubMed.
- Z. Ni, et al., Lead exposure raises superoxide and hydrogen peroxide in human endothelial and vascular smooth muscle cells, Kidney Int., 2004, 66(6), 2329–2336 CrossRef CAS.
- N. D. Vaziri and M. Khan, Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension, Clin. Exp. Pharmacol. Physiol., 2007, 34(9), 920–925 CrossRef CAS.
- Y. Fujiwara and T. Kaji, Inhibition of the repair of injured endothelial cell monolayers by lead and its possible mechanisms, Journal of Health Science, 2000, 46(1), 1–4 Search PubMed.
- L. D. White, D. A. Cory-Slechta and M. E. Gilbert, New and evolving concepts in the neurotoxicology of lead, Toxicol. Appl. Pharmacol., 2007, 225, 1–27 CrossRef CAS.
- O. Yamauchi and A. Odani, Stability constants of metal complexes of amino acids with charged side chains—Part I: Positively charged side chains, Pure Appl. Chem., 1996, 68(2), 469–496 CrossRef CAS.
- R. Dhar and M. N. Diringer, The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage, Neurocritical Care, 2008, 8(3), 404–412 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary tables. See DOI: 10.1039/c0mt00005a |
| This journal is © The Royal Society of Chemistry 2010 |
