Cytosolic metal handling in plants: determinants for zinc specificity in metal transporters and metallothioneins
Claudia A.
Blindauer
*a and
Ralf
Schmid
b
aDepartment of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: c.blindauer@warwick.ac.uk; Fax: +44 (024) 765 24112; Tel: +44-2476-528264
bDepartment of Biochemistry, Henry Wellcome Building, Lancaster Road, Leicester, LE1 9HN, UK
First published on 7th July 2010
Abstract
The handling of trace elements by plants plays a fundamental role in food security and safety. Therefore, there is a need to understand the mechanisms that govern metal ion trafficking in plants. The past decade has seen an immense expansion of knowledge on metal ion transport in a variety of biological systems including plants, but as for other organisms, the mechanisms for intracellular zinc trafficking remain enigmatic. The current report highlights recent advances in understanding zinc transport in plants and in identifying the biomolecules involved in this process. A particular focus is put on pinpointing determinants for zinc specificity, and we also highlight areas in need of development. Reliable experimental speciation data are a first step towards systems biology approaches to mineral nutrition in plants—but there is also a need to understand molecular details. One intriguing question, in particular in the context of predicting protein function, concerns how discrimination between metal ions in a biological system functions.
 Claudia A. Blindauer | Claudia Blindauer studied Chemistry at the Albert-Ludwigs University (Freiburg im Breisgau, Germany), and obtained her Ph.D. degree at the University of Basel, Switzerland, working with Prof. Helmut Sigel. In 1999, she joined the group of Prof. Peter Sadler at the University of Edinburgh, holding post-doctoral fellowships including a European Commission Marie Curie Individual Fellowship. In 2004, she took up her Royal Society Olga Kennard Fellowship, joining the Chemistry Department of the University of Warwick, where she currently is an Associate Professor. Her research centers on proteins involved in metal ion homeostasis with a particular focus on zinc. |
 Ralf Schmid | Ralf Schmid obtained his Ph.D. in Biophysical Chemistry from the Albert-Ludwigs University (Freiburg im Breisgau, Germany) working with Dr Andreas Labahn and Prof. Peter Gräber. In 2001 he moved to the University of Edinburgh to predict protein structures with Dr Dietlind Gerloff. Two years later he joined Prof. Mark Blaxter’s group in Edinburgh working in the field of Environmental Genomics and Bioinformatics. In 2006 he was appointed to a lectureship in Bioinformatics at the University of Leicester where he follows his main research interests in sequence analysis, molecular modelling and the evolution of protein families. |
1. Introduction
Plants are, either directly or indirectly, the basis of human and animal nutrition. They are of course also major contributors to global CO2 fixation. For both these reasons, it is of vital importance to understand in detail physiology and metabolism of plants, in order to solve today's and tomorrow's most pressing problems which come about as a consequence of climate change, including efforts to increase carbon sequestration, and food security.1One important factor that influences both plant growth as well as the nutritional value of field crops are “micronutrients”—otherwise known as metal ions. The metal requirements, and the abilities to take up metal ions vary considerably between different plant species, and indeed even closely related crop cultivars, but the biochemical basis of these differences is only beginning to be unravelled. Awareness for the importance of metal ion homeostasis in plant sciences is ever increasing, as a recent issue of Current Opinion in Plant Biology dedicated to “Ionomics” exemplifies.2
Amongst micronutrients, zinc has the most diverse functions in biological systems. It is a cofactor for members of all six classes of enzymes and is involved in most physiological processes. The high demand for metal ions by the photosynthetic machinery, including magnesium for light harvesting, manganese for water oxidation, and iron and copper for electron transfer processes in the light reaction, is well-known, but awareness of the requirement for zinc is less widespread. However, zinc deficiency has a direct and deleterious effect on photosynthesis, which is manifested by leaf chlorosis and a reduced number of photosystem II units per leaf.3 Moreover, various zinc-requiring carbonic anhydrases are directly involved in the actual process of CO2 fixation.4 Zinc is also crucial for a plethora of other metabolic processes in plants as well as for gene regulation.5 For example, zinc deficiency directly affects phosphate metabolism via the regulation of genes for high-affinity phosphate uptake in plant roots.6
The percentage of genes coding for zinc-binding proteins in eukaryotes is estimated conservatively at around 10%.7 A search for the keyword “zinc” in the Arabidopsis thaliana genome database TAIR8 yields 1576 loci matches with 1941 distinct gene models, and Broadley et al.9 identified, via database searches, 2367 zinc-binding proteins in 181 families in A. thaliana. Almost 1% of all genes in A. thaliana contain a C2H2 zinc finger domain, which is found in a range of transcription factors.10
It is therefore not surprising that zinc deficiency in plants leads to high plant mortality, reduced/stunted growth, chlorosis, necrosis, small leaves, delay in flowering and, in terms of agronomy, to significantly reduced crop yields.9,11 Notably, zinc deficiency is the most common crop micronutrient deficiency,12 since a large proportion of the world's soils are zinc-deficient. This has severe implications for food security in general, because crop yields depend on zinc availability. The impact of zinc deficiency in soils has been illustrated spectacularly by the 600% increase in crop yields for wheat in certain parts of Turkey achieved since the mid 1990ies through fertilisation with zinc compounds.13
Furthermore, low levels of minerals also diminish the nutritional value of field crops. As a consequence of zinc-deficient soils and cereals—the staple diet for the majority of the world's population—with correspondingly low zinc content, several billion people worldwide have a zinc-deficient diet. The importance of zinc for human nutrition has been highlighted by the Copenhagen Consensus Conference 2008, which identified “supplying vitamin A and zinc to 80 percent of the 140 million children who lack them in developing countries” as the solution with the highest priority to the ten greatest global challenges.14 Furthermore, it has recently been suggested that zinc deficiency is a far greater human health problem than is generally recognized.15
One way to improve the nutritional value of crops is biofortification.16,17 This term encompasses efforts to enhance the essential mineral (or vitamin) content of edible crops. This can, in principle, be achieved either by conventional breeding or genetic engineering. In the case of essential metal ions, it is also important to keep the levels of toxic metal ions to a minimum. Both conventional and GM approaches rely critically on a detailed understanding of processes such as mineral uptake, translocation within the plant, and in particular seed filling.18 The past decade has seen major advances in this area,9,19 spearheaded by the identification of a multitude of genes and proteins involved in metal ion homeostasis, reviewed elsewhere.20–23
However, there is still a lack of understanding of the different pathways and what constitute the rate-limiting steps involved in processes such as seed filling.24 One reason for this gap in our understanding is a widespread lack of integration of coordination chemistry into biological concepts—not only in the areas highlighted above. Considering that an estimated 25–50% of all proteins are dependent on metal binding, this lack of inter-disciplinary integration is a substantial problem. The present report will focus on coordination chemistry and structural biology in the context of interactions between zinc and proteins involved in its trafficking within plants. We will discuss the importance of meaningful structural, thermodynamic, and kinetic data for understanding not only metal ion specificity and genome annotation, but also for progress towards quantitative modelling of physiological metal ion fluxes in biological systems such as plants.
1.1 Tight control of zinc in biological systems
Zinc is often considered as a metal ion of lesser toxicity, especially since it does not display any redox activity. However, although total zinc concentrations in cells are in the high micromolar to millimolar range, uncomplexed Zn2+ is lethal at low micromolar concentrations to isolated eukaryotic cells,25 emphasising the need for tight control of zinc trafficking in organisms.The reasons for the deleterious effects of misregulated Zn2+ concentrations are manifold. Firstly, synthesis of Zn-requiring proteins, i.e. on average 10% of all synthesised proteins, must be integrated with zinc homeostasis; thus, misregulation of Zn2+ fluxes impacts on a substantial part of protein synthesis. Secondly, more and more evidence emerges that levels of Zn2+ play a regulatory role in a variety of physiological processes,26 and in animals, “zinc signals” have been compared to signalling pathways that are dependent on calcium.27 Little is known so far on zinc signalling in plants, but it has been shown that the level of zinc mediates programmed cell death in plant embryos,28 a finding with parallels in animals. Thirdly, elevated levels of zinc interfere with a variety of processes, including respiration and photosynthesis: Micromolar concentrations inhibit photosynthetic reaction centers,29 cytochrome c oxidase and mitochondrial NADH:ubiquinone oxidoreductase.30 Fourthly, although Zn2+ itself is not redox-active, its concentration in cells is coupled to the redox state, and zinc levels that are either too high or too low cause oxidative stress.31 Finally, the trends in stability constants for divalent metal ions set out by the well-known Irving-Williams series32 mean that Zn2+ needs to be prevented from occupying binding sites for, at the very least, Ca2+, Mg2+, Fe2+ and Mn2+, since Zn2+ complexes are generally more stable than the complexes of these metal ions. Therefore, ensuring that Zn2+ does not bind to proteins that require these metal ions for function must constitute a major task of the cellular machinery for its homeostasis.33
The deliberations above highlight why it is vitally important to ensure that zinc fluxes and concentrations in biological systems are controlled within well-defined boundaries. Consistent with the notion that the cytosol plays a central role in metal ion distribution,34 recent years have seen the renunciation of the concept of significant pools of free or loosely bound zinc in cytosols.35 In bacterial cells, the femtomolar sensitivity of zinc sensor proteins has been used to estimate the free Zn2+(aq) concentration at 0.5 fM,36 which translates to less than one ion per cell, and in human cells, concentrations of the free ion have been estimated in the high picomolar range,37 commensurate with the stability constants of typical cellular zinc-binding proteins. It has also been argued that pools of free Zn2+ are improbable due to the excess of available intracellular binding sites,35,37,38 which include proteins as well as small ligands with moderate affinities, e.g. amino acids, citrate, and glutathione, which are present at mM concentrations.
The numbers given above are deduced indirectly in various ways, as current methods for measurements of Zn2+ speciation in cytosols are not well enough developed, with sample preparation presenting a major obstacle. The detection of zinc in living cells is also problematic, as there are questions about what metallochromic dyes such as [[2-Methyl-8-[[(4-methylphenyl)sulfonyl]amino]-6-quinolinyl]oxy]acetic acid (zinquin) and N-6-methoxy-p-toluensulfonamide quinoline (TSQ) are actually measuring, as there are indications that some of these dyes are able to interact with and detect protein-bound Zn2+ as well.39 Furthermore, all of these probes are essentially metal ion chelators, so are inherently prone to disturb the system under study. For example, the dissociation constant of zinquin is in the high nanomolar range; thus zinquin may be able to remove zinc from sites with lower stability.
Despite these persisting problems with experimental techniques for zinc quantitation and speciation, the consensus is that free Zn2+ constitutes a very minute proportion of total cellular zinc, and this immediately raises the question of how Zn2+ is trafficked inside cells, and, crucially, how it gets incorporated into newly synthesised proteins. Similar considerations apply to copper,33,40 and it is generally accepted that cells exclude free Cu+ from the cytosol.35,40 Cu+ is transported intracellularly by a small number of dedicated “chaperones”, which transfer Cu+ to target sites in specific protein–protein interactions.41 It has been argued that a similar mechanism for intracellular zinc handling is not viable,38 mainly because of the sheer number of zinc-requiring proteins which make the existence of—as yet undiscovered—dedicated chaperones for each of them highly unlikely.
Very recently, another general mechanism emerged which exploits the location in which protein folding occurs. The two periplasmic proteins CucA and MncA42 from Synechocystis PCC6803 both have a cupin fold, but whilst the Mn-binding protein MncA folds in the cytosol and is exported in its folded form in which the release and replacement of manganese is kinetically hindered, the Cu-binding CucA is exported to the periplasm in its unfolded form, and folds and binds the copper ion there. In vitro, Zn2+ outcompetes Mn2+ in MncA, directly demonstrating that available Zn2+ concentrations in the cytosol need to be kept low, at least in Synechocystis. This study also highlights that protein trafficking/targeting, and the compartment in which protein folding occurs, may also play an important role in metal ion cofactor acquisition, and this may have relevance for zinc-binding proteins.
A model that is consistent with these ideas has recently been proposed.43 It involves a cellular zinc “buffer” and a zinc “muffler”. The “buffer” is the entirety of strong zinc binding sites in the cytosol, and the muffler may comprise intracellular compartments, from which zinc can be mobilized when required. The challenge is to identify the molecules and compartments that constitute buffer and muffler, the associated transport phenomena, and how these processes are regulated in a given biological system. Such data will make invaluable contributions towards a quantitative understanding of biological zinc transport.
1.2 Thermodynamics and kinetics of zinc binding: Importance and challenges
Molecules that are involved in metal ion transport are not only required to bind the metal ion of interest tightly enough to avoid binding to adventitious sites, but crucially, they must also be able to release it under defined conditions. This condition suggests that structures, thermodynamics, and/or kinetics of zinc sites in transport proteins are likely to differ significantly from catalytic and structural sites, in which strong binding is essential, and dissociation of Zn2+ is usually kept to a minimum. Moreover, there is a need to suppress the inherent catalytic activity of the transported ion to avoid uncontrolled adventitious reactions. Indeed, every one of the few metal transport proteins, including various zinc transporters, that have been characterised in sufficient detail, exhibits novel and unique coordination chemistry.35The previous section elaborated that free Zn2+(aq) cannot play a major role in intracellular transport. This means that transport has to be understood as a series of ligand exchange reactions and that at least some of these reactions are likely to occur through the formation of ternary complexes consisting of Zn2+, donor and acceptor molecule. Thus, the parameters that are particularly important for understanding zinc fluxes are both thermodynamics and kinetics of Zn2+ binding to the relevant biomolecules. Again, reliable techniques to measure either are not as well developed as they need to be. In cases where thermodynamic constants have been determined, there are often enormous (i.e. up to 12 orders of magnitude) discrepancies between results from different studies. These issues, that apply also to other metal ions, are discussed in more detail elsewhere, for example in ref. 33 and 44. The disarray of metal affinities of proteins is not only due to flaws in experimental design, but occasionally also to a lack of appreciation of basic coordination chemistry principles. The influences of buffers, pH, and the coupled equilibria associated with them are often under-appreciated, and as a consequence, thermodynamic constants for metalloproteins given in the literature are often ill-defined. Hence, there is a need to develop a common and standardised “language” to properly define these constants, to be able to make comparisons and apply them in quantitative modelling approaches. In particular, an awareness of the relationships between “apparent” or “conditional” constants and “intrinsic” or “stoichiometric” constants needs to become more pervasive. These are general problems in the study of metalloproteins, which are exacerbated in the case of Zn2+, as the lack of spectroscopic features of this ion makes direct measurements challenging. Recent progress in isothermal titration calorimetry (ITC) may aid in overcoming this problem,45 but the outputs from ITC, which purport to be deceptively straightforward, need a very high level of understanding of underlying equilibria to yield sensible numbers in the case of metal ion-biomolecule interactions. A further problem is presented by the fact that protein concentrations need to be very accurately known in order to determine meaningful constants. This point is surprisingly often neglected, and various reports use either theoretically calculated absorption coefficients or the results of protein assays, which—as is commonly known—can deviate from the true experimental value by several tens of percent. It should be noted here that in many cases, ICP-MS or -OES methods can be conveniently employed for accurate protein quantitation via their sulfur content,46 although these methods present their own set of problems.
It is thought that metal transport phenomena are under kinetic rather than thermodynamic control,36 therefore, we also need methods to study the kinetics of Zn2+ transfer reactions. These are generally afflicted by the same problems as thermodynamic studies, with the added problem that the reactions to be followed may be quite fast.
In summary, to advance our knowledge on the molecular mechanisms of zinc transport, bio-analytical method development with a thorough understanding of underlying principles is absolutely essential.
1.3 Whole-plant zinc distribution and transport
Uptake and distribution of iron are probably the most-studied processes in essential micronutrient metabolism, but the increasing awareness about the importance of zinc in human and plant nutrition has given rise to a number of recent dedicated research programmes.9,11,13,18In zinc-sufficient soils, plants accumulate considerable amounts of zinc in their shoots; leaf cells can contain between 10 and 100 mg Zn per gram of dry weight matter, whereas root cell levels are typically lower, at around 1 mg.9 Although zinc quotas in animals and humans are not normally expressed in this way, for the purpose of comparison, an average of ca. 50 μg per gram “dry weight” can be estimated for humans, based on 2.3 g of zinc for a person of 70 kg, and an average water content of 60%. More meaningful than total contents however, are intracellular zinc quotas, expressed in molar concentrations. For E. coli, yeast, and mammals, concentrations of 0.1–0.5 mM have been estimated.38 For the cytosol of plants, concentrations between ca. 3 mM (leaf epidermis) and 0.3 mM (roots and other leaf cells) can be estimated.9 Zinc concentrations are dependent on the developmental stage of the respective tissues; they are highest during early growth in leaves and immature fruit, then decline.47 Overall, the mobility of zinc in plants is relatively high,48,49 although the mechanisms involved are not well understood.
To understand metal transport in plants, both short (e.g. transport through membranes, and within the cytosol to sub-cellular compartments such as chloroplasts, mitochondria, and the vacuole) and long-distance transport pathways in the vasculature must be considered. In general, long-distance mineral transport (Fig. 1A) involves the following processes: (i) mobilisation and uptake from soil into roots, (ii) lateral transport to vasculature (xylem and phloem) in roots, (iii) vertical transport in vasculature from roots to shoots, (iv) lateral transport to metabolically active and storage tissues such as growth apices, leaves, flowers, and seeds, (v) remobilisation, e.g. from senescing leaves to storage organs such as seeds. These processes for long-distance transport are thought to utilise both extracellular (apoplastic) and intracellular (symplastic) transport and involve a number of dedicated membrane-bound transport proteins (Fig. 1A and B), which will be briefly introduced in the following section, as well as metal binding to low-molecular weight chelators.50,51,53 Ligands that have been proposed to bind Zn2+ in plant saps include citrate, oxalate, malate, phytate (myo-inositol-hexaphosphate), amino acids (in particular histidine), the phytosiderophore nicotianamine, and, in graminaceous plants, its derivatives mugineic acid and deoxymugineic acid.51,52 These ligands may operate in both intra- and extracelluar spaces, as well as in the vacuole, and are thought to be important in uptake into various tissues and long-distance translocation.51
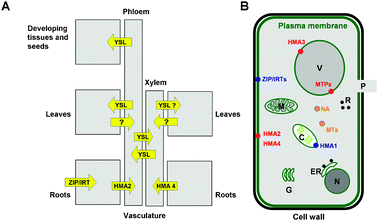 | ||
| Fig. 1 Overview of Zn pathways. (A) Schematic detailing metal pathways and known membrane-bound transporters involved in long-distance Zn trafficking. The design of this figure has been inspired by reference 19. (B) Schematic of a plant cell, detailing sub-cellular compartments (C, chloroplast, ER, endoplasmatic reticulum, G, Golgi apparatus, M, mitochondrium, P, plasmodesmum (channel to adjacent cell), R, ribosomes, V, vacuole) and molecules involved in Zn trafficking. Importers into the cytosol are depicted in blue, exporters from the cytosol in red, and intracellular chelators (metallothioneins (MTs) and nicotianamine (NA)) in orange. | ||
The concept of modelling metal ion fluxes in plants is not new, exemplifying that “Systems Biology” approaches have been around for quite some time. Estimates of speciation based on computer simulations are available,53 but results show great variations, despite models being based on the same theoretical basis.54 Hopefully, expanding our knowledge on the identity and quantitative properties of the molecules involved in metal transport will enable more accurate and reliable computations. However, whilst major advances have been made with regard to the identification of membrane-bound transport proteins (section 1.4), reviews on metal transport in plants have continuously emphasised the need for and persisting lack of knowledge and reliable qualitative and quantitative data on plant metal speciation in the various relevant fractions—xylem, phloem, cytosols, and intracellular compartments including the vacuole.9,47,48,50 This lack is due to difficulties with sample preparation as well as slow progress in instrumental techniques that have begun to be addressed only recently. Apart from the fact that it is not trivial to isolate sufficient amounts of one particular fraction without major cross-contamination, low sample stability presents major challenges. The significant increase in research activity in the field of Metallomics should help to solve these problems.
1.4 Membrane-bound transport proteins for zinc
Considerable progress has been made in recent years with the identification of transmembrane metal ion transporters, reviewed in detail elsewhere.38,55 Four main classes of transporters (Fig. 1 and 2) have been implicated in zinc transport: ZIP proteins (Pfam 02535, TC 2.A.5),56,57 P1B ATPases (TC 3.A.3),58,59 cation efflux (CE or CDF) proteins (Pfam 01545, TC 2.A.4),38,55 and YSL (for “yellow stripe like”) proteins (Pfam 03169, TC 2.A.67).74 Below, we will discuss the complement of membrane-bound zinc transporters in A. thaliana, but it should be noted that representatives for the various families are also described in a variety of other species including cereals such as rice and barley, crop plants such as soybean and tomato, and metal hyperaccumulator plants such as Thlaspi caerulescens and Arabidopsis halleri.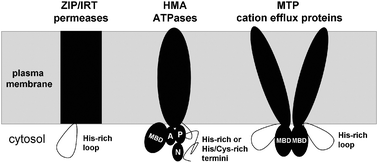 | ||
| Fig. 2 Schematic topologies of membrane-bound transporters of uncomplexed Zn2+. The presence of cytosolic domains with metal-binding capacity is a common feature for ZIP uptake proteins, HMA ATPase efflux proteins, as well as metal-tolerance proteins (MTPs) of the CDF family. MBD refers to metal-binding domains with ferredoxin-like fold, and A, P, and N designate actuator, phosphorylation, and nucleotide-binding domains of the ATPase. Metal-specific interactions between cytosolic domains may impact on ATPase activity. | ||
ZIP (for zinc/iron permease, or ZRT/IRT proteins, where ZRT or IRT stands for zinc-regulated or iron-regulated transporter, respectively) proteins are responsible for cellular metal ion uptake, for example in the roots. There are at least 18 genes for ZIP/IRT proteins in total in A. thaliana (Table 1).60 Although ZIP proteins are ubiquitous in eukaryotes, their mechanism of transport is not well understood. They are classified as secondary transporters, i.e. they do not directly use ATP. The human ZIP2 protein has been suggested to use a Zn2+:HCO3− symport mechanism,61 but other suggestions include that ZIP proteins mediate facilitated transport with the concentration gradient. ZIP proteins are not very selective23 and can transport a range of 3d and 4d divalent metal ions,62 but their transcription has been shown to be tightly regulated by zinc23,56,63,64 (ZIP1,3,4,5,9, IRT3—note that protein names do not necessarily reflect physiological function), iron65 (IRT1, IRT2), or copper63 (ZIP2 and 4), although the mechanism(s) of transcriptional regulation remain unknown.
| Accession | Common name | Cytosolic domains See section 2.4 | Metal specificity (in vivo)a |
|---|---|---|---|
| a By published in planta response to changes in metal availability; see citations in text. b N.a.: Not available. | |||
| HMA ATPases | |||
| sp|Q9M3H5|AHM1_ARATH | HMA1 | His-rich N-term; CCxxE MBD | (Zn) |
| sp|Q9SZW4|AHM3_ARATH | HMA2 | His/Cys-rich C-term; CCxxE MBD | Zn |
| sp|Q9SZW5|AHM4_ARATH | HMA3 | His/Cys-rich C-term; CCxxE MBD | Zn |
| sp|O64474|AHM2_ARATH | HMA4 | His/Cys-rich C-term; CCxxE MBD | Zn |
| sp|Q9SH30|AHM7_ARATH | HMA5 | 2x MTCxxC MBD | Cu |
| sp|Q9SZC9|AHM6_ARATH | HMA6 (Paa1) | MTCxxC MBD | Cu |
| sp|Q9S7J8|AHM5_ARATH | HMA7 (Ran1) | 2x MTCxxC MBD | Cu |
| tr|Q7Y051|Q7Y051_ARATH | HMA8 (Paa2) | MMCxxC MBD | Cu |
| MTP Cation efflux proteins | |||
| sp|Q9ZT63|MTP1_ARATH | MTP1 (Zat1) | His-rich loop; MBD | Zn |
| sp|Q9M271|MTPA1_ARATH | MTP2 | His-rich loop; MBD | |
| sp|Q9LXS1|MTPA2_ARATH | MTP3 | His-rich loop; MBD | Zn |
| sp|Q6DBM8|MTPB_ARATH | MTP4 | His-rich loop; MBD | |
| sp|Q6ICY4|MTPC2_ARATH | MTP5 | MBD | |
| sp|Q8L725|MTPC1_ARATH | MTP6 | MBD | |
| sp|Q8H1G3|MTPC4_ARATH | MTP7 | MBD | |
| sp|Q9M2P2|MTPC3_ARATH | MTP8 | MBD | |
| tr|Q9SAJ7| Q9SAJ7_ARATH | MTP9 | MBD | |
| tr|Q0WU02|Q0WU02_ARATH |
 MTP10 MTP10 |
MBD | |
| tr|Q9SA30|Q9SA30_ARATH | |||
| tr|O80632|O80632_ARATH | MTP11 | MBD | Mn |
| tr|Q9SI03|Q9SI03_ARATH | MTP12 | His-rich loop; MBD | |
| ZIP proteins | |||
| O81123|ZIP1_ARATH | ZIP1 | His-rich loop | Zn |
| Q9LTH9|ZIP2_ARATH | ZIP2 | Cu | |
| Q9SLG3|ZIP3_ARATH | ZIP3 | Zn | |
| O04089|ZIP4_ARATH | ZIP4 | His-rich loop | Cu |
| O23039|ZIP5_ARATH | ZIP5 | ||
| O64738|ZIP6_ARATH | ZIP6 | ||
| Q8W246|ZIP7_ARATH | ZIP7 | ||
| Q8S3W4|ZIP8_ARATH | ZIP8 | ||
| O82643|ZIP9_ARATH | ZIP9 | ||
| Q8W245|ZIP10_ARATH | ZIP10 | ||
| Q94EG9|ZIP11_ARATH | ZIP11 | ||
| Q9FIS2|ZIP12_ARATH | ZIP12 | ||
| Q38856|IRT1_ARATH | IRT1 | Fe | |
| Q81850|IRT2_ARATH | IRT2 | His-rich loop | Fe |
| Q8LE59|IRT3_ARATH | IRT3 | His-rich loop | Zn |
| Q8L600|Q8L600_ARATH |
 N.a. N.a. |
||
| Q9C9Z1|Q9C9Z1_ARATH | |||
| Q940Q3|Q940Q3_ARATH | N.a. | ||
| Q9LT34|Q9LT34_ARATH | N.a. | ||
| Q9M647|IAR1_ARATH | IAA-alanine re-sistance protein 1 | His-rich loop | |
Heavy-metal transporting P-type ATPases (P1B ATPases) are ubiquitous in both prokaryotes and eukaryotes; however, Zn-transporting ATPases are only known for bacteria and plants. ATPases function in efflux from the cytosol, which includes transport into compartments such as the endoplasmatic reticulum and Golgi apparatus. Plant P1B ATPases have been labelled “HMA”, for “heavy-metal associated”. They have 6 or 8 transmembrane helices and usually contain cytosolic metal-binding domains which play a role in specificity and regulate ATPase activity and thus transport. A. thaliana contains 8 HMAs (Table 1); HMA2-4 transport Zn2+ (as well as Cd2+), and HMA5-8 are Cu+ transporters.66 Information on the metal specificity of HMA1, which localises to the chloroplast envelope,67 is conflicting, but sequence analysis points towards a role in Zn2+ transport (see below), and recent careful in-planta analysis indicated a role in pumping Zn2+ out of chloroplasts, preventing toxic effects.67 In A. thaliana, HMA2 and HMA4 together are essential for Zn2+ homeostasis,66 but can also contribute to tolerance towards Cd2+.68 They are absolutely necessary for plant survival and reproduction under conditions of low zinc availability. They are expressed in vascular tissues of roots, stems, and leaves, and localise to the plasma membrane, particularly that of pericycle cells.66 They are thought to mediate Zn2+ (and Cd2+) fluxes from these cells into phloem (HMA2) and xylem (HMA4) (Fig. 1A),59 and hence translocation of both Zn2+ and Cd2+ from root to shoots, but also seed loading in cereals.18 HMA3 localises to the vacuoles of cells in roots (Fig. 1B), old rosette leaves and cauline leaves, and provides tolerance against elevated levels of zinc, cadmium, cobalt and lead, presumably by mediating sequestration of these ions into the vacuole.69 Thus, interestingly, different P1B-ATPases in A. thaliana are used to deal with both zinc excess (HMA1 and 3) and deficiency (HMA2 and 4).
The third class of ubiquitous membrane-bound transporters are the “MTPs”,55 for “metal-tolerance proteins”, which belong to the family of CDF, or CE (cation efflux), proteins. “CDF” stands for “cation diffusion facilitator”, but this commonly utilised term is certainly a misnomer, as metal transport by CDF proteins can occur against a concentration gradient. No ATP is required by the CDF proteins themselves, therefore they are classified as secondary active transporters, and appear to work as antiporters. This has been demonstrated for various CDF proteins, using either H+ or K+ as the ion transported in the other direction.38 The name “MTP” suggests a role in metal tolerance, but it is important to note that MTPs are also crucial for essential metal homeostasis, and some are also involved in seed loading in cereals.16
A. thaliana can express at least 12 MTP-type proteins (Table 1), but published functional data relating to metal specificity is only available on MTP1, MTP3 and MTP11. MTP1, which is expressed throughout the plant, but particularly in reproductive organs, and MTP3, which is mainly expressed in roots, are involved in zinc tolerance and both localise to the vacuole.70,71 MTP11, which is also expressed throughout the plant, is involved in Mn2+ trafficking and localises to pre-vacuolar compartments, i.e. trans-Golgi vesicles.73
Using a combination of phylogenetic protein sequence analysis with knowledge about metal ion specificity of some members of the various clusters, CDF proteins have recently been classified into three main groups.72 All members of group I (Zn-CDF) transport at least Zn2+, but there are also members with broader specificity that can also transport Cd2+, Co2+, Ni2+ and Cu2+, but not Mn2+ or Fe2+. According to Montanini et al., A. thaliana MTP1,2,3,4,5 and MTP12 belong to this group. Group II are the Zn/Fe-CDFs, which can transport Fe2+ and Zn2+, but not Mn2+, and only A. thaliana MTP6 appears to belong to this group. Group III are Mn-CDFs,73 and contains A. thaliana MTP8, 9, 10 and MTP11. MTP7 does not belong to any of these groups. It should be emphasised here that overall sequence similarity and metal specificity need not be commensurate, but in the case of the CDF proteins, there seems to be a correlation between primary sequences and transported metal ions. This link will be explored in more detail below (section 2.4.3).
YSL proteins74 belong to a larger family, the oligopeptide transporter proteins (OPT). Very little biochemical information is available on members of the OPT family, but YSL proteins are known proton-coupled symporters. They transport metal ion complexes of phytosiderophores such as nicotianamine, and have been shown to be important in the remobilisation of zinc and copper from senescent leaves and seed loading with iron, zinc and copper.47 YSL proteins localise to cells close to the vasculature in shoots74 and roots75 and are thought to aid in unloading metal-nicotianamine complexes from the vasculature into developing tissue. Nicotianamine (NA, Fig. 3) is a ubiquitous plant metabolite related to mugineic acid (a phytosiderophore exclusively from graminaceous plants) and is essential for inflorescence formation, pollen formation, and seed maturation,76i.e. for plant reproduction. NA is recognised to play a crucial role in the transport of iron, zinc and copper into interveinal tissues and is thought to be a symplastic (i.e. intracellular) chelator77 present in phloem, but also in vacuoles78 and the cytoplasm of other cells.76 It is also involved in metal loading of the xylem.74 In meristematic tissue, NA concentrations of up to 400 μM have been measured.79 NA can act as a hexadentate ligand, using three carboxylates and three amines for complexation (Fig. 3). The stability constants of NA with various metal ions have been determined.77,80 The stoichiometric stability constant for the Zn2+-NA complex is log K = 14.7 (Table 2), and it has been shown by comparing NA with several synthetic analogues that high stability correlates with high bioactivity.80 More thermodynamic data is summarised in Table 2.81,82 In light of the high stability of NA complexes, which exceeds that of complexes with free amino acids, malate, or citrate by five to fifteen orders of magnitude, and given indications of the ubiquitous presence of NA, complexation of Mn2+, Fe2+/3+, Zn2+, and Cu+/2+ to free amino acids or other small ligands in phloem or xylem may be negligible under regular conditions. Indeed, a recent report has demonstrated the formation of Zn2+-NA complexes in vivo, with other major contributions from His and Cys coordination, but no indication for coordination by citrate.83 Although this study used the fission yeast S. pombe, these findings, together with the considerations above, are significant for Zn2+ trafficking in plants as well.
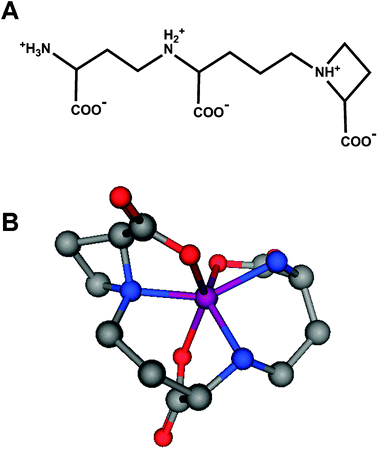 | ||
| Fig. 3 Nicotianamine as a metal ligand. (A) Chemical structure of nicotianamine. The three carboxylates, as well as all three nitrogen atoms are expected to participate in metal binding in their deprotonated form, giving rise to distorted octahedral complexes with high stability (Table 2). (B) Hypothetical 3D molecular model of a nicotianamine-Zn2+ complex. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of metal ions and small molecule chelating ligands. With the exception of the data for NA (nicotianamine),81 all values have been retrieved from ref. 82. For comparison, values for the chelators EDTA and TPEN, which are often employed in a biological context, are also included
1 complexes of metal ions and small molecule chelating ligands. With the exception of the data for NA (nicotianamine),81 all values have been retrieved from ref. 82. For comparison, values for the chelators EDTA and TPEN, which are often employed in a biological context, are also included
| Malate | Citrate | Gly | Asp | His | Cys | NA | EDTA | TPEN | |
|---|---|---|---|---|---|---|---|---|---|
| n.a.: not available. | |||||||||
| Mn2+ | 2.2 | 3.7 | 3.0 | 3.4 | 3.3 | 4.5 | 8.8 | 13.8 | 10.3 |
| Fe2+ | 2.5 | 4.4 | 4.1 | 5.3 | 5.8 | 6.2 | 12.1 | 14.3 | 14.6 |
| Co2+ | 3.1 | 4.7 | 4.6 | 5.9 | 6.8 | 9.3 | 14.8 | 16.5 | 16.6 |
| Ni2+ | 5.2 | 5.4 | 5.9 | 7.3 | 8.6 | 9.6 | 16.1 | 18.6 | 21.6 |
| Cu2+ | 4.2 | 5.9 | 8.2 | 8.8 | 10.1 | n.a. | 18.6 | 18.8 | 20.5 |
| Zn2+ | 2.9 | 4.8 | 5.3 | 5.7 | 6.5 | 9.2 | 14.7 | 16.5 | 15.6 |
| Cd2+ | 1.9 | 3.7 | 4.3 | 4.5 | 5.4 | 11.0 | n.a. | 16.5 | 16.3 |
| Pb2+ | 2.4 | 4.0 | 5.6 | 6.0 | 5.9 | 12.2 | n.a. | 18.0 | 14.0 |
| Fe3+ | 7.1 | 11 | n.a. | 11.4 | n.a. | 13.0 | n.a. | 25.1 | n.a. |
Besides the YSL/nicotianamine system, other systems for the transport of metal ion complexes may exist. Indeed recently another new vacuolar zinc transporter has been identified in A. thaliana, and has been labelled ZIF1, for zinc-induced facilitator.84 This protein belongs to the major facilitator family, and has been suggested to transport a not yet identified zinc ligand or complex.
In summary, whole plant zinc transport and partitioning involves apoplastic transport, the crossing of a series of membranes, as well as symplastic, i.e. intracellular transport, plus intracellular sequestration in sub-cellular compartments. Whilst major progress has been made in the identification of proteins involved in membrane transport, their molecular and zinc-binding properties are not well understood. Only limited information is available on xylem and phloem metal speciation, and knowledge on speciation of Zn2+ in the cytosol and sub-cellular compartments of plant cells is even more limited. The overview has also demonstrated that a particular metal ion cannot be considered in isolation, as the more or less limited selectivity of transport proteins imperatively gives rise to cross-talk between the metabolism of different metal ions.85 Below, we will discuss the idea that the cytosol is the most crucial location for discrimination between different metal ions.
2. Zinc binding in the cytosol
The overview above has shown that a considerable portion of zinc transport occurs in the symplast, i.e. in the cytosol, and that very little information is available on cytosolic metal speciation. The following questions are pertinent: (i) What happens after a Zn2+ ion (or a small molecule Zn2+ complex such as Zn2+-NA) has entered the cytosol through the transmembrane segment of an uptake protein? (ii) How is Zn2+ passed from its point of entry to other locations, such as chloroplasts, mitochondria, endoplasmatic reticulum, ribosomes, and the vacuole? (iii) What are the intracellular agents that mediate symplastic transport, and how do they interact with uptake and efflux proteins, as well as with those that mediate uptake into intracellular compartments? (iv) When, where and how does discrimination between metal ions occur?2.1 Selectivity of proteins involved in metal ion homeostasis: The importance of cytosolic domains
The question of selectivity or specificity of metal transport proteins is not only important to understand essential metal ion homeostasis, but also because a low degree of selectivity may abet the transport and accumulation of toxic metal ions in edible parts of plants.Where are the determinants for selectivity of plant metal transport proteins located? Possibilities that can be considered include extracellular domains, cytosolic domains, domains in intracellular compartments, as well as the transmembrane portions of transport proteins. There is currently no evidence for selectivity residing in extracellular protein portions. Some limited selectivity may be mediated through transmembrane sections, but it appears that intracellular portions are most suited to generate specific sites. These general thoughts are corroborated by the observation that proteins that mediate efflux from cytosols (HMAs, CDFs) are usually more specific than uptake proteins (ZIPs/IRTs).23 Intriguingly, to compensate for a lack of selectivity on the protein level, the expression of ZIPs/IRTs is highly regulated by metal ions on the transcriptional level. This transcriptional control provides a different approach to achieve control over uptake.
Thus, the cytosol is probably the most critical location for controlling metal ion distribution.34 It has been argued that in the cytosol, proteins compete with other proteins for limited amounts of metal ions—not vice versa.33 This means that in order to avoid a population of proteins that require less competitive metal ions for function bound to the wrong, more competitive metal ion, cytosols must contain sites for these metal ions that form stronger complexes than the less competitive metal ions.
Within this framework, proteins can to some extent exploit basic concepts of coordination chemistry to achieve limited selectivity85—in particular, they can make use of favouring certain coordination numbers and geometries, as well as of Pearson's HSAB principle.86 Metal sensors, which are well-studied for bacterial systems,40,87 can also display a kind of “functional selectivity” by exploiting allostery; for example, they may be able to bind the wrong metal ion even more strongly than the correct metal ion, but the wrong metal ion may not elicit the same allosteric effect as the correct one. Again, differences in preferred coordination geometry appear to be important here. Allostery may also emerge as an important selectivity filter in the case of membrane-bound transporters such as P1B ATPases, for which substrate-triggered conformational changes are an inherent part of the molecular mechanism of enzyme catalysis.
Furthermore, in cases such as zinc fingers and metallothioneins, small differences in ionic radii may have rather pronounced effects on the stability of protein folds, which in turn may have implications for the in vivo fate and turnover of a wrongly loaded protein. In addition, metal-transfer reactions can be governed by specific (metal-mediated) protein–protein interactions, as demonstrated for the interaction between copper chaperones and their targets.41,88 Whilst this is not thought to be a general mechanism for zinc trafficking, there are indications that in some instances metal-mediated protein–protein complex formation may be required for activity and thus contribute to selectivity (see 2.4.3).
2.2 Can the in vivo metal ion substrate(s) be predicted from sequence data?
As more and more genomic and transcriptomic data become available, tools for reliable functional annotation become extremely important. The correct identification of the physiological substrate is a general problem in the annotation of genomes, and metal transporters are no exception to this rule.Even if solid experimental data on metal specificity is available for a given protein, sequence and functional data alone still may not be sufficient for correct annotation of related proteins, because as mentioned before, overall sequence similarity may not correspond to substrate selectivity, as illustrated clearly in a recent work by Campbell et al. on bacterial metal sensors.89 Therefore, to be able to assign the functionally important metal ion(s) with an acceptable level of certainty, a comprehensive and detailed knowledge of the actual metal-binding residues is required. This may allow the definition of unique signatures7 that will aid more reliable annotation.
The most direct way to determine the amino acids that are important for specificity entails the structural characterisation of isolated proteins, although the expression of wild-type and specifically mutated proteins can provide some insight, too.90 However, the latter approach is time-consuming and requires prior knowledge about which residues might contribute to specificity. The generation of this required information can be significantly expedited by structural studies.
In summary, inferring specificity from sequence data requires not only reliable data on in vivo specificity of a characterised homologue, but benefits enormously from the exact knowledge of the residues which are relevant for the function of the respective protein, i.e. in particular the metal-binding side-chains. This in turn requires reliable structural information. At present, we may not be too far away from having covered all possible protein folds, but we have only rudimentary coverage of metal sites in proteins. The remediation of this lack of data requires methods for isolation and characterisation of metalloproteins with the correct complement of metal ions. Challenges in this area are discussed in ref. 85 and briefly in the following section.
2.3 Challenges associated with the characterisation of metalloproteins
Reliable quantitative data on metal-binding properties still can only be achieved by in vitro characterisation of the respective proteins, and the determination of meaningful 3D structures is required for developing our understanding of metal-binding motifs in proteins. Both of these goals require the availability of reliable methods for the isolation of proteins with metal ions bound in a physiologically relevant manner. However, there are too few interactions between the fields of coordination chemistry and protein biochemistry as well as structural biology, and therefore it is still far from trivial to reliably produce sufficient amounts of metalloproteins with the correct number and kind of metal ion(s) bound. This can be challenging even with knowledge of the natively bound metal ion, but is a serious, probably underestimated issue for structural genomics initiatives, although there have been efforts to pay particular attention to metalloproteins.91,92These problems are compounded by the scarcity of knowledge about how metal incorporation into newly synthesized proteins works. There is a general uncertainty about metal loading of heterologously expressed proteins and methods for their consistent purification. A major question remains how one can ensure that the metal complement of an over-expressed protein matches the in vivo native form. This is not only problematic with respect to the number and identity of the bound metal ion(s), but also with respect to correct identification of binding sites.
There is therefore a clear need to identify the metal ions bound by proteins in vivo. This is generally true for the entire proteome, but the discussion in section 2.2 emphasises this need for metal transport proteins themselves as well. Currently, (bio)analytical chemistry methodology is unable to match the explosion of genomic, transcriptomic and functional data, but further developments in the area of metalloproteomics will hopefully be able to remedy this.
With this set of precautions in mind, we discuss below current knowledge on cytosolic metal-binding domains and proteins.
2.4 Cytosolic metal-binding domains of membrane-bound transport proteins
In the CDF family, His-rich loops are present in AtMTP1,2,3,4, and 12 (Fig. 4), all of which belong to the Zn-CDF group as defined by Montanini.72 His-rich loops are absent in CDFs confirmed or predicted to transport manganese (AtMTP8-11) (Table 1). In yeast complementation studies, wild-type AtMTP1 does not transport either Co2+, Ni2+, or Cd2+,93 and in planta, it does not confer tolerance to those metal ions or to Mn. By partial deletion (residues 186–216), the His-rich loop in AtMTP1 has been shown to contribute to discrimination against cobalt.93 In the deletion mutant, the net affinity towards Zn2+ (KM) decreased, whereas Vmax increased. However, deletion of residues 182–232, which comprises all histidines in this loop, led to a completely non-functional protein.
 | ||
| Fig. 4 Examples of His-rich loops in metal-transporting proteins in Arabidopsis thaliana. His residues are highlighted in white on black, and carboxylates are highlighted in grey. See also Fig. 5 and 7 for His-rich sections in Zn-transporting HMA ATPases. All five MTP proteins shown are predicted to be Zn-transporting; ZIP/IRT proteins display low metal selectivity (see text for details). | ||
His-rich stretches are also found in HMA1,2 and HMA4. HMA1 contains an N-terminal His-rich stretch (see also Fig. 7), whereas HMA2 and especially HMA4 (see also next section and Fig. 5) contain His-rich C-terminal regions which are reminiscent of His-tags. For HMA4, the involvement of the C-terminal His11 stretch in metal transport activity has been established experimentally.94
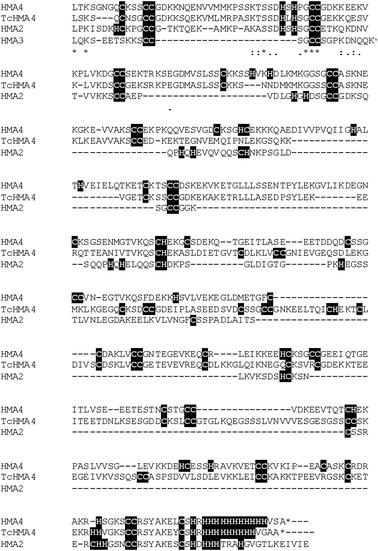 | ||
| Fig. 5 C-terminal sequences of zinc-transporting HMA ATPases with Cys- and His-rich stretches. HMA2, 3 and 4 are from A. thaliana, and TcHMA4 is from the metal hyperaccumulator Thlaspi caerulescens. The isolated C-terminus of TcHMA4 has been shown to confer resistance to Cd2+ toxicity in vivo.103 | ||
Similar loops are also present in IRT1-3 and ZIP1,4,5,9,10, and 12. As mentioned above, ZIP/IRT proteins are functionally relatively unspecific, and accordingly, a clear pattern of presence/absence of His-rich loops cannot be discerned for this family. Also considering that intracellular portions are not likely to determine substrate specificity of importers, His-rich loops in these proteins are probably not used to discriminate between transported ions. In human ZIP4, a corresponding His-rich loop mediates zinc-stimulated ubiquitination and hence protein degradation.95 Thus, in mammals, this loop is involved in regulating the levels of zinc import through regulation of the number of ZIP4 proteins per cell, and prevents uptake of excessive zinc in enterocytes. Whether this process requires zinc binding to the loop is not known, but this appears to be a likely scenario. For the related human ZIP1 protein, Milon et al. have shown that even a single mutation of the conserved loop His residues to alanines impairs zinc uptake in transfected PC-3 cells.96
Finally, small, soluble cytosolic proteins that contain His-rich stretches exist in a variety of species including bacteria,97 and in plants, a number of dehydrins also fall in this class.98 Dehydrins are involved in the response to osmotic, cold, and other abiotic stresses. They belong to the late embryogenesis abundant (LEA) proteins, are thought to have antioxidant properties, and are present in various intracellular compartments including the cytosol. Metal (Zn2+, Fe3+, Cu2+) binding to dehydrins has been demonstrated experimentally. The 20 kDa citrus dehydrin CuCOR15 appears to have two binding sites for divalent metal ions, involving the sequence HKGEHHSGDHH.
The ubiquitous presence of histidine-rich stretches in the cytosolic portions of uptake (ZIP/IRT), efflux (MTPs and HMAs) and other proteins is intriguing, raising the question of what the purpose(s) of these regions are. Judging from the findings reported above, they are likely to constitute part of the cellular zinc “buffer”. X-ray absorption studies in Thlaspi caerulescens have identified histidine as a major ligand in roots and leaves of mature plants.99,100 The EXAFS data were modeled using the Zn(His)2 complex as model compound—however, in the light of the pervasiveness of His-rich proteins, the question arises if not at least some of the detected His-bound Zn might be protein-bound after all.
In the few cases where thermodynamic data is available, micromolar dissociation constants for zinc have been determined, e.g. the dissociation constant for CuCOR15 with Zn2+ has been determined as 5 μM. Grossoehme et al. used Isothermal Titration Calorimetry (ITC) to determine the metal binding properties of the His-rich loop in A. thaliana IRT1.101 The pH and buffer-independent dissociation constant for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of the isolated loop with Zn2+ was in the order of 0.5 μM, whilst that for Mn2+ was 50 mM, that for Fe2+ was 1.5 mM, and that for Fe3+ was 29 aM. The binding constants of this loop with divalent metal ions thus follows the Irving-Williams series.
1 complex of the isolated loop with Zn2+ was in the order of 0.5 μM, whilst that for Mn2+ was 50 mM, that for Fe2+ was 1.5 mM, and that for Fe3+ was 29 aM. The binding constants of this loop with divalent metal ions thus follows the Irving-Williams series.
Comparisons with simple model complexes (Table 2) suggest that the micromolar binding constants for Zn2+ could correlate with a bidentate binding mode. Crucially, these values are in a suitable range to help avoiding inhibitory effects of Zn2+, which are thought to operate in the low micromolar to nanomolar range,102 whilst posing no danger to cytosolic zinc-requiring proteins with higher stability.
Zinc effluxers must ensure that surplus Zn2+ is eliminated from the cytosol, without removing too much Zn2+ or the wrong metal ions. This requires a module with an appropriate KD for Zn2+, and lower affinity for similar ions. The most likely metals that need to be distinguished from Zn2+ are Fe2+ and Mn2+—both with lower stabilities, thus, discrimination via a His-rich loop could be accomplished by simply exploiting the Irving-Williams series.
In the case of importers such as ZIP proteins, the loops could essentially act as “holding spaces” for incoming Zn2+ ions until they can be dealt with appropriately. In addition, the loops may function as Zn2+ sensors and thus be part of a feedback mechanism to signal sufficient metal ion, as seen in human ZIP4.
A comparison of the C-terminal portions of the four Zn-transporting ATPases of A. thaliana reveals some intriguing differences in the presence and arrangement of Cys and His residues (Fig. 5). AtHMA2 and 4 contain both His and Cys residues, whereas the C-terminus of AtHMA3 is much shorter and devoid of His residues, but contains two CC pairs as well. Apart from the natural “His-tag” mentioned above, the C-terminal portion of HMA1 is devoid of either His or Cys residues. There are also more subtle differences between HMA2 and 4; whilst HMA2 has exclusively HxH, CH, HC, and CC motifs, HMA4 in addition contains Hx(2or3)H motifs. Significantly, for the homologous TcHMA4 (Fig. 5) from the Zn2+ and Cd2+ hyperaccumulator Thlaspi caerulescens, it has been shown that heterologous expression of the 384 or 141 C-terminal residues alone conferred tolerance towards Cd2+ to yeast, demonstrating in vivo intracellular metal binding to these stretches.103
Zinc binding to the C-terminal part of AtHMA2 has been studied in vitro;104 three zinc ions were bound with nanomolar dissociation constants, and based on EXAFS data, H3CZn and H4Zn coordination has been proposed. Whilst blocking His residues completely abolished zinc binding to the C-terminus, carboxymethylation of Cys reduced zinc stoichiometry and affinity only slightly. The binding of the non-activating Co2+ ion differs qualitatively from that of Zn2+, thus the domain may contribute to selectivity.
It is likely that, once more molecular details have been elucidated, such Zn-transporting ATPases will become another example of metal selection by allostery. Conformational changes are central to the mechanism of action for ATPases, and intriguingly, zinc binding to the C-terminal metal-binding domain elicits conformational changes,104 which could alter interactions with other cytosolic domains important for catalytic activity of the ATPase. Indeed, although deletion of the C-terminal region of AtHMA2 still yields a functional protein in planta,105 the rate of enzyme turnover is decreased.104 Interestingly, these ATPases contain a further metal-binding domain at the N-terminus (see below, section 2.4.3), and deletion of this domain led to a similar reduction in turnover, whereas deletion of both N- and C-terminal metal-binding domains resulted in no further reduction. This may indicate that N- and C-terminal metal-binding domains work together, in a metal-specific fashion, to regulate enzymatic activity.
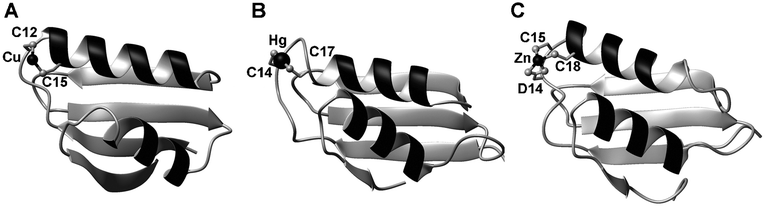 | ||
| Fig. 6 3D structures and metal-binding sites of metal-binding domains (MBDs) with a ferredoxin-like fold. (A) Human HAH1 (pdb 1fee88), a copper chaperone. (B) Shigella flexneri MerP (pdb 1afj110), a periplasmic mercury-binding protein. (C) N-terminal MBD of E. coli ZntA (pdb 1mwz),108 a Zn-transporting ATPase. | ||
Although this has been debated, these domains play a role in the selectivity of metal-transporting ATPases.112,113 Selectivity may be achieved on the one hand by the coordination chemistry imposed, and on the other hand by specific protein–protein interactions between MBDs and other proteins, such as matching cytosolic copper chaperones, or other cytosolic domains of the ATPase, such as the C-terminal region or the phosphorylation domain. Indeed, as mentioned above (2.4.2), metal-binding to the N-terminal MBD in AtHMA2 affects the rate-limiting steps in the enzymatic cycle.59 Ferredoxin-like fold MBDs are also well-known to be able to form homo- and heterodimers, which can be important for metal transfer between domains, but may also serve other functions. For example, the cyanobacterial ScAtx1, a copper chaperone with MBD fold, can interact with the cytosolic MBD domains of two membrane-bound copper transporters, PacS and CtaA, but not with that of the zinc-transporting ZiaA.41 In this case, specific protein complex formation is essential for specific metal transfer. In the case of the well-studied zinc-transporting ATPase ZntA from E. coli, the N-terminal MBD promotes homo-dimerisation,114 which is required for forming a functional transmembrane pump.
Copper-specific modules tend to bind Cu+ using two cysteines from a well-conserved GMxCxxC motif (Fig. 6A), whereas all characterised zinc- and cadmium-binding MBDs such as those found in cyanobacterial ZiaA, Staphylococcus aureus CadA108 and E. coli ZntA109 generally supply at least three ligands. ZntA (Fig. 6C) supplies two oxygens and two sulfurs from a GMDCxxC motif. This coordination environment is very unusual, and there are only two other structures in the pdb with a similar ligand set. It has been speculated that the presence of three negatively charged residues is required to suppress undesired enzymatic activity of the zinc site.115 A fourth binding site may be occupied by an external ligand—in monomeric form, this would usually be a small molecule such as water. Coordination of an amino acid side-chain from a putative partner protein or domain to form a metal-mediated protein complex can also be envisaged.
The eight HMA ATPases from A. thaliana (Table 1), as well as a number of homologous proteins from other plants all contain clearly identifiable N-terminal MBDs (Fig. 7A). The four copper-transporting HMAs (HMA5-HMA8) display the canonical GMxCxxC motif, whereas the Zn-transporting HMAs contain a novel motif, CCxxE. The essentiality of this domain and these three residues for protein function has been demonstrated for A. thaliana HMA2105,115 and HMA4.94Fig. 7B illustrates how Zn2+ might be bound in such a motif.
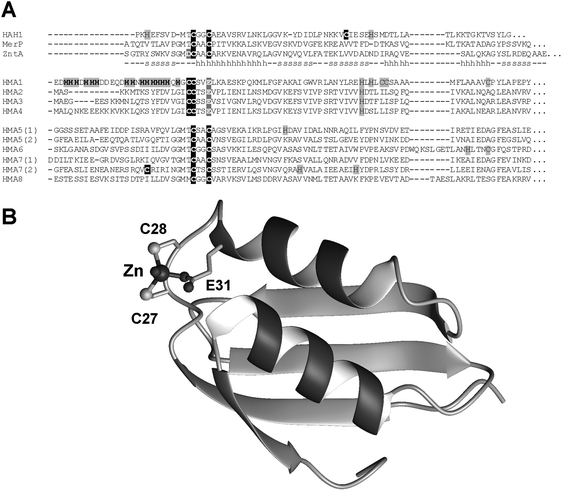 | ||
| Fig. 7 MBD metal-binding domains in plant HMA ATPases. (A) Sequential alignment of MBDs in all eight known HMA ATPases from A. thaliana, together with those of the structurally characterized MBDs shown in Fig. 6. The copper-transporting HMA5 and HMA7 comprise two MBDs each. (B) Homology model of N-terminal MBD of HMA4, based on E. coli ZntA (pdb 1mwz).108 See Methods for details on how the model was generated. | ||
Using competition experiments with the zinc-binding dye mag-fura-2, nanomolar dissociation constants have been determined for Zn-binding MBDs, for example, 21 nM for E. coli ZntA116 and 180 nM for AtHMA2.115 The structures and metal affinities of the MBDs of the Zn-transporting HMA2 and HMA4 and the copper-transporting HMA7, as well as a “chimaeric” mutant protein based on HMA7, but incorporating the ICCTSE metal binding motif found in HMA2 and HMA4, were studied recently.44 This carefully executed study established that all four proteins have a much higher affinity for Cu+ (log K = 16.6–18.2) than for Zn2+ (log K = 9.7–7.6)—which is expected for most metal binding sites that include thiolates, but was in contrast to earlier findings reported in ref. 115. Significantly however, the relative affinities of the wild-type proteins differ: the Zn-transporters have a ca. 30–50-fold lower affinity towards Cu+ than the Cu-transporter, and the affinity of the Cu-transporter towards Zn2+ is ca. 20–30-fold lower than that of the Zn-transporters. This means that in this system, binding of Cu+ to HMA7 is favoured over binding to HMA2 or HMA4, and binding of Zn2+ to HMA2 and HMA4 is favoured over binding to HMA7. These preferences are mediated by the optimal positioning of the two Cys residues for digonal or trigonal planar geometry through the CxxC motif in HMA7, and the presence of three anionic ligands in the ICCTSE motif in HMA2 and 4. Furthermore, tight-binding copper proteins in the cytosol may contribute to exclude binding of Cu+ to HMA2 and HMA4, and as a consequence, HMA2 and HMA4 are left to deal with Zn2+.
There are currently 837 plant protein sequences in Uniprot KB with annotated heavy-metal-associated domains (Pfam accession PF00403); however, the 123 sequences annotated for Arabidopsis thaliana do not include the MBDs of the four Zn-transporting ATPases. A search with the sequences in question against Pfam reveals a weak match to the PF00403 profile, however, with an e-value above the cutoff value used by the Pfam database. Furthermore, at least a quarter of these 123 sequences do not represent MBDs with a known or otherwise obvious metal-binding motif. The omission of sequences, as well as the inclusion of as yet unconfirmed subfamilies of MBDs illustrates why it is still important that sequence and annotation databases are curated manually—not only in the area of metal-binding proteins.
A remote relative of the MBD is present in proteins of the CDF family. Three structures for zinc-transporting CDF proteins are available, the C-terminal cytosolic portion of CzrB from Thermus thermophilus (pdb 3byr and 3byp),117 YiiP from E. coli (Fig. 8A and B; also termed FieF, for ferrous iron efflux facilitator, even though its role in Fe transport is controversial; pdb 2qfi and 3h90),118,119 and a putative CDF protein from Thermotoga maritima (pdb 2zzt; structure without metal ions bound).120 Both CzrB and YiiP belong to the Zn/Fe group as defined by Montanini et al.;72 the Thermotoga protein had not been included in their analysis. Zinc binding to the MBD in YiiP appears to not only enhance dimerisation of two CDF monomers via their MBDs, which is necessary to generate a functional transporter with in total 12 transmembrane helices, but is also crucial to allosterically regulate the geometry of the transmembrane metal-binding site, through the modulation of domain contacts.119 Thus the MBD acts as a metal-sensing device that regulates the transporting activity.
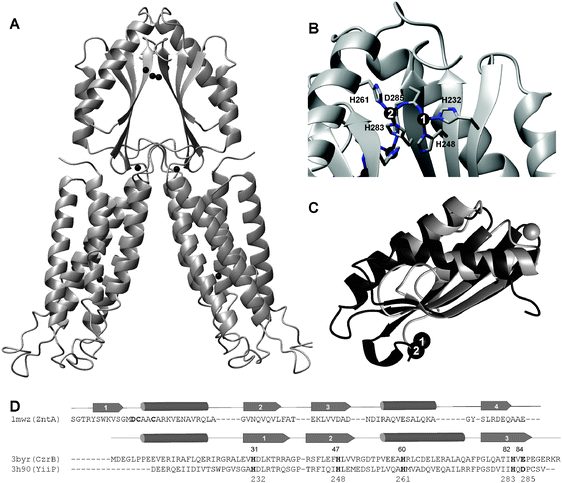 | ||
| Fig. 8 Structural features of CDF transporters. (A) Protein fold and location of zinc ions in E. coli YiiP (pdb 3h90).119 The transmembrane portion is shown in the lower section; the MBDs are at the top. Zinc ions are shown as black spheres. In total, eight Zn2+ ions are present in the dimer, one in each transmembrane region, two in the region at the boundary between transmembrane and cytosolic MBDs, and four at the dimer interface of the two MBDs. (B) Detail of zinc coordination in MBD dimers. Only one of the two pairs is labeled, but the two related zinc ions can be seen in the background as well. (C) Overlay of metal-binding domains of the N-terminal domain of the ATPase ZntA from E. coli (pdb 1mwz, grey) and the CDF protein CzrB from Thermus thermophilus (pdb 3byr, black), demonstrating the similarity in protein fold. Zinc ions are depicted as spheres. Clearly, the three β strands overlay very well, and the relative orientation of the two α helices matches well, although all five major secondary structure elements have different lengths in both proteins (see (D)). Intriguingly, CzrB (and other similar domains in CDF proteins) lacks β strand 1, and instead, β strand 3 takes up the equivalent position. The metal-binding interfaces are completely different. (D) Sequence and secondary structure comparison of metal-binding domains of E. coli ZntA and CDF proteins CzrB from Thermus thermophilus and YiiP from E. coli. Confirmed metal-binding residues are highlighted in bold, and the numbers given refer to YiiP. | ||
The sequence similarity between the MBDs from these CDF proteins and canonical MBDs is negligible (Fig. 8D), but an overlay of the protein backbone folds of CzrB and ZntA reveals their structural relationship (Fig. 8C). Intriguingly, these novel MBD-like domains use a surface for metal binding and dimerisation that is completely different from that found in ATPases or metallochaperones. The dimerisation interface in CDF MBDs comprises three β strands, and in total, four Zn2+ ions are thought to bind to this interface under physiological conditions.117–119 All residues that have been implicated in zinc binding at these sites in the structures of YiiP and CzrB, shown for YiiP in Fig. 8B and highlighted for both YiiP and CzrB sequences in Fig. 8D, are conserved between the two proteins. In the YiiP structure at a resolution of 2.9 Å, Zn1 is bound by His232, His248 and Asp285, and Zn2 by His283 and Asp285 from one monomer, and His261 from the other monomer (Fig. 8B). The CzrB protein structure, at 1.8 Å resolution, shows three zinc ions in this location. Zn1 and Zn2 (Fig. 8C) are bound in a similar fashion as the corresponding ions in YiiP, by His31, His47 and Glu84 for Zn1 (equivalent to His232, His247 and Asp285 in YiiP), and His82, Glu84 from one monomer, and His60 from the other monomer, for Zn2. The additional site, which has been deemed to be non-physiological, comprises Asp32 and His47. The presence of this additional zinc ion can be ascribed to the extremely high Zn2+ concentration that was used in the crystallisation buffer (25 mM). For the crystallisation of YiiP, also a relatively high Zn2+ concentration (5 mM), but a relatively low pH (6.0), had been employed.118
Interestingly, these C-terminal regions had not been considered in the classification of CDF proteins discussed above.72 Instead, overall sequence similarity, putative metal binding residues in transmembrane helices II and V, and the presence of His- or Ser-rich loops were presented as hallmarks for metal specificity. Our own analyses based on the C-terminal domains of sequences for 103 CDF-type proteins from 26 plant species only (Fig. 9A and B and Supplementary Fig. S1 and S2) show that the C-termini are sufficient to achieve a degree and pattern of clustering that is similar to that achieved with full length sequences, with the proteins from A. thaliana falling into four distinct groups. MTP1-5 and MTP12 (confirmed and putative Zn-CDF) on the one hand, and MTP8-11 (Mn-CDF) on the other hand are clearly separated from each other as well as from other branches. Both MTP6 (putative Zn/Fe-CDF) and MTP7 (not assigned) each form part of a separate branch. Within the branch that contains MTP1-5 and MTP12, three sub-branches can be distinguished, with the main branch containing MTP1-3. MTP4 homologues form a separate branch, and MTP5 and MTP12 and their homologues form another separate branch.
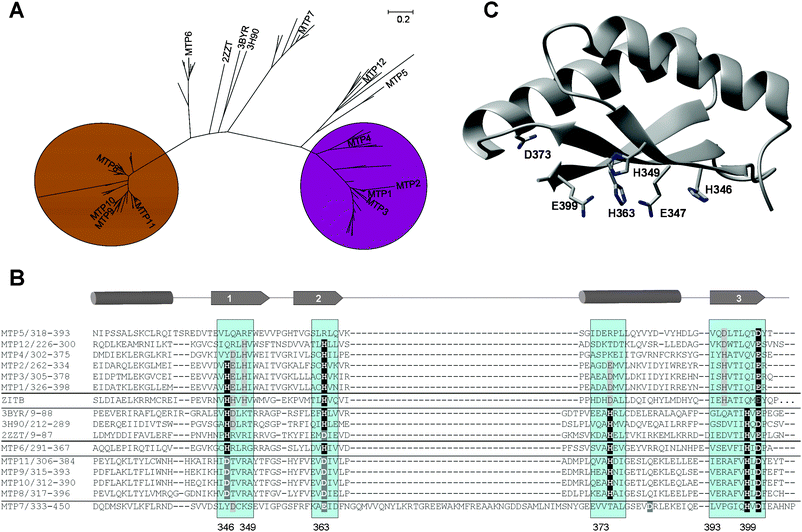 | ||
| Fig. 9 Sequence analysis of C-terminal portions of MTP proteins from green plants. (A) Neighbor-joining phylogenetic tree based on a multiple-sequence alignment of C-terminal domains of MTP proteins from plants. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. Each end of a branch corresponds to one or more of 103 proteins retrieved from UniprotKB. To enhance readability, only A. thaliana MTP proteins and bacterial proteins with 3D structure are labelled; for a fully-labelled tree and sequences, see supplementary Fig. S1 and S2. The C-terminal domains are sufficient to distinguish clearly between Zn- and Mn-transporting proteins. (B) Multiple sequence alignment of C-terminal MBDs of A. thaliana MTP proteins, including comparisons with structurally characterised proteins and E. coli ZitB, a Zn-CDF. Confirmed and putative metal-binding residues and regions are highlighted (see also Table 3). The numbers given refer to MTP1. (C) Homology model of A. thaliana MTP1, based on CzrB (pdb 3byr). Potential metal-binding residues that are (semi-)conserved between Zn-binding MTPs and the template structure (H346, H363, E399 and D373), as well as Glu347 and His349, which are conserved within several Zn-binding MTPs, are shown. | ||
The abridged multiple sequence alignment (Fig. 9B) and the summary given in Table 3 shows that all five metal-binding residues identified in YiiP and CzrB are fully conserved in MTP6, although overall sequence similarity is low. MTP8-11 and their homologues also show semi-conservation of the five metal-binding residues identified in CzrB and YiiP, with full conservation of the C-terminal HxD motif present in Zn/Fe-CDFs, but His232 and His248 (YiiP numbering, see Fig. 8B and D) are replaced by Asp residues. MTP7 and its homologues are characterised by a 42–49 residue long insertion between β-strand 2 and helix 2. Secondary structure prediction using PSIPRED suggests that the insertion adopts α-helical structure. MTP7 homologues also display the C-terminal HxD motif, and Asp or Glu residues in place of His232 and His248. There is no equivalent for the potentially inter-domain His261. In MTP proteins previously classified as Zn-CDFs (MTP1-5 and MTP12), only the residues forming site 1 are (semi-)conserved to some degree: Asp285 (YiiP numbering) is replaced by a glutamate, and His248 is conserved in MTP1,2,3,4 and MTP12, whereas His232 is conserved in MTP1,2 and MTP3 and their homologues only. Potential alternative metal-binding residues in positions 235, 247, and 279 (YiiP numbering) show a high degree of conservation between MTP1-4 and MTP12, and are also present in E. coli ZitB, a confirmed zinc transporter. Apart from the C-terminal carboxylic acid conserved in all MTPs, MTP5 and homologues, which had also been assigned to the Zn-CDF group previously, appear to have lost metal binding residues in most positions, with the exception of 261 and 279.
| Potential metal-binding amino acids in positions corresponding to (YiiP numbering, pdb 3h90) | ||||||||
|---|---|---|---|---|---|---|---|---|
| H232 | H248 | H261 (inter-domain) | H283 | D285 | Pos. 235 | Pos. 247 | Pos. 279 | |
| MTP6 | H | H | H | H | D | — | — | E |
| MTP8-11 | D | D (H) | H | H | D | — | — | — |
| MTP1-3 | H | H | D/E | — | E | H | E (D) | H |
| MTP4 | — | H | — | — | E | H | D | H (N) |
| MTP12 | — | H | — | — | E | H | — | D |
| MTP5 | — | — | E/D | — | E | — | — | D/E |
| MTP7 | — | E | — | (H) | D | — | (D) | — |
In a homology model of MTP1 based on CzrB (pdb 3byr), the three residues conserved in MTP1-3 (232, 247 and 285 in YiiP, Fig. 8B and D-corresponding to 346, 363, and 399 in MTP1, Fig. 9B), together with Glu347 and His349 may form a binuclear zinc site like Zn/Fe-CDFs (Fig. 9C). The Cys residue adjacent to His346 is only conserved in MTP1-4 and is buried in our model. It should be noted that this is a consequence of the sequence alignment used for modelling; alternative alignments are in principle possible and may produce structures with exposed Cys.
None of the six putative Zn-CDFs have the bridging His261 (YiiP numbering); instead, we find an Asp or Glu residue in this position in MTP1-3, as well as Asp or Glu residues in adjacent positions in MTP4,5 and 12, which could in principle adopt a similar role. Alternatively, His393 (MTP1 numbering), which is semi-conserved in all six putative Zn-CDFs, is too far away from the other residues in our monomeric model but might provide a different inter-domain contact.
In summary, Zn-CDFs are distinguished from all other CDFs by the absence of the C-terminal H × D motif, which is replaced by a lone Glu residue, and by the absence of the bridging His261 residue, which is replaced by a carboxylic acid or other residues. Furthermore, Mn-CDFs are distinguished from other CDFs by the replacement of two His residues by Asp residues, but the positioning of metal-binding residues appears to be similar to that of Zn/Fe-CDFs. It is highly likely that coordination geometry in these domains plays an important role in metal selectivity. Therefore it is very important that a high-resolution structure for at least one example from each sub-group is determined.
Thus, in CDFs from plants, three hallmarks for zinc specificity can be discerned. (i) Two of the four binding residues in transmembrane helices show a prevalence for His residues, (ii) zinc-transporting CDFs show a trend towards containing cytosolic His-rich loops, whilst these are not present in Mn-transporters, and finally, (iii) the strongest candidate for exercising a discriminatory role are the C-terminal cytosolic portions of CDFs.
In conclusion, for both HMAs and CDFs, metal specificity appears to be encoded mainly in cytosolic domains. Metal mediated dimerisation or domain interactions emerge as a salient feature of these exporters from the cytosol.
2.5 Metallothioneins
Metallothioneins, as the name suggests, are proteins with a high content of metal ions and sulfur. When the first plant metallothioneins (MTs) were discovered in the late 1980's,121,122 their existence was initially debated, as it was thought that the roles of MTs were fulfilled by phytochelatins in plants.123 The latter are non-ribosomally synthesised polypeptides, which do indeed play roles in resistance against cadmium toxicity,50 which was also thought to be a major role for animal MTs for several decades. Both of these notions have been revised: it is clear that MTs generally have much more fundamental roles than detoxifying cadmium, although some of them can certainly fulfil such a role, as demonstrated for MTs from the Zn and Cd hyperaccumulator Thlaspi caerulescens.103,124 Furthermore, the existence and regulated expression of metallothioneins in plants in a variety of tissues is now well-documented.50,125,126 For example, MT-2a, 2b, and 3 in A. thaliana are constitutively expressed at very high levels,63 and some plant transcriptomes are even dominated by metallothionein expression, e.g. that of ripening pineapple fruit.127One of the major proposed functions of metallothioneins in higher animals is their participation in the intracellular (re)-distribution of zinc.37 In plants, MTs have been linked to a variety of biotic and abiotic stresses, but also embryogenesis128 and pollen maturation,129 tissue formation,130 fruit ripening,127 leaf senescense,131 the desiccation and rehydration of seeds132 and other tissues in drought-tolerant and resurrection plants,133,134 and drought resistance in general.135 Thus, genetic and transcriptomic studies have demonstrated clearly that MTs play significant roles throughout the lifecycle of a variety of plants, but how they perform these functions on a molecular level remains poorly understood.
The sequence diversity of plant MTs is much greater than that of animal, fungal, or bacterial MTs. We hypothesise that although regulation by expression levels as well as access may play a role in determining which metal ion is bound in vivo, at least a portion of metal specificity may be encoded in the protein sequence—an idea that has been promoted extensively by Capdevila and colleagues.136 It should be noted that thermodynamically, Cu+ is the most competitive metal ion for pure thiolate coordination amongst the three metal ions that are commonly found in MTs (Cu+, Zn2+, Cd2+), followed by Cd2+, with Zn2+ being the least competitive. Due to the inherent high flexibility of the protein structures of metallothioneins, MTs usually can adapt to the different coordination geometries required by Cu+ on the one hand (linear or trigonal) and Zn2+ or Cd2+ on the other hand (tetrahedral). For these reasons, it is expected that any MT will be able to bind Cu+ to some extent. Nevertheless, recent work shows that metallothioneins can display preferences for a specific metal ion,136 and mostly, this preference can be accomplished through (i) employing coordinating residues other than cysteine, and (ii) differences in folding, fold stability and backbone dynamics in dependence on the bound metal ions.137
So far, four types of plant MTs have been defined in the literature,125,126 according to the number and arrangement of Cys residues, which are the major metal-binding residues in MTs. MTs 1,2, and 3 have two Cys-rich stretches joined by regions of variable length that are devoid of Cys residues, whereas MT-4 (also classified as “pec”, for plant early cysteine-labelled) has three such stretches. The classification scheme developed by Binz and Kägi, which is based on sequence similarity as well as an analysis of upstream regions of the respective MT gene, distinguishes five subdivisions,138 but this refined system has not yet been adopted widely. Fig. 10 shows the sequences of the seven MTs expressed in A. thaliana, together with metallothioneins from Oryza sativa ssp. Japonica (one of the rice species with sequenced genome), soybean as a representative for non-brassicacea dicots, and a few selected sequences from other species, for which functional data is available.
 | ||
| Fig. 10 Diversity of metallothioneins in plants. Sequences of A. thaliana, rice (Oryza sativa, ssp. japonica) and soybean metallothioneins are shown with a selection of metallothioneins from other species mentioned in the text. The four types of plant MT isoforms defined previously126 are based on overall sequence similarity, in particular on the arrangement of cysteine residues in the primary sequence. Note that, according to Binz and Kägi,138A. thaliana MT-1A and -1B are classified as p21, MT-2A and 2B belong to the p2 family, and MT-4A and -4B are classified as pec. | ||
In order to identify structure-function relationships for plant MTs, the classification system may need to be revisited, as the addition of more and more sequences has revealed more variations yet. Even the very limited selection shown in Fig. 10 demonstrates that within the four subclasses, there are significant differences in the number and spacing of metal-binding residues. These differences are prone to have an impact on metal-binding properties including specificity of these proteins. The picture is complicated by the fact that inconsistencies in classification in various protein databases are common; for example, three MTs in rice with clear similarities to MT-1s in the C-terminal part have been classified as MT-4a, b, and c, presumably in recognition of the fact that their N-terminal domains display different Cys motifs, but overlooking that MT-4 was already defined otherwise, and conversely, the “real” MT-4 in rice is listed as MT-21a. Similarly, proteins classified as MT-2 in rice have the hallmark eight Cys residue in the N-terminal stretch, but the number of Cys residues in the C-terminal stretch varies between six and nine, and there are also variations in the Cys motifs.
Recent functional studies on six A. thaliana metallothioneins have revealed that MT-1a, 2a, 2b, and 3 are likely to function as Cu-MTs, whereas MT-4a and 4b are more likely to be Zn-binding.139 The rice protein labelled OsMT-1a, in contrast, has been shown to have a role in zinc homeostasis and drought tolerance.135 Comparison of the sequences of AtMT-1a and OsMT-1a (Fig. 10) reveals that although their N-termini are extremely similar, the C-terminal portions of these proteins are quite different. AtMT-1a has seven Cys residues, whereas OsMT-1a has six Cys and one His residue, with different spacing between these putative ligands. It should also be noted that MT-1s in brassicaceae differ from other MTs of this family in the much shorter length of their “linkers”.
Proteins in the group MT-3 may also display differences in metal specificity. Functional data for oil palm MT-3a points towards a role in Zn handling,140 whereas MT-3 from A. thaliana141 is copper-responsive, and that from cotton142 has been shown to bind copper. Comparing MT-3 sequences in Fig. 10 shows that again, N-termini are well-conserved, whereas there are at least three different patterns for the C-terminal section. Significantly, the oil palm MT-3a sequence contains a His residue close to the Cys-rich stretch. This residue is also present in MT-3 sequences from fleshy fruit such as banana and pineapple.
Our knowledge on molecular properties and mechanisms of plant metallothioneins is very limited, although this lack has begun to be remedied by several groups in the past five years. Work in this field has been reviewed recently,143 and one of the most striking aspects of this review are the reported discrepancies regarding the metal ion contents of the isolated proteins. For example, reported values for zinc contents of type 1 MTs range from 4.0 to 11.5, those for MT-3 from 1.7 to 3.6, and those for MT-4 from 2.4 to 6.2. These numbers highlight once more that something as seemingly simple as determining the metal ion stoichiometry of a metalloprotein is less trivial than it may seem. Furthermore, with the exception of the MT-4 group, which are very clearly Zn-binding MTs, there is also no consensus on the natively bound metal ion(s). No complete 3D structures are available for any plant metallothionein, but a model for domain 2 of the prototype of MT-4, wheat EC-I, has been published recently.144 Structural studies of MTs are hampered by their relatively high flexibility, which makes the production of crystals for X-ray diffraction analysis very challenging. In the case of wheat EC, progress towards a definitive structure via solution NMR spectroscopy has so far been impeded by the absence of a defined protein fold for domain 2 in the presence of Cd2+,137 which is required as a structural probe for the spectroscopically silent Zn2+. Significantly, EC contains an isolated metal site with Cys2His2 coordination, and such sites are expected to prefer Zn2+ over Cd2+.145 The importance of the two His residues and the isolated site in guaranteeing an ordered protein structure in the presence of Zn2+, and a disordered one in the presence of Cd2+, has been investigated recently,146 and we suggest that these differences in metal-mediated protein folding are a direct consequence of Pearson's HSAB principle. Wheat EC is exclusively expressed in the developing embryo and pollen.128,147 We hypothesise that EC may play an important role in activating zinc-dependent processes after seed dormancy. The deleterious effects of Cd2+ incorporation into Zn-requiring proteins such as zinc fingers are well known.148 The “reluctance” of EC to fold stably in the presence of Cd2+ may be a safeguard against storage of the wrong metal ion.
3. Perspectives
In summary, what seems to be clear at this stage is that Zn2+ in plants, no matter whether extra- or intracellularly, always occurs bound to a chelator. This means that all movements of Zn2+ (as that of several other metal ions) are a series of ligand-exchange reactions, and that a true understanding of these movements must entail an understanding of these reactions on a molecular level.Therefore, we require more knowledge on (i) the start and end points of these reactions, ideally in the form of 3D structures, (ii) reliable, well-defined thermodynamic data of zinc binding to the various chelators—including membrane-bound transporters, (iii) and the kinetics of the zinc transfer reactions. At present, most of these data are not available, and it can be argued that a “systems” approach cannot truly succeed until the underlying chemistry is understood and quantified. This view is emphasized by the fact that current modelling efforts suffer from lack of reliable data, e.g. Broadley et al.9 conclude that the kinetic parameters determined for zinc fluxes into plants (e.g. KM values for Michaelis–Menten kinetics) are flawed for most experiments reported in the literature.
Hence, advanced methods to produce reliable structural and quantitative data need to be further developed—and this must include not only developments in instrumentation, but dedicated efforts to improve sample preparation. This review has shown that the acquisition of quantitative data is still far from trivial, explaining the conspicuous scarcity of reliable parameters for modelling. In such cases where data have been acquired, there often are large discrepancies between the results of various labs. A further problem is that retrieval of such data from the literature tends to be laborious. Ultimately, it would be desirable to have a concerted effort in all relevant disciplines, including information management. The clear need for quantitative data to understand metal ion homeostasis and its impacts, and the issues raised, call for an increase in research efforts as well as a new curated “Metallomics” database with such data.
It may be envisaged that this new database should be populated by (i) functional in vivo data including transcriptomics, proteomics, and tissue distributions of relevant proteins, (ii) correlated with “simple” analytical data, e.g. metal concentrations in various tissues, (iii) speciation data, both in terms of identity and concentration of species, (iv) measurements of transport in whole plants as well as cells, and (v) detailed information on metal ion–protein (or small molecule) interactions including thermodynamic and kinetic constants.
Partially, such efforts are already being realised by the excellent Purdue Ionomics Information Management System,149 but this is dedicated to functional genomics, and a more comprehensive integration of “chemical” data, especially to tackle points (iii) and (v), remains to be accomplished.
4. Methods
4.1 Sequence retrieval and analysis
Known sequences for A. thaliana HMA, ZIP and metallothionein proteins were retrieved from UniprotKB and aligned using ClustalW.150 The Transport Classification Database (TC-DB),151 TransportDB,152 and Pfam153 were also consulted to ensure that datasets were as comprehensive as possible. For the detailed analysis reported in section 2.4.3, all known A. thaliana MTP sequences were downloaded from UniprotKB and submitted to BLAST154 searches against the Viridiplantae subset of the UniprotKB database. Hits were combined, fragments and redundant sequences were removed and sequences were aligned using MUSCLE,155 followed by manual adjustments. The C-terminal domains of A. thaliana MTPs were subjected to a HHPRED156 search which confirmed putative homology to structurally characterised bacterial CDFs (pdb entries 3h90/2qfi, 3byr/3byp, 2zzt). The sequences of these pdb entries were included into the multiple sequence alignment. Phylogenetic analyses were conducted in MEGA4,157 using the Neighbor-Joining and Maximum Parsimony algorithms.158 All positions containing gaps were eliminated from the underlying alignment.4.2 Comparative modelling
Comparative modeling was carried out using Modeller version 9.7.159 Conformer 15 of pdb 1mwz (E. coli ZntA; determined by NMR)108 was used as a template for building a model of the N-terminal MBD of A. thaliana HMA4. This conformer had been identified as representative using Olderado.160 Template and model sequences were aligned manually. Sidechains and improper dihedrals were optimised with Scwrl 3.0.161 Hydrogens, the Zn ion, and metal–ligand bonds were added in MOE v. 2004.03. Energy minimisation of the final model was performed in MOE using a customised AMBER94 force-field. Thermus thermophilus CzrB (pdb 3byr, X-ray crystal structure @ 1.8 Å) served as a template for modelling of the C-terminal MBD of A. thaliana MTP1. The sequence alignment used for modelling was adjusted manually, employing insights gained from multiple sequence alignments of all available plant CDF proteins. Definitive assignment of metal ligands was not possible; therefore, no further model optimisation was carried out. Final structures were validated using the WHATIF server.162Structural images (Fig. 6–8) were generated with MOLMOL v.2 K.2.163 The structural alignment for Fig. 8C was carried out in Swiss pdb viewer, v. 3.7.
List of abbreviations
| CDF | cation-diffusion facilitator |
| CE | cation efflux |
| HMA | Heavy-metal associated (abbreviation for both ATPases and metal-binding domains) |
| IRT | iron-regulated transporter |
| MBD | metal binding domain (usually referring to those with ferredoxin fold) |
| MTP | metal tolerance protein (usually of the CDF family) |
| NA | nicotianamine |
| OPT | oligopeptide transporter |
| YSL | yellow-stripe-like |
| ZIP | Zinc/iron permease or ZRT/IRT protein |
| ZRT | zinc-regulated transporter |
References
- Anon, Mol. Plant, 2008, 1, 561–563 Search PubMed.
- L. Williams and D. E. Salt, Curr. Opin. Plant Biol., 2009, 12, 247–249 CrossRef CAS.
- W. R. Chen, X. Yang, Z. L. He, Y. Feng and F. H. Hu, Physiol. Plant., 2008, 132, 89–101 CAS.
- M. R. Badger and G. D. Price, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1994, 45, 369–392 CrossRef CAS.
- R. Hänsch and R. R. Mendel, Curr. Opin. Plant Biol., 2009, 12, 259–266 CrossRef CAS.
- C. Huang, S. J. Barker, P. Langridge, F. W. Smith and R. D. Graham, Plant Physiol., 2000, 124, 415–422 CrossRef CAS.
- I. Bertini and A. Rosato, Eur. J. Inorg. Chem., 2007, 2546–2555 CrossRef CAS.
- www.arabidopsis.org/ .
- M. R. Broadley, P. J. White, J. P. Hammond, I. Zelko and A. Lux, New Phytol., 2007, 173, 677–702 Search PubMed.
- S. Ciftci-Yilmaz and R. Mittler, Cell. Mol. Life Sci., 2008, 65, 1150–1160 CrossRef CAS.
- G. Hacisalihoglu and L. V. Kochian, New Phytol., 2003, 159, 341–350 Search PubMed.
- Micronutrient deficiencies in global crop production, ed. B.J. Alloway, Springer, 2008, p. 27 Search PubMed.
- I. Cakmak, Proceedings of the International Fertilizer Society, 2004, 552, 1–26 Search PubMed.
- http://www.copenhagenconsensus.com/Projects/Copenhagen%20Consensus%202008/Outcome.aspx..
- W. Maret and H. H. Sandstead, Exp. Gerontol., 2008, 43, 378–381 CrossRef CAS.
- Z. Peleg, Y. Saranga, A. Yazici, T. Fahima, L. Ozturk and I. Cakmak, Plant Soil, 2008, 306, 57–67 CrossRef CAS.
- M. L. Guerinot and D. E. Salt, Plant Physiol., 2001, 125, 164–167 CrossRef CAS.
- M. G. Palmgren, S. Clemens, L. E. Williams, U. Kraemer, S. Borg, J. K. Schjorring and D. Sanders, Trends Plant Sci., 2008, 13, 464–473 CrossRef CAS.
- C. M. Palmer and M. L. Guerinot, Nat. Chem. Biol., 2009, 5, 333–340 CrossRef CAS.
- N. Grotz and M. L. Guerinot, Biochim. Biophys. Acta, Mol. Cell Res., 2006, 1763, 595–608 CrossRef CAS.
- E. P. Colangelo and M. L. Guerinot, Curr. Opin. Plant Biol., 2006, 9, 322–330 CrossRef CAS.
- J. L. Hall and L. E. Williams, J. Exp. Bot., 2003, 54(393), 2601–2613 CrossRef CAS.
- U. Krämer, I. N. Talke and M. Hanikenne, FEBS Lett., 2007, 581, 2263–2272 CrossRef.
- R. P. Sankaran, T. Huguet and M. A. Grusak, Theor. Appl. Genet., 2009, 119, 241–253 CrossRef CAS.
- C. A. Blindauer, I. Harvey, K. E. Bunyan, A. J. Stewart, D. Sleep, D. J. Harrison, S. Berezenko and P. J. Sadler, J. Biol. Chem., 2009, 284, 23116–23124 CrossRef CAS.
- T. Hirano, M. Murakami, T. Fukada, K. Nishida, S. Yamasaki and T. Suzuki, Adv. Immunol., 2008, 97, 149–176 Search PubMed.
- C. J. Frederickson, J. Y. Koh and A. I. Bush, Nat. Rev. Neurosci., 2005, 6, 449–462 CrossRef CAS.
- A. Helmersson, S. von Arnold and P. V. Bozhkov, Plant Physiol., 2008, 147, 1158–1167 CrossRef CAS.
- H. L. Axelrod, E. C. Abresch, M. L. Paddock, M. Y. Okamura and G. Feher, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1542–1547 CrossRef CAS.
- K. Muramoto, K. Hirata, K. Shinzawa-Itoh, S. Yoko-O, E. Yamashita, H. Aoyama, T. Tsukihara and S. Yoshikawa, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7881–7886 CrossRef CAS.
- W. Maret, BioMetals, 2009, 22, 149–157 Search PubMed.
- H. Irving and R. J. P. Williams, J. Chem. Soc., 1953, 3192–3210 RSC.
- K. J. Waldron and N. J. Robinson, Nat. Rev. Microbiol., 2009, 7, 25–35 Search PubMed.
- K. J. Waldron, J. C. Rutherford, D. Ford and N. J. Robinson, Nature, 2009, 460, 823–830 CrossRef CAS.
- L. A. Finney and T. V. O'Halloran, Science, 2003, 300, 931–936 CrossRef CAS.
- C. E. Outten and T. V. O'Halloran, Science, 2001, 292, 2488–2492 CrossRef CAS.
- A. Krezel and W. Maret, JBIC, J. Biol. Inorg. Chem., 2006, 11, 1049–1062 CrossRef CAS.
- D. J. Eide, Biochim. Biophys. Acta, Mol. Cell Res., 2006, 1763, 711–722 CrossRef CAS.
- D. H. Petering, personal communication.
- S. Tottey, D. R. Harvie and N. J. Robinson, Acc. Chem. Res., 2005, 38, 775–783 CrossRef CAS.
- L. Banci, I. Bertini, S. Ciofi-Baffoni, N. G. Kandias, N. J. Robinson, G. A. Spyroulias, X. C. Su, S. Tottey and M. Vanarotti, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8320–8325 CrossRef CAS.
- S. Tottey, K. J. Waldron, S. J. Firbank, B. Reale, C. Bessant, K. Sato, T. R. Cheek, J. Gray, M. J. Banfield, C. Dennison and N. J. Robinson, Nature, 2008, 455, 1138–U1117 CrossRef CAS.
- R. A. Colvin, A. I. Bush, I. Volitakis, C. P. Fontaine, D. Thomas, K. Kikuchi and W. R. Holmes, Am. J. Physiol.: Cell Physiol., 2008, 294, C726–C742 CrossRef CAS.
- M. Zimmermann, O. Clarke, J. M. Gulbis, D. W. Keizer, R. S. Jarvis, C. S. Cobbett, M. G. Hinds, Z. Xiao and A. G. Wedd, Biochemistry, 2009, 48, 11640–11654 CrossRef CAS.
- D. E. Wilcox, Inorg. Chim. Acta, 2008, 361, 857–867 CrossRef CAS.
- A. Prange and D. Proefrock, J. Anal. At. Spectrom., 2008, 23, 432–459 RSC.
- B. M. Waters and M. A. Grusak, New Phytol., 2008, 177, 389–405 Search PubMed.
- Z. Rengel, Xylem and phloem transport of micronutrients, in Dev. Plant Soil Sci., 2001, vol. 92, pp. 628–629 Search PubMed.
- O. Riesen and U. Feller, J. Plant Nutr., 2005, 28, 421–430 CrossRef CAS.
- W. E. Rauser, Cell Biochem. Biophys., 1999, 31, 19–48 CrossRef CAS.
- M. J. Haydon and C. S. Cobbett, New Phytol., 2007, 174, 499–506 Search PubMed.
- R. D. Graham and J. C. R. Stangoulis, J. Nutr., 2003, 133, 1502S–1505S CAS.
- M. C. White, A. M. Decker and R. L. Chaney, Plant Physiol., 1981, 67, 292–300 CrossRef CAS.
- G. L. Mullins, L. E. Sommers and T. L. Housley, Plant Soil, 1986, 96, 377–391 CrossRef CAS.
- P. Mäser, S. Thomine, J. I. Schroeder, J. M. Ward, K. Hirschi, H. Sze, I. N. Talke, A. Amtmann, F. J. M. Maathuis, D. Sanders, J. F. Harper, J. Tchieu, M. Gribskov, M. W. Persans, D. E. Salt, S. A. Kim and M. L. Guerinot, Plant Physiol., 2001, 126, 1646–1667 CrossRef CAS.
- N. Grotz, T. Fox, E. Connolly, W. Park, M. L. Guerinot and D. Eide, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 7220–7224 CrossRef CAS.
- B. H. Eng, M. L. Guerinot, D. Eide and M. H. Saier, Jr., J. Membr. Biol., 1998, 166, 1–7 CrossRef CAS.
- L. E. Williams and R. F. Mills, Trends Plant Sci., 2005, 10, 491–502 CrossRef CAS.
- J. M. Argüello, E. Eren and M. Gonzalez-Guerrero, BioMetals, 2007, 20, 233–248 Search PubMed.
- http://www.membranetransport.org/ .
- L. A. Gaither and D. J. Eide, J. Biol. Chem., 2000, 275, 5560–5564 CrossRef CAS.
- Y. O. Korshunova, D. Eide, W. G. Clark, M. L. Guerinot and H. B. Pakrasi, Plant Mol. Biol., 1999, 40, 37–44 CrossRef CAS.
- H. Wintz, T. Fox, Y. Y. Wu, V. Feng, W. Q. Chen, H. S. Chang, T. Zhu and C. Vulpe, J. Biol. Chem., 2003, 278, 47644–47653 CrossRef CAS.
- Y. F. Lin, H. M. Liang, S. Y. Yang, A. Boch, S. Clemens, C. C. Chen, J. F. Wu, J. L. Huang and K. C. Yeh, New Phytol., 2009, 182, 392–404 Search PubMed.
- C. Varotto, D. Maiwald, P. Pesaresi, P. Jahns, F. Salamini and D. Leister, Plant J., 2002, 31, 589–599 CrossRef CAS.
- D. Hussain, M. J. Haydon, Y. Wang, E. Wong, S. M. Sherson, J. Young, J. Camakaris, J. F. Harper and C. S. Cobbett, Plant Cell, 2004, 16, 1327–1339 CrossRef CAS.
- Y. Y. Kim, H. Choi, S. Segami, H. T. Cho, E. Martinoia, M. Maeshima and Y. Lee, Plant J., 2009, 5, 5.
- C. K. E. Wong and C. S. Cobbett, New Phytol., 2009, 181, 71–78 Search PubMed.
- M. Morel, J. Crouzet, A. Gravot, P. Auroy, N. Leonhardt, A. Vavasseur and P. Richaud, Plant Physiol., 2008, 149, 894–904 CrossRef.
- J. L. Gustin, M. E. Loureiro, D. Kim, G. Na, M. Tikhonova and D. E. Salt, Plant J., 2009, 57, 1116–1127 CrossRef CAS.
- S. Arrivault, T. Senger and U. Krämer, Plant J., 2006, 46, 861–879 CrossRef CAS.
- B. Montanini, D. Blaudez, S. Jeandroz, D. Sanders and M. Chalot, BMC Genomics, 2007, 8, 107 CrossRef.
- E. Delhaize, B. D. Gruber, J. K. Pittman, R. G. White, H. Leung, Y. S. Miao, L. W. Jiang, P. R. Ryan and A. E. Richardson, Plant J., 2007, 51, 198–210 CrossRef CAS.
- C. Curie, G. Cassin, D. Couch, F. Divol, K. Higuchi, M. Jean, J. Misson, A. Schikora, P. Czernic and S. Mari, Ann. Bot., 2008, 103, 1–11 CrossRef.
- G. Schaaf, A. Schikora, J. Haberle, G. Vert, U. Ludewig, J. F. Briat, C. Curie and N. von Wiren, Plant Cell Physiol., 2005, 46(5), 762–774 CrossRef CAS.
- M. Takahashi, Y. Terada, I. Nakai, H. Nakanishi, E. Yoshimura, S. Mori and N. K. Nishizawa, Plant Cell, 2003, 15, 1263–1280 CrossRef CAS.
- N. von Wiren, S. Klair, S. Bansal, J. F. Briat, H. Khodr, T. Shioiri, R. A. Leigh and R. C. Hider, Plant Physiol., 1999, 119, 1107–1114 CrossRef CAS.
- A. Pich, S. Hillmer, R. Manteuffel and G. Scholz, J. Exp. Bot., 1997, 48, 759–767 CrossRef CAS.
- U. W. Stephan, I. Schmidke, V. W. Stephan and G. Scholz, BioMetals, 1996, 9, 84–90 CrossRef CAS.
- G. Anderegg and H. Ripperger, J. Chem. Soc., Chem. Commun., 1989, 647–650 RSC.
- I. Benes, K. Schreiber, H. Ripperger and A. Kircheiss, Experientia, 1983, 39, 261–262 CrossRef CAS.
- IUPAC Stability Constants Database, Data version 4.56 compiled by L.D. Pettitt and H.K.J. Powell, Timble, Otley, UK, 2005.
- A. Trampczynska, H. Küpper, W. Meyer-Klaucke, H. Schmidt and S. Clemens, Metallomics, 2010, 2, 57–66 RSC.
- M. J. Haydon and C. S. Cobbett, Plant Physiol., 2007, 143, 1705–1719 CrossRef CAS.
- W. Maret, Metallomics, 2010, 2, 117–125 RSC.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3543 CrossRef CAS.
- D. P. Giedroc and A. I. Arunkumar, Dalton Trans., 2007, 3107–3120 RSC.
- A. K. Wernimont, D. L. Huffman, A. L. Lamb, T. V. O'Halloran and A. C. Rosenzweig, Nat. Struct. Biol., 2000, 7, 766–771 CrossRef CAS.
- D. R. Campbell, K. E. Chapman, K. J. Waldron, S. Tottey, S. Kendall, G. Cavallaro, C. Andreini, J. Hinds, N. G. Stoker, N. J. Robinson and J. S. Cavet, J. Biol. Chem., 2007, 282, 32298 CrossRef CAS.
- E. E. Rogers, D. J. Eide and M. L. Guerinot, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 12356–12360 CrossRef CAS.
- F. Arnesano, L. Banci, I. Bertini, F. Capozzi, S. Ciofi-Baffoni, S. Ciurli, C. Luchinat, S. Mangani, A. Rosato, P. Turano and M. S. Viezzoli, Coord. Chem. Rev., 2006, 250, 1419–1450 CrossRef CAS.
- I. Ascone, R. Fourme, S. Hasnain and K. Hodgson, J. Synchrotron Radiat., 2004, 12, 1–3 CrossRef.
- M. Kawachi, Y. Kobae, T. Mimura and M. Maeshima, J. Biol. Chem., 2008, 283, 8374–8383 CrossRef CAS.
- F. Verret, A. Gravot, P. Auroy, S. Preveral, C. Forestier, A. Vavasseur and P. Richaud, FEBS Lett., 2005, 579, 1515–1522 CrossRef CAS.
- X. Q. Mao, B. E. Kim, F. Wang, D. J. Eide and M. J. Pettis, J. Biol. Chem., 2006, 282, 6992–7000 CrossRef.
- B. Milon, Q. Wu, J. Zou, L. C. Costello and R. B. Franklin, Biochim. Biophys. Acta, Biomembr., 2006, 1758, 1696–1701 CrossRef CAS.
- R. G. Ge, Y. Zhang, X. S. Sun, R. M. Watt, Q. Y. He, J. D. Huang, D. E. Wilcox and H. Z. Sun, J. Am. Chem. Soc., 2006, 128, 11330–11331 CrossRef CAS.
- M. Hara, M. Fujinaga and T. Kuboi, J. Exp. Bot., 2005, 56, 2695–2703 CrossRef CAS.
- H. Küpper, A. Mijovilovich, W. Meyer-Klaucke and P. M. H. Kroneck, Plant Physiol., 2004, 134, 748–757 CrossRef.
- D. E. Salt, R. C. Prince, A. J. M. Baker, I. Raskin and I. J. Pickering, Environ. Sci. Technol., 1999, 33, 713–717 CrossRef CAS.
- N. E. Grossoehme, S. Akilesh, M. L. Guerinot and D. E. Wilcox, Inorg. Chem., 2006, 45, 8500–8508 CrossRef CAS.
- A. Krezel and W. G. Maret, JBIC, J. Biol. Inorg. Chem., 2008, 13, 401–409 CrossRef CAS.
- A. Papoyan and L. V. Kochian, Plant Physiol., 2004, 136, 3814–3823 CrossRef CAS.
- E. Eren, D. C. Kennedy, M. J. Maroney and J. M. Argüello, J. Biol. Chem., 2006, 281, 33881–33891 CrossRef CAS.
- C. K. E. Wong, R. S. Jarvis, S. M. Sherson and C. S. Cobbett, New Phytol., 2009, 181, 79–88 Search PubMed.
- Z. Ma, F. E. Jacobsen and D. P. Giedroc, Chem. Rev., 2009, 109, 4644–4681 CrossRef CAS.
- L. Banci, I. Bertini, S. Ciofi-Baffoni, X. C. Su, G. P. M. Borrelly and N. J. Robinson, J. Biol. Chem., 2004, 279, 27502–27510 CrossRef CAS.
- L. Banci, I. Bertini, S. Ciofi-Baffoni, X. C. Su, R. Miras, N. Bal, E. Mintz, P. Catty, J. E. Shokes and R. A. Scott, J. Mol. Biol., 2006, 356, 638–650 CrossRef CAS.
- L. Banci, I. Bertini, S. Ciofi-Baffoni, L. A. Finney, C. E. Outten and T. V. O'Halloran, J. Mol. Biol., 2002, 323, 883–897 CrossRef CAS.
- R. A. Steele and S. J. Opella, Biochemistry, 1997, 36, 6885–6895 CrossRef CAS.
- F. Arnesano, L. Banci, I. Bertini, S. Ciofi-Baffoni, E. Molteni, D. L. Huffman and T. V. O'Halloran, Genome Res., 2002, 12, 255–271 CrossRef CAS.
- O. Lewinson, A. T. Lee and D. C. Rees, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4677–4682 CrossRef CAS.
- G. P. M. Borrelly, S. A. M. Rondet, S. Tottey and N. J. Robinson, Mol. Microbiol., 2004, 53, 217–227 CrossRef CAS.
- Z. Hou and B. Mitra, J. Biol. Chem., 2003, 278, 28455–28461 CrossRef CAS.
- E. Eren, M. Gonzalez-Guerrero, B. M. Kaufman and J. M. Argüello, Biochemistry, 2007, 46, 7754–7764 CrossRef CAS.
- J. B. Liu, S. J. Dutta, A. J. Stemmler and B. Mitra, Biochemistry, 2006, 45, 763–772 CrossRef CAS.
- V. Cherezov, N. Hofer, D. M. E. Szebenyi, O. Kolaj, J. G. Wall, R. Gillilan, V. Srinivasan, C. P. Jaroniec and M. Caffrey, Structure, 2008, 16, 1378–1388 CrossRef CAS.
- M. Lu and D. Fu, Science, 2007, 317, 1746–1748 CrossRef CAS.
- M. Lu, J. Chai and D. Fu, Nat. Struct. Mol. Biol., 2009, 16, 1063–1081 CrossRef CAS.
- T. Higuchi, M. Hattori, Y. Tanaka, R. Ishitani and O. Nureki, Proteins: Struct., Funct., Bioinf., 2009, 76, 768–771 Search PubMed.
- B. Lane, R. Kajioka and T. Kennedy, Biochem. Cell Biol., 1987, 65, 1001–1005 Search PubMed.
- I. M. Evans, L. N. Gatehouse, J. A. Gatehouse, N. J. Robinson and R. R. D. Croy, FEBS Lett., 1990, 262, 29–32 CrossRef CAS.
- N. J. Robinson and P. J. Jackson, Physiol. Plant., 1986, 67, 499–506 CrossRef CAS.
- N. Roosens, R. Leplae, C. Bernard and N. Verbruggen, Planta, 2005, 222, 716–729 CrossRef CAS.
- N. J. Robinson, A. M. Tommey, C. Kuske and P. J. Jackson, Biochem. J., 1993, 295, 1–10 CAS.
- C. Cobbett and P. Goldsbrough, Annu. Rev. Plant Biol., 2002, 53, 159–182 CrossRef CAS.
- R. Moyle, D. J. Fairbairn, J. Ripi, M. Crowe and J. R. Botella, J. Exp. Bot., 2005, 56, 101–112 CAS.
- I. Kawashima, T. D. Kennedy, M. Chino and B. G. Lane, Eur. J. Biochem., 1992, 209, 971–976 CrossRef CAS.
- T. L. Reynolds and R. L. Crawford, Plant Mol. Biol., 1996, 32, 823–829 CrossRef CAS.
- S. Fujimaki, E. Akahoshi, K. Takahashi, H. Kim, T. Fujiwara and M. Chino, Soil Sci. Plant Nutr., 2003, 49, 379–385 Search PubMed.
- V. Buchanan-Wollaston, Plant Physiol., 1994, 105, 839–846 CrossRef CAS.
- B. G. Lane, FASEB J., 1991, 5, 2893–2901 CAS.
- H. Collett, A. Shen, M. Gardner, J. M. Farrant, K. J. Denby and N. Illing, Physiol. Plant., 2004, 122, 39–53 CrossRef CAS.
- M. J. Oliver, S. E. Dowd, J. Zaragoza, S. A. Mauget and P. R. Payton, BMC Genomics, 2004, 5, 89 CrossRef.
- Z. Yang, Y. R. Wu, Y. Li, H. Q. Ling and C. C. Chu, Plant Mol. Biol., 2009, 70, 219–229 CrossRef CAS.
- R. Bofill, M. Capdevila and S. Atrian, Metallomics, 2009, 1, 229–234 RSC.
- O. I. Leszczyszyn, R. Schmid and C. A. Blindauer, Proteins: Struct., Funct., Bioinf., 2007, 68, 922–935 Search PubMed.
- P. A. Binz and J. H. R. Kägi, in Metallothionein IV, ed. C. D., Birkhäuser Verlag, Basel, 1999, pp. 7–13 Search PubMed.
- W. J. Guo, M. Meetam and P. B. Goldsbrough, Plant Physiol., 2008, 146, 1697–1706 CrossRef CAS.
- S. N. A. Abdullah, S. C. Cheah and D. J. Murphy, Plant Physiol. Biochem., 2002, 40, 255–263 CrossRef CAS.
- W. J. Guo, W. Bundithya and P. B. Goldsbrough, New Phytol., 2003, 159, 369–381 Search PubMed.
- R. H. Jordan, R. B. Turley, S. L. Defauw and M. Steele, DNA Sequence, 2005, 16, 96–102 Search PubMed.
- E. Freisinger, Dalton Trans., 2008, 6663–6675 RSC.
- E. A. Peroza, R. Schmucki, P. Güntert, E. Freisinger and O. Zerbe, J. Mol. Biol., 2009, 387, 207–218 CrossRef CAS.
- B. A. Krizek, D. L. Merkle and J. M. Berg, Inorg. Chem., 1993, 32, 937–940 CrossRef CAS.
- O. I. Leszczyszyn, C. R. J. White and C. A. Blindauer, Mol. BioSyst., 2010 10.1039/c002348e.
- T. L. Reynolds and R. L. Crawford, Plant Mol. Biol., 1996, 32, 823–829 CrossRef CAS.
- D. Beyersmann and A. Hartwig, Arch. Toxicol., 2008, 82, 493–512 CrossRef CAS.
- http://www.ionomicshub.org/ .
- M. A. Larkin, G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson and D. G. Higgins, Bioinformatics, 2007, 23, 2947–2948 CrossRef CAS.
- http://www.tcdb.org/tcdb/ .
- http://www.membranetransport.org/ .
- http://pfam.sanger.ac.uk/ .
- S. F. Altschul, W. Gish, W. Miller, E. W. Myers and D. J. Lipman, J. Mol. Biol., 1990, 215, 403–410 CrossRef CAS.
- R. C. Edgar, Nucleic Acids Res., 2004, 32, 1792–1797 CrossRef CAS.
- J. Söding, A. Biegert and A. N. Lupas, Nucleic Acids Res., 2005, 33, W244–W248 CrossRef (Web Server issue).
- K. Tamura, J. Dudley, M. Nei and S. Kumar, Mol. Biol. Evol., 2007, 24, 1596–1599 CrossRef CAS.
- N. Saitou and M. Nei, Mol. Biol. Evol., 1987, 4, 406–425 CAS.
- M. A. Marti-Renom, A. C. Stuart, A. Fiser, R. Sanchez, F. Melo and A. Sali, Annu. Rev. Biophys. Biomol. Struct., 2000, 29, 291–325 CrossRef CAS.
- http://www.ebi.ac.uk/msd-srv/olderado/ .
- A. A. Canutescu, A. A. Shelenkov and R. L. Dunbrack, Jr., Protein Sci., 2003, 12, 2001–2014 CrossRef CAS.
- http://swift.cmbi.ru.nl/servers/html/index.html .
- R. Koradi, M. Billeter and K. Wüthrich, J. Mol. Graphics, 1996, 14, 51–55 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
