Remarkable effect of mobile phase buffer on the SEC-ICP-AES derived Cu, Fe and Zn-metalloproteome pattern of rabbit blood plasma†‡
Elham Zeini
Jahromi
,
Wade
White
,
Qiao
Wu
,
Raghav
Yamdagni
and
Jürgen
Gailer
*
Department of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, AB, T2N 1 N4, Canada. E-mail: jgailer@ucalgary.ca; Fax: +1 403-289-9488; Tel: +1 403-210-8899
First published on 27th May 2010
Abstract
The development of an analytical method to quantify the major Cu, Fe and Zn-containing metalloproteins in mammalian plasma has been recently reported. This method is based on the separation of plasma proteins by size exclusion chromatography (SEC) followed by the on-line detection of the metalloproteins by an inductively coupled plasma atomic emission spectrometer (ICP-AES). To assess whether the mobile phase buffer can affect the SEC-ICP-AES-derived metalloproteome pattern, thawed rabbit plasma was analyzed using phosphate buffered saline (PBS)-buffer (0.15 M, pH 7.4), Tris-buffer (0.1 and 0.05 M, pH 7.4), Hepes-buffer (0.1 M, pH 7.4) or Mops-buffer (0.1 M, pH 7.4). In contrast to the Cu-specific chromatograms, the Fe and Zn-specific chromatograms that were obtained with Tris, Hepes and Mops-buffer were considerably different from those attained with PBS-buffer. The Tris, Hepes and Mops-buffer mediated redistribution of ∼25% plasma Zn2+ from <100 kDa to >100–600 kDa plasma proteins and to a smaller extent to a <10 kDa (Tris)2Zn2+-complex can be rationalized in terms of the abstraction of Zn2+ from the weak binding site on albumin. In contrast, only Hepes and Mops-buffer redistributed ∼20% of plasma Fe3+ from the <100 kDa to the >600 kDa elution range. Based on these results and considering that the utilization of PBS-buffer has previously resulted in the detection of a number of Cu, Fe and Zn-containing metalloentities in rabbit plasma that was most consistent with literature data, this mobile phase buffer is recommended for metallomic studies regarding mammalian blood plasma.
Introduction
Metallomics represents an exciting new research area which can be broadly defined as the entirety of research efforts that are currently underway to better understand the biomolecular mechanisms that govern metal-dependent life processes.1 In order to visualize dynamic processes inside organisms, however, fast bioanalytical techniques are needed that are capable of extracting relevant information from complex biological fluids, such as blood plasma2 and/or bile.3 To this end, we have recently demonstrated that by hyphenating size exclusion chromatography (SEC) to an inductively coupled plasma atomic emission spectrometer (ICP-AES), it is possible to analyze rabbit plasma for the major Cu, Fe and Zn-containing metalloproteins/peptides.4 Using a Superdex 200 SEC-column (30 × 1.0 cm I.D.) and 0.15 M phosphate buffered saline (PBS) as the mobile phase, ∼12 Cu, Fe and Zn-metalloproteins/peptides were detected in <25 min. Unsurprisingly, the analysis of human plasma with SEC-ICP-AES resulted in the detection of a similar number of Cu, Fe and Zn-containing metalloproteins/peptides.5 Interestingly, the relative intensities and retention times of some metalloprotein peaks were different in human as compared to rabbit plasma, which indicates that SEC-ICP-AES gives biochemically relevant information. Even though the detected metalloproteins had to be qualitatively identified in the column effluent by immunoassays, the developed analytical method nevertheless offers several unique advantages compared to other proteomic techniques, which makes it an interesting tool for metallomic studies.5 During our previous studies we noted that the number of Cu, Fe and Zn peaks that were detected in fresh rabbit plasma (∼5 Cu-peaks, ∼5 Zn-peaks, 2 Fe-peaks)4 was larger than what had been reported for human serum by others using similar instrumental analytical techniques (on average 2 Cu-peaks, 3 Zn-peaks, 1.5 Fe-peaks were reported).4 Even though the differences with regard to Cu can be explained by the loss of labile Cu-proteins (e.g. coagulation factor V is a Cu-metalloprotein that rapidly decomposes in plasma4), the difference in the number of Zn peaks could be due to the utilization of Tris-buffer in these studies.4 It has in fact long been known that Tris can interact with Zn2+ ions in aqueous solution.6 Since in general not much is known about a possible mobile phase buffer-mediated abstraction of metal ions from plasma metalloproteins during liquid chromatographic separations, we systematically investigated the effect of four physiological mobile phase buffers (all pH 7.4) on the results that are obtained when rabbit plasma is analyzed for the major Cu, Fe and Zn-containing metalloproteins by SEC-ICP-AES.Using the previously established SEC-ICP-AES system,4,7 rabbit plasma was analyzed with PBS-buffer (0.15 M, pH 7.4), Tris-buffer (0.1 and 0.05 M, pH 7.4), Hepes-buffer (0.1 M, pH 7.4) and Mops-buffer (0.1 M, pH 7.4). In view of the previously noted considerable variation of plasma metalloprotein concentrations between animals,4 the present study was conducted with a homogenous stock of frozen rabbit plasma, aliquots (1.0 ml) of which were thawed 45 min prior to analysis (the obtained results were corroborated by analyzing fresh rabbit plasma). Specifically, we wanted to assess if the Cu, Fe and Zn-metallproteome pattern that is obtained when plasma is analyzed by SEC-ICP-AES differs between the investigated mobile phase buffers. Since the SEC-based separation of plasma proteins should not be significantly affected by the employed mobile phase buffers,8 the expected data were intended to allow us to classify the investigated mobile phase buffers into those that should be avoided because they generate speciation artifacts and those that produce results (i.e. the Cu, Fe and Zn-metallproteome) that are in accord with literature data and are therefore to be preferred. Considering that metallomics research represents a rapidly evolving field, such investigations must be regarded as particularly timely since only mobile phase buffers which produce clinically meaningful data should be employed (e.g. the determination of a particular plasma metalloprotein concentration for diagnostic purposes). In fact, several research groups have employed SEC-ICP-AES or SEC-inductively coupled plasma mass spectrometry (ICP-MS) in conjunction with different mobile phase buffers to analyze various mammalian body fluids for metalloproteins in order to gain new insights into human disease processes.9–14 Thus, the results that are reported in the present study represent another step in our efforts to establish a robust analytical method which can be employed to analyze mammalian plasma for Cu, Fe and Zn-metallproteins.
Experimental
Chemicals
Phosphate-buffered saline (PBS) tablets, tris(hydroxymethyl)aminomethane (Tris), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes), 3-(N-morpholino)propanesulfonic acid (Mops), N-(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)glycine (Tricine), and blue dextran were purchased from Sigma-Aldrich (St. Louis, MO, USA). PBS-buffer (10 mM phosphate, 2.7 mM KCl and 137 mM NaCl) was prepared by dissolving PBS tablets in the appropriate volume of de-ionized water from a Simplicity water purification system (Millipore, Billerica, MA, USA). 0.1 M Tris, Hepes and Mops-buffer solutions were prepared by dissolving the appropriate amount of each reagent in de-ionized water and the adjustment of the pH to 7.4 by the addition of either HCl or NaOH (4.0 M). All mobile phases were filtered through 0.45 μm nylon-filter membranes (Mandel Scientific, Guelph, ON, Canada) before use. A mixture of protein standards which contained thyroglobulin (670 kDa), γ-globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa), and vitamin B12 (1.35 kDa) was purchased from Bio-Rad Laboratories (Hercules, CA, USA) to calibrate the Superdex 200 SEC column. A heparin solution for coating the syringe with which blood was collected from rabbits (see below) was obtained from Hepalean-LOK (Organon Canada Ltd., Toronto, ON, Canada).SEC-ICP-AES system
The SEC-ICP-AES system which was employed in this study was described previously.7 In brief, this system consisted of a Smartline 1000 HPLC pump (Knauer, Berlin, Germany), a Rheodyne 9010 PEEK injection valve (Rheodyne, Rhonert Park, CA, USA) equipped with a PEEK injection loop (0.5 mL) and a pre-packed Superdex™ 200 10/300 GL Tricorn™ high performance size-exclusion chromatography column (30.0 × 1.0 cm I.D., separates globular proteins between ∼600 and ∼10 kDa; GE Healthcare, Piscataway, NJ, USA). The exit of the SEC column was connected to the Meinhard concentric glass tube nebulizer of the ICP-AES with FEP Teflon tubing (30 cm, I.D. 0.5 mm). The mobile phase flow rate was 1.0 mL min−1 (column temperature: 22 °C). Simultaneous multielement-specific detection of C (193.091 nm), S (180.731 nm), P (213.618 nm), Cu (324.754 nm), Fe (259.940 nm), and Zn (213.856 nm) in the column effluent was achieved with a Prodigy, high-dispersion, radial-view ICP-AES (Teledyne Leeman Labs, Hudson, NH, USA) at an Ar gas-flow rate of 19 L min−1, an RF power of 1.3 kW and a nebulizer gas pressure of 35 psi. The detector technology utilized in the Prodigy (a charge injection device or CID) allows the simultaneous measurement of the peak and the background emissions to generate the net emission intensity. This capability is critical in experiments where the background emission intensity changes (e.g. when a major protein peak reaches the ICP-AES) so the operator is not mislead into believing that an analytically significant event has occurred when in fact it has not. This advantage, together with the ability of ICP-AES to handle salt-containing solutions, makes the Prodigy well suited for the LC analysis of metal or metalloid-containing compounds with salt-containing mobile phases. Time scans were performed using the time-resolved analysis (TRA) mode (Salsa software version 3.0) and a data acquisition rate of 1 data point per 2 s was used. The raw data were imported into Sigmaplot 11 and smoothed using the bisquare algorithm. According to the void volume of the Superdex 200 column [which was determined by the injection of blue dextran and C-specific detection (7.95 mL or 477 s)], a 7.0 min delay was implemented between injection and the beginning of data acquisition and a 1000 s acquisition window was used.SEC-ICP-AES analysis of rabbit plasma
The Animal Care Committee of the University of Calgary approved the procedure to collect blood from New Zealand white rabbits (Protocol Approval #BI 08R-05). Male New Zealand white rabbits were purchased from Riemens Fur Ranches Ltd. (St. Agatha, ON, Canada), fed ad libitum on a “high fiber” diet (Lab Diet 5321, Canadian Lab Diets, Leduc, AB, Canada) and fasted 4.5 h before blood collection. Both fresh and frozen rabbit plasma were analyzed in this study. Fresh rabbit plasma was prepared according to the procedure described previously,7 but 6 mL heparinized trace metal testing blood collection tubes (Vacuette, Greiner bio-one North America, Monroe, NC, USA) were used. A frozen rabbit plasma stock was prepared by drawing an adequate amount of blood (∼60 ml) from a rabbit with a 60 mL syringe (heart puncture) into 10 heparinized trace metal testing blood collection tubes. In order to prevent blood coagulation in the syringe, the syringe was coated with 1.0 mL heparin solution. The obtained blood was centrifuged at 1,100 g (4 °C) for 10 min and the supernatant plasma was removed, gently mixed and aliquots (1.0 mL) transferred into plastic cryovials and frozen at −30 °C. Frozen plasma aliquots were thawed at room temperature for 45 min and subsequently injected onto the SEC-ICP-AES system. Four plasma injections were conducted for each buffer unless otherwise stated. The obtained Cu, Fe and Zn-specific chromatograms were integrated to obtain the peak area either of individual peaks (or of all detected peaks in a cluster) of a particular metal using Sigmaplot 11.Atmospheric Pressure Chemical Ionization-Mass Spectrometry (APCI-MS)
A Bruker Esquire 3000 instrument was used to detect positively charged ions in the collected SEC column fraction when 0.1 M Tris-buffer was used as the mobile phase. The Zn-peak that eluted in the <10 kDa elution range was collected, diluted 5 times with methanol and the obtained solution was introduced into the APCI source at a flow rate of 240 μL h−1. The capillary voltage was 3200 kV and the corona current was 6000 nA. The experiment was run with an APCI temperature of 350 °C.Results and discussion
In the context of advancing human health, one of the most important challenges that remains to be solved in the post-genomic era is to establish functional connections between genes, proteins, metabolites and mineral ions.15–17 To this end, a more detailed understanding of the bioinorganic chemistry of environmentally abundant toxic metals and metalloid compounds that enter the mammalian bloodstream has been recently identified as a promising research strategy to possibly establish links between the chronic exposure of human populations to low levels of inorganic pollutants and specific diseases.18 Such a strategy, however, inherently relies on bioanalytical techniques, which must allow one to probe/visualize interactions between toxic metals/metalloid compounds and plasma proteins/metalloproteins and to study these interactions in a physiological context (i.e. in whole plasma)19 and preferably in a systems-biology oriented manner.17,20–22 Using SEC-ICP-AES, we have recently demonstrated that the major Cu, Fe and Zn-containing metalloproteins and peptides can be detected in rabbit and human plasma in <25 min.4,5,7 Since this method was intended to be potentially employed for the diagnosis of human diseases (e.g. serum from Wilson’s disease patients contains negligible concentrations of the Cu-metalloprotein ceruloplasmin),23 0.15 M PBS-buffer (pH 7.4) was chosen as the mobile phase to avoid unnecessary pH-induced stress of the plasma metalloproteins during the analytical separation process.4 Tris-buffer (0.02–0.1 M), however, has also been frequently employed for the separation of metalloproteins that are contained in various biological fluids.4,11,12,14 In view of the fact that no systematic studies were found in the literature that addressed the potentially adverse effect that Tris-buffer or other mobile phase buffers may have on the analytical result, we systematically investigated the effect of PBS-buffer (0.15 M), Tris-buffer (0.1 and 0.05 M), Hepes-buffer (0.1 M) and Mops-buffer (0.1 M) on the Cu, Fe and Zn-specific chromatograms that are obtained when thawed rabbit plasma is analyzed by SEC-ICP-AES. We corroborated these results by analyzing fresh rabbit plasma (data not shown). Among the investigated mobile phase buffers, only Mops-buffer produced different results for thawed versus fresh rabbit plasma (see discussion below).The combined area of all detected Cu, Fe and Zn-peaks that were obtained after the analysis of thawed plasma with the aforementioned mobile phase buffers are displayed in Table 1. The total Cu peak area that was obtained for PBS and Tris-buffer was marginally higher (∼12%) than that for Hepes and Mops-buffer. With regard to Fe, PBS and Tris-buffer resulted in smaller total peak areas (∼14%) compared to those attained for Hepes and Mops-buffer, whereas the total peak area for Zn was slightly smaller with PBS-buffer (∼11%) compared to the peak areas obtained with the other buffers. Since a homogenous stock of rabbit plasma was used, the observed small differences in the Cu, Fe and Zn-peak areas for the investigated mobile phase buffers must be attributed to the effect of their notably different salt content (0.1 M Mops and Hepes-buffer contain ∼22 g of buffer salt/L, whereas 0.1 M Tris and 0.15 M PBS-buffer contain ∼11 g of buffer salt/L) on the inductively coupled plasma.24
| Emission Line | Total Area Counts | |||
|---|---|---|---|---|
| 0.15 M PBS-buffer | 0.1 M Tris-buffer | 0.1 M Hepes-buffer | 0.1 M Mops-buffer | |
| a N = 3. | ||||
| Cu 324.754 nm | 41176 ± ±679 | 43467 ± 831 | 38095 ± 1650 | 36653 ± 1236 |
| Fe 259.837 nm | 29217 ± 246 | 30008 ± 394 | 34805 ± 899 | 34989 ± 770 |
| Zn 213.856 nm | 32020 ± 800 | 39007 ± 2566a | 35680 ± 3295 | 34461 ± 3834 |
Using PBS-buffer, the Cu, Fe and Zn-specific chromatograms which were obtained from the analysis of thawed rabbit plasma (Fig. 1) were essentially identical to the ones that were previously attained for 2 h-aged rabbit plasma.4 The Cu-specific chromatogram revealed a single Cu-peak (the retention time and peak area of this and all other Fe and Zn peaks for all mobile phases can be found in the supplementary material), which displayed considerable tailing.§ The detected Cu-peak (Fig. 1) had a retention time similar to the most intense Cu-peak that was previously identified in 2 h-aged rabbit plasma as ceruloplasmin (Cp).4 The Fe-specific chromatogram revealed 2 baseline-separated Fe-peaks (peak 1 ∼13% and peak 2 ∼87% of total Fe), which were previously identified as ferritin (Ft, peak 1) and transferrin (Tf, peak 2).4 The Zn-specific chromatogram that was obtained with PBS-buffer contained a cluster of Zn-peaks (peak 1 and 2 ∼43% of total peak area) followed by a more pronounced Zn-peak (peak 3 ∼57% of total peak area), which likely corresponds to albumin bound-Zn2+. Based on these results it can be concluded that freezing/thawing rabbit plasma did not appreciably affect the Cu, Fe and Zn-specific chromatograms compared to those that were previously derived for 2 h-aged rabbit plasma.4
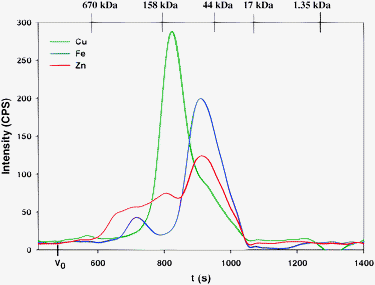 | ||
| Fig. 1 Representative simultaneous Cu, Fe and Zn-specific chromatogram of rabbit plasma on a Superdex 200 10/300 GL (30 × 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using phosphate buffered saline buffer (PBS, pH 7.4) as the mobile phase. Flow-rate 1.0 mL min−1, Injection volume 500 μL, Detector: ICP-AES at 324.754 nm (Cu), 259.940 nm (Fe) and 213.856 nm (Zn). The retention times of the molecular weight markers are depicted on top of the figure. | ||
When Tris-buffer was used for the analysis of thawed rabbit plasma, the Cu, Fe and Zn-specific chromatograms (Fig. 2) were either slightly (Cu and Fe) or notably different (Zn) from those obtained with PBS-buffer (qualitatively similar results were obtained with 0.05 M Tris-buffer with pH 7.4, data not shown).¶ In the Zn-specific chromatogram (Fig. 2 and 3) and to a first approximation three major Zn-peaks were observed, which is similar to what was previously reported for the analysis of mammalian serum or plasma by SEC using 0.1 or 0.05 M Tris-buffer.4 Upon closer scrutiny, however, a total of 5 Zn-peaks were detected in the present study (peaks 1-3 59%, peak 4 37%, and peak 5 4% of total Zn). The fact that Zn-peaks 1 and 5 (oval inset and arrow in Fig. 3) were essentially absent from the corresponding chromatogram with PBS-buffer (Fig. 1) implies that the presence of these Zn-peaks must be attributed to a Tris-buffer mediated redistribution of a fraction of the protein-bound Zn2+-ions from plasma protein binding sites during the chromatographic separation process. The detection of Zn-peak 5 (arrow in Fig. 2 and 3) is particularly noteworthy since its retention time corresponds to an entity with a molecular weight of <10 kDa. The analysis of a collected fraction of this peak revealed the presence of a (Tris)2Zn2+ complex (Fig. 4). In contrast, no Zn-peak was detected in this elution range when PBS-buffer was used (Fig. 1), which is—quite importantly—in accord with the concentration of free Zn2+ (2.10−10 M) that has been estimated for mammalian plasma.25,26 Taken together, these findings substantiate results that were previously reported in a study in which LC-ICP-MS and a 0.05 M Tris-buffer mobile phase (pH 7.4) resulted in a speciation artefact with regard to Zn, when human serum was analyzed.11
 | ||
| Fig. 2 Representative simultaneous Cu, Fe and Zn-specific chromatogram of rabbit plasma on a Superdex Peptide 10/300 GL (30 × 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using 0.1 M Tris-buffer (pH 7.4) as the mobile phase. Flow-rate: 1.0 mL min−1, Injection volume: 500 μL, Detector: ICP-AES at 324.754 nm (Cu), 259.940 nm (Fe) and 213.856 nm (Zn). The retention times of the molecular weight markers are depicted on top of the figure. | ||
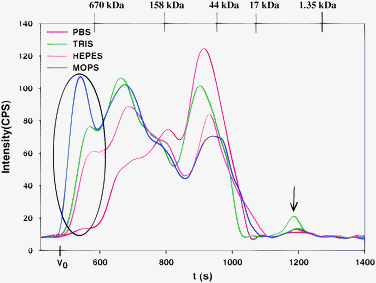 | ||
| Fig. 3 Zn-specific chromatograms of rabbit plasma on a Superdex Peptide 10/300 GL (30 × 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using 0.15 M PBS-buffer, 0.1 M Tris-buffer, 0.1 M Hepes-buffer or 0.1 M Mops-buffer (all pH 7.4) as the mobile phase. Flow-rate 1.0 mL min−1, Injection volume: 500 μL, Detector: ICP-AES at 213.856 nm (Zn). The retention times of the molecular weight markers are depicted on top of the figure. Note the different y-scale compared to the previous figures. | ||
 | ||
| Fig. 4 APCI-MS identification of the Zn-entity that eluted in the <10 kDa elution range after the analysis of rabbit plasma by SEC using 0.1 M Tris-buffer (pH 7.4). | ||
Using Hepes-buffer for the analysis of thawed rabbit plasma (Fig. 5), slightly different Cu and notably different Fe and Zn-specific chromatograms were obtained compared to those attained with PBS-buffer (Fig. 1).|| Interestingly, the Fe-specific chromatogram revealed 3 distinct peaks (peak 1 ∼15%, peak 2 ∼14%, and peak 3 ∼71% of total Fe), which is one more than what was observed with PBS and Tris-buffer (Fig. 1 and 2). The fact that the Fe-peak area that corresponds to Tf was ∼20% smaller with Hepes than with PBS and Tris-buffer suggests that Fe was abstracted from this plasma protein to yield the additional Fe-peak that eluted in the >600 kDa elution range (arrow in Fig. 5). The Zn-specific chromatogram that was obtained with Hepes-buffer (peak 1–3 ∼66%, and peak 4 ∼34% of total Zn) resembled that obtained with Tris-buffer, but did not contain the <10 kDa Zn-peak (Fig. 3).
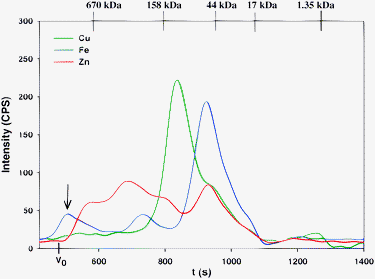 | ||
| Fig. 5 Representative simultaneous Cu, Fe and Zn-specific chromatogram of rabbit plasma on a Superdex Peptide 10/300 GL (30 × 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using 0.1 M Hepes-buffer (pH 7.4) as the mobile phase. Flow-rate 1.0 mL min−1, Injection volume: 500 μL, Detector: ICP-AES at 324.754 nm (Cu), 259.940 nm (Fe) and 213.856 nm (Zn). The retention times of the molecular weight markers are depicted on top of the figure. | ||
When Mops-buffer was utilized to analyze thawed rabbit plasma, the obtained Cu, Fe and Zn-specific chromatograms (Fig. 6) closely resembled those that were attained with Hepes-buffer (Fig. 5).** Importantly, the SEC-ICP-AES analysis of fresh rabbit plasma with this mobile phase buffer did not result in the elution of an Fe-peak in the >600 kDa elution range. The reason for this behavior is not understood at present.
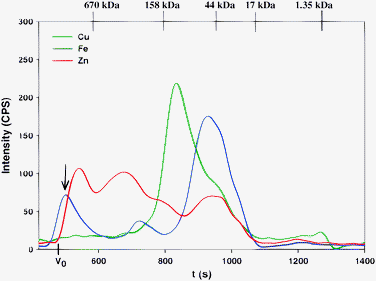 | ||
| Fig. 6 Representative simultaneous Cu, Fe and Zn-specific chromatogram of rabbit plasma on a Superdex Peptide 10/300 GL (30 × 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using 0.1 M Mops-buffer (pH 7.4) as the mobile phase. Flow-rate 1.0 mL min−1, Injection volume: 500 μL, Detector: ICP-AES at 324.754 nm (Cu), 259.940 nm (Fe) and 213.856 nm (Zn). The retention times of the molecular weight markers are depicted on top of the figure. | ||
In view of the fact that all investigated buffers had pH 7.4, the observed differences in the SEC-ICP-AES-derived plasma Cu, Fe and Zn-metalloproteome patterns between the investigated mobile phase buffers (Fig. 1–3, 5 and 6) must be attributed either to differences in their ionic strength and/or the chemical nature of the buffer components.14 Considering that several of the detected metal-plasma protein complexes (otherwise referred to as metalloproteins throughout this manuscript) must remain un-dissociated in vivo (in the presence of ∼0.9% salt, ∼55 g albumin/L and 37 °C) in order to deliver their essential trace metal cargo to internal organs (e.g. Fe3+-loaded transferrin binds to transferrin receptors on the surface of target cells and the formed transferrin-transferrin receptor complex is subsequently internalized via receptor-mediated endocytosis),27 it seems unlikely that the buffer-mediated changes in the observed Cu, Fe and Zn-metalloproteome pattern were caused by the different ionic strengths, but it cannot be entirely excluded. Considering that there is direct experimental evidence that Zn2+ and Cu2+ can form complexes with Tris6,28,29 and Hepes30,31, it is chemically more likely that the observed differences (Fig. 1–3, 5 and 6) are predominantly based on the buffer-mediated partial abstraction of Fe and Zn2+ from plasma protein binding sites during the chromatographic separation process (this may involve subtle buffer-induced conformational changes to facilitate metal-abstraction) and their subsequent elution in the >100 kDa elution range. Since qualitatively similar Cu, Fe and Zn-specific chromatograms were obtained after the analysis of fresh rabbit plasma with Tris and Hepes-buffer, the different elution pattern of Fe and Zn (compared to PBS-buffer) cannot be caused by freezing plasma.
In order to rationalize the findings displayed in Fig. 1–3, 5 and 6, it is instructive to scrutinize the buffer molecules that are actually present at pH 7.4. Hepes and Mops are both zwitterions and each contain one negatively charged SO3− group and a positively charged tertiary amine (pKa2 Hepes: 7.5,32 Mops: 7.033), whereas Tris (pKa 8.06) is mostly positively charged and contains ∼25% of uncharged Tris (Fig. 7). From a coordination chemistry point of view, Tris contains 3 OH-groups, which could (albeit weakly) coordinate to Zn2+ ions.29 Conversely, Hepes contains two potential binding sites for Zn2+ ions because zwitterions and anions are both present at pH 7.4. In the zwitterion, Zn2+ could potentially coordinate to the SO3− group and the adjacent amine (Fig. 7), whereas in the Hepes anion, Zn2+ could coordinate to the OH-group and the deprotonated tertiary amine (Fig. 7). These molecular characteristics of the buffer constituents have to be considered if we strive to explain why the investigated mobile phase buffers produced the results that were obtained (see discussion below). In order to do so, however, it is useful to discuss the qualitative differences that were observed for the analysis of thawed rabbit plasma with the different mobile phase buffers for each metal separately.
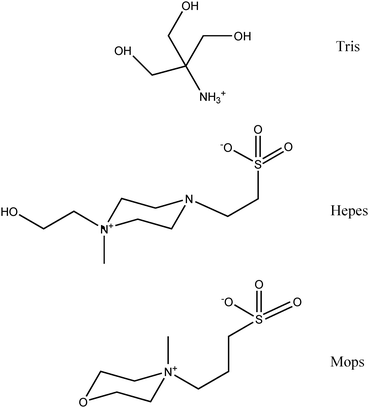 | ||
| Fig. 7 Molecular structure and protonation state of Tris, Hepes and Mops-buffer constituents at pH 7.4. With regard to Tris the protonated form is shown, whereas for Hepes and Mops the zwitterionic molecules are depicted. | ||
The Cu-specific chromatograms that were obtained with Tris, Hepes and Mops-buffer (Fig. 2, 5 and 6) essentially contained one Cu-peak (Cp) and were qualitatively similar, but were subtly different from the chromatogram obtained with PBS-buffer since the elevated Cu-baseline between 480 s and 730 s was considerably smaller with PBS buffer compared to the other buffers (Fig. 1). These results clearly demonstrate that the elution of Cp was virtually unaffected by the investigated mobile phase buffers, which is in agreement with the fact that all 6 Cu atoms in Cp are tightly bound (they are integrated into the structure of this metalloprotein) and therefore unavailable to chelation by dithiocarbamate.34 The elution of Cp in all of our chromatograms may therefore be regarded as an internal standard.
With regard to the Fe-specific chromatograms (Fig. 8), the results that were obtained with PBS and Tris-buffer were essentially identical, but remarkably different from those obtained with Hepes and Mops-buffer, which both contained an additional Fe-peak at ∼500 s (oval inset in Fig. 8). These results demonstrate that Hepes and Mops-buffer display a similar apparent selectivity for ∼20% of plasma protein-bound Fe (Table 2). This redistribution of Fe from a <100 kDa plasma protein to >600 kDa plasma proteins can be rationalized in terms of an abstraction of Fe3+ from Tf, which has a molecular mass of 78 kDa (Table 2).35 Owing to the possibility that Fe may have been abstracted from another Fe-containing protein with a similar hydrodynamic radius than Tf (this cannot be entirely excluded in view of the limited resolution of the SEC-column employed), it is impossible to unequivocally identify the plasma protein from which Fe was abstracted. Based on the virtually identical results that were obtained after the analysis of rabbit plasma with Tris and Tricine-buffer (0.1 M, pH 7.4, data not shown; Tricine represents a Tris-derivative in which one hydrogen atom from the amine is replaced with a carboxyl group) it can be concluded that the abstraction of Fe requires mobile phase buffers that are zwitterionic at pH 7.4 and contain a SO3− moiety. A more detailed understanding of this rather unexpected mobile phase buffer-mediated shift in the elution of ∼20% of plasma Fe from <100 kDa to >600 kDa plasma proteins would require further studies.
 | ||
| Fig. 8 Fe-specific chromatograms of rabbit plasma on a Superdex Peptide 10/300 GL (30 x 1.0 cm I.D., 13 μm particle size) SEC column at 22 °C using 0.15 M PBS-buffer, 0.1 M Tris-buffer, 0.1 M Hepes-buffer or 0.1 M Mops-buffer (all pH 7.4) as the mobile phase. Flow-rate 1.0 mL min−1, Injection volume: 500 μL, Detector: ICP-AES at 259.940 nm (Fe). The retention times of the molecular weight markers are depicted on top of the figure. Note the different y-scale compared to Fig. 1, 2, 3, 5 and 6. | ||
The Zn-specific chromatograms that were obtained with Tris, Hepes and Mops-buffer were—to a first approximation and neglecting the ∼10 kDa Zn-peak that was obtained with Tris-buffer—rather similar, but considerably different from that obtained with PBS-buffer (Fig. 3). Notably, a considerable redistribution of ∼25% plasma Zn2+ from <100 kDa to >100 kDa plasma proteins was observed (Table 2). In view of the fact that albumin is the major Zn-binding protein in mammalian plasma,4,20,36,37 the plasma protein from which Zn2+ was abstracted likely is albumin. Considering that the interaction between metal cations and Tris molecules are known to be rather weak,38 our results imply that Hepes, Mops and Tris-buffer ions abstracted Zn2+-ions from comparatively weak plasma protein binding sites. This buffer constituent-mediated redistribution of plasma Zn2+ (during the chromatographic analysis) is consistent with the previously reported Tris-buffer-mediated transfer of Zn2+-ions from Zn2+-proteins to metallothionein39 and with studies which demonstrated that ∼70% of total plasma Zn in human plasma is labile.40 In our study and using rabbit plasma, however, only ∼25% of plasma Zn were found to be labile (Table 2). The overall similarity of the Zn-metalloproteome patterns that were obtained with Hepes, Mops and Tris-buffer (Fig. 3) indicates that these buffer constituents have a rather similar selectivity/affinity for plasma protein bound Zn2+-ions, which can be explained by the fact that these buffer constituents contain potential Zn2+ coordination sites (Fig. 7). The putative Zn2+-coordination site on Tris is likely comprised of two (or possibly three) OH-groups.29 With regard to Hepes, two possible Zn2+ binding sites must be considered. The first one is comprised of the OH-group and the unprotonated amine-group (at pH 7.4 at least 50% of Hepes is present in this form), since the related borderline metal Cu2+ has been demonstrated to bind to this binding site by EPR-spectroscopy.31 The second potential binding site for Zn2+ on Hepes is the SO3− group and the other tertiary amine (Fig. 7). The utilization of Mops-buffer produced results that were somewhat similar to those obtained for Tris and Hepes-buffer. These findings indicate that Mops-buffer molecules contain a Zn2+ binding site, which likely is the SO3− group (Fig. 7). Considering the structural difference of the putative Zn2+-coordination sites on the buffer constituents, these results seem somewhat surprising, but are in agreement with studies which have shown that Zn2+ has a similar affinity for several eukaryotic Zn-proteins even though these proteins have different numbers and types of ligands.20 Taken together, the observed Tris, Hepes, and Mops-buffer mediated abstraction of Zn2+-ions from albumin and the subsequent elution of more intense Zn-peaks in the >100 kDa elution range and to a smaller extent the elution of a (Tris)2Zn2+-peak in the ∼10 kDa fractionation range (with Tris-buffer) can be rationalized based on the fact that albumin contains a strong and a weak Zn2+-binding site.41−43 In human serum albumin (HSA), for instance, the strong Zn2+ binding site (KD 45 nM) has been demonstrated to involve 2 N donors of His-67 and 247, O donors of Asn-99 and Asp-249, as well as a water molecule.36,37 In the context of interpreting the aforementioned results, however, the weak Zn2+ binding site on albumin42,43 is more relevant, since Tris, Hepes and Mops-buffer most likely removed Zn2+ ions from this binding site (Table 2, Fig. 3). Even though details regarding the molecular abstraction mechanism are currently unknown, our findings indicate that the weak Zn2+-binding site on rabbit serum albumin is solvent accessible and could therefore play an important part in the transport of gastrointestinally absorbed Zn2+ to internal organs. Unfortunately, the exact location of this weak Zn2+-binding site on albumin is currently unknown. Finally, the <10 kDa Zn-peak which was identified as a (Tris)2Zn2+-complex in the column effluent when Tris-buffer was used as the mobile phase, deserves mention (Fig. 2 and 3). The detection of this Zn2+-complex implies that it must have a somewhat smaller affinity for functional groups on >100 kDa plasma proteins compared to the complexes that were presumably also formed between the abstracted Zn2+ ions and the Hepes/Mops-buffer ions (Fig. 3). This smaller affinity can be explained by the fact that in a previously reported 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex between Zn2+ and Tris,29 most OH and NH2-groups of the latter were directed toward the Zn2+ ion at the core of the complex. Thus, the affinity of this complex for functional groups on the surface of proteins may be diminished. The detection of this <10 kDa Zn-peak underlines the capability of SEC-ICP-AES to rapidly visualize biochemically relevant interactions between small molecules (e.g.Tris) and plasma metalloproteins (e.g. Zn2+-metalloproteins) in mammalian plasma in vitro.
2 complex between Zn2+ and Tris,29 most OH and NH2-groups of the latter were directed toward the Zn2+ ion at the core of the complex. Thus, the affinity of this complex for functional groups on the surface of proteins may be diminished. The detection of this <10 kDa Zn-peak underlines the capability of SEC-ICP-AES to rapidly visualize biochemically relevant interactions between small molecules (e.g.Tris) and plasma metalloproteins (e.g. Zn2+-metalloproteins) in mammalian plasma in vitro.
In summary, the analysis of thawed rabbit plasma with SEC-ICP-AES revealed an additional Fe-peak when Hepes and Mops-buffer were used (compared to PBS and Tris-buffer). In addition, a <10 kDa Zn-peak was detected when Tris-buffer was used as the mobile phase, but not when any of the other buffers were employed. These results clearly demonstrate that Tris, Hepes and Mops-buffer each adversely affected the Cu, Fe and Zn-metalloproteome pattern that was obtained by SEC-ICP-AES compared to the results that were obtained with PBS-buffer.
Conclusion
The analysis of thawed rabbit plasma with SEC-ICP-AES revealed that Tris, Hepes and Mops-buffer (0.1 M, pH 7.4) resulted in a considerably different Fe and Zn plasma metalloproteome pattern compared to the results that were attained with PBS-buffer (0.15 M, pH 7.4). Hepes and Mops-buffer shifted the elution of ∼20% of Fe from the <100 kDa to the >600 kDa elution range, which resulted in the detection of an additional Fe-peak. The Zn-specific chromatograms that were obtained with Tris, Hepes and Mops-buffer revealed that ∼25% of plasma Zn2+ were redistributed from <100 kDa to >100 kDa plasma proteins. With Tris-buffer, a (Tris)2Zn2+-complex eluted in the <10 kDa elution range. Combined with our previous results in which the analysis of rabbit plasma with SEC-ICP-AES using PBS-buffer resulted in the detection of a total number of Cu, Fe and Zn-containing entities that was in accord with literature data,4,44 PBS-buffer does not appear to affect the chemical equilibria between metal ions and plasma proteins during the chromatographic separation of plasma proteins. Thus, Tris, Hepes and Mops-buffer—owing to their documented chelation properties with regard to the abstraction of Fe and Zn from plasma metalloproteins—should be avoided in metallomics studies involving the analysis of plasma even though they are widely regarded to be among the most appropriate all-purpose buffers for biological research. Therefore, and considering that human plasma contains 0.81-3.58 mM45 phosphate (compared to 10 mM phosphate in the PBS-buffer that was used)—which is also known to stabilize the structure of some proteins46—PBS-buffer emerges as the buffer of choice for the determination of mammalian plasma Cu, Fe and Zn-metalloproteins by SEC-ICP-AES. In addition, the results from the present study provide further evidence that SEC-ICP-AES is a useful bioanalytical tool to visualize potentially health relevant bioinorganic chemistry related reactions in blood plasma (e.g. the abstraction of essential metal ions from parent plasma protein binding sites by chelating agents) in a systems biology-minded manner. Considering that the displacement of Zn2+ from its binding site on 5-aminolaevulinic acid dehydratase by Pb2+ (which ultimately disrupts haeme-biosynthesis) has been previously demonstrated to represent the biomolecular mechanism for the chronic toxicity of Pb2+ in mammals,47 the application of SEC-ICP-AES is likely to play an important role in better understanding the biomolecular mechanisms that underly the chronic toxicity of inorganic pollutants in mammals.18 Therefore, bioinorganic chemists should consider to utilize SEC-ICP-AES to study dynamic processes that involve metalloproteins in the bloodstream in order to ultimately contribute to improve human health in the 21st century.18Acknowledgements
This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada. The staff of the Animal Health Unit (LESACC) at the University of Calgary is gratefully acknowledged for the maintenance of and the drawing of blood from the rabbits.References
- S. Mounicou, J. Szpunar and R. Lobinski, Chem. Soc. Rev., 2009, 38, 1119–1138 RSC.
- K. Cottingham, Anal. Chem., 2005, 77, 197A–200A CAS.
- J. Gailer, S. Madden, G. A. Buttigieg, M. B. Denton and H. S. Younis, Appl. Organomet. Chem., 2002, 16, 72–75 CrossRef CAS.
- S. A. Manley, S. Byrns, A. W. Lyon, P. Brown and J. Gailer, JBIC, J. Biol. Inorg. Chem., 2009, 14, 61–74 CrossRef CAS.
- S. A. Manley and J. Gailer, Expert Rev. Proteomics, 2009, 6, 251–265 Search PubMed.
- B. E. Fischer, U. K. Haering, R. Trobolet and H. Sigel, Eur. J. Biochem., 1979, 94, 523–530 CrossRef CAS.
- K. L. Pei and J. Gailer, Metallomics, 2009, 1, 403–408 RSC.
- H. G. Barth, B. E. Boyes and C. Jackson, Anal. Chem., 1994, 66, 595–620R CrossRef.
- M. Gonzalez-Fernandez, T. Garcia-Barrera, A. Arias-Borrego, J. Jurado, C. Pueyo, J. Lopez-Barea and J. L. Gomez-Ariza, Biochimie, 2009, 91, 1311–1317 CrossRef CAS.
- M. Sulyok, S. Hann, C. G. Hartinger, B. K. Keppler, G. Stingeder and G. Koellensperger, J. Anal. At. Spectrom., 2005, 20, 856–863 RSC.
- C. S. Muniz, J. M. M. Gayon, J. I. G. Alonso and A. Sanz-Medel, J. Anal. At. Spectrom., 2001, 16, 587–592 RSC.
- B. Michalke, A. Berthele, P. Mistriotis, M. Ochsenkuehn-Petropoulou and S. Halbach, J. Trace Elem. Med. Biol., 2007, 21(S1), 4–9 CrossRef CAS.
- J. Ellis, E. Del Castillot, M. M. Bayon, R. Grimm, J. F. Clark, G. Pyne-Geithman, S. Wilbur and J. A. Caruso, J. Proteome Res., 2008, 7, 3747–3754 CrossRef CAS.
- S. A. Rodriguez, E. B. Gonzalez, G. A. Llamas, M. Montes-Bayon and A. Sanz-Medel, Anal. Bioanal. Chem., 2005, 383, 390–397 CrossRef.
- B. Lahner, J. Gong, M. Mahmoudian, E. L. Smith, K. B. Abid, E. E. Rogers, M. L. Guerinot, J. F. Harper, J. M. Ward, L. McIntyre, J. I. Schroeder and D. E. Salt, Nat. Biotechnol., 2003, 21, 1215–1221 CrossRef CAS.
- L. Hood, in Physical Biology, from Atoms to Medicine, ed. A. H. Zewail, Imperial College Press, London, 2008, pp. 337–366 Search PubMed.
- L. Hood, J. R. Heath, M. E. Phelps and B. Lin, Science, 2004, 306, 640–643 CrossRef CAS.
- E. Zeini Jahromi and J. Gailer, Dalton Trans., 2010, 39, 329–336 RSC.
- L. M. Gierasch and A. Gershenson, Nat. Chem. Biol., 2009, 5, 774–777 CrossRef CAS.
- W. Maret and Y. Li, Chem. Rev., 2009, 109, 4682–4707 CrossRef CAS.
- J. K. Nicholson and J. C. Lindon, Nature, 2008, 455, 1054–1056 CrossRef CAS.
- J. van der Greef, P. Stroobant and R. van der Heijden, Curr. Opin. Chem. Biol., 2004, 8, 559–565 CrossRef CAS.
- N. E. Hellman and J. D. Gitlin, Annu. Rev. Nutr., 2002, 22, 439–458 CrossRef CAS.
- S. J. Hill, Inductively Coupled Plasma Spectrometry and its Applications, Blackwell Publishing Ltd., 2007 Search PubMed.
- G. R. Magneson, J. M. Puvathingal and W. J. J. Ray, J. Biol. Chem., 1987, 262, 11140–11148 CAS.
- R. A. Colvin, C. P. Fontaine, M. Laskowski and D. Thomas, Eur. J. Pharmacol., 2003, 479, 171–185 CrossRef CAS.
- W. R. Harris, Z. Wang, C. Brook, B. Yang and A. Islam, Inorg. Chem., 2003, 42, 5880–5889 CrossRef CAS.
- D. Masi, C. Mealli, M. Sabat, A. Sabatini, A. Vacca and F. Zanobini, Helv. Chim. Acta, 1984, 67, 1818–1826 CrossRef CAS.
- R. L. Dotson, J. Inorg. Nucl. Chem., 1972, 34, 3131–3138 CrossRef CAS.
- Z. M. Anwar, J. Chin. Chem. Soc., 2005, 52, 863–871 CAS.
- M. Sokolowska and W. Bal, J. Inorg. Biochem., 2005, 99, 1653–1660 CrossRef CAS.
- J. Wouters, L. Haeming and G. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, 52, 1687–1688 CrossRef.
- R. N. Roy, D. R. Mrad, P. A. Lord, J. A. Carlsten, W. S. Good, P. Allsup, L. N. Roy, K. M. Kuhler, W. F. Koch and Y. C. Wu, J. Solution Chem., 1998, 27, 73–87 CrossRef CAS.
- P. F. Lindley, in Handbook of Metalloproteins, ed. A. Messerschmidt, R. Huber, T. Poulos and K. Wieghardt, John Wiley & Sons, Ontario, 2001, vol. 2, pp. 1369–1379 Search PubMed.
- X. S. Liu, L. D. Patterson, M. J. Miller and E. C. Theil, J. Biol. Chem., 2007, 282, 31821–31825 CrossRef CAS.
- A. J. Stewart, C. A. Blindauer, S. Berezenko, D. Sleep and P. J. Sadler, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3701–3706 CrossRef CAS.
- C. A. Blindauer, I. Harvey, K. E. Bunyan, A. J. Stewart, D. Sleep, D. J. Harrison, S. Berezenko and P. J. Sadler, J. Biol. Chem., 2009, 284, 23116–23124 CrossRef CAS.
- J.-M. Pfefferle and J.-C. G. Buenzli, Helv. Chim. Acta, 1989, 72, 1487–1494 CrossRef.
- C. Jacob, W. Maret and B. L. Vallee, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 3489–3494 CrossRef CAS.
- P. Zalewski, A. Truong-Tran, S. Lincoln, D. Ward, A. Shankar, P. Coyle, L. Jayaram, A. Copley, D. Grosser, C. Muriga, C. Lang and R. Ruffin, BioTechniques, 2006, 40, 509–520 CrossRef CAS.
- M. S. N. Rao and H. Lal, J. Am. Chem. Soc., 1958, 80, 3222–3226 CrossRef CAS.
- S. Ostojic, V. Dragutinovic, M. Kicanovic and B. R. Simonovic, J. Serb. Chem. Soc., 2007, 72, 331–337 CrossRef CAS.
- C. Andre and Y. C. Guillaume, Talanta, 2004, 63, 503–508 CrossRef CAS.
- W. Y. Craig, T. B. Ledue and R. F. Ritchie, Plasma proteins. Clinical Utility and Interpretation, Dade Behring Inc., Newark, 2000 Search PubMed.
- M. Lehman and F. Mimouni, Am. J. Dis. Child, 1989, 143, 1340–1341 Search PubMed.
- R. Kumar and A. G. Mauk, J. Phys. Chem. B, 2009, 113, 12400–12409 CrossRef CAS.
- M. J. Warren, J. B. Cooper, S. P. Wood and P. M. Shoolingin-Jordan, TIBS, 1998, 23, 217–221 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1 - Retention time and peak area data for the Cu, Fe and Zn peaks of all mobile phases. See DOI: 10.1039/c003321a |
| ‡ Throughout this manuscript a metalloprotein is defined as a protein which contains either a weakly or strongly bound metal irrespective of the biochemical role that the metalloprotein serves in the context of biology. |
| § As discussed previously,4 a much smaller number of Cu peaks is observed when 2 h-aged plasma is analyzed (∼2 Cu-peaks) compared to fresh rabbit plasma (∼5 Cu-peaks), because Cu is lost from labile metalloproteins, such as blood coagulation factor V, transcuprein and small molecular weight Cu after 30 min at room temperature.4 To rationalize the detection of essentially a single Cu-peak after the analysis of thawed plasma in the present study, it is chemically feasible that all Cu2+-ions that were liberated from labile plasma Cu-metalloproteins formed an insoluble precipitate with the phosphate ions from the PBS-buffer when the plasma was injected onto the SEC-column (this can explain why labile Cu-metalloproteins were essentially absent in the column effluent). |
| ¶ With regard to Cu, the major Cu-peak had a marginally shorter retention time (13 s) than that obtained with PBS-buffer. Importantly, an elevated Cu-baseline was observed between 485 s and 710 s (rectangular inset in Fig. 2), which was much less pronounced in the chromatogram attained with PBS-buffer (Fig. 1). The elevated baseline that was obtained with Tris-buffer (peak area: 5484 ± 396) corresponded to ∼12% of the total Cu-peak area. In the Fe-specific chromatogram, both Fe-peaks (peak 1 ∼10%, and peak 2 ∼90% of total Fe) had slightly shorter retention times (by ∼17 s) compared to PBS-buffer and the peak corresponding to Tf (peak 2) had a shoulder on its long retention end. |
| || The Cu-specific chromatogram was similar to that obtained with Tris-buffer and displayed an elevated baseline after 470 s (∼14% of total Cu) followed by a pronounced Cu-peak. Compared to the results that were obtained for PBS and Tris-buffer, the smaller intensity of the major Cu-peak can be explained by the effect that the higher salt content of the Hepes-buffer had on the inductively coupled plasma. |
| ** The Cu-specific chromatogram was very similar to that obtained with Hepes and Tris-buffer (Fig. 2). An elevated baseline was observed between 490 s and 700 s (∼9% of total Cu) and a pronounced Cu-peak eluted thereafter. In the Fe-specific chromatogram, the same number of Fe-peaks (arrow in Fig. 6) was detected as with the Hepes-buffer (peak 1 ∼21%, peak 2 ∼9%, and peak 3 ∼70% of total Fe). The Zn-specific chromatogram revealed peaks with similar retention times compared to those obtained with Hepes-buffer (peak 1–3 ∼76% and peak 4 ∼24% of total Zn). |
| This journal is © The Royal Society of Chemistry 2010 |
