DOI:
10.1039/B906709D
(Paper)
Metallomics, 2010,
2, 67-73
Received
2nd April 2009
, Accepted 24th September 2009
First published on 16th October 2009
Abstract
We investigated the effects of divalent alkaline earth and first-row transition metal and zinc ions on α-glucosidase activityin vitro and in vivo. CuSO4 and ZnSO4 exhibited a high α-glucosidase inhibitory effect in vitro. The IC50 values of CuSO4 were 0.77 ± 0.01 (substrate; maltose) and 0.78 ± 0.01 (substrate; sucrose), and those of ZnSO4 were 5.49 ± 0.14 (substrate; maltose) and 4.70 ± 0.06 (substrate; sucrose) for yeast α-glucosidase. On the basis of Lineweaver–Burk plots, both CuSO4 and ZnSO4 exhibited different modes of inhibition against α-glucosidase. Subsequently, oral glucose and sucrose tolerance tests (OGTT and OSTT) were performed on non-diabetic ddY mice to examine the effect of the metal ions on their blood glucose levels. As a result of single oral administration of CuSO4 in non-diabetic ddY mice, a significant and potent lowering of the blood glycemic response toward disaccharide, sucrose, ingestion was observed at 45 min after doses of 0.08 and 0.24 mmol kg−1 body weight. In contrast, the CuSO4 administration showed no suppression of the elevation of blood glucose levels in mice after a monosaccharide, glucose, administration. These results indicate that CuSO4 suppresses disaccharide digestion by inhibiting α-glucosidase activity in the epithelium of the small intestine, suggesting that antidiabetic Cu complexes with some ligands have a similar action mechanism to that of α-glucosidase inhibitor, acarbose, currently used for clinical purposes.
Introduction
According to the World Health Organization’s prediction in 2006, the number of type 2 diabetic patients in the world could increase to 360 million by 2030.1 The goal of diabetes treatment is to control the blood glucose levels, body weight, blood pressure, and cholesterol and triglyceride levels, and prevent the development of complications.2 Combinations of alimentotherapy, ergotherapy, oral antidiabetic medicines, and/or insulin have been used to treat diabetes mellitus. Among the oral antidiabetic medicines, α-glucosidase inhibitors inhibit α-glucosidase, which metabolizes disaccharides into monosaccharides in the small intestine. The inhibition of α-glucosidase delays the digestion and absorption of carbohydrates, resulting in suppression of postprandial hyperglycemia and excessive insulin secretion. Thus, α-glucosidase inhibitors lower the insulin requirement resulting from less absorption of glucose, and lead to less strain on pancreatic β-cells in insulin production. Hyperinsulinemia is considered as a potential risk factor for arteriosclerosis. Reduced requirement for insulin secretion can reduce the risk of arteriosclerosis.3
α-Glucosidase inhibitors including acarbose,4–7 voglibose,8,9 and miglitol10–13 have been available for clinical use since 2005 (Fig. 1). Such α-glucosidase inhibitors have been approved not only as medicines but also as a food for specified health uses (FOSHU), that is available without prescription.14–16
 |
| | Fig. 1 Structures of clinically used α-glucosidase inhibitors. | |
Several metal ions and their complexes exhibit antidiabetic effects.17–22 A chromium-containing material extracted from pig spleen improved glucose tolerance in vivo.17 Manganese plays an important role in glucose metabolism.18 Zinc exerts an insulin-like effect in rat adipocytes.19 In addition, some metal ions, such as tungsten,23 vanadium,24 and selenium25 lower high blood glucose levels in the diabetic state. It appears attractive to many researchers to study the relationship between diabetes mellitus and metal ions. The action mechanism of these metal ions, however, remains unclear. We have examined the mechanism of some transition metal ions and their complexes since 2006 and demonstrated that they activate the insulin signaling pathway followed by glucose transporter 4 (GLUT4) translocation to the plasma membrane and the enhancement of glucose utilization.26–29 Furthermore, in 2004, it was reported that insulin/C-peptide were closely associated with metal ions.30
In this study, we have focused on the inhibitory activity of these metals on α-glucosidase to analyze alternative action mechanisms of these metal ions. We have evaluated the α-glucosidase inhibitory activity of several divalent first-row transition metal ions, whose antidiabetic effects have been reported, such as vanadium, manganese, iron, cobalt, nickel, and copper, and a non-transition metal ion, zinc,24,31,32 together with divalent alkaline earth metal ions such as magnesium, calcium, strontium, and barium.33 In addition, we have compared their inhibitory activities with α-glucosidase inhibitor, acarbose, used for clinical purposes in both in vitro and in vivo experimental systems, and then evaluated the mode of α-glucosidase inhibition.
Materials and methods
Materials and animals
MgSO4, CaSO4, SrSO4, BaSO4, VOSO4, MnSO4, FeSO4, CoSO4, NiSO4, CuSO4, ZnSO4, α-glucosidase (from a Saccharomyces sp.), sucrose, maltose, acarbose, and a Glucose CII Test kit were purchased from Wako Pure Chemical Industries (Osaka, Japan). Rat small intestine acetone powder was obtained from Sigma Chemical Co., (St. Louis, MO, USA). Six-week-old ddY mice, which are non-inbred mice maintained in a closed colony, were purchased from SHIMIZU Laboratory Supplies Co. (Kyoto, Japan). All the mice were maintained on a 12-h light/dark cycle in our temperature-controlled central animal facility. The animal study was approved by the Experimental Animal Research Committee at the Kyoto Pharmaceutical University (KPU) and was performed according to the Guidelines for Animal Experimentation.
Yeast-derived α-glucosidase was adjusted to a concentration of 5 unit ml−1. The rat small intestine acetone powder was suspended in a 0.01 M (mol L−1) sodium phosphate buffer (pH 6.8). The suspension was homogenized, sonicated for 30 min and followed by the addition of 2% Triton X buffer (pH 7.0) containing 3 mM EDTA-2Na and 1 mM dithiothreitol (DTT) and by cold centrifugation for 60 min at 20000 × g. The supernatant was subjected to ammonium sulfateprecipitation, and its precipitate dialyzed against a 10 mM potassium phosphate buffer (pH 7.0). This was followed by column chromatography using a Sephadex G-100 column. Each fraction collected was assayed for the enzyme activity and used in further experiments.34
In vitro α-glucosidase inhibitory effect
Solutions of metal ions at various concentrations were prepared, and their α-glucosidase inhibitory effects evaluated using a modified Dahlqvist method.35 In brief, 0.1 ml of the test solution in 0.15 M HEPES buffer (pH 6.8) and 0.1 ml of 5 units/ml α-glucosidase in 0.015 M HEPES buffer were added to 0.1 ml of the substrate (0.1 M sucrose or maltose in 0.15 M HEPES buffer) and then incubated at 37 °C for 60 min. After incubation, the reactions were terminated by heating in boiling water. The glucose concentration was determined using a Glucose CII Test Wako kit. Acarbose was used as a positive control. The inhibition of α-glucosidase activity was calculated by using the following equation:
where Ac is the glucose concentration in the control solution (α-glucosidase and substrate, glucose or sucrose) and At is the glucose concentration in the test solutions (α-glucosidase, substrate, and test compounds).
In vivo α-glucosidase inhibitory effect
Seven-week-old ddY mice were fasted overnight. For in vivo experiments, we prepared 5.75 and 23 mM Cu solutions. Further, ddY mice were orally administered 0.2 mL of either one of the two solutions.36 The 5.75 and 23 mM Cu solutions corresponded to 0.08 and 0.24 mmol kg−1 body weight, respectively. After 30 min, a saline solution of glucose (2.0 g kg−1 body weight) or sucrose (2.0 g kg−1 body weight) was orally administered. Blood samples were obtained from the lateral tail vein at several points at 0, 30, 60, 90 and 120 min. The blood glucose levels were measured by a glucose oxidase method (Glucocard; Arkray, Kyoto, Japan).
The inhibition mode of the metal ions was determined as reported elsewhere.37,38 Briefly, 0.1 ml of the test solution in a 0.15 M HEPES buffer (pH 6.8) and 0.1 ml of the enzyme solution (5 units mL−1) in a 0.015 M HEPES buffer were added to 0.1 ml of the substrate (0.1 M sucrose in a 0.15 M HEPES buffer). The mixture was incubated at 37 °C for 1 min, and the reaction was terminated by heating at 90 °C. The amount of glucose formed was determined by the glucose oxidase method using a Glucose CII Test Wako kit. Substrate solutions with sucrose at 1, 0.5, 0.4, 0.3, 0.2, 0.1, 0.05, and 0.025 M were prepared. Solutions of α-glucosidases derived from the yeast and rat small intestine were adjusted to contain 5 units mL−1 for use in the assays . When yeast-derived α-glucosidase was used as the test enzyme, Cu and Zn ions, which showed high activity in vitro, were used to determine the IC75 and IC50 concentrations in vitro. For experiments using rat-derived α-glucosidase, Cu ion, which showed high activity in vitro, was used to estimate the IC75 and IC50 concentrations. A 0.15 M HEPES buffer (pH 7.0) was used to prepare the solutions.
Statistical analysis
All experimental data are expressed as mean values ± standard deviation (SD). Statistical analyses were performed using one-way analysis of variance (ANOVA), followed by Dunnett multiple comparison post-hoc tests. Differences were considered to be statistically significant when p values were <0.001, 0.01, or 0.05.
Results
Purification of the enzyme from the rat small intestine acetone powder
The purification of α-glucosidase derived from the rat small intestine resulted in an increase in its specific activity in comparison with the yeast α-glucosidase. The α-glucosidase solution derived from the rat intestine was separated into 3 fractions by column chromatography. Fraction 1 showed approximately 100-fold higher activity than the crude extract (Fig. 2 and Table 1), and was used for further study.
α-Glucosidase (from Saccharomyces sp.) inhibitory effect
First, we checked whether the metals directly affected the substrates, i.e., maltose and sucrose (in the absence of the enzyme). We found that the metals did not have any effects on the substrates because of the inhibitory action of the metal ions on α-glucosidase (data not shown). In the in vitro experiment involving yeast α-glucosidase, the order of α-glucosidase-inhibitory activities of divalent metal ions was Cu > Zn > VO > Ni > Fe (=acarbose) > Co > Mn, Mg, Ca, Sr, Ba (= 0) (Fig. 3A and B and Table 2).
 |
| | Fig. 3 Inhibition of the yeast α-glucosidase activity ((A) maltase and (B) sucrase) by metal compounds. Maltose and sucrose (0.1 M) were used as the substrates. Data are represented as mean ± SD of 3 experiments. The symbols denote the following compounds: ●, CuSO4; ○, ZnSO4; ▲, VOSO4; ◇, NiSO4; ■, FeSO4; △, acarbose; ×, CoSO4. | |
Table 2 Estimated IC50 values of metal compounds and acarbose for yeast α-glucosidase (maltase and sucrase)
| Compound |
IC50 (μM) |
|
Maltose
a
|
Sucrose
b
|
|
Maltose (0.1 M) used as substrate. Data are presented as the mean ± SD of 3 experiments.
Sucrose (0.1 M) used as substrate. Data are presented as the mean ± SD of 3 experiments.
Significance: p < 0.001 vs.acarbose.
Significance: p < 0.01 vs.acarbose.
|
|
CuSO4
|
0.77 ± 0.01c |
0.78 ± 0.01c |
|
ZnSO4
|
5.49 ± 0.14c |
4.70 ± 0.06c |
| VOSO4 |
189 ± 3d |
201 ± 5d |
|
NiSO4
|
251 ± 4d |
211 ± 8d |
|
FeSO4
|
595 ± 2 |
612 ± 24 |
| CoSO4 |
3560 ± 57 |
4900 ± 33 |
| MnSO4 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| MgSO4 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
CaSO4
|
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| SrSO4 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
BaSO4
|
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
Acarbose
|
582 ± 25 |
747 ± 7 |
α-Glucosidase (from rat small intestine) inhibitory effect
Some transition metal ions, which showed inhibitory activity against the yeast α-glucosidase, were tested for inhibitory activity against the rat intestine-derived enzymes. The rat is a more highly evolved species than the yeast. Cu, Zn, and VO ions exhibited different α-glucosidase-inhibitory activities, in which the activity order was as follows: acarbose > Cu > Zn > VO > Ni, Fe, Co, Mn (= 0) (Fig. 4A and B and Table 3). In addition, these test compounds inhibited the activities of sucrase and maltase to a similar extent (Fig. 4A and B and Table 3).
 |
| | Fig. 4 Inhibition of the rat small intestine α-glucosidase activity ((A) maltase and (B) sucrase) by metal compounds. Maltose and sucrose (0.1 M) were used as the substrates. Data are represented as the mean ± SD of 3 experiments. The symbols denote the following compounds: ●, CuSO4; ○, ZnSO4; ▲, VOSO4; △, acarbose. | |
Table 3 Estimated IC50 values of metal compounds and acarbose for rat small intestine α-glucosidase (maltase and sucrase)
| Compound |
IC50 (μM) |
|
Maltose
a
|
Sucrose
b
|
|
Maltose (0.1 M) used as substrate. Data are presented as the mean ± SD of 3 experiments.
Sucrose (0.1 M) used as substrate. Data are presented as the mean ± SD of 3 experiments.
|
|
CuSO4
|
5.10 ± 1.20 |
2.25 ± 0.85 |
|
ZnSO4
|
56.2 ± 2.85 |
30.0 ± 2.90 |
| VOSO4 |
1200 ± 90 |
956 ± 58 |
|
NiSO4
|
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
FeSO4
|
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| CoSO4 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| MnSO4 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
Acarbose
|
2.98 ± 0.54 |
1.38 ± 0.14 |
The glucose- and sucrose-loading tests were performed on the ddY mice to compare the α-glucosidase-inhibitory activities of acarbose in clinical use and Cu ions, which showed a particularly high inhibitory activity against the yeast and rat small intestine α-glucosidases.35 In the glucose-loading test, there was no significant difference between the Cu ion- and acarbose-administered group (Fig. 5). In contrast to the sucrose-loading test, the postprandial blood glucose levels in the Cu ion-administered group were significantly lower than those in the control group (Fig. 6A and B), similar to those in the acarbose-administered groups. Moreover, the values of the area under the curve (AUC) were lower in both Cu ion- and acarbose-administered groups than that in the control group. All test compounds inhibited α-glucosidase in a dose-dependent manner (Table 4 and Fig. 6).
 |
| | Fig. 5 Inhibitory effects of Cu ions (●) and acarbose (□) on the postprandial blood glucose level in glucose-loaded ddY mice. Male ddY mice weighing 33–45 g were starved for 12 h. The test compounds (0.24 mmol kg−1 of body weight) and saline (control: ×) were orally administered to the mice. Thirty minutes later, glucose (2 g kg−1 of body weight) was orally administered. Data are expressed as mean ± SD for 5–6 animals. | |
 |
| | Fig. 6 Inhibitory effects of Cu ions (●) and acarbose (□) on the postprandial blood glucose level in sucrose-loaded ddY mice. Male ddY mice weighing 33–45 g were starved for 12 h. The test compounds ((A) 0.08 mmol kg−1 of body weight, (B) 0.24 mmol kg−1 of body weight) and saline (control: ×) were orally administered to the mice. Thirty minutes later, sucrose (2 g/kg of body weight) was orally administered. Data are expressed as mean ± SD for 5–6 animals. Significance: *p < 0.05 vs. untreated mice. | |
| ΔAUC0–2h (mg h/dL) |
| Compound |
Glucose loading tests |
Sucrose loading tests |
| 0.09 mmol/kg body weight |
0.24 mmol/kg body weight |
| Data are represented as the mean ± SD of 5–6 animals. The AUC0–2h values were calculated as the area under the mean blood glucose concentration–time curve for 2 h after glucose or sucrose loading. Significance: p < 0.01 vs. control. |
| Control |
332 ± 53 |
243 ± 65 |
243 ± 65 |
| Cu ion |
311 ± 45 |
219 ± 30 |
167 ± 13a |
|
Acarbose
|
323 ± 35 |
207 ± 33 |
147 ± 19a |
The inhibitory modes of the yeast α-glucosidase by Cu and Zn ions were studied. Lineweaver–Burk plots showed that Zn and Cu ions acted on the yeast α-glucosidase in competitive and non-competitive modes, respectively (Fig. 7A and B). The mode of inhibition of the rat small intestine α-glucosidase by Cu ion, was also determined. Cu ion acted as a non-competitive inhibitor against the rat small intestine α-glucosidase (Fig. 8).
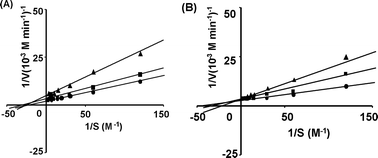 |
| | Fig. 7 Lineweaver–Burk plots of Cu ions (A) and Zn ions (B). Sucrose was used as the substrate. The enzyme used was yeast α-glucosidase (5 units ml−1). The symbols represent the following values: ●, IC0; ■, IC50; and ▲, IC75. | |
 |
| | Fig. 8 Lineweaver–Burk plots of Cu ion. Sucrose was used as the substrate. The enzyme used in the experiment was rat small intestine-α-glucosidase (5 units ml−1). The symbols represent the following values: ●, IC0; ■, IC50; and ▲, IC75. | |
Discussion
In the average diet, 60% of total calories are derived from carbohydrates, of which approximately 65% and 25% of calories are derived from starch and sucrose, respectively.39Polysaccharides, such as starch, are degraded by salivary and pancreatic amylases into oligosaccharides, which are hydrolyzed by α-glucosidase in the brush borders of the small intestine to form glucose.40 The disaccharides sucrose and maltose are not affected by amylase, but cleaved by α-glucosidase to generate glucose which is absorbed from the small intestine.41Glucose is then taken up by Na/glucose co-transporters and glucose transporters.42
α-Glucosidase inhibitors, as antidiabetic medicines, inhibit α-glucosidase to suppress carbohydrate digestion and delay its absorption resulting in a subsequent improvement of postprandial hyperglycemia.42
A continued hyperglycemic state results in impaired insulin secretion and decreased insulin sensitivity.43 In the present study, the inhibitory effects of Cu, Zn, VO, and Ni ions on the yeast α-glucosidase were approximately 1000-, 100-, 4-, and 2-fold higher than that of acarbose, respectively (Table 2). Furthermore, the inhibitory effects of Cu and Zn ions on the rat small intestine α-glucosidase were about 0.6- and 0.05-fold higher than that of acarbose, respectively (Table 3). The inhibitory effects of the metal ions on the activity of yeast α-glucosidase were in the following order; Ba, Sr, Ca, Mg, Mn < Co < Fe < Ni < VO < Cu > Zn, and those on the activity of the rat small intestine α-glucosidase were in the order of Mn, Co, Fe, Ni < VO < Cu > Zn. These orders of metal ions are roughly consistent with the Irving–Williams’ stability order; Ba < Sr < Ca < Mg < Mn < Fe < Co < Ni < Cu > Zn.44 This observation suggests that the α-glucosidase-inhibitory activities of the metal ions are related to their stability constants with general ligands.45
The reversible inhibition of enzymes is generally expressed as competitive, non-competitive, or uncompetitive, by the Lineweaver–Burk plot analysis. The inhibitory modes of the yeast α-glucosidase by Cu and Zn ions were non-competitive and competitive, respectively (Fig. 7A and B). The inhibition mode of the rat-derived α-glucosidase by Cu ions, as determined by the Lineweaver–Burk plots, was similarly indicative of non-competitive inhibition (Fig. 8).
The active center of the α-glucosidases used in this study remains unknown, but it has been reported that cysteine (Cys) involved in the active center of bacterial α-glucosidase, and the surrounding histidine (His) residues also play a role in the reaction.46 The intrinsic stability constant (log K1) of Cu–His (28.0) is higher than that of Cu–Cys (16.0).45 Therefore, Cu ion binds weakly with the Cys of the active center, which may be responsible for the non-competitive inhibition mode. In contrast, log K1 values of Zn–His and Zn–Cys are 12.0 and 18.2, respectively.45 Zn ion binds more strongly with Cys than with His, which follows the competitive inhibition mode.
In support of the present results, Cu complexes with 3-hydroxypyridine-2-carboxylic acid47 and picolinic acid48 have recently been proposed to be potent insulin-mimetic and antidiabetic compounds, in which the α-glucosidase inhibitory effect may contribute to the anti-diabetic activity.
In conclusion, we have shown that the divalent metal ions examined in the present study exhibited inhibitory effects on α-glucosidasein vitro. Cu ion showed not only the highest α-glucosidase-inhibitory activity in vitro but also inhibitory activity in vivo similar to that of acarbose. In addition, we have identified the action mechanism of the anti-diabetic metal ions, which suggests the contribution of an α-glucosidase-inhibitory effect in the mechanism.
Acknowledgements
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (Grant-in-Aid for Scientific Research, Scientific Research on Priority Areas, and Specially Promoted Research to H. S.). This research was partially supported by a Grant-in-Aid for Young Scientists (B), 17790042, 2005–2006 from the Ministry of Education, Science, Sports and Culture to Y. Y.
References
- WHO Study Group, Diabetes mellitus, Geneva, Fact Sheets, 2006, 312.
- O. Pinhas-Hamiel and P. Zeitler, Lancet, 2007, 369, 1823–1831 CrossRef.
- S. Lehto, T. Ronnemaa, K. Pyorala and M. Laakso, Diabetologia, 2000, 43, 148–155 CrossRef CAS.
- W. Creutzfeldt, Diabetes/Metab. Res. Rev., 1999, 15, 289–296 Search PubMed.
- A. J. Scheen, Diabetes Metab., 1998, 24, 311–320 CAS.
- W. F. Caspary and S. Graf, Res. Exp. Med., 1979, 175, 1–6 CrossRef CAS.
- I. Suehiro, M. Otsuki and T. Yamasaki, Clin. Chim. Acta, 1979, 117, 145–152.
- X. Chen, Y. Zheng and Y. Shen, Curr. Med. Chem., 2006, 13, 109–116 CrossRef CAS.
- M. Koyama, R. Wada, H. Mizukami, H. Sakuraba, H. Odaka, H. Ikeda and S. Yagihashi, Metab., Clin. Exp., 2000, 49, 347–352 Search PubMed.
- K. Tsukamoto, Y. Nakayama and T. Mitsuzono, Jpn. Pharmacol. Ther., 2001, 29, 623–633 Search PubMed.
- H. J. Ahr, M. Boberg, E. Brendel, H. P. Krause and W. Steinke, Drug Res., 1997, 47, 734–745 Search PubMed.
- A. Lee, P. Patrick, J. Wishart, M. Horowitz and J. E. Morley, Diabetes, Obes. Metab., 2002, 4, 329–335 CrossRef CAS.
- F. A. Van de Laar, P. L. Lucassen, R. P. Akkermans, E. H. Van de Lisdonk, G. E. Rutten and C. Van Weel, Diabetes Care, 2005, 28, 166–175.
- T. Oku, M. Yamada, M. Nakamura, N. Sadamori and S. Nakamura, Br. J. Nutr., 2006, 95, 933–938 CrossRef CAS.
- T. Kimura, K. Nakagawa, Y. Saito, K. Yamagishi, M. Suzuki, K. Yamaki, H. Shinmoto and T. Miyazawa, J. Agric. Food Chem., 2004, 52, 1415–1418 CrossRef CAS.
- S. Onal, S. Timur and B. Okutucu, Prep. Biochem. Biotechnol., 2005, 35, 29–36 CrossRef.
- K. Schwarz and W. Mertz, Arch. Biochem. Biophys., 1959, 85, 292–295.
- A. H. Rubenstein, N. W. Levin and G. A. Elliott, Nature, 1962, 194, 188–189 CrossRef CAS.
- L. Coulston and P. Dandona, Diabetes, 1980, 29, 665–667 CrossRef CAS.
- C. E. Heyliger, A. G. Thahiliani and J. H. McNeil, Science, 1985, 227, 1474–1476 CrossRef CAS.
- H. Sakurai, K. Tsuchiya, M. Nukatsuka, M. Sofue and J. Kawada, J. Endocrinol., 1990, 126, 451–459 Search PubMed.
- Y. Yoshikawa, E. Ueda, K. Kawabe, H. Miyake, H. Sakurai and Y. Kojima, Chem. Lett., 2000, 874–875 CrossRef.
- J. E. Dominguez, M. C. Munoz, D. Zafra and I. R. Sanchez-Perez, J. Biol. Chem., 2003, 278, 42785–42794 CrossRef CAS.
- C. E. Heyliger, A. G. Tahiliani and J. H. McNeill, Science, 1985, 227, 1474–1477 CrossRef CAS.
- O. Esaki, J. Biol. Chem., 1990, 265, 1124–1128 CAS.
- W. Basuki, M. Hiromura, Y. Adachi, K. Tayama, M. Hattori and H. Sakurai, Biochem. Biophys. Res. Commun., 2006, 349, 1163–1170 CrossRef CAS.
- W. Basuki, M. Hiromura and H. Sakurai, J. Inorg. Biochem., 2007, 101, 692–699 CrossRef CAS.
- M. Hiromura, A. Nakayama, Y. Adachi, M. Doi and H. Sakurai, JBIC, J. Biol. Inorg. Chem., 2007, 12, 1275–1287 CrossRef CAS.
- A. Nakayama, M. Hiromura, Y. Adachi and H. Sakurai, J. Biol. Inorg. Chem., 2008, 13, 675–684 CrossRef CAS.
- J. A. Meyer and D. M. Spence, Metallomics, 2009, 1, 32–41 RSC.
- C. Hadrzynski, J. Trace Elem. Exp. Med., 1999, 12, 367–374 CrossRef CAS.
- H. Sakurai, Y. Kojima, Y. Yoshikawa, K. Kawabe and H. Yasui, Coord. Chem. Rev., 2002, 226, 187–198 CrossRef CAS.
- D. E. Baker and R. K. Campbell, Diabetes Educ., 1992, 18, 420–427 CrossRef CAS.
- H. Okada, S. Onodera and N. Shiomi, J. Rakuno Gakuen Univ., 2001, 26, 31–38 Search PubMed.
- A. Dahlqvist, Anal. Biochem., 1964, 7, 18–25 CrossRef CAS.
- T. Hanamura, C. Mayama, H. Aoki, Y. Hirayama and M. Shimizu, Biosci., Biotechnol., Biochem., 2006, 70, 1813–1820 CrossRef CAS.
- K. Suresh Babu, A. K. Tiwari, P. V. Srinivas, A. Z. Ali, B. China Raju and J. M. Rao, Bioorg. Med. Chem. Lett., 2004, 14, 3841–3845 CrossRef CAS.
- T. Oku, M. Yamada, M. Nakamura, N. Sadamori and S. Nakamura, Br. J. Nutr., 2006, 95, 933–938 CrossRef CAS.
- Y. Furuhashi, A. Yagi and E. Sakai, J. Integrated Study Dietary Habits, 2006, 17, 130–140 Search PubMed.
- W. F. Caspary and S. Graf, Res. Exp. Med., 1979, 175, 1–6 CrossRef CAS.
- A. Dahlqvist, Acta. Med. Scand. Suppl., 1972, 542, 13–18 Search PubMed.
- The Diabetes Control and Complications Trial Group, N. Engl. J. Med., 1993, 329, 977–986 CrossRef.
- Y. Ohkubo, H. Kishikawa, E. Araki, T. Miyata, S. Isami and S. Motoyoshi, Diabetes Res. Clin. Pract., 1995, 28, 103–117 CrossRef CAS.
- R. J. Williams, J. Inorg. Biochem., 2002, 88, 241–50 CrossRef CAS.
- The Chemical Society London: Stability Constants of Metal-Ion Supplement No.1, special publication No. 25, 1971.
- J. A. Lodge, T. Maier, W. Liebl, V. Hoffmann and N. Sträter, J. Biol. Chem., 2003, 278, 19151–19158 CrossRef CAS.
- M. Nakai, F. Sekiguchi, M. Obata, C. Ohtsuki, Y. Adachi, H. Sakurai, C. Orvig, D. Rehder and S. Yano, J. Inorg. Biochem., 2005, 99, 1275–1282 CrossRef CAS.
- N. Yasumatsu, Y. Yoshikawa, Y. Adachi and H. Sakurai, Bioorg. Med. Chem., 2007, 15, 4917–4922 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000



