Cytotoxic sugar analogues of an optimized novobiocin scaffold†
Alison C.
Donnelly‡
,
Huiping
Zhao‡
,
Bhaskar
Reddy Kusuma
and
Brian S. J.
Blagg
*
Department of Medicinal Chemistry, 1251 Wescoe Hall Drive, Malott 4070, The University of Kansas, Lawrence, Kansas 66045-7563, U.S.A. E-mail: bblagg@ku.edu; Fax: +1 (785) 864-5326; Tel: +1 (785) 864-2288
First published on 30th July 2010
Abstract
Studies on the natural product, novobiocin, have elucidated specific modifications that increase Hsp90 inhibition. Through diversification of the sugar appendage, coumarin core and benzamide side chain of novobiocin, structurally unique scaffolds have been synthesized. These structural adaptations have produced potent cytotoxic agents, such as KU135, which are prepared more simply than those that contain the noviose sugar. These analogues have been evaluated against two cancer cell lines and demonstrated low micromolar anti-proliferative activity.
Introduction
Although many novobiocin analogues have been synthesized, the noviose sugar requires the most synthetic steps, as assembly of this pyranose is not trivial. Even the most efficient syntheses of noviose require more than 10 synthetic procedures, and the overall yield is less than desirable.1 It was proposed that while the sugar moiety plays a critical role in the presentation of binding interactions to Hsp90, not all functionalities are essential.2,3 To further elucidate structure–activity relationships and identify key interactions, while simplifying overall inhibitor synthesis, a library of sugar and non-sugar analogues was prepared.Simplified sugars and related azasugars were designed to conserve structural motifs present in noviose, while allowing identification of essential interactions with the putative binding pocket. These analogues were designed to probe potential hydrogen bonds as well as identify spatial constraints. In addition to these simplified or modified sugars, alternative groups were attached to the 7-position of the coumarin ring, wherein noviose is typically attached. These non-sugar analogues incorporate heteroatoms alongside steric bulk, to probe both the hydrogen bonding capabilities and pocket dimensions, respectively. Exploration of these diverse moieties provides the opportunity to simplify the novobiocin scaffold, while maintaining or improving solubility, absorption and activity.4–6 Identification of a simplified noviose surrogate will enable the expeditious synthesis of novobiocin-derived Hsp90 inhibitors.
KU135 (Fig. 1) was designed based on studies of the novobiocin coumarin core, which revealed that an alkoxy substituent at the 6-position improved activity.8,9 In addition, the noviose sugar was replaced with an acetylated phenol, capable of participating in hydrogen bonding, but lacking complexity of the sugar. This compound was synthesized and tested against a panel of cancer cell lines, revealing it to be a potent derivative that manifested the following IC50 values: 5.72 ± 0.03 μM against SKBr3 cells, 1.50 ± 0.30 μM against MCF7 cells, 0.52 ± 0.01 μM against HCT-116 cells, 4.10 ± 2.20 μM against PL45 cells, 0.42 ± 0.27 μM against LNCaP cells, 0.97 ± 0.68 μM against PC-3 cells and 0.42 μM against Jurkat T-cells. Subsequent studies in Jurkat T-cells confirmed a unique mechanism through which this compound exhibits anti-proliferative activity. Moreover, it was confirmed, through three complementary approaches, that KU135 binds the Hsp90 C-terminus and induces Hsp90-dependent client protein degradation.9 The attachment of an acetyl group in lieu of noviose resulted in a remarkable 10-fold improvement in activity against SKBr3 cells versus the noviosylated counterpart (KU144). Because of this novel and exciting activity, it was proposed that complementary analogues, built upon an optimized novobiocin scaffold, could lead to more efficacious compounds. The design and synthesis of these non-sugar analogues are discussed in this communication, as well as the anti-proliferative activities manifested by these molecules.
 | ||
| Fig. 1 Structures of novobiocin, noviosylated analogues and KU135.7–10 | ||
Results and discussion
Design of sugar analogues of novobiocin
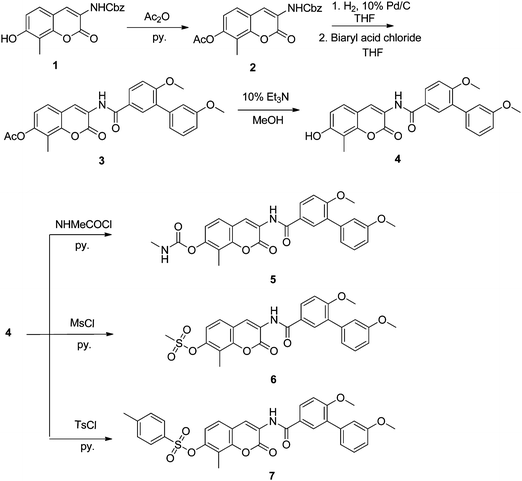
To extend upon our knowledge of KU135 and its SAR, several simplified non-sugar moieties were appended to the 7-position of the coumarin ring. Chemistry developed by our lab was used to construct the coumarin core and attach the desired biaryl benzamide, while acetylation protected the phenol until subsequent modification.8 Phenol 4 (Scheme 1) was chosen as the scaffold upon which to build these analogues, because of its expeditious synthesis and to explore whether the activity of KU135 can be further attenuated.7 Most analogues were designed with a sugar replacement that maintained interactions proposed to be manifested by noviose. In addition to probing the dimensions and electronic environment, functionalities that exhibit improved solubility profiles were incorporated. Denoviosylated phenol 4, which lacks the sugar functionality and corresponds to a potential metabolite upon cleavage of the glycosidic bond, was also evaluated. Many bioactive natural products bearing carbohydrates are often rendered inactive upon removal of their sugar moieties.11–16 However, Le Bras and co-workers recently reported that disruption of the Hsp90 heteroprotein complexes results from treatment with a compound bearing a free phenol at the 7-position of a novobiocin-derived scaffold.17 Although this compound did not demonstrate remarkable activity, it was notable that it maintained Hsp90 inhibition despite removal of noviose.For direct comparison to KU135, the acetylated scaffold 3, was also evaluated. A methylated carbamate was chosen to complement the size of the acetate group, while providing additional stability, as carbamates are not readily cleaved in vivo. The methylated carbamate was installed on phenol 4, via the carbamoyl chloride, to further probe dimensions of the pocket. Methyl and toluene sulfonic esters were also appended to the 7-position to explore hydrogen bonding interactions, while simultaneously probing pocket dimensions. It was envisioned that the sulfonic ester is capable of participating as a hydrogen bond acceptor, while the alkyl/aryl appendages probe the ability of the pocket to encapsulate hydrophobic bulk. Moreover, the 7-tolylsulfonate ester has been shown by the Renoir group to maintain Hsp90 inhibition and demonstrate modest potency when attached to a novobiocin-derived scaffold.18 The desired functional group was installed onto the 7-phenol using the requisite sulfonyl chloride in the presence of pyridine.
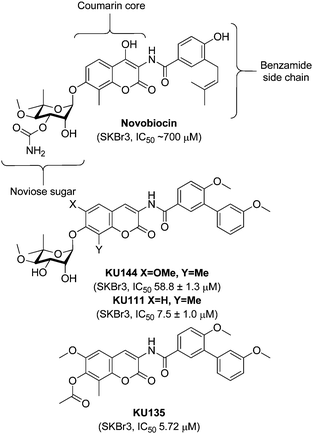
While the planar aromatic tolyl group offers a rigid six-membered ring, the pyranose of noviose represents a flexible ring system. Thus, saturated heterocyclic six-membered rings were investigated to serve as simplified mimics of noviose. Substituted piperidines were synthesized as simplified azasugars to probe essential substituents on the noviose ring. Azasugars were selected due to the prevalence of N-heterocycles in a wide variety of bioactive compounds with diverse therapeutic applications.19–21 An unsubstituted 3-piperidine represents an aza-surrogate capable of probing essential hydrogen bonds, such as those potentially made by the noviose diol. In contrast, the acetylated 3-piperidine, while still capable of maintaining hydrogen bonds, introduces steric bulk. It is proposed that this acetylated amine, which introduces bulk into the gem-dimethyl region on noviose, should be tolerated. Finally, a methylated 4-piperidine was introduced to replace the sugar. The methylated amine was introduced to project this moiety into the same cavity as the 4′-methoxy group on noviose.
As outlined in Scheme 2, Mitsunobu conditions were employed to prepare the protected amine, 9, from Boc-protected commercially available 3-piperidine 8 and phenol 4.22 The alkene of 9 was reduced to give 10 and the Boc-group was subsequently removed upon exposure to 10% TFA/CH2Cl2 to furnish 11. In contrast, the Boc-group could be directly removed from amine 9 to afford 12, which was acetylated to furnish 13, and then subsequently reduced with 10% Pd/C and hydrogen to give acetylated amine, 14. Alternatively, commercially available Boc-protected 4-piperidine 15 was coupled with phenol 4 using Mitsunobu conditions to yield 16.23 Subsequent deprotection led to compound 17, which was methylated to afford 18 in good yield.
 | ||
| Scheme 2 Synthesis and attachment of azasugar analogues. | ||
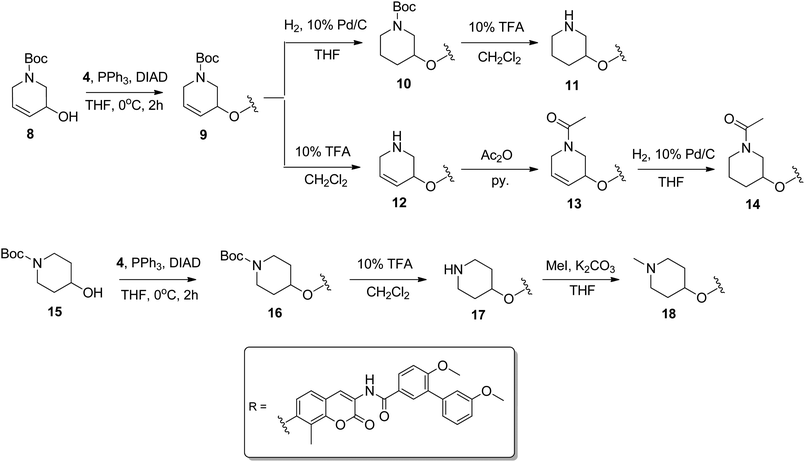
In addition to the piperidines, simplified pyranoses were appended in lieu of noviose. Pyranoses are attached to several clinically prescribed anti-cancer drugs, such as bleomycin, doxorubicin and etoposide, in addition to novobiocin.22 The simple 2- and 3-hydroxy pyranoses were chosen, as each could maintain similar hydrogen bonds as found in noviose. Through systematic removal of each hydroxyl, identification of which hydroxy group is essential could be ascertained. Once attached, the α anomer of each of the pyranoses was evaluated, as this is the anomer present in novobiocin.24,25 Finally, a syn-diol-containing cyclohexane was chosen as the final non-sugar moiety. This sugar alternative maintains the diol of noviose, but does not contain the labile hemi-acetal linkage.
The pyranose analogues were prepared through a series of coupling and deprotection procedures. Compounds 20 and 23 were synthesized as a mixture of diastereomers by coupling protected sugars 19 and 22 with coumarin 4 under standard Mitsunobu conditions.6,26,27 Removal of the benzoyl group produced a diastereomeric mixture of 20 in 10 min, yielding the desired α anomer 21 along with the β anomer in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio.5 Similarly, removal of the tri-isopropyl silyl protecting group of 23 gave a mixture of diastereomers, from which compound 24 was isolated via silica gel chromatography (Scheme 3). The dihydroxylated cyclohexane was synthesized from α,β-unsaturated ketone 25 using Luche conditions to yield 26. The resulting cyclohexene was coupled using Mitsunobu conditions and subsequently dihydroxylated to give diol 28.
3 ratio.5 Similarly, removal of the tri-isopropyl silyl protecting group of 23 gave a mixture of diastereomers, from which compound 24 was isolated via silica gel chromatography (Scheme 3). The dihydroxylated cyclohexane was synthesized from α,β-unsaturated ketone 25 using Luche conditions to yield 26. The resulting cyclohexene was coupled using Mitsunobu conditions and subsequently dihydroxylated to give diol 28.
 | ||
| Scheme 3 Synthesis and attachment of hydroxylated pyranoses and cyclohexane. | ||
Biological evaluation of sugar analogues of novobiocin
Upon construction of the non-sugar and modified sugar analogues of novobiocin, the compounds were evaluated for anti-proliferative activity against SKBr3 (estrogen receptor negative, Her2 overexpressing breast cancer cells) and MCF-7 (estrogen receptor positive breast cancer cells) (Table 1).| SKBr3 | MCF-7 | |
|---|---|---|
| a Values represent mean ± standard deviation for at least two separate experiments performed in triplicate. | ||
| KU144 | 58.80 ± 1.3a | >100 |
| KU135 | 5.72 | 1.50 ± 0.3 |
| KU111 | 7.50 ± 1.0 | 18.70 ± 1.8 |
| 3 | 0.98 | 1.40 ± 0.0 |
| 4 | 8.88 ± 0.6 | 6.93 ± 0.6 |
| 5 | 2.40 ± 0.2 | 1.72 ± 0.2 |
| 6 | 7.33 ± 1.2 | 8.85 ± 1.1 |
| 7 | >100 | >100 |
| 11 | 1.16 ± 0.2 | 1.63 ± 0.4 |
| 14 | 10.79 ± 0.1 | 9.18 ± 0.8 |
| 18 | 1.34 ± 0.2 | 1.51 ± 0.3 |
| 21 | >100 | >100 |
| 24 | 9.28 ± 0.1 | 11.20 ± 0.6 |
| 28 | >100 | >100 |
Upon examination of the non-sugar analogues, some interesting trends were observed. Despite complete excision of the noviose sugar, the free phenol 4 demonstrated comparable activity to the corresponding noviosylated scaffold (KU111). Also, the acetylated and methylated carbamate scaffolds, 3 and 5, respectively, demonstrated comparable activity to KU135, and the latter represents an analogue that maintains activity without the labile acetate group. While the methyl sulfonic ester 6 maintained similar activity to its noviosylated counterpart, the toluene sulfonic ester 7 was inactive, indicating the toluene-containing compound exceeded the limits tolerated by the binding pocket.
Much like the non-sugar analogues, the piperidine scaffolds manifested a broad range of activities. While the unsubstituted 3-piperidine 11 demonstrated low-micromolar activity, acetylation (14) compromised its activity. Transposition of the amine to the 4-position and subsequent methylation (18) returned low-micromolar activity. The various hydroxylated pyranoses demonstrated intriguing activity. While the 3′-hydroxylated pyranose 24 maintained the activity of noviosylated KU111, the 2′-hydroxylated pyranose 21 was completely inactive. Similarly, the syn-diol-containing cyclohexane 28 did not display measurable activity in the anti-proliferation assay, indicating a hydrogen-bond acceptor adjacent to the phenolic oxygen is essential.
The SAR trends observed for the non-sugar analogues offer insight into the nature of the binding pocket. As mentioned previously, sugars are known to play important roles in both solubility and transport.24,25 The potency of compounds containing a hydrogen bond donor, such as the carbamate and unsubstituted piperidine, affirms the need for hydrogen bonds with the pocket for favorable binding. Furthermore, activity of the 3-piperidine correlates well with the data from the set of pyranoses, supporting an essential hydrogen bond donor at the 3-position of the ring, while a hydrogen-bond at the 2-position is dispensable. In contrast, it appears that within this hydrogen bonding network, a simple syn-diol cyclohexane system cannot take advantage of such interactions, suggesting an essential role of the hemi-acetal linkage made by the pyranose systems. It is possible that the endocyclic oxygen of the pyranose ring provides interactions with the binding pocket that properly orients the hydroxy groups. Another important observation from this series of sugar mimics is that steric bulk is selectively tolerated. When comparing the methylated 4-piperidine and acetylated 3-piperidine, it becomes clear that bulk is much better tolerated at the 4-position than the 3-position. Moreover, when comparing the methyl and toluene sulfonic ester, the methyl-containing compound, despite its poor solubility, maintains activity, while the corresponding toluene derivative does not. SAR for this series of compounds is summarized in Fig. 2.
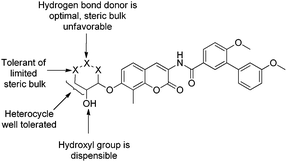 | ||
| Fig. 2 SAR summary for modified sugar analogues of novobiocin | ||
To confirm that the anti-proliferative activities exhibited by the sugar and non-sugar analogues result from Hsp90 inhibition, analogues 5 and 18 were evaluated by Western blot analyses (Fig. 3). It was essential that these analogues demonstrate selective Hsp90-dependent client protein degradation, versus a loading control to confirm anti-Hsp90 activity. Fig. 3 shows that in MCF-7 cells, the Hsp90-dependent client proteins Akt, Raf, and Her2 were degraded in a concentration-dependent manner upon treatment with the compounds. Hsp90 client protein degradation occurred at concentrations that paralleled the observed anti-proliferative IC50 values of 1.72 ± 0.2 μM, for 5, and 1.51 ± 0.3 μM, for 18, confirming that Hsp90-dependent client protein degradation is causative for inhibition of cell growth. Moreover, Hsp90 remained constant at all concentrations tested, which is a hallmark of C-terminal Hsp90 inhibition. Since the non-Hsp90-dependent protein, actin, was not affected by these analogues, selective degradation of Hsp90-dependent proteins occurred directly through Hsp90 inhibition.
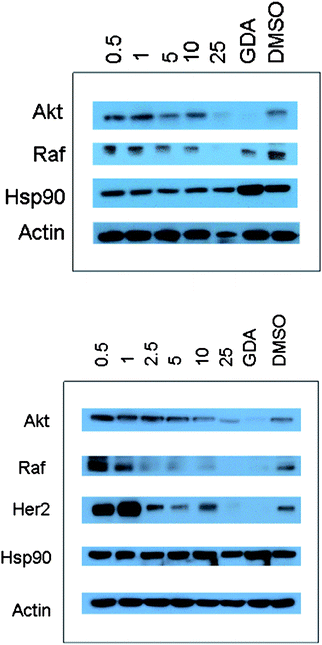 | ||
| Fig. 3 Western blot analyses of MCF-7 cell lysates for Hsp90 client protein degradation after 24 h incubation. Concentrations (in μM) of 5 (top blot) and 18 (lower blot) are indicated above each lane. GDA (geldanamycin, 500 nM) and DMSO were respectively employed as positive and negative controls. | ||
Conclusion
The studies described sought to elucidate the potential role of the noviose sugar in binding of novobiocin-derived compounds to Hsp90. SAR for the noviose appendage has been presented and important interactions highlighted. Essential interactions, which can be maintained by simplified scaffolds, as well as those that are dispensable were identified. In addition, the steric environment into which the sugar is presented was explored and insight was gained into which groups are best tolerated. Small changes in substitution resulted in significant changes in activity, reflecting a sensitive steric environment within this binding pocket. Capitalization on key interactions and steric bulk resulted in the preparation of several low-micromolar Hsp90 inhibitors that exhibit improved activity versus the parent noviosylated compounds and lack complexity. The analogues described benefit from facile preparation, and offer the potential for the more efficient synthesis of Hsp90 C-terminal inhibitors.Acknowledgements
Authors gratefully acknowledge support of this project by the NIH/NCI (CA120458) and the ACS Division of Organic Chemistry Fellowship (A.D.).References
- X. M. Yu, G. Shen and B. S. J. Blagg, J. Org. Chem., 2004, 69, 7375 CrossRef CAS.
- X. M. Yu, G. Shen, L. Neckers, H. Blake, J. Holzbeierlein, B. Cronk and B. S. J. Blagg, J. Am. Chem. Soc., 2005, 127, 12778–12779 CrossRef CAS.
- J. A. Burlison, L. Neckers, A. B. Smith, A. Maxwell and B. S. J. Blagg, J. Am. Chem. Soc., 2006, 128, 15529–15536 CrossRef CAS.
- H. Garg, N. Francella, K. A. Tony, L. A. Augustine, J. J. Barchi, J. Fantini, A. Puri, D. R. Mootoo and R. Blumenthal, Antiviral Res., 2008, 80, 54–61 CrossRef CAS.
- F. Anizon, P. Moreau, M. Sancelme, W. Laine, C. Bailly and M. Prudhomme, Bioorg. Med. Chem., 2003, 11, 3709–3722 CrossRef CAS.
- Y. M. Yu, H. Han and B. S. J. Blagg, J. Org. Chem., 2005, 70, 5599 CrossRef CAS.
- J. A. Burlison, C. Avila, G. Vielhauer, D. J. Lubbers, J. Holzbeierlein and B. S. J. Blagg, J. Org. Chem., 2008, 73, 2130–2137 CrossRef CAS.
- A. C. Donnelly, J. R. Mays, J. A. Burlison, J. T. Nelson, G. Vielhauer, J. Holzbeierlein and B. S. J. Blagg, J. Org. Chem., 2008, 73, 8901–8920 CrossRef CAS.
- S. N. Shelton, M. E. Shawgo, S. B. Comer, Y. Lu, A. C. Donnelly, K. Szabla, M. Tanol, G. A. Vielhauer, R. A. Rajewski, R. L. Matts, B. S. Blagg and J. D. Robertson, Mol. Pharmacol., 2009, 76, 1314–1322 CrossRef CAS.
- A. Donnelly and B. S. Blagg, Curr. Med. Chem., 2008, 15, 2702–2717 CrossRef CAS.
- A. C. Weymouth-Wilson, Nat. Prod. Rep., 1997, 14, 99–110 RSC.
- J. S. Thorson, T. J. Hosted, J. Jiang, J. B. Biggins and J. Ahlert, Curr. Org. Chem., 2001, 5, 139–167 CrossRef CAS.
- K. C. Nicolaou and H. J. Mitchell, Angew. Chem., Int. Ed., 2001, 40, 1576–1624 CrossRef CAS.
- In Carbohydrates in Chemistry and Biology, ed. B. Ernst, G. W. Hart and P. Sinay, Wiley-VCH, Weinheim, 2000 Search PubMed.
- Glycochemistry: Principles, Synthesis and Applications, ed. P. G. Wang and C. R. Bertozzi, Marcel Dekker, Inc., New York, 2001 Search PubMed.
- P. Vogel, in Glycoscience, ed. B. O. Fraser-Reid, K. Tatsuta, J. Thiem, Springer, Berlin, 2001, vol. 2 Search PubMed.
- G. Le Bras, C. Radanyi, J.-F. Peyrat, J.-D. Brio, M. Alami, V. Marsaud, B. Stella and J.-M. Renoir, J. Med. Chem., 2007, 50, 6189–6200 CrossRef CAS.
- C. Radanyi, G. Le Bras, S. Messaoudi, C. Bouclier, J.-F. Peyrat, J.-D. Brion, V. Marsaud, J.-M. Renoir and M. Alami, Bioorg. Med. Chem. Lett., 2008, 18, 2495–2498 CrossRef CAS.
- R. J. Capon, C. Peng and C. Dooms, Org. Biomol. Chem., 2008, 6, 2765–2771 RSC.
- N. Kolocouris, G. Zoidis, G. B. Foscolos, G. Fytas, S. R. Prathalingham, J. M. Kelly, L. Naesens and E. De Clercq, Bioorg. Med. Chem. Lett., 2007, 17, 4358–4362 CrossRef CAS.
- S. Y. Ke, X. H. Qian, F. Y. Liu, N. Wang, Q. Yang and Z. Li, Eur. J. Med. Chem., 2009, 44, 2113–2121 CrossRef CAS.
- A. J. Ross, O. V. Sizova and A. V. Nikolaev, Carbohydr. Res., 2006, 341, 1954 CrossRef CAS.
- G. Shen, X. M. Yu and B. S. J. Blagg, Bioorg. Med. Chem. Lett., 2004, 14, 5903–5906 CrossRef CAS.
- I. Huber-Ruano and M. Pastor-Anglada, Curr. Drug Metab., 2009, 10, 347–358 Search PubMed.
- A. Bernardi and P. Cheshev, Chem.–Eur. J., 2008, 14, 7434–7441 CrossRef CAS.
- M. V. Powers and P. Workman, FEBS Lett., 2007, 581, 3758–3769 CrossRef CAS.
- D. Mahalingam, R. Swords, J. S. Carew, S. T. Nawrocki, K. Bhalla and F. J. Giles, Br. J. Cancer, 2009, 100, 1523–1529 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, characterisation data and NMR spectra. See DOI: 10.1039/c0md00063a |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |