Small molecule modulation of stem cells in regenerative medicine: recent applications and future direction
Timothy E.
Allsopp†
*a,
Mark E.
Bunnage†
*b and
Paul V.
Fish†
*c
aPfizer Regenerative Medicine, UCB Building, Granta Park, Great Abington, Cambridge, CB21 6GS, UK. E-mail: timothy.allsopp@pfizer.com; Tel: +44 (0)1304 643483
bRegenerative Medicine Chemistry, Pfizer Global Research and Development, Sandwich Laboratories, Sandwich, Kent CT13 9NJ, UK. E-mail: mark.bunnage@pfizer.com; Tel: +44 (0)1304 648974
cRegenerative Medicine Chemistry, Pfizer Global Research and Development, Sandwich Laboratories, Sandwich, Kent CT13 9NJ, UK. E-mail: paul.fish@pfizer.com; Tel: +44 (0)1304 644589
First published on 22nd June 2010
Abstract
Regenerative medicine research is focussed on the discovery of novel therapies (small molecules, biologics or cells) that restore function in damaged or aging tissues and organs. The field is underpinned by developments in stem cell science and the modulation of stem cells using small molecules is now providing unique insights into stem cell regulation and developmental biology. It has been shown that small molecules can be used to help drive somatic cell reprogramming, maintain induced pluripotent states and also directly control lineage specification and proliferation events. Small molecules have the potential to help enable the development of viable cell therapies and small molecule oral drugs can be envisaged that can control endogenous cell populations to support regeneration. This review seeks to illustrate the key role small molecules have to play in modulation of stem cells for regenerative medicine and describes some future potential directions in the field.
 Timothy E. Allsopp | Tim is a biology graduate from University College London and performed doctorate studies in developmental neurobiology at King’s College, University of London. He has publications in the fields of neurobiology, apoptosis and stem cells. Nine years’ valuable experience was gained in the stem cell biotechnology sector, as CSO of Stem Cell Sciences, at which he led the successful development of a number of product lines. He joined Pfizer Regenerative Medicine in March 2009 as Head of External Research. Tim has reviewed for and acted as consultant to a number of internationally funded RM programmes and is a member of management boards for the UK SCB and National Network. |
 Mark E. Bunnage | Mark read chemistry at the University of Durham, completed his DPhil with Professor S.G. Davies at the University of Oxford, and then moved to The Scripps Research Institute to work with Professor K.C. Nicolaou as a NATO postdoctoral fellow. Mark joined Pfizer in the UK as a medicinal chemist in 1996 and since then has assumed a number roles of increasing responsibility. Mark is currently Executive Director, Head of Chemistry for Regenerative Medicine, Pfizer’s New Opportunities Unit and Lead Discovery Technologies. Mark has broad interests in medicinal chemistry and is an author or inventor on over 50 publications and patents. |
 Paul V. Fish | Paul received his BSc in Chemistry from the University of Nottingham and he then undertook PhD studies in synthetic organic chemistry at Nottingham with Professor G. Pattenden working on the total synthesis of marine natural products. Subsequently, he moved to the USA where he performed postdoctoral research with Professor E. J. Corey at Harvard University and then with Professor W. S. Johnson at Stanford University. Paul joined Pfizer as a medicinal chemist in 1994 and through his research he has helped to co-discover and deliver drug candidates across several therapeutic disease areas, some of which have gone on to advanced clinical studies. Paul has published 60 scientific papers and patents. In 2009, Paul joined the Regenerative Medicine unit where he leads the exploratory chemistry team. |
Introduction
Regenerative medicine is defined as the reconstruction of diseased or injured tissue by activation of endogenous cells or by cell transplantation. Although it will be many years before sophisticated restoration of organ function becomes routine in clinical practise, significant progress has been made in developing first generation therapies, the majority of which involve transplantation of a patient's own cells (autologous) to partially restore tissue function. Substantial advancements have also taken place in the understanding of the science of cell and tissue replacement sufficient for the major challenges to be defined.1 In order to provide for the eventual deployment of safe, effective therapies these challenges include: identification and high-fidelity production of cells with tissue repair capacity for cell therapy; controlled mobilization and systemic release of often quiescent cells from tissue compartments for endogenous tissue repair; knowing the precise requirements for sustained, efficient tissue engraftment; strategies to safely subvert a patient's immune response to the cells of a donor. The impact of chemical biology as the nascent discipline of regenerative medicine expands is unquestionable. Indeed, this is to be expected, as there is now a greater comprehension of the signalling pathways regulating cell growth and expansion, the mobilization characteristics of tissue resident progenitor cells, and the mechanisms promoting nascent cell survival and engraftment.There are currently many well characterised examples of regenerative medicine products for which the living cell components are more specialized fibroblast, skin, cartilage or endothelial cells2–8 and these function to ‘catalyze’ an intrinsic repair process. Progress in this area has been extensively reviewed in the recent past and therefore will not be considered further.9 The successful and beneficial manipulation of fundamental cell features by small molecules has already had significant impact in regenerative medicine. The field is exemplified by a growing number of recent reports in which small molecules have improved ex vivo cell production, cell stability, mobilization and integration from tissue niches and modulation of the host response to foreign cell grafts. For anchorage dependent cells more efficient cell production processes can be achieved via anoikis (apoptosis) inhibition using Rho kinase (ROCK) inhibitors.10,11 The integrity and stability of tissue constructs can be enhanced using small molecule stimulators of extracellular matrix production.12–14 Antagonism of the chemokine receptor CXCR4 acts to mobilize haematopoietic progenitors from the human bone marrow niche15 and inhibitors of protein kinase C(ζ) act as potent suppressors of anti-inflammatory T lymphocyte activation.16 Progress in this area will continue to be dynamic and will be the subject of future reviews.
This article will focus instead on a contemporary review of the way in which chemistry is facilitating the harnessing of fundamental stem cell biology, namely a choice of cell fate. Stem cells, unspecialized resident cells in embryonic and adult somatic tissues, can continuously produce unaltered progeny (self-renewal) or progeny that have distinct, more restricted properties linked to commitment to specialized cell fates. Stem cells afford an unsurpassed platform for drug discovery.17 Human pluripotent stem cells can renew indefinitely in tissue culture whereas somatic, more restricted stem cells isolated ex vivo show more limited expansion capacity. Human stem cells, however, can be grown stably, akin to conventional cell lines used in pharmaceutical research. There have been several timely reviews on small molecule mediators of stem cell fate.18–29 It is notable that for normal stem cells, proportionally less has been described regarding small molecule mediators of intermediate and later stages of differentiation for the major tissue lineages, other than neuro-ectoderm, although some recent examples have been described.30,31 Why this may be the case will be examined later in the review.
The molecular events associated with lineage decisions are contemporary topics regarding both normal and abnormal stem cells. Cancer stem cells (CSC) likely possess deregulation of these decision processes and brief mention will be made of the prospects that a chemical biology approach will bring to the development of cancer therapeutics. Ultimately, it can be anticipated that this research will culminate in the effective harnessing of normal stem cells, for example an eventual understanding of how to replenish pools of endogenous tissue repair cells or how to prepare a defined population of differentiated cells from stem cells in the laboratory. From this basic research, many novel regenerative medicine therapies will inevitably emerge, from small molecule and biological drugs to the development of cell therapies as medicines. However there is no more logical a starting point to review small molecule modulation than with the ‘master’ of all stem cell states – pluripotency.
Small molecules can harness the most cardinal of stem cells
The most fundamental choice of fate is made by the first stem cells of the embryo and the mechanism underpinning this choice is being elucidated experimentally in the laboratory. The decision to self-renew or differentiate is arguably most striking for embryonic stem cells (ES), a pluripotent population established artificially in culture from pre-implantation embryos (Fig. 1) (see Table 1 for a glossary of terms). An explanation of the initial molecular events of commitment has been proposed, with observations derived predominantly from experiments using small molecular inhibitors. Many of the difficulties in growing pluripotent stem cells, such as spontaneous differentiation and quantitative inconsistencies in specification to certain lineages, are coherent with the premise that ES cells in culture are continuously exposed to extrinsic signals driving specification. This is in part due to sub-optimal culture methodology and this technical limitation leads to cells intermittently lapsing through a range of transcriptional states (Box 1).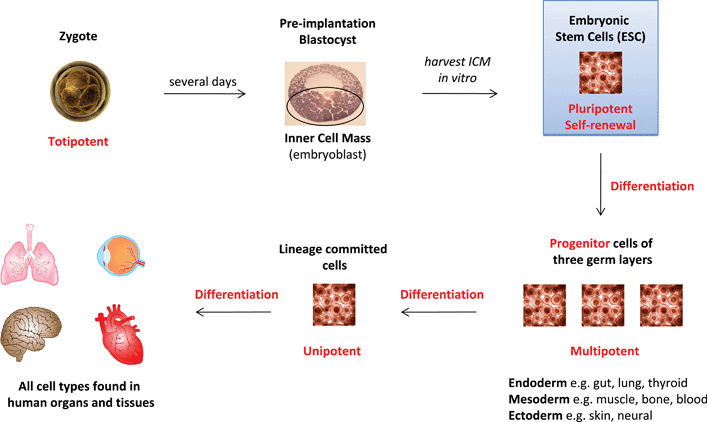 | ||
| Fig. 1 Origin of embryonic stem cells and their utility in forming all cell types found in human organs and tissue. (Terms in red are defined in Table 1.) | ||
| Potency | The range of commitment options available to a cell |
|---|---|
| Totipotent | Sufficient to form entire organism, including extra-embryonic tissues of the placenta. Described for zygote and not demonstrated for any vertebrate stem cell |
| Pluripotent | Able to form all the body's cell lineages, including germ cells and certain extraembryonic tissues. |
| Multipotent | Can form multiple lineages that constitute an entire tissue or tissues e.g haematopoietic stem cells |
| Oligopotent | Able to form two or more lineages within a tissue e.g. neural stem cells |
| Unipotent | Forms a single lineage e.g. spermatogonial stem cells |
| Cancer stem cell | Self renewing cell responsible for sustaining a cancer and for producing differentiated progeny that form the bulk of the cancer. |
| Reprogramming | An increase in potency. A process which occurs naturally in regenerative organisms. Induced experimentally in mammalian cells by in vitro culture, genetic manipulation, cell fusion or nuclear transfer. |
| Differentiation | Commitment to a program leading to specification of functional cell types and represents exit from self-renewal for a stem cell. |
| Progenitor | General term for any dividing cell with capacity to differentiate and can encompass a putative stem cell for which self-renewal has not yet been defined. |
| Self-renewal | Cycles of cell division that repeatedly generate at least one daughter equivalent to the mother cell with latent capacity for differentiation (a defining property of stem cells). |
Box 1: The highs and lows of human pluripotent stem cell technologyPros • in vitro system for modelling human development or disease pathology • conventional druggable target categories when cells differentiated • signalling pathways germane for tissue regeneration when cells are undifferentiated • tunable approach: production of homogenous cell types for screens or mixtures for cell therapy
Cons • non standard culture conditions; diverse serum products and cell feeders often used by different laboratories • predominant commitment to certain lineages upon differentiation • inconsistent mixture of progeny upon spontaneous differentiation • quantitative variability in fate choice due to unique genetic and epigenetic constitution • temporal sequence of unknown paracrine factors constantly modifies cell fate during differentiation |
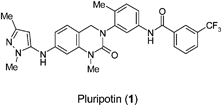
The use of small molecules has contributed to the description of various pluripotent stem cell states [e.g. pre- or post-implantation epiblast, epistem cells, induced pluripotency] evoking the concept of pluripotency categories that at individual cell level may be reflected by an inherent lineage bias even though the stem cells in culture appear to be uncommitted.32 The transcriptional organizers, Oct4 and Nanog, define pluripotency in ES cells.33 In mouse, the first ES cells were described and in this well characterised system Oct4 perversely specifies lineage commitment by inducing expression of the specification factor fibroblast growth factor 4 (FGF4). Nanog is expressed in a dynamic and heterogeneous fashion in cells and it is likely that a fraction of the cells are transiently primed for commitment as a result.34 The combination of small molecule pluripotin (1) and leukaemia inhibitory factor (LIF) furnished a highly efficient protocol for the derivation of mouse ES cells by selectively maintaining Oct4 positive cells in outgrowths.35
Recently a small molecule cocktail has been formulated that stringently harnesses mouse ES cells in culture. Using chemical inhibitors of the autocrine FGF4 signal, the multifunctional enzyme glycogen synthase kinase-3 (GSK-3; itself a negative regulator both of canonical Wnt signalling and of a broad range of biosynthetic and transcriptional activities), and mitogen activated protein kinase (MEK; extra-cellular signal regulated kinase) which have been collectively termed the ‘3i’ (three inhibitors), a ‘ground state’ of pluripotency can be established.36 In this state there is no spontaneous differentiation and heterogeneity in Nanog protein expression is greatly reduced. By extension of the premise, FGF signalling likely induces fluctuation of Nanog expression making cells permissive for progression to commitment and eventual exit from the self renewing state (Box 2).36
Box 2: Features of ‘ground state’ cultured ES cells• express key markers (rex-1, stella, Klf4), • no X chromosome inactivation in female cells • cells differentiate in LIF alone or with activin/FGF2 • BMP4 with LIF stimulates self-renewal • cells self-renew in presence of MEK inhibitor, GSK3 inhibitor, and FGF receptor tyrosine kinase inhibitor • competent to incorporate into pre-implantation embryos (demonstrated for rodents) • contribute to formation of balanced chimaeras during embryogenesis (demonstrated for rodents) |
Critical factors central to sustaining pluripotency in ES cells are similar to those for reprogramming somatic cells to make induced pluripotent stem (iPS) cells (Fig. 2). Yamanaka and Takahashi first demonstrated the propensity for four genetic factors (Oct3/4, Sox2, c-Myc, and Klf4) to induce pluripotency in murine somatic cells37 and significant recent effort has focussed on discovering small molecule substitutes for these transcription factors in attempts to make the overall process more efficient, easier and safer.
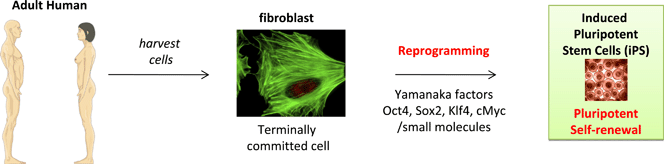 | ||
| Fig. 2 Source of induced pluripotent stem cells by reprogramming terminally committed cells. (Terms in red are defined in Table 1.) | ||
Recent findings, for example, indicate that exogenous Sox2 and c-Myc can be replaced by small molecule inhibitors of Tgf-β signalling.38,39 Studies have concluded that establishment of a pluripotent state via reprogramming requires (i) deconstruction of the differentiated state by repression of lineage-specific genes, (ii) establishment of an “open” chromatin stage, and (iii) reactivation of endogenous pluripotency-related genes. Use of the ‘3i’ small molecules facilitates progression of dedifferentiated intermediates to acquire pluripotency.40 A chemical biology and systems biology approach is thus enabling comprehension and control of the mechanisms underlying the fundamental choice that ‘master’ stem cells continuously make.
Small molecules influence stem cell fate
Harnessing cell fate; deprogrammed, reprogrammed and cancer stem cell states
The differentiated status of a cell is determined by genetic, epigenetic and chromatin structure influences. Lineage specification appears not to be an irreversible consequence of progressive restrictions in potency imposed by patterns of gene repression and activation, as evidence indicates it is possible for cells to switch their lineage relationships and this can be facilitated by manipulations of key transcription factor networks. From recent studies the sequence of molecular events required to reprogramme cells is being defined. There are now a number of examples of defined transcription factor induced switches in cell lineage that are providing insight into the mechanism of reprogramming. None more so striking than the direct conversion of mouse skin cells to neurons by a defined, transcription factor combination, surely the harbinger of direct fate switching by small molecules. Initiation of a pluripotent state however requires a more complex orchestration of events. Firstly, a deconstruction of the differentiated state via repression of lineage-specific genes and this is linked with establishment of an “open” chromatin stage. Secondly, endogenous genes associated with pluripotency are reactivated giving rise to partially reprogrammed, intermediate or metastable states. A significant number of examples exist of small molecule mediators that are able to shift apparently stable, balanced epigenetic states. In a similar context small molecule screens have demonstrated that epithelial cancer stem cells can be induced to differentiate, heralding important insights for the mechanism of tumour formation and prospects for therapeutics development.Lineage reprogramming and the reversal of commitment by small molecule mediators
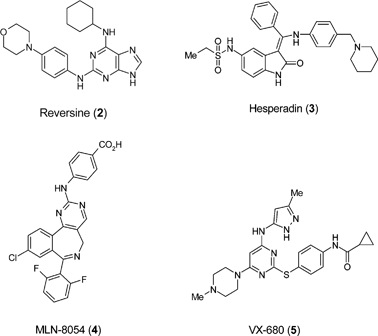
Dedifferentiation of lineage-committed cells by a small molecule was first demonstrated by Schultz and Ding in 2004.41 Reversine (2) was shown to induce myogenic lineage-committed cells (murine myoblast C2C12) to become multipotent progenitor cells with osteo- and adipogenic capacity. Mechanism of action studies suggest that reversine can function as a dual inhibitor of non-muscle myosin II and MEK1, although other activities are possible.42 Several well-characterised inhibitors of Aurora A or B, including reversine, are capable of dedifferentiating C2C12 cells.43 Reversine and hesperadin (3) were potent inducers of dedifferentiation whereas MLN-8054 (4) and VX-680 (5) were only active at significantly higher concentrations suggesting that Aurora B kinase (rather than A) might be the target of reversine in dedifferentiation. As continuously cultured cell lines frequently present unorthodox mechanisms of regulation it was important that the effect of reversine was corroborated and treatment of primary murine and human fibroblasts has been shown to induce skeletal myogenic potential both in vitro and in vivo.44 It can be postulated that the ability of reversine to reprogramme somatic cells is linked with its property of generating metastable states, allowing cells to be directed in their differentiation into a range of lineages, including neural, under appropriate conditions.45Replacement of the exogenously expressed reprogramming genes by small molecules in order to induce pluripotency
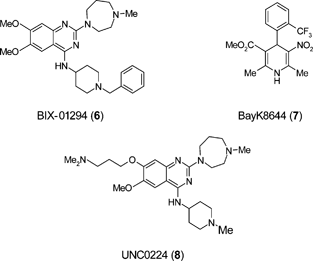
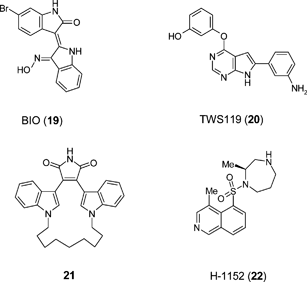
Independent screening strategies have been adopted in attempts to elucidate the mechanism of induced pluripotency and these are providing important insights into the overall process and associated molecular pathways. Systematic searches are being performed to identify individual small molecule replacements of Oct3/4, Sox2, c-Myc, and Klf4 in their ability to induce pluripotency. Cell types that retain endogenous expression of one or more of the genes have been treated with small molecules to induce reprogramming. Examples are neural progenitor cells (NPC), which express Sox2 endogenously and epistem cells (EpiSC) which express Oct3/4, Sox2 and c-Myc but not Klf4.46,47 Other studies have shown that mouse and human fibroblasts are reprogrammed in the absence of c-Myc, albeit with reduced efficiency.48,49The small molecule BIX-01294 (6) improved reprogramming efficiency in NPC transduced with Oct3/4 and Klf4 to a level comparable to transduction with all four factors.50 BIX-01294 is an inhibitor of G9a, a histone methyl-transferase (HMT) that represses transcription of several target genes by di-methylating the histone on the H3K9 residue.51 BIX-01294 combined with the potassium channel blocker BayK8644 (7) promoted reprogramming with Oct4 and Klf4 genes in mouse embryonic fibroblasts.52 The crystal structure of the SET domain of G9a-like protein (GLP) in complex with BIX-01294 and cofactor product S-adenosyl-L-homocysteine (SAH) shows the inhibitor bound in the substrate peptide groove which is occupied by H3K4-H3R8.53 The structure of the catalytic domain of G9a in complex with SAH has also been solved.54 BIX-01294 is reported to have similar inhibition activity for both G9a and GLP when assayed under linear reaction conditions (IC50 1.9 μM and 0.7 μM respectively).53 Structure activity relationship (SAR) exploration of this 2,4-diaminoquinazoline template led to the discovery of UNC0224 (8) as a potent and selective inhibitor of G9a.55 A high resolution X-ray structure of the G9a–UNC0224 complex was obtained with the template residing in the peptide groove and the 7-dimethylaminopropoxy side chain occupying the lysine binding channel of G9a.55 These crystal structures have the potential to guide further structure-based optimisation of the 2,4-diaminoquinazoline template to yield inhibitors with improved activity against G9a, or related HMTs, that could be useful in reprogramming.
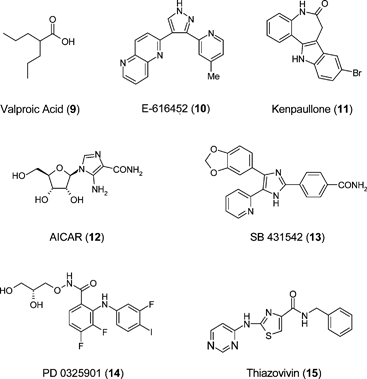
Melton has reported that DNA methyltransferase and histone deacetylase (HDAC) inhibitors improve reprogramming efficiency in mouse embryonic fibroblasts.56 Valproic acid (9), a HDAC inhibitor, improves reprogramming efficiency by more than 100-fold and enables efficient induction of pluripotent stem cells without introduction of c-Myc. A number of new small molecules have recently been reported to replace transcription factors in reprogramming of adult cells to pluripotency. High-content chemical screening has been used to identify small molecules that can replace Sox2 in reprogramming.39 E-616452 (10) combined with exogenously encoding Oct4, Klf4 and c-Myc, could reprogramme mouse embryonic fibroblasts (MEF) with comparable efficiency to that gene combination containing Sox2. Compound E-616452, a Tgf-β receptor pathway blocker, enables reprogramming via the induction of endogenous Nanog transcription thereby facilitating the conversion of partial to completely reprogrammed cells. The contribution of Klf4 to reprogramming mouse cells can be replaced by the small molecule kenpaullone (11).57 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR: 12) has been shown to activate the molecular circuitry of pluripotency, being able to induce Klf4, Klf2 and c-Myc expression in mouse cells.58 The mechanism of induced pluripotency from fibroblasts is manifest as a mesenchyme to epithelium transition and the efficiency of maintenance of this phenotype can be exacerbated using a small molecule cohort consisting of an ALK5 inhibitor SB431542 (13), a MEK inhibitor PD0325901 (14) and an inhibitor of anoikis, thiazovivin (15).59
Lineage specification of normal stem cells and a role for small molecule mediators
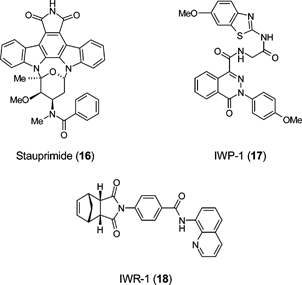
The number of examples of small molecule mediators of lineage specification is relatively modest compared with the described procedures of in vitro specification using growth factor cocktails and is clearly associated with the better characterised in vitro differentiation paradigms. The molecule stauprimide (16), when used with other specification cues, significantly increased the efficiency of directed ES cell differentiation.60 Stauprimide was identified from a screen performed in the presence of low activin A concentrations, an effort focussed on identifying molecules inducing definitive endoderm from mouse ES cells. The molecule promoted efficient induction of Sox17 in both mouse and human ES cells and also helped induce extensive endoderm differentiation but also appeared to generally lower the threshold for specification of a range of lineages. Down-regulation of c-Myc and the inhibition of NME2 (c-myc activating factor) nuclear localisation is proposed as the likely mechanism by which this molecule primes cells for differentiation. Small molecules that disrupt Wnt pathway responses offer the advantage of targeting discrete regulatory steps in the pathway. IWP-1 (17) is an example of an inhibitor of Porcupine, an acyltransferase that is essential to the production of Wnt proteins and IWR-1 (18) cancels the destruction of Axin proteins, which are suppressors of Wnt/b-catenin pathway activity.61A number of studies have implicated GSK-3 in regulating ES cell self-renewal. The GSK-3 inhibitor BIO (19) has been reported to promote both mouse ES cell and human ES cell self-renewal62 whereas a structurally discrete GSK-3 inhibitor TWS119 (20) directs neuronal differentiation.63 Bisindolylmaleimides (e.g.21) selectively inhibit GSK-3 in mouse ES cells and robustly enhance self-renewal in the presence of LIF and serum, but not in the absence of LIF. However, these compounds have effects on other pathways, making a clear definition of the role of this enzyme in ES cell biology problematic.64 Likewise screening of 41 known kinase inhibitors for their ability to modulate the differentiation of mouse ES cells has led to the finding that the Rho kinase inhibitor H-1152 (22) has the potential to enhance ES cell differentiation towards dopaminergic neurons.65 Caution was placed in ascribing the phenotypic screening outcome to the Rho kinase inhibitory activity of H-1152 due to the polypharmacology of the molecule.
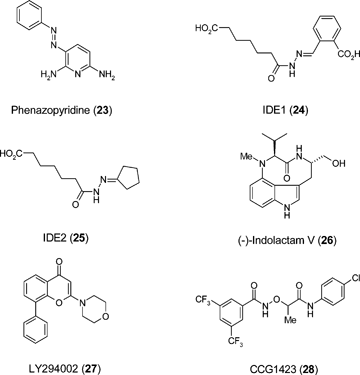
Phenazopyridine (23) enhances derivation of multipotent neural progenitors from human ES cells66 and a well defined procedure for quantitative generation of motoneuron fates from cultured ES cells using small molecule agonists of the sonic hedgehog pathway has been known for some time.67 Conversely astro- and neurogenesis from ES cell-derived neuro-progenitors is suppressed by agonists of the mu, enkephalin, and kappa opioid receptors, processes driven via extracellular-signal regulated kinase (ERK) and p38 mitogen-activated protein kinase pathways respectively.68 The generation of definitive endoderm and efficient production of Pdx-1-expressing pancreatic progenitors has been described using a combination of the small molecules IDE1 (24) and IDE2 (25), produced by de novo chemical synthesis from a library of putative HDAC inhibitors, and (-)-indolactam V (26).69,70 Higher yields of pancreatic progenitors can be generated compared to a growth factor-based approach and the Pdx1-expressing cells express other pancreatic markers and contribute to endocrine, exocrine and duct cells, in vitro and in vivo. Recent studies have demonstrated the specification of intermediate mesoderm from mouse ES cells, a stage in kidney development.40 A small molecule combination of Janus-associated tyrosine kinase 1 (JAK1) inhibitor, LY294002 (27), and CCG1423 (28) induces BMP7, a morphogen specifying the intermediate mesoderm at the expense of ectoderm and endoderm.
Induced differentiation of cancer stem cells by small molecule mediators
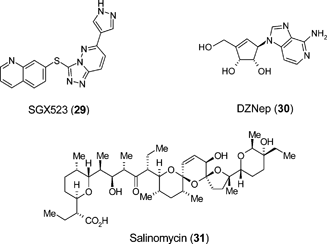
There is accumulating evidence in support of a cancer stem cell hypothesis from studies of the self-renewal mechanisms in normal and cancer stem cells. Common strands linking regenerative medicine and cancer reside within the molecular architecture of stem cells and important lessons for future regenerative therapy strategies can be learnt from characterising small molecule modifiers of the cancer stem cell state. For example antagonists of indefinite self-renewal of cancer stem cells may yield pro-differentiating signals for normal tissue stem cells.Many screening strategies have led to discovery of small molecules that target tumour cell growth and malignancy. For example a small molecule kinase inhibitor of c-Met, SGX523 (29), at nanomolar concentrations was shown to inhibit c-Met brain tumor cell activation, c-Met-dependent malignancy, and in vivo glioma xenograft growth.71 However screens for agents that specifically kill the CSC rather than the bulk of the tumour are difficult to implement as the cells lack definitive markers, are rare and relatively unstable once isolated in tissue culture. A growing number of recent reports indicate however that it is possible, with appropriate design, to identify small molecules that target the basic pathways underlying aberrant CSC behaviour. Over-expression of the polycomb group protein enhancer of zeste homologue 2 (EZH2) occurs in diverse malignancies, including prostate cancer, breast cancer, and glioblastoma multiforme (GBM). Targeted pharmacologic disruption of EZH2 by the S-adenosylhomocysteine hydrolase inhibitor 3-deazaneplanocin A (DZNep: 30), strongly impairs GBM CSC self-renewal in vitro and tumor-initiating capacity in vivo.72 In an unbiased screening strategy compounds were systematically identified, with a success rate of approximately 30%, that specifically target epithelial CSC by focussing on the differentiation status of the cells rather than molecular targets. One such compound, Salinomycin (31) which is a highly selective potassium ionophore facilitating bidirectional ion flux, selectively induced increased epithelial differentiation of tumour cells and impaired the viability of cells with features of cancer stem cells.73 The feasibility of targeting CSC self-renewal, migration, apoptosis and differentiation with a small molecule approach was also exemplified recently using an adherent GBM CSC system.74 Intriguingly, of the 23 drugs that killed all GBM-CSC lines, 7 are known to modulate the monoamine-signalling pathways.
Cell therapy and oral drug approaches for small molecules in regenerative medicine
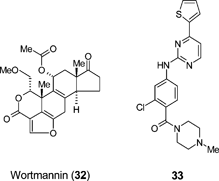
Small molecules will continue to play a significant role in the precise orchestration of making transplantable precursors from stem cells. Small molecules have a number of potential advantages over protein and peptide factors in this context. For example, small molecules are stable, synthesisable on scale with high quality control, and since they are discrete and well-defined entities, processes using small molecules should be highly reproducible. Finally, small molecules can be designed that are cell permeable and thus access intracellular pathways – providing additional opportunities for cell regulation beyond the use of exogeneous factors that act at the cell surface.A key regenerative medicine opportunity is in the treatment of diabetes. Diabetes mellitus type 1 (insulin-dependent diabetes, or juvenile diabetes) is caused by a self destruction of insulin-producing β-cells in the pancreas. The standard of care for type 1 diabetes is currently daily insulin injection. Failure to adequately control blood sugar level can be fatal. Partial respite can be achieved by donor pancreatic islet transplantation and significant research is focussed on generating insulin-producing β-cells from renewable resources such as stem cells, since donor islets are in scarce supply. Stem cell derived insulin-producing β-cells have been validated in preclinical rodent models of diabetes.75 In addition to the small molecules already discussed (24–26), the well known inhibitor of Phosphatidyl inositol-3-K (PI3K), wortmannin (32), a known promoter of cell survival, can enhance the overall differentiation of hES and iPS cells through to insulin-positive β-cells.76 The ability to then expand β-cell populations through small molecules that drive β-cell proliferation could also be a key requirement to make large scale cell therapy for diabetes viable. In this regard, Schultz et al. have reported a high-throughput cell-based screening approach that identified two classes of compounds, exemplified by 33 and BayK8644 (7), that demonstrate the ability to stimulate the replication of mouse β-cells in vitro.77 Initial studies suggest that 33 may exert this effect through activation of the Wnt signalling pathway, via inhibition of GSK3-β (a kinase that negatively regulates Wnt signalling), whereas the mechanism of action for BayK8644 could be due to activation of L-type calcium channels. Strategies to identify small molecules that facilitate cell production in the laboratory may in the future lead to the discovery of oral small molecule anti-diabetic drugs that act through regeneration of β-cell mass directly within the pancreas itself.
Neurodegenerative disorders such as Parkinson's and Alzheimer's disease continue to represent a significant and growing area of unmet medical need. Multipotent neural stem cells (NSCs) were first demonstrated in the adult mammalian brain by means of an in vitro functional assay termed the neurosphere assay. Under the appropriate conditions, NSCs proliferate to form spherical clusters of neural cells termed “neurospheres” (Fig. 3). Neurospheres can be dissociated and expanded without limit, thereby providing an abundant source of neural cells. When transferred to differentiative conditions, individual NSC-derived neurospheres generate the three major central nervous system (CNS) cell types: neurons, astrocytes and oligodendroctyes (Fig. 4). The discovery of NSCs has sparked considerable interest in the potential for regenerative medicine approaches in the treatment of neurodegeneration, for example through small molecules that regulate differentiation and proliferation to promote neurogenesis and restore brain function.
 | ||
| Fig. 3 Neural stem cells proliferate to form heterogenous clusters of cells termed “neurospheres”. Typically, after 5 days in culture, a neurosphere is comprised of approximately 2000 cells. An individual neurosphere labeled with the marker PSA-NCAM. © Dr Rodney Rietze, Pfizer Regenerative Medicine. | ||
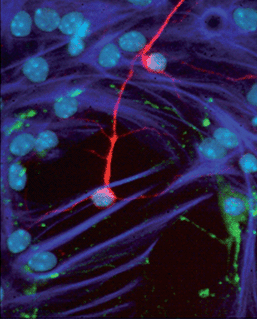 | ||
| Fig. 4 When transferred to differentiating conditions, NSC-derived neurospheres generate neurons (red), astrocytes (dark blue), and oligodendrocytes (green). Individual cells are identified by their DAPI+ve nuclei (light blue). © Dr Rodney Rietze, Pfizer Regenerative Medicine. | ||
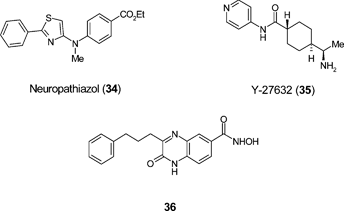
Ding and Schultz78 have described the discovery of neuropathiazol (34) which is reported to induce adult rat hippocampal neuroprogenitor cells (NPC) to differentiate primarily to neurons with more than 90% specificity. The precise mechanism of action of neuropathiazol has not been elucidated. Other studies79 have also reported that Rho kinase inhibitor Y27632 (35) induces the generation of multipotent neural crest-like cells from human NSCs and the non-specific HDAC inhibitor valproic acid (9) has been shown to promote neuronal differentiation of multipotent adult rat NPC in vitro80 and neurogenesis in rat brain in vivo.81 Few examples have been reported of the ability of small molecules to generate physiologically active neurons from non-NSCs. A recent screening approach in which over 20 000 compounds were tested for their ability to promote neuronal differentiation from mesenchymal stem cells (MSC) has identified quinoxaline 36.82 Importantly, these neuron-like cells also exhibited functional electrophysiology and cholinergic activity. This is believed to be the first report of a small molecule that has the capacity to commit MSCs to neuron-like cells in a quantitative manner, though the mechanism(s) remain to be determined.
It is clear from the above that small molecules have an important role to play in modulating neurogenesis and that research in this area is moving apace. Progress in this field could potentially lead to the discovery of oral, brain-penetrant small molecule drugs that promote the differentiation, proliferation and integration of endogenous cells derived from NPC as part of a neuroregenerative paradigm in the treatment of neurological disease. Selective serotinin reuptake inhibitors (SSRI), which are safe and well-tolerated in treatment of depression, have been shown to increase neurogenesis in preclinical rodent models and humans.83 This precedent suggests that safe, well-tolerated small molecules that promote neurogenesis and restore neuronal function in neurodegenerative disease could ultimately be achievable.
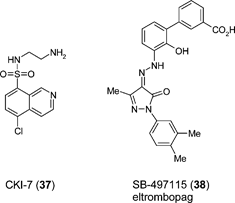
Other small molecule modulators of stem cell types in the context of regenerative medicine have been reported. The in vitro differentiation of retinal cells from both human ES cells and iPS cells following initial treatment with Rho kinase inhibitor Y27632 (35), and subsequent exposure to the casein kinase I inhibitor CKI-7 (37) and ALK4 inhibitor SB-431542 (13) has been described.84 CKI-7 and SB-431542 serve to inhibit the Wnt and Nodal signalling pathways respectively, suggesting that blockade of these pathways is important to direct pluripotent cells towards a retinal cell specification. The use of human ES cell-derived retinal pigmented epithelial cells (RPEs) is attracting significant interest as a potential cell therapy for treatment of diseases of the eye, such as age-related macular degeneration (AMD) which is a common cause of blindness. The discovery of small molecules that improve the control of RPE generation could significantly enable such cell therapy advances.
Additional examples of small molecule stem cell modulators include eltrombopag (SB-497115: 38), an orally-bioavailable nonpeptide agonist of the thrombopoietin receptor for treatment of thrombocyopenia. Preclincal studies with eltrombopag have show that the agent increases the proliferation and differentiation of human bone marrow progenitor cells into megakaryoctyes and leads to increased platelet production.85
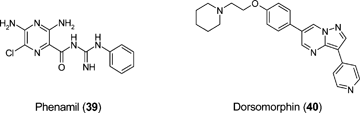
Bone morphogenic proteins (BMPs) that induce osteoblast generation are used in the clinic to drive bone formation in the treatment of human fractures. Recently, Tontonoz et al. have shown that the small molecule phenamil (39) helps induce osteoblast differentiation from mouse mesenchymal stem cells (MSCs).86 Interestingly, phenamil appears to act cooperatively with BMPs on osteoblast generation and mineralization endpoints, suggesting that small molecules may ultimately be useful in a therapeutic setting for enhancing BMP-driven bone healing. Dorsomorphin (40) is an example of small molecule that has been reported to negatively regulate BMP signalling, through inhibition of BMP type I receptors ALK2, ALK3 and ALK6.87 Dorsomorphin has also been used in mouse ES cells models to promote cardiac myocyte differentiation.88
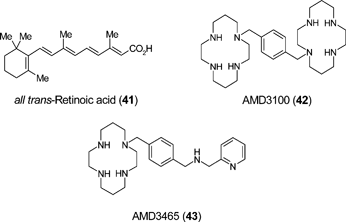
Retinoic acid (RA: 41) is the oxidised form of vitamin A and has been shown to have pleiotropic activity when used experimentally in a wide variety of embryonic model systems. RA is a known morphogen during embryonic development and its activity is precisely regulated. Treatment of mouse ES cells with RA to specify cell lineages in tissue culture is a well characterised procedure. Exposure to varying concentrations can enhance differentiation through ectodermal and mesodermal lineages.89 RA facilitates specification of mesodermal lineage through inhibiting BMP expression and activating the Wnt/β-catenin signalling pathway.89 Retinoic acid is thus a key small molecule that may prove useful in a variety of settings to help control ES cell differentiation in support of cell therapy applications.
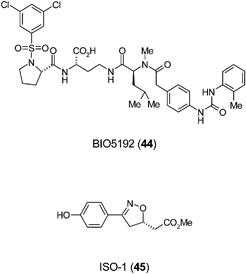
The best studied system for determining factors that regulate mobilization of intrinsic tissue repair cells is that of the bone marrow niche from which originates blood, vascular and musculo-skeletal progenitors. Haematopoietic stem cell (HSC) transplantation is a treatment option for haematological malignancies and improved regimes for stem cell mobilization would increase the efficiency of HSC harvest for therapy. The chemokine receptor CXCR4 and ligand SDF-1 are integrally involved in HSC homing and mobilization.90 Plerixafor (AMD3100: 42), a selective inhibitor of CXCR4, is a promising new agent and clinical trials have demonstrated that AMD3100 rapidly mobilizes HSC into peripheral blood.91 Furthermore, mobilization by the combination of AMD3100 and granulocyte colony-stimulating factor (G-CSF) results in the collection of more progenitor cells than G-CSF alone.91 Second generation cyclam inhibitors of CXCR4 such as AMD3465 (43) offer enhanced potency against the CXCR4 receptor. AMD3465 caused leukocytosis in mice and dog indicating that AMD3465 has the potential to mobilize HSC.92
A second pathway has been shown to be critical in HSC mobilization involving the α4β1 integrin VLA-4 with its ligand VCAM-1. BIO5192 (44), a small molecule inhibitor of VLA-4, has been shown to increase mobilization of murine HSC by 30-fold over basal levels.93 Small molecule inhibitors of VLA-4 mobilize HSC alone or with an additive effect when used in combination with G-CSF or AMD3100.93 A new modulator of vertebrate HSC number has been discovered using a chemical genetic screen in zebrafish. Prostaglandin E2 (PGE2) increases HSCs in the embryo and enhances marrow recovery following irradiation of adult fish.94 These results suggest that modulation of this pathway may have utility in treating patients undergoing bone marrow transplant.
The small molecule ISO-1 (45) was employed to identify the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF) as a potent inhibitor of MSC migration in vitro. ISO-1, a MIF antagonist, increased MSC migration by four-fold to bronchial epithelial cells and demonstrates that antagonists of MIF may have utility in controlling MSC homing during tissue repair.95
Future directions
The field of stem cell modulation by small molecules is currently largely based on phenotypic screening paradigms. Through these approaches, as illustrated in this review, a number of small molecules have been identified that exhibit a powerful influence on stem cell behaviour. There is undoubtedly room for improvement and the virtues of a greater comprehension of the range of defined tissue resident stem cell types that can be isolated are the prospects for enhanced level of detail from phenotypic screens. This will be linked with detailed information of ‘stem cell signatures’ provided by genomic and proteomic approaches. This is paramount in studies with CSC in which more precise markers of putative tumour forming stem cells can be linked with functional definition, to a single cell level, in xenograft studies in which the tumour phenotype is accurately reproduced.Evidence in support of the competency of cells to convert from one lineage to another via partial reprogramming is substantial. This is best illustrated from studies with altered transcription factor status in the blood lineage. For example the transcription factor C/EBPa, required for the formation of granulocyte-macrophage precursors can convert committed B- and T-cell progenitors into functional macrophages.96 Genetic ablation of Pax5 in B-cell precursors activates expression of genes from alternative haematopoietic lineages and after transplantation these cells can differentiate into both myeloid and lymphoid lineages.97 Conditional Pax5 deletion in mice triggers the dedifferentiation of mature B cells in vivo back to early uncommitted progenitors in the bone marrow and rescued T lymphopoiesis in the thymus of T-cell-deficient mice.98 This is not a peculiarity of the blood system as co-expression of PU.1 and C/EBPa in fibroblasts converted them into macrophage-like cells;99 inactivation of PRDM16 in brown fat cells promotes muscle differentiation with loss of brown fat characteristics;100 expression of the genes Ngn3, Pdx1 and Mafa in pancreatic exocrine cells reprogrammes them into cells closely resembling pancreatic beta-cell;101 and mouse fibroblasts can be rapidly and efficiently converted into functional neurons in vitro using the three genes, Ascl1, Brn2 and Myt1.102 It is to be anticipated that experimental approaches using genes to re-tune cell lineages will give rise to the imminent development of small molecule alternatives. Technical enhancements in the homogeneity of adherent stem cell culture, via the use of chemically defined or animal component free culture medium supplements, will also improve assay design and implementation of screening on scale.
One of the challenges with phenotypic screens is linking screen outcomes to the underlying mechanism of action of the small molecules. This is important to develop a deeper understanding of the targets and pathways that control stem cell self-renewal and differentiation events. Current phenotype screens are aided by using assays frequently based on genetic loss or gain of function modification to introduce reporter genes and it is anticipated that time resolved, non-invasive collection of image information on stem cell state changes, such as shape, size and orientation of division planes from in vitro screens will afford novel definition of mechanism.
Although the small molecules that are identified usually have some degree of pharmacological characterisation, it should not be concluded that the known pharmacology is always responsible for the observed behaviour – as indeed many authors in the field acknowledge. To address this challenge, it is anticipated that this area of research will see a greater emphasis on the use of chemical genetic and chemical proteomic methods in the future. The use of chemogenomic screening paradigms in phenotypic assay systems has attracted much attention in recent years103 and this approach is ideally suited for probing stem cell biology. For example, in our laboratories at Pfizer, we are now applying a chemogenomic library platform in a number of stem cell proliferation and differentiation assays.104,105 This library contains up to five small molecules for every target for which there is available screening data. Importantly, the molecules selected for inclusion in the library have been chosen based on their orthogonality in structure and potency/selectivity fingerprints. This orthogonality ensures a high level of confidence in associating a phenotypic outcome to a given mechanism of action, especially in cases where all the compounds for a given target show the same phenotypic activity. The Pfizer chemogenomic library currently covers over 700 proteins and it is envisaged that use of this library will aid the discovery of new molecules that influence stem cell behavior and, importantly, provide useful insights into the underlying mechanistic pathways (Fig. 5). Results from such investigations will be reported in due course.
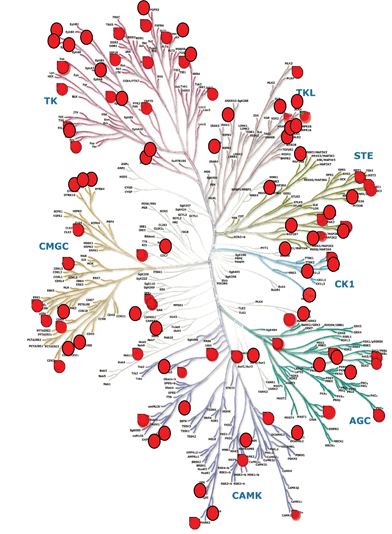 | ||
| Fig. 5 Chemogenomic coverage. Taking the kinase gene family as an example, this section of the Pfizer chemogenomic library contains 756 compounds covering 196 kinase mechanisms (red circles). Human Kinome image provided courtesy of Cell Signaling Technology, Inc. (Science 2002) with modifications by Dr Andrew Cook, Pfizer Regenerative Medicine Chemistry. | ||
A more detailed understanding of the targets and pathways that control stem cell self-renewal, progenitor pool expansion and cell differentiation will also guide strategies for the targeted delivery of drugs to safely regenerate tissue function and avoid undesired effects such as hyperplasia, fibrosis and cancer. Drug candidates that act to augment an existing repair process or alter the balance of functional integration rather than stimulate stem cell asymmetric self-renewal could be more effective and safer than those designed to stimulate the generation of nascent progenitors. Strategies will also involve topical versus systemic delivery and controlled release mechanisms for drug candidates to avoid inappropriate activation of common stem cell pathways expressed in a broad range of tissues.
It should also be anticipated that in the future the role of small molecule epigenetic modulators will have a key role to play in stem cell biology and regenerative medicine. Indeed, in addition to the crucial role of transcription factors, it is increasingly clear that control of gene expression at the epigenetic level can influence lineage specification and reprogramming.106,107 As described above, the data for the HDAC inhibitor valproic acid (9) in neurogenesis provides just one example of how epigenetic modulation through small molecules can influence stem cell behaviour. In addition to histone acetylation state, other epigenetic modifications of both histones and DNA are also implicated in stem cell regulation.106,107 However, progress in this area is hampered by the relative lack of small molecule tools available to regulate epigenetic pathways. Encouragingly, the development of chemical probes for epigenetic enzymes is now attracting increasing attention and it is envisaged that increased availability of such tools will significantly enable stell cell research and enable new approaches in regenerative medicine.
Conclusion
This review illustrates the important role small molecules can play in the modulation of stem cell behaviour and the potential this may offer in enabling development of future regenerative medicine therapies. In the last 2–3 years in particular, there has been a dramatic increase in the number of publications in this arena, and in this regard this review should be viewed as illustrative rather than exhaustive. It is clear that the transformational therapies that regenerative medicine approaches could offer will continue to drive increasing interest in the field, both within acadamic laboratories and increasingly also the biotechnology and pharmaceutical sectors. It is anticipated that small molecules will have a pivotal role to play in regenerative medicine, through enabling novel cell therapy approaches and via small molecule drugs that control the differentiation and proliferation of endogenous cells to therapeutic benefit.Acknowledgements
The authors would like to thank Dr Rodney Rietze (Pfizer Regenerative Medicine) for provision of Fig. 3 and 4. We are grateful to Eric Scharf (Cell Signaling Technology, Inc.) for permission to use the image of the ‘human kinome’ and to Dr Andrew Cook (Pfizer Regenerative Medicine Chemistry) for the creation of Fig. 5.Notes and references
- A. Atala, Engineering organs, Curr. Opin. Biotechnol., 2009, 20(5), 575–92 CrossRef CAS.
- R. White and C. McIntosh, A review of the literature on topical therapies for diabetic foot ulcers. Part 2: Advanced treatments, J. Wound Care, 2009, 18(8), 335–41 Search PubMed.
- D. Stavrou, Neovascularisation in wound healing, J. Wound Care., 2008, 17(7), 298–300 Search PubMed.
- J. Wang, N. Xing, X. Zhang, Y. Yan, J. Zhang, X. Li, W. Zhang and D. Guan, Orthotopic ileal neobladder reconstruction in patients with recurring bladder cancer after renal transplantation–a report of two cases and a review of the literature, Clinical Transplantation, 2009, 23(5), 700–4 Search PubMed.
- M. B. Davidson, K. Mustafa and R. W. Girdwood, Tracheal replacement with an aortic homograft, Ann. Thorac. Surg., 2009, 88(3), 1006–8 CrossRef.
- M. S. Conte, H. M. Nugent, P. Gaccione, I. Guleria, P. Roy-Chaudhury and J. H. Lawson, Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: the V-HEALTH study, J. Vasc. Surg., 2009, 50(6), 1359–68 CrossRef.
- H. M. Nugent, Y. S. Ng, D. White, A. Groothius, G. Kanner and E. R. Edelman, Delivery site of perivascular endothelial cell matrices determines control of stenosis in a porcine femoral stent model, J. Vasc. Interv. Radiol., 2009, 20(12), 1617–24 CrossRef.
- R. S. Kellar, L. K. Landeen, B. R. Shepherd, G. K. Naughton, A. Ratcliffe and S. K. Williams, Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue, Circulation, 2001, 104(17), 2063–8 CrossRef CAS.
- T. Wong, J. A. McGrath and H. Navsaria, The role of fibroblasts in tissue engineering and regeneration, Br. J. Dermatol., 2007, 156(6), 1149–55 CrossRef CAS.
- K. Watanabe, M. Ueno, D. Kamiya, A. Nishiyama, M. Matsumura, T. Wataya, J. B. Takahashi, S. Nishikawa, K. Muguruma and Y. Sasai, A ROCK inhibitor permits survival of dissociated human embryonic stem cells, Nat. Biotechnol., 2007, 25(6), 681–6 CrossRef CAS.
- B. C. Heng, Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells, Tissue Cell, 2009, 41(5), 376–80 CrossRef CAS.
- S. K. Gunasekar, M. Asnani, C. Limbad, J. S. Haghpanah, W. Hom, H. Barra, S. Nanda, M. Lu and J. K. Montclare, N-terminal aliphatic residues dictate the structure, stability, assembly, and small molecule binding of the coiled-coil region of cartilage oligomeric matrix protein, Biochemistry, 2009, 48(36), 8559–67 CrossRef CAS.
- M. Funahashi, T. Nakamura, I. Kakizaki, H. Mizunuma and M. Endo, Stimulation of small proteoglycan synthesis by the hyaluronansynthesis inhibitor 4-methylumbelliferone in human skin fibroblasts, Connect. Tissue Res., 2009, 50(3), 194–202 CrossRef CAS.
- D. S. Benoit, M. P. Schwartz, A. R. Durney and K. S. Anseth, Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells, Nat. Mater., 2008, 7(10), 816–23 CrossRef CAS.
- C. Martin, G. J. Bridger and S. M. Rankin, Structural analogues of AMD3100 mobilise haematopoietic progenitor cells from bone marrow in vivo according to their ability to inhibit CXCL12 binding to CXCR4 in vitro, Br. J. Haematol., 2006, 134(3), 326–9 CrossRef CAS.
- D. H. Boschelli, Small molecule inhibitors of PKCθ as potential antiinflammatory therapeutics, Curr. Top. Med. Chem., 2009, 9(7), 640–54 CrossRef CAS.
- J. D. McNeish, Stem cells as screening tools in drug discovery, Curr. Opin. Pharmacol., 2007, 7(5), 515–20 CrossRef CAS.
- S. Ding and P. G. Schultz, A role for chemistry in stem cell biology, Nat. Biotechnol., 2004, 22(7), 833–840 CrossRef.
- S. Ding and P. G. Schultz, Small molecules and future regenerative medicine, Curr. Top. Med. Chem., 2005, 5(4), 383–395 CrossRef CAS.
- S. Chen, S. Hilcove and S. Ding, Exploring stem cell biology with small molecules, Mol. BioSyst., 2006, 2(1), 18–24 RSC.
- N. Emre, R. Coleman and S. Ding, A chemical approach to stem cell biology, Curr. Opin. Chem. Biol., 2007, 11(3), 252–258 CrossRef CAS.
- Y. Xu, Y. Shi and S. Ding, A chemical approach to stem-cell biology and regenerative medicine, Nature, 2008, 453(7193), 338–344 CrossRef CAS.
- J. Clark, Y. Xu, S. Hilcove and S. Ding, Exploring stem cell biology with small molecules and functional genomics, Chemical and Functional Genomic Approaches to Stem Cell Biology and Regenerative Medicine, 2008, 187–206 Search PubMed.
- A. Kochegarov, Small molecules for stem cells, Expert Opin. Ther. Pat., 2009, 19(3), 275–281 Search PubMed.
- S. Hilcove, Y. Xu and S. Ding, Stem cell fates: control by small molecules, Wiley Encyclopedia of Chemical Biology, 2009, 4, 376–384 Search PubMed.
- A. I. Lukaszewicz, M. K. McMillan and M. Kahn, Small molecules and stem cells. Potency and lineage commitment: The new quest for the fountain of youth, J. Med. Chem., 2010, 53(9), 3439–3453 CrossRef CAS.
- V. S. Nirmalanandhan and G. S. Sittampalam, Stem cells in drug discovery, tissue engineering, and regenerative medicine: emerging opportunities and challenges, J. Biomol. Screening, 2009, 14(7), 755–768 CrossRef CAS.
- W. Li and S. Ding, Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming, Trends Pharmacol. Sci., 2010, 31(1), 36–45 CrossRef CAS.
- A. J. Firestone and J. K. Chen, Controlling destiny through chemistry: Small-molecule regulators of cell fate, Chemical Biology, 2010, 5(1), 15–34 Search PubMed.
- M. Borowiak, R. Maehr, S. Chen, A. E. Chen, W. Tang, J. L. Fox, S. L. Schreiber and D. A. Melton, Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells, Cell Stem Cell, 2009, 4(4), 348–358 CrossRef CAS.
- S. I. Mae, S. Shirasawa, S. Yoshie, F. Sato, Y. Kanoh, H. Ichikawa, T. Yokoyama, F. Yue, D. Tomotsune and K. Sasaki, Combination of small molecules enhances differentiation of mouse embryonic stem cells into intermediate mesoderm through BMP7-positive cells, Biochem. Biophys. Res. Commun., 2010, 393(4), 877–882 CrossRef CAS.
- J. Nichols and A. Smith, Naive and primed pluripotent states, Cell Stem Cell, 2009, 4(6), 487–92 CrossRef CAS.
- S. Masui, Y. Nakatake, Y. Toyooka, D. Shimosato, R. Yagi, K. Takahashi, H. Okochi, A. Okuda, R. Matoba, A. A. Sharov, M. S. Ko and H. Niwa, Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells, Nat. Cell Biol., 2007, 9(6), 625–35 CrossRef CAS.
- J. Silva and A. Smith, Capturing pluripotency, Cell, 2008, 132(4), 532–6 CrossRef CAS.
- W. Yang, W. Wei, C. Shi, J. Zhu, W. Ying, Y. Shen, X. Ye, L. Fang, S. Duo, J. Che, H. Shen, S. Ding and Ho Deng, Pluripotin combined with leukemia inhibitory factor greatly promotes the derivation of embryonic stem cell lines from refractory strains, Stem Cells, 2009, 27(2), 383–389 Search PubMed.
- Q. L. Ying, J. Wray, J. Nichols, L. Batlle-Morera, B. Doble, J. Woodgett, P. Cohen and A. Smith, The ground state of embryonic stem cell self-renewal, Nature, 2008, 453(7194), 519–23 CrossRef CAS.
- K. Takahashi and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 2006, 126(4), 663–76 CrossRef CAS.
- N. Maherali and K. Hochedlinger, Tgfβ signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc, Curr. Biol., 2009, 19(20), 1718–23 CrossRef CAS.
- J. K. Ichida, J. Blanchard, K. Lam, E. Y. Son, J. E. Chung, D. Egli, K. M. Loh, A. C. Carter, F. P. Di Giorgio, K. Koszka, D. Huangfu, H. Akutsu, D. R. Liu, L. L. Rubin and K. Eggan, A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog, Cell Stem Cell, 2009, 5(5), 491–503 CrossRef CAS.
- J. Silva, J. Nichols, T. W. Theunissen, G. Guo, A. L. van Oosten, O. Barrandon, J. Wray, S. Yamanaka, I. Chambers and A. Smith, Nanog is the gateway to the pluripotent ground state, Cell, 2009, 138(4), 722–37 CrossRef CAS.
- S. Chen, Q. Zhang, X. Wu, P. G. Schultz and S. Ding, Dedifferentiation of lineage-committed cells by a small molecule, J. Am. Chem. Soc., 2004, 126(2), 410–411 CrossRef CAS.
- S. Chen, S. Takanashi, Q. Zhang, W. Xiong, S. Zhu, E. C. Peters, S. Ding and P. G. Schultz, Reversine increases the plasticity of lineage-committed mammalian cells, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(25), 10482–10487 CrossRef CAS.
- G. Amabile, A. M. D'Alise, M. Iovino, P. Jones, S. Santaguida, A. Musacchio, S. Taylor and R. Cortese, The Aurora B kinase activity is required for the maintenance of the differentiated state of murine myoblasts, Cell Death Differ., 2009, 16(2), 321–330 CrossRef CAS.
- L. Anastasia, M. Sampaolesi, N. Papini, D. Oleari, G. Lamorte, C. Tringali, E. Monti, D. Galli, G. Tettamanti, G. Cossu and B. Venerando, Reversine - treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle, Cell Death Differ., 2006, 13(12), 2042–2051 CrossRef CAS.
- E. K. Lee, G.-U. Bae, J. S. You, J. C. Lee, Y. J. Jeon, J. W. Park, J. H. Park, S. H. Ahn, Y. K. Kim, W. S. Choi, J.-S. Kang, G. Han and J.-W. Han, Reversine increases the plasticity of lineage-committed cells toward neuroectodermal lineage, J. Biol. Chem., 2009, 284(5), 2891–2901 CAS.
- Y. Shi, C. Desponts, J. T. Do, H. S. Hahm, H. R. Scholer and S. Ding, Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds, Cell Stem Cell, 2008, 3(5), 568–574 CrossRef CAS.
- G. Guo, J. Yang, J. Nichols, J. S. Hall, I. Eyres, W. Mansfield and A. Smith, Klf4 reverts developmentally programmed restriction of ground state pluripotency, Development, 2009, 136(7), 1063–1069 CrossRef CAS.
- M. Wernig, A. Meissner, J. P. Cassady and R. Jaenisch, c-Myc is dispensable for direct reprogramming of mouse fibroblasts, Cell Stem Cell, 2008, 2(1), 10–12 CrossRef CAS.
- M. Nakagawa, M. Koyanagi, K. Tanabe, K. Takahashi, T. Ichisaka, T. Aoi, K. Okita, Y. Mochiduki, N. Takizawa and S. Yamanaka, Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts, Nat. Biotechnol., 2008, 26(1), 101–106 CrossRef CAS.
- Y. Shi, J. T. Do, C. Desponts, H. S. Hahm, H. R. Scholer and S. Ding, A combined chemical and genetic approach for the generation of induced pluripotent stem cells, Cell Stem Cell, 2008, 2(6), 525–528 CrossRef CAS.
- S. Kubicek, R. J. O'Sullivan, E. M. August, E. R. Hickey, Q. Zhang, M. L. Teodoro, S. Rea, K. Mechtler, J. A. Kowalski, C. A. Homon, T. A. Kelly and T. Jenuwein, Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase, Mol. Cell, 2007, 25(3), 473–481 CrossRef CAS.
- Y. Shi, C. Desponts, J. T. Do, H. S. Hahm, H. R. Scholer and S. Ding, Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds, Cell Stem Cell, 2008, 3(5), 568–574 CrossRef CAS.
- Y. Chang, X. Zhang, J. R. Horton, A. K. Upadhyay, A. Spannhoff, J. Liu, J. P. Snyder, M. T. Bedford and X. Cheng, Structural basis for G9a-like protein lysine methyltransferase inhibition by BIX-01294, Nat. Struct. Mol. Biol., 2009, 16(3), 312–317 CrossRef CAS.
- H. Wu, J. Min, V. V. Lunin, T. Antoshenko, L. Dombrovski, H. Zeng, A. Allali-Hassani, V. Campagna-Slater, M. Vedadi, C. H. Arrowsmith, A. N. Plotnikov and M. Schapira, Structural biology of human H3K9 methyltransferases, PLoS One, 2010, 5(1), e8570 CrossRef.
- F. Liu, X. Chen, A. Allali-Hassani, A. M. Quinn, G. A. Wasney, A. Dong, D. Barsyte, I. Kozieradzki, G. Senisterra, I. Chau, A. Siarheyeva, D. B. Kireev, A. Jadhav, J. M. Herold, S. V. Frye, C. H. Arrowsmith, P. J. Brown, A. Simeonov, M. Vedadi and J. Jin, Discovery of a 2,4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a, J. Med. Chem., 2009, 52(24), 7950–7953 CrossRef CAS.
- D. Huangfu, R. Maehr, W. Guo, A. Eijkelenboom, M. Snitow, A. E. Chen and D. A. Melton, Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds, Nat. Biotechnol., 2008, 26(7), 795–797 CrossRef CAS.
- C. A. Lyssiotisa, R. K. Foreman, J. Staerk, M. Garcia, D. Mathur, S. Markoulaki, J. Hanna, L. L. Lairson, B. D. Charette, L. C. Bouchez, M. Bollong, C. Kunick, A. Brinker, C. Y. Cho, P. G. Schultz and R. Jaenisch, Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(22), 8912–8917 CrossRef CAS.
- L. Adamo, Y. Zhang and G. Garcia-Cardena, AICAR activates the pluripotency transcriptional network in embryonic stem cells and induces KLF4 and KLF2 expression in fibroblasts, BMC Pharmacol., 2009, 9, 2 Search PubMed.
- T. Lin, R. Ambasudhan, X. Yuan, W. Li, S. Hilcove, R. Abujarour, X. Lin, H. S. Hahm, E. Hao, A. Hayek and S. A. Ding, Chemical platform for improved induction of human iPSCs, Nat. Methods, 2009, 6(11), 805–8 CrossRef CAS.
- S. Zhu, H. Wurdak, J. Wang, C. A. Lyssiotis, E. C. Peters, C. Y. Cho, X. Wu and P. G. Shultz, A small molecule primes embryonic stem cells for differentiation, Cell Stem Cell, 2009, 4(5), 416–426 CrossRef CAS.
- B. Chen, M. E. Dodge, W. Tang, J. Lu, Z. Ma, C.-W. Fan, S. Wei, W. Hao, J. Kilgore, N. S. Williams, M. G. Roth, J. F. Amatruda, C. Chen and L. Lum, Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer, Nat. Chem. Biol., 2009, 5(2), 100–107 CrossRef CAS.
- N. Sato, L. Meijer, L. Skaltsounis, P. Greengard and A. H. Brivanlou, Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor, Nat. Med., 2004, 10(1), 55–63 CrossRef CAS.
- S. Ding, T. Y. H. Wu, A. Brinker, E. C. Peters, W. Hur, N. S. Gray and P. G. Schultz, Synthetic small molecules that control stem cell fate, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(13), 7632–7637 CrossRef CAS.
- H. K. Bone, T. Damiano, S. Bartlett, A. Perry, J. Letchford, Y. S. Ripoll, A. S. Nelson and M. J. Welham, Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides, Chem. Biol., 2009, 16(1), 15–27 CrossRef CAS.
- K.-C. Hwang, J. Y. Kim, W. Chang, D.-S. Kim, S. Lim, S.-M. Kang, B.-W. Song, H.-Y. Ha, Y. J. Huh, I.-G. Choi, D.-Y. Hwang, H. Song, Y. Jang, N. Chung, S.-H. Kim and D.-W. Kim, Chemicals that modulate stem cell differentiation, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(21), 7467–7471 CrossRef CAS.
- D. M. Suter, O. Preynat-Seauve, D. Tirefort, A. Feki and K. H. Krause, Phenazopyridine induces and synchronizes neuronal differentiation of embryonic stem cells, J. Cell. Mol. Med., 2009, 13(9b), 3517–27 CrossRef.
- P. Soundararajan, G. B. Miles, L. L. Rubin, R. M. Brownstone and V. F. Rafuse, Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons, J. Neurosci., 2006, 26(12), 3256–68 CrossRef CAS.
- J. W. Hahn, S. Jagwani, E. Kim, V. R. Rendell, J. He, L. A. Ezerskiy, R. Wesselschmidt, C. J. Coscia and M. M. Belcheva, Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases, J. Neurochem., 2010, 112(6), 1431–1441 CrossRef CAS.
- S. Chen, M. Borowiak, J. L. Fox, R. Maehr, K. Osafune, L. Davidow, K. Lam, L. F. Peng, S. L. Schreiber, L. L. Rubin and D. Melton, A small molecule that directs differentiation of human ESCs into the pancreatic lineage, Nat. Chem. Biol., 2009, 5(4), 258–265 CrossRef CAS.
- M. Borowiak, R. Maehr, S. Chen, A. E. Chen, W. Tang, J. L. Fox, S. L. Schreiber and D. A. Melton, Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells, Cell Stem Cell, 2009, 4(4), 348–58 CrossRef CAS.
- F. Guessous, Y. Zhang, C. Dipierro, L. Marcinkiewicz, J. Sarkaria, D. Schiff, S. Buchanan and R. Abounader, An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth, Anticancer Agents Med. Chem., 2010, 10(1), 28–35 CAS.
- M. L. Suvà, N. Riggi, M. Janiszewska, I. Radovanovic, P. Provero, J. C. Stehle, K. Baumer, M. A. Le Bitoux, D. Marino, L. Cironi, V. E. Marquez, V. Clément and I. Stamenkovic, EZH2 is essential for glioblastoma cancer stem cell maintenance, Cancer Res., 2009, 69(24), 9211–8 CrossRef CAS.
- P. B. Gupta, T. T. Onder, G. Jiang, K. Tao, C. Kuperwasser, R. A. Weinberg and E. S. Lander, Identification of selective inhibitors of cancer stem cells by high-throughput screening, Cell, 2009, 138(4), 645–659 CrossRef CAS.
- S. M. Pollard, K. Yoshikawa, I. D. Clarke, D. Danovi, S. Stricker, R. Russell, J. Bayani, R. Head, M. Lee, M. Bernstein, J. A. Squire, A. Smith and P. Dirks, Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens, Cell Stem Cell, 2009, 4(6), 568–80 CrossRef CAS.
- E. Kroon, L. A. Martinson, K. Kadoya, A. G. Bang, O. G. Kelly, S. Eliazer, H. Young, M. Richardson, N. G. Smart, J. Cunningham, A. D. Agulnick, K. A. D'Amour, M. K. Carpenter and E. E. Baetge, Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo, Nat. Biotechnol., 2008, 26(4), 443–452 CrossRef CAS.
- D. Zhang, W. Jiang, M. Liu, X. Sui, X. Yin, S. Chen, Y. Shi and H. Deng, Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells, Cell Res., 2009, 19(4), 429–438 CrossRef CAS.
- W. Wang, J. R. Walker, X. Wang, M. S. Tremblay, J. W. Lee, X. Wu and P. G. Schultz, Identification of small-molecule inducers of pancreatic β -cell expansion, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(5), 1427–1432 CrossRef CAS.
- M. Warashina, K. H. Min, T. Kuwabara, A. Huynh, F. H. Gage, P. G. Schultz and S. Ding, A synthetic small molecule that induces neuronal differentiation of adult hippocampal neural progenitor cells, Angew. Chem., Int. Ed., 2006, 45(4), 591–593 CrossRef CAS.
- R. Hotta, L. Pepdjonovic, R. B. Anderson, D. Zhang, A. J. Bergner, J. Leung, A. Pebay, H. M. Young, D. F. Newgreen and M. Dottori, Small-molecule induction of neural crest-like cells derived from human neural progenitors, Stem Cells, 2009, 27(12), 2896–2905 Search PubMed.
- J. Hsieh, K. Nakashima, T. Kuwabara, E. Mejia and F. H. Gage, Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells, Proc. Natl. Acad. Sci. U. S. A., 2004, 101(47), 16659–16664 CrossRef CAS.
- Y. Hao, T. Creson, L. Zhang, P. Li, F. Du, P. Yuan, T. D. Gould, H. K. Manji and G. Chen, Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis, J. Neurosci., 2004, 24(29), 6590–6599 CrossRef CAS.
- N. R. Kim, S. K. Kang, H. H. Ahn, S. W. Kwon, W. S. Park, K. S. Kim, S. S. Kim, H. J. Jung, S. U. Choi, J. H. Ahn and K. R. Kim, Discovery of a new and efficient small molecule for neuronal differentiation from mesenchymal stem cell, J. Med. Chem., 2009, 52(24), 7931–7933 CrossRef CAS.
- M. Boldrini, M. D. Underwood, R. Hen, G. B. Rosoklija, A. J. Dwork, J. Mann and V. Arango, Antidepressants increase neural progenitor cells in the human hippocampus, Neuropsychopharmacology, 2009, 34(11), 2376–2389 CrossRef CAS.
- F. Osakada, Z.-B. Jin, Y. Hirami, H. Ikeda, T. Danjyo, K. Watanabe, Y. Sasai and M. Takahashi, In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction, J. Cell Sci., 2009, 122(17), 3169–3179 CrossRef CAS.
- C. L. Erickson-Miller, E. Delorme, S.-S. Tian, C. B. Hopson, A. J. Landis, E. I. Valoret, T. S. Sellers, J. Rosen, S. G. Miller, J. I. Luengo, K. J. Duffy and J. M. Jenkins, Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist, Stem Cells, 2009, 27(2), 424–430 Search PubMed.
- K. W. Park, H. Waki, W.-K. Kim, B. S. J. Davies, S. G. Young, F. Parhami and P. Tontonoz, The small molecule phenamil induces osteoblast differentiation and mineralization, Mol. Cell. Biol., 2009, 29(14), 3905–3914 CrossRef CAS.
- C. C. Hong and P. B. Yu, Applications of small molecule BMP inhibitors in physiology and disease, Cytokine Growth Factor Rev., 2009, 20(5–6), 409–418 CrossRef CAS.
- J. Hao, M. A. Daleo, C. K. Murphy, P. B. Yu, J. N. Ho, J. Hu, R. T. Peterson, A. K. Hatzopoulos and C. C. Hong, Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells, PLoS One, 2008, 3(8), e2904 CrossRef.
- K. A. M. Kennedy, T. Porter, V. Mehta, S. D. Ryan, F. Price, V. Peshdary, C. Karamboulas, J. Savage, T. A. Drysdale, S.-C. Li, S. A. L. Bennett and I. S. Skerjanc, Retinoic acid enhances skeletal muscle progenitor formation and bypasses inhibition by bone morphogenetic protein 4 but not dominant negative β-catenin, BMC Biol., 2009, 7, 67 CrossRef.
- S. P. Fricker, A novel CXCR4 antagonist for hematopoietic stem cell mobilization, Expert Opin. Invest. Drugs, 2008, 17(11), 1749–1760 Search PubMed.
- A. F. Cashen, B. Nervi and J. DiPersio, AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent, Future Oncol., 2007, 3(1), 19–27 Search PubMed.
- V. Bodart, V. Anastassov, M. C. Darkes, S. R. Idzan, J. Labrecque, G. Lau, R. M. Mosi, K. S. Neff, K. L. Nelson, M. C. Ruzek, K. Patel, Z. Santucci, R. Scarborough, R. S. Y. Wong, G. J. Bridger, R. T. MacFarland and S. P. Fricker, Pharmacology of AMD3465: A small molecule antagonist of the chemokine receptor CXCR4, Biochem. Pharmacol., 2009, 78(8), 993–1000 CrossRef CAS.
- P. Ramirez, M. P. Rettig, G. L. Uy, E. Deych, M. S. Holt, J. K. Ritchey and J. F. DiPersio, BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells, Blood, 2009, 114(7), 1340–1343 CrossRef CAS.
- T. E. North, W. Goessling, C. R. Walkley, C. Lengerke, K. R. Kopani, A. M. Lord, G. J. Weber, T. V. Bowman, I.-H. Jang, T. Grosser, G. A. FitzGerald, G. Q. Daley, S. H. Orkin and L. I. Zon, Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis, Nature, 2007, 447(7147), 1007–1011 CrossRef CAS.
- B. L. Barrilleaux, D. G. Phinney, B. W. Fischer-Valuck, K. C. Russell, G. Wang, D. J. Prockop and K. C. O'Connor, Small-molecule antagonist of macrophage migration inhibitory factor enhances migratory response of mesenchymal stem cells to bronchial epithelial cells, Tissue Eng. A, 2009, 15(9), 2335–2346 Search PubMed.
- H. Xie, M. Ye, R. Feng and T. Graf, Stepwise reprogramming of B cells into macrophages, Cell, 2004, 117, 663–676 CrossRef CAS.
- C. V. Laiosa, M. Stadtfeld, H. Xie, L. de Andres-Aguayo and T. Graf, Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBPa and PU.1 transcription factors, Immunity, 2006, 25, 731–744 CrossRef CAS.
- C. Cobaleda, W. Jochum and M. Busslinger, Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors, Nature, 2007, 449(7161), 473–477 CrossRef CAS.
- R. Feng, S. C. Desbordes, H. Xie, E. S. Tillo, F. Pixley, E. R. Stanley and T. Graf, PU.1 and C/EBPα/β convert fibroblasts into macrophage-like cells, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(16), 6057–6062 CrossRef CAS.
- S. Kajimura, P. Seale, T. Tomaru, H. Erdjument-Bromage, M. P. Cooper, J. L. Ruas, S. Chin, P. Tempst, M. A. Lazar and B. M. Spiegelman, Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex, Genes Dev., 2008, 22(10), 1397–1409 CrossRef CAS.
- Q. Zhou, J. Brown, A. Kanarek, J. Rajagopal and D. A. Melton, In vivo reprogramming of adult pancreatic exocrine cells to beta-cells, Nature, 2008, 455(7213), 627–32 CrossRef CAS.
- T. Vierbuchen, A. Ostermeier, Z. P. Pang, Y. Kokubu, T. C. Suedhof and M. Wernig, Direct conversion of fibroblasts to functional neurons by defined factors, Nature, 2010, 463(7284), 1035–1041 CrossRef CAS.
- M. Bredel and E. Jacoby, Chemogenomics: an emerging strategy for rapid target and drug discovery, Nat. Rev. Genet., 2004, 5(4), 262–275 CrossRef CAS.
- A. Wood. Chemical Biology for Drug Discovery. 8,9th December 2009; Oxford UK Search PubMed.
- Y. Sabnis, J. L. Klug-McLeod, A. Stobie, A. Cook, manuscript in preparation.
- X. Li and X. Zhao, Epigenetic regulation of mammalian stem cells, Stem Cells Dev., 2008, 17(6), 1043–1052 CrossRef CAS.
- V. V. Lunyak and M. G. Rosenfeld, Epigenetic regulation of stem cell fate, Hum. Mol. Genet., 2008, 17(Rev. Iss. 1), R28–R36 CrossRef CAS.
Footnote |
| † All three authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2010 |