Tripod amphiphiles for membrane protein manipulation
Pil Seok
Chae
a,
Philip D.
Laible
*b and
Samuel H.
Gellman
*a
aDepartment of Chemistry, University of Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706, USA. E-mail: gellman@wisc.edu; Fax: +1 608-265-4534; Tel: +1 608-262-3303
bBiosciences Division Argonne National Laborotory, 9700 South Cass Avenue, Argonne, IL 60439, USA. E-mail: laible@anl.gov; Fax: +1 630-252-3387
First published on 14th October 2009
Abstract
Integral membrane proteins (IMPs) are crucial biological components, mediating the transfer of material and information between cells and their environment. Many IMPs have proven to be difficult to isolate and study. High-resolution structural information on this class of proteins is limited, largely because of difficulties in generating soluble forms of such proteins that retain native folding and activity, and difficulties in generating high-quality crystals from such preparations. Isolated IMPs typically do not dissolve in aqueous solution, a property that arises from the large patches of hydrophobic surface necessary for favorable interactions with the core of a lipid bilayer. Detergents are generally required for IMP solubilization: hydrophobic segments of detergent molecules cluster around and shield from water the hydrophobic protein surfaces. The critical role played by detergents in membrane protein manipulation, and the fact that many IMPs are recalcitrant to solubilization and/or crystallization with currently available detergents, suggest that it should be valuable to explore new types of amphiphiles for these purposes. This review constitutes a progress report on our long-term effort to develop a new class of organic molecules, collectively designated “tripod amphiphiles,” that are intended as alternatives to conventional detergents for membrane protein manipulation. One long-range goal of this research is to identify new types of amphiphiles that facilitate IMP crystallization. This review should help introduce an important biochemical need to organic chemists, and perhaps inspire new approaches to the problem.
1. Introduction
Integral membrane proteins (IMPs) account for about 25% of all open reading frames in the genomes of living organisms and play vital roles in diverse cellular processes, such as signal transduction, transport, and cell recognition and communication. More than 50% of drugs target IMPs.1 Therefore, structural and functional knowledge of IMPs is essential for fundamental biological understanding as well as for rational drug design. High-resolution structural data are available for <0.5% of IMPs despite extensive effort; the few hundred IMP crystal structures currently available stand in contrast to the tens of thousands of structures that have been determined for soluble proteins.2–4 This scarcity of IMP structural information is mainly attributable to the difficulty of manipulating IMPs. It is often impossible to generate aqueous solutions of functional IMPs without the assistance of low-molecular-weight amphiphilic agents. Conventional detergents are widely used for this purpose. IMPs must display large patches of hydrophobic surface in order to reside in a membrane, and detergent molecules apparently coat these hydrophobic surfaces, rendering the protein compatible with an aqueous environment.5,6 However, conventional detergents are not compatible with all IMPs.7,8 Therefore, new types of amphiphile merit exploration for the ability to maintain IMPs in a native-like state after removal from the membrane.9–12 Ultimately, such amphiphiles might facilitate IMP crystallization.Over the past two decades, a few research groups have examined unusual synthetic amphiphiles for the ability to stabilize IMPs in aqueous solution. Examples include peptitergents,13 amphiphilic polymers (amphipols),14–16 hemifluorinated amphiphiles,17,18lipopeptidedetergents (LPDs),19 and deoxycholate-based facial amphiphiles.20,21 Peptitergents are peptides designed to be lipophilic on one side and hydrophilic on the other upon folding to an α-helical conformation; this design has its roots in earlier fundamental exploration of amphiphilic secondary structures.22 Amphipols are co-polymers that have been shown to stabilize a number of IMPs after they have been extracted from the membrane with conventional detergents.23 Some hemifluorinated amphiphiles were observed to be mild relative to non-fluorinated homologues for stabilization of a few of IMPs, particularly delicate assemblies composed of multiple subunits, such as cytochrome b6f complexes.17,18 LPDs, featuring an amphiphilic α-helix, have been reported to keep several IMPs (e.g., bacteriorhodopsin, PagP, Lac permease-cytochromeb562 fusion protein) soluble in water while maintaining native structure.19 However, few of these novel amphiphiles are commercially available, and there is no report of their use for IMP crystallization so far. Thus, there is a continuing need for synthetically accessible amphiphiles that can serve as tools for manipulating IMPs.
Organic chemists can readily envision new types of low-molecular-weight amphiphiles and plan synthetic routes to generate such molecules, but there have been relatively few efforts of this type to date. In our view, this situation arises for several reasons. (1) Very few organic chemists are aware of the problem. (2) Specialized biochemical skills are required to work with IMPs, and few, if any, organic chemistry groups have the necessary skills. Therefore, pursuing the development of new amphiphiles for use with IMPs requires long-term collaborations. (3) Assessing structural and functional integrity of an IMP in an amphiphile-solubilized state is often challenging. (4) Each integral membrane protein has its own peculiarities as a subject of experimental analysis, and expertise developed with one particular IMP does not necessarily transfer directly to the study of other IMPs. Most laboratories that explore IMP structure and function are focused on just one or a few examples. This situation makes it challenging to assess the extent to which new agents for membrane protein manipulation might have broad utility.24,25
2. Tripod amphiphile design
Classical detergents, as exemplified by n-dodecyl-β-D-maltoside (DDM) and lauryl dimethylamine-N-oxide (LDAO) (Fig. 1), have a relatively simple architecture in which a hydrophilic “head group” is attached to a long lipophilic “tail” (the latter is usually a straight-chain alkyl group).12 The classical detergent motif is generalized in structure I of Fig. 2. A few widely used detergents have different architectures (e.g., 3-[(3-cholamindopropyl)dimethylammonio]-1-propanesulfonate (CHAPS),26,27 shown in Fig. 1). CHAPS is an instructive example. This non-classical and commercially available detergent is widely used in protein science. CHAPS has been used to promote or assist crystallization of several solubleproteins, and occasionally specific CHAPS molecules are observed in the resulting structures.28–31 To our knowledge, however, there is no example of an integral membrane protein that has been crystallized from a CHAPS-solubilized state.32 One IMP, lactose permease LacY from Escherichia coli, has been crystallized from a DDM-solubilized state with the use of CHAPS as an additive; the protein structure was determined from these crystals (3.5 Å resolution). In general, it remains an open question whether amphiphiles with non-classical architectures can promote IMP crystallization. In our view, this uncertainty arises at least in part because relatively few non-classical amphiphiles have received careful attention from membrane protein scientists.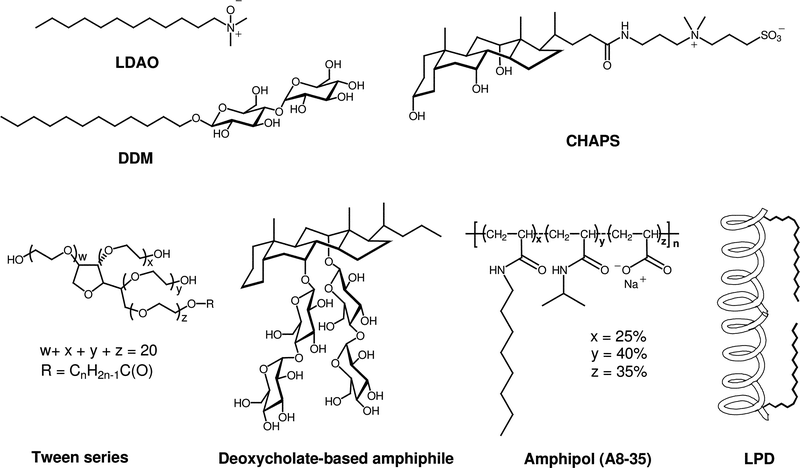 | ||
| Fig. 1 Chemical structures of representative classical detergents (LDAO, DDM) and selected non-classical amphiphiles (CHAPS, tween detergents, deoxycholate-based amphiphile, amphipol (A8-35; n ≈ 80), and LPD). | ||
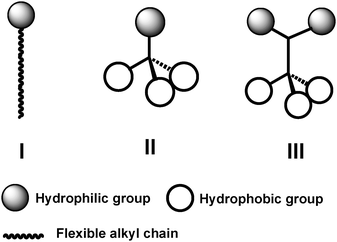 | ||
| Fig. 2 Schematic representation of conventional detergents (I) and tripod amphiphiles (TPAs; II and III). | ||
Tripod amphiphile architecture,33–35 illustrated in structures II and III of Fig. 2, differs fundamentally from that of classical detergents in that the tripods contain a branch point within the hydrophobic portion (II) or within both the hydrophobic and hydrophilic portions (III). A branch point, particularly a quarternary center, provides a subtle restriction on conformational mobility relative to linear molecular fragments,36–38 such as the n-alkyl tails common among conventional detergents. (Of course, a steroidal structure, as in CHAPS, has several branch points, but in this case conformational restriction is more severe, resulting from the cyclic constraints.) Our motivation for including branch points was to try to decrease the flexibility that characterizes most conventional detergents, which we believed might contribute to the difficulty of maintaining membrane proteins in an active state in solution and, perhaps, to the crystallization of membrane protein–detergent complexes.
3. Synthetic approaches to tripod amphiphiles
Evaluation of new amphiphiles for solubilization and stabilization of membrane proteins requires access to multi-gram quantities of these compounds. Widespread use by biochemists would require kilogram quantities. Conventional detergents such as DDM, along with cholate derivatives such as CHAPS, can be readily prepared in these amounts, but more exotic amphiphiles such as LPDs and peptitergents cannot. Our reduction of the tripod amphiphile design to practice has been guided by the importance of synthetic accessibility. The synthetic scheme summarized in Fig. 3 shows how a short sequence of reactions gives rise to an intermediate tripod-carboxylic acid33,34 that can then be converted into a variety of different tripod amphiphiles. We have focused on uncharged polar moieties (N-oxide or carbohydrate) because these polar groups are dominant among conventional detergents employed for membrane protein manipulation.32 (Detergents with polar groups that bear a net charge, such as SDS, tend to denature proteins.) The modularity of the synthetic approach illustrated in Fig. 3 supports a broad survey of structure–property relationships among tripod amphiphiles. Fig. 3 shows variation of only the polar group, but straightforward modifications of this route (e.g., use of a different ketone starting material or a different Grignard reagent in step b) allow alteration of the hydrophobic portion. The tuning of hydrophile–lipophile balance (HLB)39,40 and conformational flexibility41 enabled by this modularity should be important in the search for optimal performance with specific proteins. (HLB has been defined as 20 times the molecular weight of the hydrophilic portion divided by the molecular weight of the compound.39,40)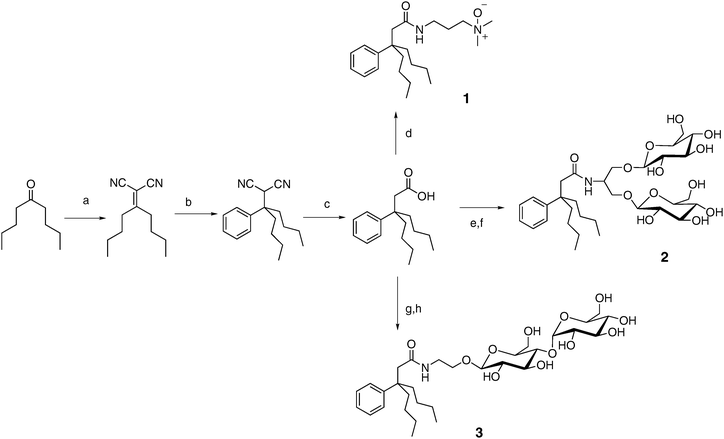 | ||
| Fig. 3 Modular synthesis of tripod amphiphiles: (a) CH2(CN)2, AcOH, NH4OAc, benzene, reflux; (b) PhMgBr, CuCN, THF, 0 °C; (c) KOH, ethylene glycol, reflux; (d) EDC·HCl, HOBt, 3-(dimethylamino)-1-propylamine, DMF, then m-CPBA, CHCl3; (e) EDC·HCl, HOBt, serinol, DMF; (f) AgOTf, 2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl bromide, DCM, then NaOMe, MeOH; (e) EDC·HCl, HOBt, ethanolamine, DMF; (h) 1,2-trans-peracetylated maltose, BF3·Et2O, DCM, then NaOMe, MeOH. | ||
The ease with which tripod amphiphile structure may be varied is important because it is unlikely that any single version will be a “magic bullet” that succeeds with all or even a large fraction of membrane proteins. The relationship between conventional detergent HLB and efficacy in extraction of IMPs from their native membranes illustrates this point.42–46Detergents within a narrow range of HLB values (12 to 15) display high efficiency and selectivity for the extraction of human adenosine A3 receptor in a functional state.47 Comparable optimal HLB ranges have been found for other IMPs from prokaryotic and eukaryotic sources. For instance, detergents with HLB ≈ 13 successfully extracted D-alanine carboxypeptidase from Bacillus subtilis cells.45 A similar value was found for protein extraction from mitochondrial membrane from bovine heart.48 It is notable that optimum HLB values vary somewhat from one IMP to the next. For instance, mitochondrial porin solubilization from bovine heart was more effective with detergents that have a smaller HLB, while total membrane protein solubilization from the same source was better achieved with the detergents of higher HLB.48
4. IMP solubilization with N-oxide tripod amphiphiles
Our initial tripod amphiphile studies focused on solubilization of bacteriorhodopsin (bR) and bovine rhodopsin (Rho) from their native membranes. These IMPs are attractive testbeds because the native membranes are relatively easy to obtain, and the quality of the extracted protein can be assessed via simple optical spectroscopic measurements. bR occurs naturally in a two-dimensional crystalline protein–lipid array (“purple membrane”) in Halobacterium salinarum. The high degree of order in the purple membrane assembly renders bR resistant to solubilization by many detergents, and only a few classical detergents (e.g., octyl glucoside, Triton X-100) have been successful.49,50 Triton X-100 is the most effective among classical detergents, but 20 hours are required for complete solubilization.51 In contrast, more than 95% of the bR was solubilized from purple membrane by N-oxide tripod amphiphiles 1 and 4 within 30 min (Fig. 4).33,34 In addition, these two tripod amphiphiles proved to be effective for solubilization of Rho, a G-protein coupled receptor, from bovine rod outer segments.33,34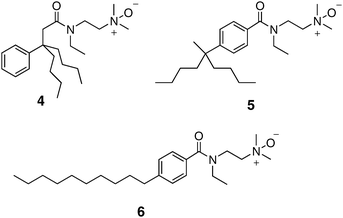 | ||
| Fig. 4 Chemical structures of tripod amphiphile 4 and isomeric amphiphiles 5 and 6, which have more classical architectures. | ||
We were initially surprised by the success of N-oxides 1 and 4 for bR solubilization, because treatment of purple membrane with LDAO (Fig. 1), a widely used conventional N-oxide detergent, causes rapid bR denaturation, as indicated by a loss of the characteristic purple color.52 This contrast enabled us to probe the functional importance of amphiphile architecture. We prepared 5 and 6, isomers of tripod amphiphile 4, in which the lipophilic portion is incrementally transformed into a conventional detergent “tail”. Both 5 and 6 behaved similarly to LDAO, causing rapid bR denaturation.33 This early result provided strong support for our hypothesis that the tripod amphiphile architecture represents an advantageous complement to conventional detergent architecture.
Comparisons among a small set of N-oxide tripod amphiphiles revealed that 1 was somewhat superior to 4 in terms of behavior with bR and Rho. Solubilized forms of each protein could be readily purified (including removal of endogenous lipids), and the resulting preparations were stable for several weeks, as assessed by optical absorbance.34 Stability of solubilized membrane proteins on this time scale is essential for crystallization efforts. Two proteins have been crystallized from the 1-solubilized state, bR53 and a form of the potassium channel from Streptomyces lividans;33 however, structure-determination has not been carried out in either case.
5. IMP solubilization with glyco-tripod amphiphiles
The promising behavior of N-oxide TPAs with bR and Rho encouraged us to expand the application of tripod amphiphiles to more delicate systems. We focused on the transmembrane protein superassembly formed by the light harvesting-I (LHI) complex and the reaction center (RC) complex of the photosynthetic bacterium Rhodobacter capsulatus. This LHI–RC superassembly has not been crystallized, but its architecture is believed to be similar to that of the LHI–RC superassembly from Rhodopseudomonas palustris, previously solved to 4.8 Å resolution,54 and the LHI–RC superassemblies from other purple non-sulfur bacteria.55–57 The LHI–RC superassembly represents a challenging system. To solubilize the superassembly in a native form, an amphiphile should be mild enough to preserve the tertiary structures of at least five different component proteins as well as the quaternary association of ∼30–40 protein molecules. The fragile nature of the R. capsulatus LHI–RC superassembly may underlie the lack of crystallographic analysis for this system. Unique spectral signatures arising from cofactors enable one to detect the native state of the LHI–RC superassembly and partially denatured states; this capability is very useful for evaluation of superassembly solubilization and stability over time.Attempted solubilization of the LHI–RC superassembly from R. capsulatus membranes with N-oxide tripod amphiphile 1 led to extensive disruption.35 However, tripod amphiphile 2, which has a branched diglucoside head group, could extract the intact LHI–RC superassembly from the native membrane. Tripod amphiphile 3 is very similar to 2, but there is no branch point in the hydrophilic portion of 3. This subtle structural difference leads to a substantial functional difference: considerable degradation of LHI was observed when solubilization was undertaken with 3. The contrast between 2 and 3 suggests that a branch point in the hydrophilic portion can complement a branch point in the hydrophobic portion in terms of membrane protein stabilization in aqueous solution. Interestingly, this functional distinction does not arise from HLB, which is quite similar for the two tripod amphiphiles, nor is the distinction reflected in critical micelle concentrations (CMC), which are almost identical for 2 and 3 (3.6 and 4.0 mM). It should be pointed out that 3, lacking a branch point in the hydrophilic portion, is significantly less soluble than analogue 2.
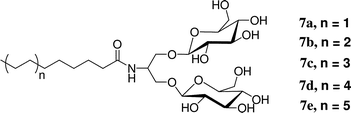
We prepared a series of detergents (7a–e) bearing the branched diglucoside head group found in 2 connected to a linear alkyl tail. Among this series, the version with a 12-carbon tail (7c) was most effective at extracting the LHI–RC superassembly from R. capsulatus membranes. However, the extraction efficiency of this detergent was much lower than that observed with tripod amphiphile 2. This comparison seems to complement the comparison of N-oxides 4–6 discussed above in suggesting that the tripod architecture displays distinctive advantages in the context of membrane protein solubilization and stabilization. Interestingly, some conventional detergents feature branching in the hydrophilic portion, as exemplified by the tween series (Fig. 1). Among non-classical amphiphiles, amphipols and LPDs (Fig. 1) can be viewed as containing branch points. We could not compare 7c with the analogue bearing a maltose (i.e., non-branched) head group because the latter was insoluble. Thus, even in the context of a conventional hydrophobic tail, hydrophilic group branching seems to confer favorable behavior. Collectively, these observations raise the possibility that new amphiphile architectures, featuring different placements of internal branch points and manifesting useful properties for IMP manipulation, remain to be discovered by imaginative chemists.
A recent comparison of >120 commercially available detergents in terms of LHI–RC superassembly solubilization from R. capsulatus membranes indicates that DDM is one of the most effective among conventional detergents.58 This demonstration of the value of DDM as a tool for membrane protein manipulation complements results obtained from studies with other IMPs, including Rho,59 diacyl glycerol kinase,60lactose permease,61 and human apelin receptor.62 In this context, it is noteworthy that we found DDM to be less effective than 2 for long-term stabilization of the R. capsulatus LHI–RC superassembly in aqueous solution;35 this comparison highlights the promise of tripod amphiphiles for membrane protein research.
6. Conclusions
The results summarized above suggest that a simple molecular design strategy, summarized by cartoons II and III in Fig. 2, represents a useful addition to the toolkit available for the study of integral membrane proteins. Three tripod amphiphiles have recently become commercially available, which should enable a full-fledged test of their utility. In our view, the most important conclusion to be drawn so far from this effort involves branch points: their placement in either the hydrophobic portion or the hydrophilic portion can be productive, and incorporation in both portions can be synergistic, with regard to membrane protein solubilization and stabilization.A molecular design that is conducive to facile variation enables exploration of structure–property relationships. Comparisons among tripod amphiphiles having closely related hydrophilic portions, as reported in our publications,34,35 have shown that performance is quite sensitive to these variations. More recently we have explored the impact of variations in the hydrophobic portions and observed profound effects on function. The two TPAs shown in Fig. 5, related structurally to 2, proved to be inferior to 2 for solubilization of the LHI–RC superassembly from R. capsulatus (unpublished results). These findings show that variations in the TPA hydrophobic group can have a significant influence on performance.
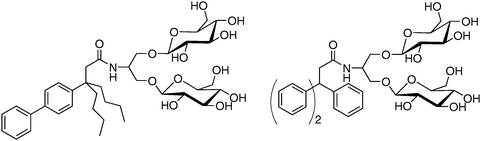 | ||
| Fig. 5 Tripod amphiphiles with variations in the hydrophobic portion relative to 2. These amphiphiles are inferior to 2 for R. capsulatus LHI–RC superassembly solubilization. | ||
The many integral membrane proteins that remain difficult to isolate and purify, or that are refractory to crystallization, constitute a strong impetus for the invention of new synthetic amphiphiles. This work requires the creativity and skill set of the organic chemist, which must be deployed in concert with the techniques and insights of membrane protein biochemists. In addition to solubilization, stabilization and ultimately crystallization of membrane proteins, novel amphiphiles can be applied to solution NMR spectroscopy63–65 and might support exciting new methods for study of membrane proteins, such as mass spectrometry.66 It is hoped that this review will inspire more chemists to apply their imaginations to the development of new types of amphiphilic agents for applications in membrane protein science.
Acknowledgements
This work was supported by NIH grant P01 GM75913.References
- J. Liu and B. Rost, Protein Sci., 2001, 10, 1970–1979 CrossRef CAS.
- P. J. Loll, J. Struct. Biol., 2003, 142, 144–153 CrossRef CAS.
- S. H. White, Protein Sci., 2004, 13, 1948–1949 CrossRef CAS.
- For a continuously updated database of membrane protein structures, see: http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html.
- C. Tanford and J. A. Reynolds, Biochim. Biophys. Acta, 1976, 457, 133–170 CrossRef CAS.
- J. V. Moller and J. le Maire, J. Biol. Chem., 1993, 268, 18659–18672 CAS.
- J. U. Bowie, Curr. Opin. Struct. Biol., 2001, 11, 397–402 CrossRef CAS.
- Y. Zhou, F. W. Lau, S. Nauli, D. Yang and J. U. Bowie, Protein Sci., 2001, 10, 378–383 CrossRef CAS.
- A. M. Seddon, P. Curnow and P. J. Booth, Biochim. Biophys. Acta, 2004, 1666, 105–117 CAS.
- P. Nollert, Prog. Biophys. Mol. Biol., 2005, 88, 339–357 CrossRef CAS.
- K. Lundstrom, Cell. Mol. Life Sci., 2006, 63, 2597–2607 CrossRef CAS.
- G. G. Privé, Methods, 2007, 41, 388–397 CrossRef CAS.
- C. E. Schafmeister, L. J. W. Meircke and R. M. Stroud, Science, 1993, 262, 734–738 CAS.
- C. Tribet, R. Audebert and J.-L. Popot, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 15047–15050 CrossRef CAS.
- C. Prata, F. Giusti, Y. Gohon, B. Pucci, J.-L. Popot and C. Tribet, Biopolymers, 2001, 56, 77–84 CAS.
- M. Gorzelle, A. K. Hoffman, M. H. Keyes, D. N. Gray, D. G. Ray and C. R. Sanders, J. Am. Chem. Soc., 2002, 124, 11594–11595 CrossRef.
- C. Breyton, E. Chabaud, Y. Chaudier, B. Pucci and J.-L. Popot, FEBS Lett., 2004, 564, 312–318 CrossRef CAS.
- F. Lebaupain, A. G. Salvay, B. Olivier, G. Durand, A.-S. Fabiano, N. Michei, J.-L. Popot, C. Ebel, C. Breyton and B. Pucci, Langmuir, 2006, 22, 8881–8890 CrossRef CAS.
- C.-L. McGregor, L. Chen, N. C. Pomroy, P. Hwang, S. Go, A. Chakrabartty and G. G. Privé, Nat. Biotechnol., 2003, 21, 171–176 CrossRef CAS.
- Q. Zhang, X. Ma, A. Ward, W.-X. Hong, V.-P. Jaakola, R. C. Stevens, M. G. Fin and G. Chang, Angew. Chem., Int. Ed., 2007, 119, 7153–7155 CrossRef.
- Y. Cheng, D. M. Ho, C. R. Gottlieb, D. Kahne and M. A. Bruck, J. Am. Chem. Soc., 1992, 114, 7319–7320 CrossRef CAS.
- E. T. Kaiser and F. J. Kezdy, Science, 1984, 223, 249–255 CAS.
- J.-L. Popot, E. A. Berry, D. Charvlin, C. Creuzenet, C. Ebel, D. M. Engelman, M. Flötenmyer, F. Giusti, Y. Gohon, P. Hervé, Q. Hong, J. H. Lakey, K. Leonard, H. A. Shuman and P. Timmnins, Cell. Mol. Life Sci., 2003, 60, 1559–1574 CrossRef CAS.
- D. A. P. Gutmann, E. Mizohata, S. Newstead, S. Ferrandon, P. J. F. Henderson, H. W. Van Veen and B. Byrne, Protein Sci., 2007, 16, 1422–1428 CrossRef CAS.
- S. Eshaghi, Methods Mol. Biol., 2009, 498, 265–271 CAS.
- L. M. Hjelmeland, D. W. Nebert and J. C. Osborne, Anal. Biochem., 1983, 130, 72–82 CAS.
- L. M. Hjelmeland, Proc. Natl. Acad. Sci. U. S. A., 1980, 77, 6368–6370 CAS.
- F. F. Vajdos, M. Ultsch, M. L. Schaffer, K. D. Deshayes, J. Liu, N. J. Skelton and A. M. de Vos, Biochemistry, 2001, 40, 11022–11029 CrossRef CAS.
- H. Itou, M. Yao, I. Fujita, N. Watanabe, M. Suzuki, J. Nishihira and I. Tanaka, J. Mol. Biol., 2002, 316, 265–276 CrossRef CAS.
- A.-L. Gall, M. Ruff and D. Moras, Acta Crystallogr., Sect. D, 2003, 59, 603–606 CrossRef.
- M. Kvansakul, O. Bogin, E. Hohenester and A. Yayon, Matrix Biol., 2003, 22, 145–152 CrossRef CAS.
- http://www.mpibp-frankfurt.mpg.de/michel/public/memprotstruct.html .
- D. T. McQuade, M. A. Quinn, S. M. Yu, A. S. Polans, M. P. Krebs and S. H. Gellman, Angew. Chem., Int. Ed., 2000, 39, 758–761 CrossRef CAS.
- S. M. Yu, D. T. McQuade, M. A. Quinn, C. P. R. Hackenberger, M. P. Krebs, A. S. Polans and S. H. Gellman, Protein Sci., 2000, 9, 2518–2527 CrossRef CAS.
- P. S. Chae, M. J. Wander, A. P. Bowling, P. D. Laible and S. H. Gellman, ChemBioChem, 2008, 9, 1706–1709 CrossRef CAS.
- R. W. Alder, C. M. Maunder and A. G. Orpen, Tetrahedron Lett., 1990, 31, 6717–6720 CrossRef CAS.
- R. W. Hoffmann, Angew. Chem., Int. Ed. Engl., 1992, 31, 1124–1134 CrossRef.
- R. W. Hoffmann, M. Stahl, U. Schopfer and G. Frenking, Chem.–Eur. J., 1998, 4, 559–566 CrossRef CAS.
- W. C. Griffin, J. Soc. Cosmet. Chem., 1949, 1, 311–326 Search PubMed.
- W. C. Griffin, J. Soc. Cosmet. Chem., 1954, 5, 249–256 Search PubMed.
- L. M. Hjelmeland and A. Chrambach, Methods Enzymol., 1984, 104, 305–318 Search PubMed.
- G. Guillon, C. Roy and S. Jard, Eur. J. Biochem., 1978, 92, 341–348 CrossRef CAS.
- V. Depinto, R. Benz and F. Palmieri, Eur. J. Biochem., 1989, 183, 179–187 CrossRef CAS.
- E. Slinde and T. Flatmark, Biochim. Biophys. Acta, 1976, 455, 796–805 CAS.
- J. N. Umbreit and J. L. Strominger, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 2997–3001 CrossRef CAS.
- W. L. Stauss, G. Ghai, C. M. Fraser and J. C. Venter, Arch. Biochem. Biophys., 1979, 196, 566–573 CrossRef.
- B. W. Berger, R. Y. Garcia, A. M. Lenhoff, E. W. Kaler and C. R. Robinson, Biophys. J., 2005, 89, 452–464 CrossRef CAS.
- V. D. Pinto, R. Benz and F. Palmieri, Eur. J. Biochem., 1989, 183, 179–187 CrossRef CAS.
- S. J. Milder, T. E. Thorgeirsson, L. J. W. Miercke, R. M. Stroud and D. S. Kliger, Biochemistry, 1991, 30, 1751–1761 CrossRef CAS.
- G. F. X. Schertler, H. D. Bartunik, H. Michel and D. Oesterhelt, J. Mol. Biol., 1993, 234, 156–164 CrossRef CAS.
- N. A. Dencher and M. P. Heyn, FEBS Lett., 1978, 96, 322–326 CrossRef CAS.
- H. G. Khorana, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1166–1171 CrossRef CAS.
- M. J. Theisen, T. B. Potocky, D. T. McQuade, S. H. Gellman and M. L. Chiu, Biochim. Biophys. Acta, 2005, 1751, 213–216 CAS.
- W. Roszak, T. D. Howard, J. Southall, A. T. Gardiner, C. J. Law, N. W. Isaacs and R. J. Cogdell, Science, 2003, 302, 1969–1972 CrossRef CAS.
- C. Jungas, J. L. Ranck, J. L. Rigaud, P. Joliot and A. Verméglio, EMBO J., 1999, 18, 534–542 CrossRef CAS.
- C. A. Siebert, P. Qian, D. Fotiadis, A. Engel, C. N. Hunter and P. A. Bullough, EMBO J., 2004, 23, 690–700 CrossRef CAS.
- S. Scheuring, J. Busselez and D. Levy, J. Biol. Chem., 2005, 280, 1426–1431 CAS.
- M. J. Wander, A. P. Bowling, and P. D. Laible, manuscript in preparation.
- W. De Grip, Methods Enzymol., 1982, 81, 256–265 Search PubMed.
- Y. Zhou and J. U. Bowie, J. Biol. Chem., 2000, 275, 6975–6979 CrossRef CAS.
- C. K. Engel, L. Chen and G. G. Privé, Biochim. Biophys. Acta, 2002, 1564, 47–56 CAS.
- A. I. Alexandrov, M. Mileni, E. Y. T. Chien, M. A. Hanson and R. C. Stevens, Structure, 2008, 16, 351–359 CrossRef CAS.
- P. A. Mcdonnell and S. J. Opella, J. Magn. Reson., Ser. B, 1993, 102, 120–125 CrossRef CAS.
- M. Zoones, J. C. Laurent, G. Fabrice and J.-L. Popot, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 8893–8898 CrossRef.
- Q. Zhang, R. Horst, M. Geralt, X. Ma, W.-X. Hong, M. G. Finn, R. C. Stevens and K. Wüthrich, J. Am. Chem. Soc., 2008, 130, 7375–7363.
- N. P. Barrera, N. D. Bartolo, P. J. Booth and C. V. Robison, Science, 2008, 321, 243–246 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |