Temperature and salt concentration alter base-sequence selectivity of a duplex DNA-bindingprotein†
Satoru
Nagatoishi
,
Yoshikazu
Tanaka
,
Motonori
Kudou
and
Kouhei
Tsumoto
*
Department of Medical Genome Sciences, Graduate School of Frontier Sciences, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan. E-mail: tsumoto@k.u-tokyo.ac.jp; Fax: +81-4-7136-3601; Tel: +81-4-7136-5402
First published on 19th October 2009
Abstract
A structural polymorphism of nucleic acids, which depends on the concentration of cations and the conditions of hydration, are strongly involved with interactions between DNA and proteins. In this paper, we report that different DNA sequences bound to hyperthermostable TATA-box-binding protein (PhoTBP) at different combinations of temperature and salt concentration in in vitro selection experiments. As a result of the interaction of-these selected DNAs with PhoTBP, characteristic changes in the numbers of water molecules and ions occurred under each condition of the selection experiment. This finding could help us to understand the solvent environment-dependent preference for base sequences in protein–DNA interactions.
Nucleic acids have a structural polymorphism that depends on the presence of a solvent environment (e.g. salt concentrations or cosolutes).1,2 The structural deformability or flexibility of the duplex in nucleic acids depends on the base sequences, the concentration of cations, and the conditions of hydration.1,3–5 These polymorphic conformational properties of nucleic acids are strongly involved with interactions between DNA and proteins such as transcription factors, polymerases, or nucleosomes, which play important biological roles.4,6 However, almost all of these interactions between DNA and proteins have been studied under standard physiological conditions. In some protein–DNA interactions, the protein recognizes the target DNA by its conformational properties, as well as directly interacting with bases.7–10 We focused here on the relationship between the solvent environment–dependent structural polymorphism of DNA and the specificity of DNA interactions with proteins recognizing characteristic DNA conformations.
TATA-box promoter DNAs have an A/T-rich sequence that interacts with TATA-box-binding protein (TBP), which is a minor groove–binding protein interacting with a hydrophobic interface of the minor groove in bending DNA. TATA-boxDNAs have various A/T-rich consensus sequences11–13 and appropriate conformational flexibility and deformability, which favor their interaction with TBP.14–16 It is therefore likely that the TATA-box base sequences preferential for binding to TBP alter in accordance with the solvent environment (e.g. temperature, salt concentration, presence of cosolutes) in which the TATA-boxDNA binds selectively to the TBP. To test this hypothesis, we performed in vitro selection experiments on TBP to identify selective DNA sequences at different temperatures or in different salt concentrations, and we characterized the interactions between TBP and the in vitro—selected DNAs.
We chose a hyperthermostable TBP from Pyrococcus horikoshii (PhoTBP),17 which lives under intracellular conditions of high temperature and high salt concentration. The melting transition temperature, Tm, of this protein is 100 °C in 20 mM sodium phosphate (pH 8.0), 1 M NaCl (data not shown). Because PhoTBP is also halophilic, the interaction with its target DNA is favorable at high salt concentrations.18 As reported previously,18PhoTBP interacts with its target DNA between 25 and 55 °C with a binding constant (Ka) of 105 to 107 M−1 in 1 M NaCl; the interaction is driven by favorable entropy change (data not shown), which is a typical thermodynamic property of TBP.19,20
The strategy used in the in vitro selection experiment is described in detail in the Supplementary Information.† In brief, as a DNA pool, a 68-nucleotide (nt) DNA template containing a 24-nt randomized sequence in its central position was amplified to DNA duplexes by DNA polymerase. The PCR fragments of the DNA duplex (50 pmol) were incubated with resin-bound PhoTBP (5 nmol) in a binding buffer (20 mM Tris-HCl [pH 7.7], 1 M or 150 mM NaCl, 10 mM imidazole) for 30 min. at either 50 or 25 °C. After being washed with the binding buffer, the PhoTBP–DNA complexes were eluted with elution buffer containing 500 mM imidazole. The bound DNA fragments were eluted by phenol–chloroform extraction and ethanolprecipitation and then amplified by PCR. The PCR products were used as a DNA pool for the next cycle of selection. This process was repeated to a total of 7 cycles, and selected DNA sequences of the random region were then analyzed.
In the in vitro selection when 1 M NaCl (high salt concentration) was used in the binding buffer, characteristic DNA sequences were obtained at both 50 and 25 °C. The selected DNA fragments contained 6- to 8-nt A/T-rich sequences (Supplementary Fig. S1 and Fig. S2† ). We therefore considered that, at the high salt concentration used in these in vitro selection experiments, these A/T-rich DNAs were not selected by nonspecific electrostatic interaction. At 50 °C, A/T-rich sequences containing T-tracts (e.g. TTTA, TTTT) were selected (Supplementary Fig. S1† ). In contrast, at 25 °C, A/T-rich sequences containing TA sequences (e.g. TATA, TTAT) were selected (Supplementary Fig. S2† ). Motifs with the consensus sequences of the DNAs selected in each in vitro selection were identified by using the motif search program MEME.21 The sequence g/tCTTTTAA (ODN50H) was found at 50 °C, and the sequence Ca/tt/aTATAa/t (ODN25H) at 25 °C (Fig. 1A and B).22
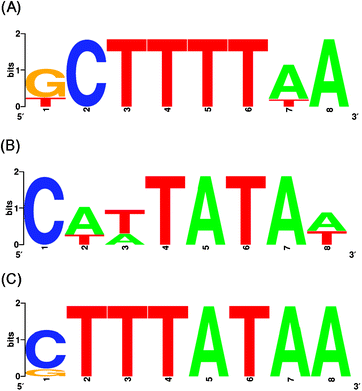 | ||
| Fig. 1 Motif consensus sequences of DNAs selected by PhoTBP in in vitro selection experiments at different temperatures or salt concentrations. (A) 1 M NaCl, 50 °C; (B) 1 M NaCl, 25 °C; (C) 150 mM NaCl, 50 °C. These motif consensus sequences are presented as sequence logos generated with WebLogo (http://weblogo.berkeley.edu/). The overall height of each letter stack indicates the sequence conservation at that position, and the height of each symbol within the stack reflects the relative frequency of the corresponding nucleic acid at that position. These selected sequences are shown in Supplementary Fig. S1–S3,† and are aligned according to the results of the MEME analysis (http://meme.sdsc.edu/meme4_1/intro.html). The MEME program for the occurrences of one motif presenting on each sequence was selected. The “any number of repetitions per sequence” mode was used, and motif width was restricted to 8 nt. | ||
When 150 mM NaCl (low salt concentration) was used in the binding buffer, characteristic DNA sequences were obtained at 50 °C, but not at 25 °C. At 50 °C, the selected DNA fragments contained 6- to 8-nt A / T-rich sequences (Supplementary Fig. S3† ). MEME analysis21 revealed that the motif with the consensus sequence of the DNAs selected in this selection experiment was CTTTATAA (ODN50L; Fig. 1C).22 At 25 °C, the DNA fragments obtained were random (Supplementary Fig. S4† ), suggesting that specificity of PhoTBP for DNA occurred at high temperature or high salt concentration.
We characterized the interaction between PhoTBP and ODN50H, ODN25H or ODN50L, which were represented by MEME analysis (Fig. 2). We measured the binding constants (Ka values) in isothermal titration calorimetry (ITC) experiments at various NaCl concentrations in order to determine the changes occurring in the numbers of water molecules and ions as a result of the interaction with PhoTBP. The experimental procedures are described in the Supplementary Information.†23,24 The representative ITC profile was shown in Fig. 3. The interaction between PhoTBP and ODN50H had the Ka value on the order of 106 M−1 (2.47 ± 0.37 × 106 M−1), and was driven by favorable entropy changes (ΔHo = 17.4 ± 0.28 kcal mol−1 and ΔSo = 83.0 cal mol−1 K−1) under the condition of SELEX experiment. The interactions of all the selected DNAs with PhoTBP were also driven by favorable entropy changes (Supplementary Fig. S5 and Table S1† ), which were very similar to the thermodynamic properties of the interaction between PhoTBP and its target DNA.18 The dependence of Ka on NaCl concentration can be determined by using the following relationship:25,26
| log Ka = log Kref,1M − Alog[NaCl] + 0.016B[NaCl] | (1) |
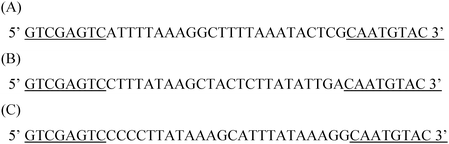 | ||
| Fig. 2 Sequences of the selected DNA used in this study. (A) ODN50H, (B) ODN25H, (C) ODN50L. These sequences contained consensus motifs of DNAs selected by PhoTBP in each in vitro selection experiment. Common sequences are underlined. | ||
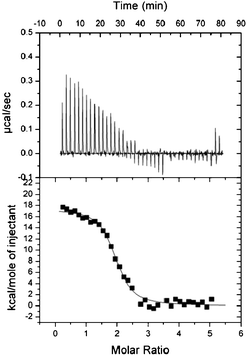 | ||
| Fig. 3 Top panel: Representative raw ITC profile for the interaction between 250 μM PhoTBP and 10 μM ODN50H at 50 °C, 1 M NaCl in 20 mM Tris-HCl (pH 7.5) and 1 mM EDTA. Bottom panel: Integration plot of data calculated from the ITC profile after correction for the heat of PhoTBP dilution. Solid line corresponds to the best fit curve obtained by least-squares deconvolution. Experimental procedures are described in the Supplementary Information.† | ||
The slope of logKa against log[NaCl] was positive upon the interaction of ODN50H or ODN25H with PhoTBP (Fig. 4). At low salt concentrations (<1 M NaCl), Ka of ODN50H was weakly affected by salt concentration at 50 °C and strongly affected at 25 °C. In contrast, the Ka of ODN25H was strongly affected by salt concentration at 50 °C and weakly affected at 25 °C (Fig. 4). These plots of log Ka against log[NaCl] were used to calculate A and B values by fitting to eqn (1) (Table 1). ODN50H had a large positive B value at 50 °C but not 25 °C, and ODN25H had a large positive B value at 25 °C but not 50 °C, indicating that the interaction of ODN50H with PhoTBP at 50 °C was accompanied by the release of large numbers of water molecules, as was the interaction of ODN25H with PhoTBP at 25 °C. Under these conditions, the A values were nearly zero. In contrast, ODN50H and ODN25H had relatively large negative A values at 25 °C and 50 °C, respectively, indicating that the interactions were accompanied by the uptake of ions. Large positive B values have been obtained in interactions between TBPs in various organisms and target DNAs, in which A values distinctly differ among different organisms;26 these large B values have resulted from dehydration of the binding interface upon formation of the TBP–DNA complex. Our results therefore suggest that ODN50H and ODN25H exerted a favorable dehydration effect in their interaction with PhoTBP at 50 °C and 25 °C, respectively.
![Plots of log Ka against log[NaCl] for ODN50H at 50 °C (red circles) and 25 °C (orange squares), for ODN25H at 50 °C (green diamonds) and 25 °C (blue triangles), and for ODN50L at 50 °C (purple inverted triangles), interacting with PhoTBP in 20 mM Tris-HCl (pH 7.5) and 1 mM EDTA. Solid lines correspond to the best-fit curves obtained by fitting eqn (1) with a nonlinear least-squares algorithm. Error bars represent standard deviation of data sets obtained from at least three runs.](/image/article/2010/MB/b914828k/b914828k-f4.gif) | ||
| Fig. 4 Plots of log Ka against log[NaCl] for ODN50H at 50 °C (red circles) and 25 °C (orange squares), for ODN25H at 50 °C (green diamonds) and 25 °C (blue triangles), and for ODN50L at 50 °C (purple inverted triangles), interacting with PhoTBP in 20 mM Tris-HCl (pH 7.5) and 1 mM EDTA. Solid lines correspond to the best-fit curves obtained by fitting eqn (1) with a nonlinear least-squares algorithm. Error bars represent standard deviation of data sets obtained from at least three runs. | ||
| Sample | Ion (A) | Water (B) | log Kref, 1M | |
|---|---|---|---|---|
| ODN50H | 50 °C | −0.51 ± 0.5 | 28 ± 13 | 5.97 ± 0.2 |
| 25 °C | −2.13 ± 0.3 | 4 ± 9 | 5.53 ± 0.2 | |
| ODN25H | 50 °C | −2.34 ± 0.3 | −11 ± 7 | 6.53 ± 0.1 |
| 25 °C | 0.005 ± 0.8 | 46 ± 21 | 4.98 ± 0.4 | |
| ODN50L, 50 °C | 2.83 ± 1.9 | 69 ± 65 | 4.84 ± 1.1 | |
In the interaction of ODN50L with PhoTBP at 50 °C, the slope of log Ka against log[NaCl] was negative at low salt concentrations (<1 M NaCl; Fig. 4). Use of the plots of log Ka against log[NaCl] and eqn (1) to derive A and B values revealed that ODN50L had relatively large positive A and B values (Table 1), indicating that the interaction of ODN50L with PhoTBP at 50 °C was accompanied by the release of ions and large numbers of water molecules. These results are similar to those for a mesophilic TBP (that of Saccharomyces cerevisiae),26 the A and B values of which are 3.45 and 71, respectively. Therefore, release of ions is an important event in the interaction between TBP and DNA at low salt concentrations.
From these in vitro selection analyses of PhoTBP, we obtained different A/T-rich sequences under different conditions of temperature or salt concentration (Fig. 5). The DNAs selected in these selection analyses had large positive B values as a common feature, suggesting that the interaction between TBP and selective DNA is exerted through favorable dehydration. Sequence of TATA-elements has appropriate conformational flexibility and deformability, which are favorable for its interaction with TBP.14–16 Conformational flexibility of biomolecules becomes generally high with increasing temperature. Structural properties of DNA are strongly affected by the concentration of cations and by the conditions on hydration.1 Conformational flexibility or deformability of DNAs depends on the types of components in the base sequence, which has been investigated in molecular dynamics simulations.3,4 Under the condition of high salt concentration in the selection experiments, A/T-rich sequences containing T-tracts and TA sequences were selected at 50 °C and 25 °C, respectively, with large B values at each temperature. Moreover, selection analysis of the conditions of high salt and low salt concentration at 50 °C revealed that the selected DNAs demonstrated a different ion behavior under each condition. It has been proposed that the conformational properties of A/T-rich DNAs containing TA sequences differ from those containing T-tract sequences, which is conformationally more rigid than that of TA sequence.3,27 The structural properties of ODN50H, ODN25H, and ODN50L for TBP binding might therefore differ, resulting in temperature- or salt concentration- dependent differences in water or ion release. In addition, binding interface of PhoTBP probably play an important role in the interaction with the selected DNAs. The amino acid sequence of PhoTBP is 92% identical to that of Pyrococcus woesei TBP (PwTBP),18,19 suggesting that the biophysical binding properties of PhoTBP is similar to those of PwTBP. PwTBP have the cation binding capacity (large positive B values) on the interaction with the target DNA.26,28 This uptake of ions is thermodynamically unfavorable at low salt concentration on the interaction between protein and DNA.25,28 Since T-tract sequences are more conformationally rigid than those of TA sequences,3,27 ODN50H is therefore probably required for the structural flexibility with increasing temperature and the uptake of cations at high salt concentration for the stable complex with PhoTBP, but not ODN50L due to its appropriate conformational flexibility. A positive A value (ion release) in the interaction between PhoTBP and ODN50L might be attributed to the favorable entropic effect on the interaction between TBP and DNA. ODN25H is probably conformationally more flexible than that of ODN50H and ODN50L, which was consequently selected at low temperature and high salt concentration.
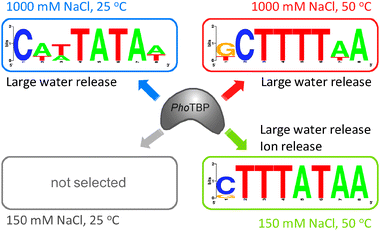 | ||
| Fig. 5 Schematic illustration of the interaction between PhoTBP and the preferential base sequence of DNA selected in vitro under each condition. | ||
The consensus sequences of TATA elements in the genomic DNA of thermophilic species are T-tract sequences (e.g., TTTTTAAA in the sulfolobales and TTTATA in the thermococcales)11–13; these sequences are similar to the DNA sequences selected here under high temperature and high salt concentration. On the other hand, halophilic or mesophilic species have TATA sequences (e.g., TTt/aa/tAN in halobacteria, TATAa/tAa/tN in eukaryotes)11–13 that are similar to the DNA sequences selected here under low temperature or low salt concentration. M. Suzuki et al. have proposed an adaptation of archaeal genomic DNAs to temperatures for the conformational flexibility in DNA molecules.29 Therefore, the DNA elements selected here may be representative of the genomic sequences of organisms that live under conditions of high temperature, high salt concentration, or low salt concentration.
In conclusion, in vitro selection experiments on PhoTBP gave different DNA sequences binding to PhoTBP at different combinations of temperature and salt concentration. As a result of the interaction of these selected DNAs with PhoTBP, large numbers of water molecules were released as a common feature, or ions were released under the condition of low salt concentration. These findings could help us to understand the presence of solvent environment-dependent preferential base sequences in protein–DNA interactions and the biological significance of adaptation of the base sequences in genomic DNAs to growth environments.
This work was supported in part by Grant-in-Aid for General Research from the Japan Society for the Promotion of Science. S.N. thanks the Japan Society for the Promotion of Science for its research fellowship.
References
- W. Saenger, Principles of Nucleic Acid Structure, Springer-Verlag, New York, 1984 Search PubMed.
- D. Miyoshi and N. Sugimoto, Biochimie, 2008, 90, 1040–1051 CrossRef CAS.
- M. J. Packer, M. P. Dauncey and C. A. Hunter, J. Mol. Biol., 2000, 295, 85–103 CrossRef CAS.
- S. Fujii, H. Kono, S. Takenaka, N. Go and A. Sarai, Nucleic Acids Res., 2007, 35, 6063–6074 CrossRef CAS.
- T. E. Cheatham, III, M. F. Crowley, T. Fox and P. A. Kollman, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 9626–9630 CrossRef.
- A. A. Travers, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 1423–1438 CrossRef CAS.
- R. K. Allemann and M. Egli, Chem. Biol., 1997, 4, 643–650 CrossRef CAS.
- J. R. Williamson, Nat. Struct. Biol., 2000, 7, 834–837 CrossRef CAS.
- F. V. Murphy and M. E. A. Churchill, Structure, 2000, 8, R83–R89 CrossRef.
- C. A. Bewley, A. M. Gronenborn and G. M. Clore, Annu. Rev. Biophys. Biomol. Struct., 1998, 27, 105–131 CrossRef CAS.
- J. R. Palmer and C. J. Daniels, J. Bacteriol., 1995, 177, 1844–1849 CAS.
- J. van der Oost, M. Ciaramella, M. Moracci, F. M. Pisani, M. Rossi and W. M. de Vos, Adv. Biochem. Eng. Biotechnol., 1998, 61, 87–115 CAS.
- J. N. Reeve, Mol. Microbiol., 2003, 48, 587–598 CrossRef CAS.
- A. Bareket-Samish, I. Cohen and T. E. Haran, J. Mol. Biol., 2000, 299, 965–977 CrossRef CAS.
- Z. S. Juo, T. K. Chiu, P. M. Leiberman, I. Baikalov, A. J. Berk and R. E. Dickerson, J. Mol. Biol., 1996, 261, 239–254 CrossRef CAS.
- O. N. de Souza and R. L. Ornstein, Biopolymers, 1998, 46, 403–415 CrossRef CAS.
- Y. Kawarabayashi, M. Sawada, H. Horikawa, Y. Haikawa, Y. Hino, S. Yamamoto, M. Sekine, S. Baba, H. Kosugi, A. Hosoyama, Y. Nagai, M. Sakai, K. Ogura, R. Otsuka, H. Nakazawa, M. Takamiya, Y. Ohfuku, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, K. Aoki and H. Kikuchi, DNA Res., 1998, 5, 55–76 CrossRef.
- S. Nagatoishi, Y. Tanaka, M. Kudou and K. Tsumoto, Mol. BioSyst., 2009, 5, 957–961 RSC.
- R. O'Brien, B. DeDecker, K. G. Fleming, P. B. Sigler and J. E. Ladbury, J. Mol. Biol., 1998, 279, 117–125 CrossRef CAS.
- V. Petri, M. Hsieh, E. Jamison and M. Brenowitz, Biochemistry, 1998, 37, 15842–15849 CrossRef CAS.
- T. L. Bailey and C. Elkan, Proc. Int. Conf. Intell. Syst. Mol. Biol., 1994, 2, 28–36 Search PubMed.
- G. E. Crooks, G. Hon, J. M. Chandonia and S. E. Brenner, Genome Res., 2004, 14, 1188–1190 CrossRef CAS.
- P. Giri and G. S. Kumar, Mol. BioSyst., 2008, 4, 341–348 RSC.
- P. Giri and G. S. Kumar, Arch. Biochem. Biophys., 2008, 474, 183–192 CrossRef CAS.
- J. H. Ha, M. W. Capp, M. D. Hohenwalter, M. Baskerville and M. T. Record, J. Mol. Biol., 1992, 228, 252–264 CrossRef CAS.
- S. Bergqvist, M. A. Williams, R. O'Brien and J. E. Ladbury, Structure, 2002, 10, 629–637 CrossRef CAS.
- H. Faiger, M. Ivanchenko and T. E. Haran, Nucleic Acids Res., 2007, 35, 4409–4419 CrossRef CAS.
- S. Bergqvist, R. O'Brien and J. E. Ladbury, Biochemistry, 2001, 40, 2419–2425 CrossRef CAS.
- T. Kawashima, N. Amano, H. Koike, S. Makino, S. Higuchi, Y. Kawashima-Ohya, K. Watanabe, M. Yamazaki, K. Kanehori, T. Kawamoto, T. Nunoshiba, Y. Yamamoto, H. Aramaki, K. Makino and M. Suzuki, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14257–14262 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental. See DOI: 10.1039/b914828k |
| This journal is © The Royal Society of Chemistry 2010 |
