Optoelectrofluidic platforms for chemistry and biology†
Hyundoo
Hwang
a and
Je-Kyun
Park
*ab
aDepartment of Bio and Brain Engineering, Korea Advanced Institute of Science and Technology (KAIST), 335 Gwahangno, Yuseong-gu, Daejeon 305–701, Republic of Korea. E-mail: jekyun@kaist.ac.kr; Fax: +82-42-350-4310; Tel: +82-42-350-4315
bKAIST Institute for the NanoCentury, 335 Gwahangno, Yuseong-gu, Daejeon 305-701, Republic of Korea
First published on 14th October 2010
Abstract
Extraordinary advances in lab on a chip systems have been made on the basis of the development of micro/nanofluidics and its fusion with other technologies based on electrokinetics and optics. Optoelectrofluidic technology, which has been recently introduced as a new manipulation scheme, allows programmable manipulation of particles or fluids in microenvironments based on optically induced electrokinetics. Herein, the behaviour of particles or fluids can be controlled by inducing or perturbing electric fields on demand in an optical manner, which includes photochemical, photoconductive, and photothermal effects. This elegant scheme of the optoelectrofluidic platform has attracted attention in various fields of science and engineering. A lot of research on optoelectrofluidic manipulation technologies has been reported and the field has advanced rapidly, although some technical hurdles still remain. This review describes recent developments and future perspectives of optoelectrofluidic platforms for chemical and biological applications.
 Hyundoo Hwang | Hyundoo Hwang received his B.S., M.S., and Ph.D. degrees in bio and brain engineering from the Korea Advanced Institute of Science and Technology (KAIST), in 2006, 2007, and 2010, respectively. His Ph.D. thesis was on optoelectrofluidic manipulation and detection of biomolecules under the direction of Prof. Je-Kyun Park. He is also interested in nanotechnology and microfluidic devices for analysis of biochemical molecules and elements. He received a National Science Scholarship (2003–2006) from the Korea Science and Engineering Foundation, and a Ph.D. Scholarship Award (2008–2010) from the Seoam Scholarship Foundation in Korea. |
 Je-Kyun Park | Je-Kyun Park is the director of the NanoBiotech Laboratory and a Professor of Bio and Brain Engineering at the Korea Advanced Institute of Science and Technology (KAIST). He has been an editorial board member of several international journals. In 2010, he became an editorial board member for Lab on a Chip, and the Chair of the BioMEMS and Lab on a Chip committee at the Korean BioChip Society. His research focuses on the microfluidic lab on a chip platform for biological sample processing and detection, including optoelectrofluidic manipulation, hydrophoretic separation, magnetophoretic assay, and cell-based assay. |
1. Introduction
Miniaturized systems have made extraordinary advances in analytical chemistry1,2 and biomedical applications such as clinical diagnostics3,4 and drug discovery,5,6 since the development of micro/nanofluidics began in the late 1900s. On the progress of the microfluidic lab-on-a-chip, electrokinetics has been an important role in manipulating molecules and fluids in micro/nanoscale devices.7,8 The sample flow and other operations in the electrokinetic microfluidic devices are driven by applying electric fields into microfluidic channels with electrodes and electronic circuits. Much effort has been devoted to understanding the complex characteristics of the electrokinetic phenomena and for developing more efficient and powerful lab-on-a-chip devices.In recent years, optofluidics, the marriage of optics and microfluidics, has emerged as one of the most advanced technologies for achieving more efficient and more functional lab-on-a-chip systems.9,10 The combination of optical fields and microfluidic devices yields many advantages in both perspectives; fluidics for optics and optics for fluidics.11 In particular, numerous optical methods provide simple and powerful way for controlling and sensing biochemical samples in a lab-on-a-chip.
Most recently, a technology called optoelectrofluidics, has attracted significant attention for its fascinating concept, which implies optical control of the electrokinetic phenomena in microfluidic environments, in various fields of science and engineering. Here, we will review the birth and the progress of optoelectrofluidic technologies. The fundamental principles and history of optoelectrofluidic platforms will be presented at first. Some typical cases and potential applications of this technology will be then summarized. Then, current challenges and future perspectives of optoelectrofluidic technologies will be discussed.
2. Optoelectrofluidics
2.1. Principles and history of optoelectrofluidics
Optoelectrofluidics is based on the electrokinetic motions of particles or fluids under an electric field, which is induced or perturbed by light. There are two typical approaches to control the electrokinetic behaviour of particles or fluids in an optical manner: (1) using light directly to change properties of the liquid; and (2) using light to change the conductivity of surfaces. The former was first demonstrated through electrothermal (ET) vortex due to the laser-induced thermal gradient in the liquid by Mizuno et al. in 1995.12 The optoelectrothermal vortex, due to the increase of local temperature of the liquid by a strong infrared laser, has been applied to rapidly concentrate microparticles and to stretch single deoxyribonucleic acid (DNA) molecules (Fig. 1a).13 The latter concept was first utilized for patterning of colloidal particles using selective exposure of ultraviolet (UV) onto indium tin oxide (ITO) surface. In 2000, Hayward et al. demonstrated the electrokinetic patterning of microparticles in a non-uniform electric field induced by projecting a UV pattern onto an ITO electrode as shown in Fig. 1b.14 The local increase of current by UV illumination at an ITO–water interface could promote a perturbation of an applied electric field, resulting in the electrokinetic migration of particles.15,16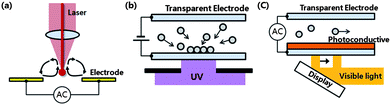 | ||
| Fig. 1 Three typical types of optoelectrofluidic platforms. (a) Electrothermal vortices due to the optical increase of local temperature of the liquid. Electrokinetic particle manipulation using optically induced virtual electrodes formed by (b) an ultraviolet light pattern projected onto indium tin oxide or (c) an image projected onto a photoconductive layer in an optoelectronic tweezers device. | ||
Recently, both approaches have been noted due to the desires of researchers, which is to optically manipulate fluids or individual particles in a programmable manner. In particular, the methodology based on the optical control of surface conductivity has achieved much advance since the development of optoelectronic tweezers (OETs) in 2005. Chiou et al. deposited a photoconductive layer on a plate electrode and made it possible to control an electric field only with a weak conventional light source and to spatially modulate the light pattern with a display device in a simple and easy way (Fig. 1c).17 This OET platform has further served as a momentum to attract much attention to the optoelectrofluidic technologies. The OET device provided a solution for disposability and interconnection issues in the parallel manipulation of multiple cells using a microelectrode array.18,19 Moreover, this method required much lower optical power and offered much larger manipulation area than the typical optical manipulation technique called optical tweezers20 and the laser-induced ET vortex.12
2.2. Essential components for optoelectronic tweezers
OET platforms require special configuration, which includes a photoconductive surface and a programmable display device. In this section, therefore, we focus on reviewing the essential components for constructing an OET platform. To construct an OET platform, a device composed of a photoconductive layer and a sample solution, a light source projected into the device to induce or perturb an electric field in the sample solution by forming virtual electrodes, a display device for modulating the light pattern, and a power supply for applying a voltage are essential. Optical components such as lenses and mirrors also play a crucial role in the optoelectrofluidic system, but it depends on the display device and the light source.The OET device, which was first introduced in 2005, was composed of ITO plate electrodes for the application of a voltage and hydrogenated amorphous silicon (a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H) as a photoconductive layer deposited on the plate electrode.17 Since the intrinsic a-Si
H) as a photoconductive layer deposited on the plate electrode.17 Since the intrinsic a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H has shorter carrier diffusion length and higher optical absorption coefficient than crystalline silicon, it is a good photoconductive material to make virtual electrode patterns of high resolution.21 The OET device based on a-Si
H has shorter carrier diffusion length and higher optical absorption coefficient than crystalline silicon, it is a good photoconductive material to make virtual electrode patterns of high resolution.21 The OET device based on a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H is simple and easy to fabricate using a plasma enhanced chemical vapour deposition (PECVD) method. In general, a triple layer of (i) heavily doped a-Si
H is simple and easy to fabricate using a plasma enhanced chemical vapour deposition (PECVD) method. In general, a triple layer of (i) heavily doped a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H for lowering contact resistance, (ii) intrinsic a-Si
H for lowering contact resistance, (ii) intrinsic a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H for high photoconductivity, and (iii) silicon nitride (or silicon oxide) for passivation was sequentially deposited onto the ITO-coated glass substrate in a single chamber reactor.22,23 Finally, a bare ITO-coated glass substrate as a ground electrode is turned upside down and put on the fabricated photoconductive layer as sandwiching a sample solution containing target materials with a certain gap height using spacers as shown in Fig. 2a. The first layer for Ohmic contact was sometimes removed24,25 or replaced by other materials such as zinc oxide26 and molybdenum.27 A device composed of two photoconductive layers, in which the ground electrode was exchanged into another photoconductive layer, has also been applied to manipulate microparticles with vertical focusing by three-dimensional (3D) virtual electrodes as shown in Fig. 2b.28 The conventional OET devices have two-parallel-plate configuration, in which a sample solution is sandwiched between two parallel layers. Single-sided OET devices, which are based on interdigitated electrodes patterned on a single plate substrate, have also been developed (Fig. 2c).29
H for high photoconductivity, and (iii) silicon nitride (or silicon oxide) for passivation was sequentially deposited onto the ITO-coated glass substrate in a single chamber reactor.22,23 Finally, a bare ITO-coated glass substrate as a ground electrode is turned upside down and put on the fabricated photoconductive layer as sandwiching a sample solution containing target materials with a certain gap height using spacers as shown in Fig. 2a. The first layer for Ohmic contact was sometimes removed24,25 or replaced by other materials such as zinc oxide26 and molybdenum.27 A device composed of two photoconductive layers, in which the ground electrode was exchanged into another photoconductive layer, has also been applied to manipulate microparticles with vertical focusing by three-dimensional (3D) virtual electrodes as shown in Fig. 2b.28 The conventional OET devices have two-parallel-plate configuration, in which a sample solution is sandwiched between two parallel layers. Single-sided OET devices, which are based on interdigitated electrodes patterned on a single plate substrate, have also been developed (Fig. 2c).29
 | ||
| Fig. 2 Typical types of optoelectronic tweezers (OET). (a) A conventional OET device composed of a photoconductive layer and a ground electrode layer. (b) A three-dimensional OET device composed of two photoconductive layers. (c) A single-sided OET device composed of interdigitated electrodes, on which a photoconductive layer is deposited, on a single plate substrate. | ||
Recently, different types of photoconductive layers have also been utilized for the optoelectrofluidic device—single-crystalline bipolar junction transistor (BJT),30 bulk-heterojunction (BHJ) polymer,31 and dye-sensitized solar cells (DSSC).32 When compared to the typical a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H-based OET device, the photoconductivity of the BJT type was about 500-fold higher and it was applicable to high-conductivity media (> 1 S m−1). However, the fabrication process for the BJT type device was much more complicated than the conventional one. In the case of the BHJ polymer, it is advantageous that the cost and the temperature for manufacturing are relatively low. However, it requires complicated chemical processes as well as is very sensitive to water and oxygen, which may collapse the polymer layer. The DSSC type provides simple and rapid fabrication processes (< 40 min), but its long-term stability problem still remained as one of challenging issues.
H-based OET device, the photoconductivity of the BJT type was about 500-fold higher and it was applicable to high-conductivity media (> 1 S m−1). However, the fabrication process for the BJT type device was much more complicated than the conventional one. In the case of the BHJ polymer, it is advantageous that the cost and the temperature for manufacturing are relatively low. However, it requires complicated chemical processes as well as is very sensitive to water and oxygen, which may collapse the polymer layer. The DSSC type provides simple and rapid fabrication processes (< 40 min), but its long-term stability problem still remained as one of challenging issues.
The type of photoconductive layer can affect the configuration of whole optical system as well. There are two types of optical system for optoelectrofluidic platforms—transmissive and reflective. In the transmissive system, an illumination for the manipulation is located on the opposite position to an objective lens for observation. On the other hand, an illumination for manipulation is projected through an objective lens for observation in the reflective system. In the case of the device based on a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H and ITO, for example, both transmissive and reflective optical systems are applicable, while only the reflective system can be utilized for the phototransistor-based device fabricated on a silicon substrate.
H and ITO, for example, both transmissive and reflective optical systems are applicable, while only the reflective system can be utilized for the phototransistor-based device fabricated on a silicon substrate.
The display device is also one of the important components in the optoelectrofluidic platform. A photomask with a fixed pattern,14 a diaphragm,33 or only a focused laser spot26,34 has also been applied for projecting a light onto a partial area of the photoconductive layer. A display device, however, is necessary for programmable manipulation of the light-activated virtual electrodes. There are three types of display device, which have been used for operating an OET device (Table 1): (i) a digital micro-mirror device (DMD);17 (ii) a beam projector;24 and (iii) a liquid crystal display (LCD).22,23 The OET platforms based on a DMD and a beam projector always require well-aligned and relatively complicated optical setup for generating and focusing a light pattern, limiting the system integration for user-friendly and portable applications. In an LCD-based optoelectrofluidic platform, called lab-on-a-display, a light pattern generated from an LCD is directly transferred onto an OET device without any optical components between an LCD and an OET device.22 This LCD-based OET offers the simplest structure and the largest manipulation area among the previously-reported optoelectrofluidic platforms. In addition, the lab-on-a-display platform is very thin and tolerant to vibrations due to the elimination of lens and optical alignment, providing more suitable form for portable applications. The lens-less structure, however, causes a blurred image due to diffraction of light, limiting the minimum size of virtual electrodes and the performance of particle manipulation. To overcome those limitations of each platform, Hwang et al. proposed a lens-integrated type of an LCD-based OET system.23 In this system, an LCD module is installed on an illumination of a conventional microscope. A condenser lens, which is integrated in the microscope, focuses a light pattern from the LCD module onto the photoconductive layer of an OET device on the microscope stage. This platform based on a conventional microscope provides much simpler and easier way to practically use OET in a laboratory for chemical and biological research than a DMD- and a projector-based platforms, as well as much higher manipulation performances than a lens-less lab-on-a-display platform.
| Display device | Year of the first demonstration | Optical setup | Portability | Manipulation performance | Minimum pixel size/μm | Ref. |
|---|---|---|---|---|---|---|
| DMD | 2005 | Complicated | No | Excellent | 1.52 | Chiou et al.17 |
| Projector | 2005 | Complicated | No | Excellent | 5–35 | Lu et al.24 |
| LCD | 2007 | No | Yes | Bad | 200 | Choi et al.22 |
| LCD | 2008 | Conventional microscope (One condenser lens) | Yes | Excellent | 1–2.8 | Hwang et al.23 |
The OET platform requires a light source, whose intensity is much lower than that for typical optical tweezers system.17 Therefore, we do not have to seriously care about photonic and thermal damages of biochemical samples. Practically, however, a light source is closely connected with the optical components in a whole system and a display device. When a laser source is applied, numerous optical components are required to project, to spatially modulate, and to focus a light pattern onto the photoconductive surface whatever a display device is used.26,34–36 On the other hand, in the case of a conventional light source such as a halogen lamp, optical components are not always required or not so complicated if an LCD is utilized as a display device.22,23 This simple configuration provides higher flexibility on the optoelectrofluidic system according to the target application.
2.3. Physical phenomena in an optoelectrofluidic device
The main driving force for particle manipulation in an optoelectrofluidic device includes conventional electrokinetic mechanisms such as electrophoresis, dielectrophoresis (DEP), ac electroosmosis (ACEO), ET effect, and electro-orientation, which are induced by an optical method. In addition, the electrostatic interactions due to the polarization of dielectric particles could also be observed.Electrophoresis, the movement of charged objects in an electric field, is originated from the Coulomb force, which is defined by:
| FColumb = qE | (1) |
DEP, one of the most widely applied principles for the optoelectrofluidic manipulation, is the movement of dielectric objects under a non-uniform electric field driven by forces arising from the interaction between an induced electric dipole of the particle and the applied electric field.38 The DEP force acting on a spherical particle is given by:
| FDEP = 2πr3εmRe[fCM]∇|E|2 | (2) |
 | (3) |
The DEP force is proportional to the volume of particle and the square of the electric field gradient. This nature of DEP force limits the rapid manipulation of submicro-/nanoscale particles existing far from the edge of the virtual electrodes. Due to the limitation of DEP, the optically induced ACEO, which is a fluidic motion generated by the motion of ions within the electric double layer due to the tangential electric field, have been applied for rapid concentration of microparticles, nanoparticles and molecules using the optoelectrofluidic device. When an image generated from a display device was projected onto the photoconductive layer, the particles and molecules suspended around the image pattern are rapidly moved to the illuminated area by the optically induced ACEO flow. The particles, which have been staying far away from the virtual electrodes, are also driven by the globally occurred flows. The fluids around the partially illuminated area in the optoelectrofluidic device flow along the surface of the photoconductive layer with a rectified slip velocity defined as:
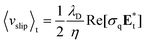 | (4) |
The frequency-dependent phenomena of those two mechanisms, DEP and ACEO, has been applied for rapid and selective concentration of microparticles using an optoelectrofluidic platform.40 At 10 kHz frequency, 1 μm diameter polystyrene beads were concentrated into the illuminated area by ACEO flows, while 6 μm diameter polystyrene beads were repelled from the area due to relatively strong negative DEP forces. As a consequence, the 1 μm beads were simultaneously separated from the mixture as shown in Fig. 3a. At ac frequencies below 1 kHz, both different sized particles were concentrated into the illuminated area due to the hydrodynamic drag forces by ACEO flows, which is much stronger than the DEP forces. At such an extremely low frequency, it has been known that not only the global flows by ACEO around the light pattern, but also local induced-charge electroosmosis along the surface of particles,41 Faradaically-coupled electroosmosis beneath the particles,42 and the electrostatic particle–particle interactions43 significantly affect the behavior of particles in concert within the illuminated area, where a stagnation region is formed by converging ACEO flows.44 Those mechanisms have been utilized for two-dimensional (2D) patterning of 3 μm diameter polystyrene microparticles with a certain distance among them, which is tunable by adjusting the applied ac frequency as shown in Fig. 3b. At 100 Hz, the forces, which assemble and closely pack the particles, acting on the 6 μm beads, became larger than that acting on the 1 μm beads. As a result, only the 6 μm beads were concentrated and closely packed within the illuminated area, and the 1 μm beads were pushed out from the area and swept away by the vortices around there as shown in the right panel of Fig. 3c.
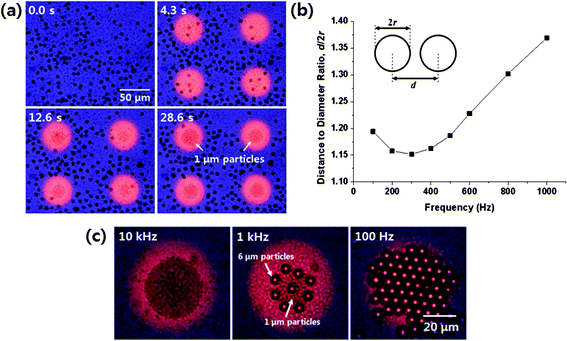 | ||
| Fig. 3 Frequency-dependent phenomena in an optoelectrofluidic platform. (a) Optoelectrofluidic simultaneous separation of microparticles using DEP and ACEO at 10 kHz (Hwang and Park40—reproduced by permission of The Royal Society of Chemistry). (b) Change of distances among 3 μm diameter microparticles concentrated within the illuminated area (adapted with permission from Hwang et al.44 Copyright 2009 American Chemical Society). (c) Microscopic pictures of the 1 μm and 6 μm particles patterned within the illuminated area at the frequency conditions of 10 kHz (left), 1 kHz (center) and 100 Hz (right) (Hwang and Park40—reproduced by permission of The Royal Society of Chemistry). | ||
The electrostatic interactions generated by induced dipole of dielectric particles also affect the particle behaviour in an optoelectrofluidic device.43 The electrostatic force among the polarized particles can be governed by:45
 | (5) |
The ET effect, which is due to a temperature gradient created by a strong light source, has also been observable in the optoelectrofluidic device at high optical power intensities. The thermal gradient in the fluid results in a gradient in the fluid permittivity and conductivity, thus a fluidic motion is induced by a body force due to an electric field, which is defined by:46
 | (6) |
 | ||
| Fig. 4 Patterning of colloidal particles using optically induced electrothermal (ET) effects. The temperature gradients in a fluid for ET vortices can be induced (a) by a highly focused laser between two parallel plate electrodes (adapted with permission from Kumar et al.49 Copyright 2010 American Chemical Society) or (b) by Joule heating in an OET device (adapted with permission from Jamshidi et al.36 Copyright 2009 American Chemical Society). | ||
When non-spherical particles immersed in a solution of different permittivity are exposed to a uniform electric field, they are aligned along the direction of electric field with a torque defined as:51
 | (7) |
 | ||
| Fig. 5 Electro-orientation in an optoelectrofluidic device. (a) Red blood cells and (b) nanowires (adapted by permission from Jamshidi et al.35 Copyright Macmillan Publishers Ltd 2008). | ||
Non-electrical force, which induces non-specific particle adhesion on the device surface, has also been observed. Such the surface-particle interaction could be promoted by influences of gravity, electrostatic attraction, vertical component of electrokinetic force such as DEP, and surface property of target particle. For reducing those effects, several approaches have been demonstrated: (i) 3D focusing using two photoconductive layers;28 (ii) cancellation of gravity force by pulling-up DEP force;53 and (iii) chemical treatment of device surface.54 The method, which is based on two photoconductive layers, makes it available to completely prevent the particle-surface interactions by focusing particles in 3D, and changing number or direction of the photoconductive layer is relatively simple and does not require additional processes for chemical treatment. However, the opaque photoconductive layer may interfere with the optical observation of the samples or require some modifications of optical pathways.
3. Optoelectrofluidic manipulation
Manipulation of many kinds of materials including both biological and non-biological things has been demonstrated using optoelectrofluidics since it had appeared. Some typical cases are summarized in Table 2.| Type | Target | Platform | Light source/pattern generator | Manipulation principles | Function | Ref. |
|---|---|---|---|---|---|---|
| Cells | Blood cells | Optoelectronic tweezers (OET) | Microscope illumination/liquid crystal display (LCD) | Dielectrophoresis (DEP) | Interactive manipulation | Hwang et al.23 |
| Laser/spot or digital micro-mirror device (DMD) | DEP | Concentration | Ohta et al.55 | |||
| HeLa cells and Jurkat cells | OET | Laser/DMD | DEP | Separation | Ohta et al.29 | |
| HepG2 cells | OET | Mercury lamp/DMD | DEP | Patterning | Yang et al.32 | |
| Porcine oocytes | Inverted OET | Microscope illumination/LCD | DEP | Separation | Hwang et al.53 | |
| Tetrahymena Pyriformis | Grayscale OET | Microscope illumination/LCD | Electro-orientation | Trapping | Choi et al.52 | |
| Molecules | DNA | IR-induced electrothermal (ET) vortices | Laser/spot | ET flow | Stretching | Nakano et al.13 |
| OET | Laser/spot | DEP | Concentration | Hoeb et al.26 | ||
| Microscope illumination/diaphragm | Ac electroosmosis (ACEO) | Concentration | Chiou et al.62 | |||
| DNA-attached microbeads | OET | Projector | DEP | Stretching | Lin et al.60 | |
| Dextrans, Bovine serum albumin, Fluorecein, and Bisbenzimide | OET | Microscope illumination/diaphragm | DEP, ACEO, electrostatic interactions | Concentration, dispersion | Hwang and Park33 | |
| Non-biological things | Polystyrene beads | OET | Microscope illumination/LCD | DEP, ACEO, electrostatic interactions | Separation | Hwang and Park40 |
| ACEO, electrostatic interactions | Concentration, patterning | Hwang et al.44 | ||||
| IR-induced ET vortices | Laser/spot | ET flow | Concentration, patterning | Williams et al.48 Williams et al.63 | ||
| Separation | Williams et al.84 | |||||
| Quantum dots | OET | Microscope illumination or laser/diaphragm | ACEO | Concentration | Chiou et al.62 | |
| Metal nanoparticles | OET | Laser/spot or projector | DEP, ET flow | Concentration, Patterning | Jamshidi et al.36 | |
| Nanowires | OET | Laser/DMD | DEP | Trapping, separation | Jamshidi et al.35 | |
| Carbon nanotubes | OET | Projector | DEP | Trapping, separation | Lee et al.64 | |
| Laser/spot | DEP | Trapping | Pauzauskie et al.65 | |||
| InGaAsP microdisk | Single-sided OET | Projector | DEP | Positioning | Tien et al.66 | |
| Two-phase systems | Water-in-oil | Channel-integrated OET | Microscope illumination/LCD | DEP | Generation, transporting, merging | Lee et al.67 |
| Floating-electrode OET | Projector | DEP | Transporting, merging | Park et al.70 | ||
| Projector or LCD | Electrowetting | Transporting, merging | Park et al.78 | |||
| Water-in-air | OET | Laser/scanning mirror | Electrowetting | Transporting | Chiou et al.75 | |
| Single-sided OET | Laser/spot | Electrowetting | Transporting, merging | Chuang et al.77 | ||
| Oil-in-water | OET | Projector | DEP | Separation | Hung et al.68 |
3.1. Biological materials: cells and molecules
Optoelectrofluidic technologies have been applied to manipulate several types of biological materials such as cells and molecules. Since most cells show positive DEP motion in low-conductivity media, they are trapped within and moved along dynamic image patterns in an optoelectrofluidic device. Based on this phenomenon, the trapping and manipulation of blood cells,23,55 HeLa cells,29,34,54 and HepG2 cells32 have been demonstrated using a programmable image and an OET device (Fig. 6a) | ||
| Fig. 6 Optoelectrofluidic trapping and manipulation of cells. (a) Jurkat cells and HeLa cells were manipulated using a conventional optoelectronic tweezers (OET) device (adapted with permission from Ohta et al.29 Copyright 2007 IEEE). (b) Heavy and sticky cells such as oocytes were manipulated using a pulling-up dielectrophoretic force in an OET device turned upside down (adapted with permission from Hwang et al.53 Copyright 2009, American Institute of Physics). (c) Swimming bacteria, which have an ellipsoidal structure, were trapped by optically induced electro-orientation (adapted with permission from Choi et al.52 Copyright 2008, American Institute of Physics). | ||
The DEP characteristics of cells can be utilized as a criterion for judging the state of the cells—death,56 toxicants treatment,57fertilization,58 or health.59 On the basis of this knowledge, an OET device has been applied for automated selection of normal oocytes for in vitrofertilization in a non-contact manner.53 In this study, the heavy and sticky cells could be effectively manipulated by applying pulling-up DEP force induced by an LCD image and an OET device turned upside down—the photoconductive layer was on the upper position—as shown in Fig. 6b.
Not only the non-motile cells such as blood cells and oocytes, but also motile bacteria have been trapped using optically controlled electro-orientation (Fig. 6c).52 The high-motility of the ciliates interferes with effective trapping and manipulation of them using optically induced DEP. In this study, therefore, a different electrokinetic mechanism, electro-orientation, not for trapping swimming bacteria by force, but for changing their moving direction, has been applied.
The manipulation of DNA molecules using optically induced DEP in an OET device has also been demonstrated as shown in Fig. 7a.26 Biological molecules generally show positive DEP motions in low-conductivity media like cells, thus they were attracted toward a light pattern projected onto the photoconductive layer. However, rapid and effective manipulation of individual molecules using DEP is more difficult than that of cells because of their tiny volume and dominant thermal motion. To overcome this limitation, a microbead, on which DNA molecules were immobilized, has been applied to control elongation and rotation of a single DNA molecule using OET as shown in Fig. 7b.60 The stretching of a DNA molecule has also been demonstrated by applying laser-induced ET vortices.61 In addition, ACEO vortices induced by a partial illumination of the photoconductive layer in an OET device has been applied to concentrate DNA molecules.62
 | ||
| Fig. 7 Optoelectrofluidic manipulation of molecules. (a) Concentration of DNA toward a laser spot (adapted from Hoeb et al.26 Copyright 2007, with permission from Elsevier). (b) Elongation of DNA bounded onto a polymer microbead using a programmed image (adapted with permission from Lin et al.60 Copyright 2009, Optical Society of America). (c) Temporal and (d) spatial control of local chemical concentration of FITC-dextran molecules in a solution using an optoelectrofluidic fluorescence microscopy (adapted with permission from Hwang and Park,33 Copyright 2009 American Chemical Society). | ||
The optically induced ACEO flow has been applied to concentrate proteins and polysaccharides as well.33 In that report, not only the concentration, but also the control of local chemical concentration in a solution droplet have been demonstrated using a conventional fluorescence microscope. Rapid switching and precise control of chemical concentration within an illuminated area has been possible based on the combination of frequency-dependent electrokinetic mechanisms such as ACEO, electrostatic interactions, and DEP (Fig. 7c). Spatial control of local molecular concentration in a fluid was also possible by controlling a light pattern as shown in Fig. 7d.
3.2. Non-biological materials
Many types of non-biological materials have also been manipulated using an optoelectrofluidic device. Polymer microbeads, which are well-understood, cheap, and easy to prepare, have been frequently applied for both understanding fundamental principles occur in optoelectrofluidic devices and testing device performance. In parallel plate ITO electrodes, concentration and patterning of polymer micro/nanoparticles have been demonstrated using laser-induced vortices.48,49,63 In an OET device, structures of 2D crystals composed of polymer microbeads have been controlled using optoelectrofluidic mechanisms occur at the ac frequencies below 1 kHz and image patterns generated from a conventional beam projector.44 Image-driven concentration of quantum dots62 and metal nanoparticles36 have also been demonstrated using optically induced ACEO and ET flows in an OET device, respectively. Trapping and separation of nanowires35 and carbon nanotubes64,65 have been performed using an optically induced DEP force. Microdisk lasers, which were released from a substrate after the fabrication, have been reassembled on a silicon substrate using a single-sided OET device.663.3. Two-phase systems
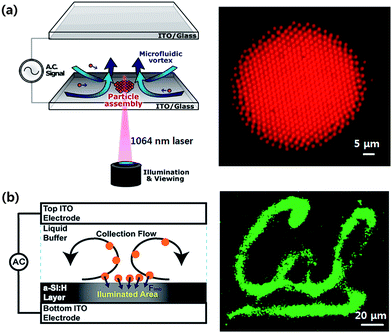
Liquid phase has also been manipulated using an optoelectrofluidic device. Both water-in-oil67 and oil-in-water68 droplets could be manipulated using optically induced DEP in an OET device. Microfluidic channels have also been integrated using a soft-lithographic method for continuous generation and manipulation of picolitre volume water-in-oil emulsions as shown in Fig. 8a.67 | ||
| Fig. 8 Optoelectrofluidic manipulation of liquid droplets. (a) Continuous generation and manipulation of picolitre volume droplets in a channel-integrated OET device (adapted with permission from Lee et al.67 Copyright 2009, American Institute of Physics). (b) Floating electrode OET device for light-induced dielectrophoretic droplet manipulation (adapted with permission from Park et al.69 Copyright 2008, American Institute of Physics). (c) Optoelectrowetting-based droplet manipulation in an open environment (adapted with permission from Chuang et al.77 Copyright 2008, American Institute of Physics). | ||
For droplet manipulation, devices of which configurations are different from that of the conventional OET devices for particle manipulation have also been developed. For example, a floating electrode OET (FEOET) device, in which a lateral electric field formed by two separated electrodes on a photoconductive layer is perturbed by an illumination, has been reported for DEP-based droplet manipulation as shown in Fig. 8b.69 Although the FEOET platform requires high-power electrical source above several hundreds of volts for operation, it provides a flexible interface with other microfluidic components such as tubing, microwell arrays and closed channels as well as an image-controlled parallel processing for transportation, merging, mixing, and sorting nanolitre volume droplets.70
A different mechanism, electrowetting, has also been applied for droplet manipulation. Electrowetting is a phenomenon that an interfacial tension of small volumes of liquid is altered by an electric field and thus the contact angle is changed or the bulk liquid motion is appeared.71 This mechanism has been applied to parallel manipulation of droplets using a microelectrode array for several fields of biology and chemistry.72–74 In an OET device, the virtual electrodes for electrowetting of liquid droplets can be generated by partial illumination of the photoconductive layer either. The technology, which is called optoelectrowetting, has been applied for manipulation of picolitre-volume droplets with a light.75,76
For the electrowetting-based droplet manipulation in open environments, single-sided OET (Fig. 8c)77 and FEOET78 devices have been applied. Those open-optoelectrowetting systems have configurations similar to each other, in which the photoconductive layer is deposited on or beneath the separated electrodes. Here the voltage conditions for operation depend on the thickness of the photoconductive layer and the gap between the electrodes. The open-optoelectrowetting technologies are very useful for sample injection and collection and for integration with other fluidic components.
4. Integration issues
The optoelectrofluidic platforms are simple and flexible as well as programmable. However, integration of other components—fluidic or optical—is sometimes required for allowing more flexibility, higher performance, and more complicated processes. The OET basically requires no fluidic components such as tubing or pumps. This simple structure, however, sometimes makes trouble in performing complicated processes, which require injection or collection of multiple samples, change of buffer media, and continuous sample processing.Integration of microfluidic channels into an OET device has allowed it to perform such the complicated processes. For example, continuous sorting of microbeads,27,79 and in situ generation and manipulation of water droplets67 have been demonstrated with an OET device, into which microfluidic channels made from photoresist and polydimethylsiloxane (PDMS), respectively, were integrated.
In addition, integration of optical components such as optical fibers, lenses, and mirrors is also required for in situ measurement of experimental results or higher manipulation performances. Optical fibers have been integrated into an OET device for continuous counting of microbeads27 and cells80 as shown in Fig. 9a.
 | ||
| Fig. 9 Integration of OET device with other components. (a) Integration of optical fibers for continuous counting of particles (adapted from Lin and Lee,27 Copyright 2008, with permission from Elsevier). (b) Integration with electrowetting device for independent manipulation of droplets and suspended particles (Shah et al.81—reproduced by permission of The Royal Society of Chemistry). | ||
In the case of the single-sided OET device, it is easy to integrate other components because of its simple and open configuration. A device for electrowetting-based droplet manipulation has been integrated into an single-sided OET device as shown in Fig. 9b.81 In this system, microbeads were manipulated using OET, and droplets, which contain the microbeads, were manipulated by controlling an electrode array. Simultaneous manipulation of microparticles and droplets containing them has also been demonstrated on an open-optoelectrowetting device using two laser sources; one for manipulating droplets based on optoelectrowetting and the other for concentrating microparticles based on optoelectrothermal effect.82 These hybrid systems could make it possible to perform more complicated chemical and biological processes, which require several types of buffer media.
5. Applications in chemistry and biology
Based on the optoelectrofluidic manipulation of various materials, numerous potential applications have been suggested in several fields including chemistry and biology. Some typical examples, which show specific applications of the optoelectrofluidic technologies in chemistry and biology, are summarized in Table 3.| Types of application | Specific application | Scheme | Light source/pattern generator | Typical operating principles | Target | Ref. |
|---|---|---|---|---|---|---|
| Manipulation | Particle patterning | UV-induced electrokinetics | UV/photomask | Electrophoresis | Polymer particles | Hayward et al.14 |
| IR-induced electrothermal vortices | Laser/spot | Electrothermal (ET) flow | Polymer particles | Williams et al.48,63 | ||
| Electrothermal vortices in optoelectronic tweezers (OET) | Laser/spot or Projector | Dielectrophoresis (DEP), ET flow | Metal nanoparticles | Jamshidi et al.36 | ||
| Combination of electrokinetic phenomena in OET | Projector | DEP, ac electroosmosis (ACEO), electrostatic interactions | Polymer particles | Hwang et al.44 | ||
| Negative DEP in OET | Halogen lamp/DMD | DEP | HepG2 cells | Yang et al.32 | ||
| Particle sorting | Combination of electrokinetics with IR-induced ET flow | Laser/spot | ET flow, ACEO, electrostatic interactions | Polymer particles | Williams et al.84 | |
| Combination of electrokinetics in OET | Halogen lamp/liquid crystal display (LCD) | DEP, ACEO, electrostatic interactions | Polymer particles | Hwang and Park40 | ||
| Continuous sorting with flows in fiber-integrated OET | Projector | DEP | Polymer particles | Lin and Lee27 | ||
| Light-scanning in OET | Projector | DEP | Polymer particles | Lin et al.85 | ||
| Laser/digital micro-mirror device (DMD) | DEP | HeLa and Jurkat cells | Ohta et al.29 | |||
| Halogen lamp/LCD | DEP | Porcine oocytes | Hwang et al.53 | |||
| Electric stimulation | Cell electroporation | Local enhancement of electric field in OET | Projector | — | HeLa cells | Valley et al.90 |
| Cell lysis | Local enhancement of electric field in OET | Projector | — | Skin fibroblasts | Lin and Lee80 | |
| Measurement and analysis | Diffusion analysis | Local molecular depletion in OET at low ac frequency | Halogen lamp/diaphragm | ACEO, electrostatic interactions | Dextran molecules | Hwang and Park93 |
Optoelectrofluidics is a technology originating on the basis of the manipulation of micro-scale objects. Patterning or sorting of microbeads, which can be a model of biological cells, are basic potential applications, which can be most easily and simply embodied with such manipulation technologies. For patterning microbeads, 2D colloidal assembly has been demonstrated using several optoelectrofluidic techniques such as UV-induced electrokinetics on ITO,14 optically induced ET vortices (Fig. 4a and 4b),48,63 and frequency-dependent optoelectrofluidic mechanisms in an OET device.44 Recently, optoelectrofluidic patterning of HepG2 cells has been demonstrated.32 However, those patterning techniques have never been applied for practical biological applications such as cell-based assays or tissue engineering. Even if cellular patterning can be done soon, a way for using the cellular pattern on an OET device or on an electrode to improve traditional methods for biologists should be found for practical usages of these technologies. This issue is a challenge not only for the optoelectrofluidic techniques, but also for the other conventional electrokinetic patterning methods.83
Several separation skills using an optoelectrofluidic platform have also been reported: (1) continuous separation based on optically induced DEP with pressure-driven flows in a microfluidic channel (Fig. 9a);27 (2) simultaneous separation and concentration using frequency-dependency of optically induced electrokinetics and electrostatic interactions in a static droplet (Fig. 3a);40,84 and (3) separation based on optically induced DEP using a light-scanning method without flows in a static droplet (Fig. 6a).29,53,55,85 Among these separation techniques, the second and the third ones have unique advantages: only a tiny droplet of sample is necessary; no fluidic components are required; all processes are automatically controllable with a display device. However, separation performances such as throughput, purity, and resolution have never been investigated and compared to conventional technologies such as fluorescence-activated,86 magnetic-activated,87 and DEP-activated88cell sorters. Collection of the separated samples with high recovery is also one of the challenging problems.
In an OET device, an electric field distribution can be controlled by an illumination and a voltage source. When a strong light is focused to a cell in an OET device, a dense electric field is formed around the cell, thus the cell membrane becomes destabilized.89 Based on this technique, selective electroporation of cells has been possible as shown in Fig. 10a.90 Control of electrical cell lysis has also been demonstrated by adjusting the applied voltage and the light intensity.80 This optoelectrical stimulation of cell membrane, optoelectroporation, can be applied to single cell-based studies about drug or gene delivery. However, more studies should follow to investigate how more useful these techniques on the basis of virtual electrodes is in practice, when compared to conventional methods based on microelectrodes91 or microfluidic devices.92
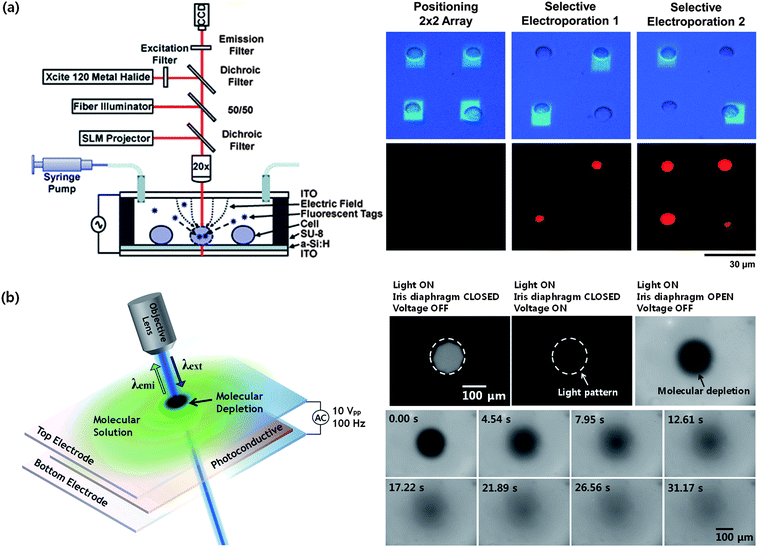 | ||
| Fig. 10 Potential applications of optoelectrofluidics. (a) Optically controlled selective electroporation of HeLa cells for propidium iodide uptake (Valley et al.90–reproduced by permission of The Royal Society of Chemistry). (b) Measurement of molecular diffusion coefficient using fluorescence recovery after optoelectrofluidic local molecular depletion at 100 Hz ac frequency (adapted with permission from Hwang and Park.93 Copyright 2009 American Chemical Society). | ||
Recently, the first analytical tool based on the optoelectrofluidics has been developed.93 In the report, an optoelectrofluidic platform was applied to measure molecular mobility in a fluid. After rapid molecular depletion within a localized region by optoelectrofluidic phenomena at extremely low ac frequency around a few hundred Hz, the recovery of fluorescence signal due to the molecular diffusion was measured according to the time (Fig. 10b). This measurement scheme is very similar to the conventional technique called fluorescence recovery after photobleaching (FRAP).94 Compared to other optical methods such as FRAP95,96 and fluorescence correlation spectroscopy,97,98 this optoelectrofluidic technique, which requires no high-power lasers, no high-speed camera, no photobleaching, no fluidic components, and a few optical components, provides an easier and simpler way to measure the molecular diffusion coefficient in solution. In addition, tuning of optimal operation range for more accurate measurement is easily possible by controlling the light pattern. However, the optoelectrofluidic method has also disadvantages compared to the other optical techniques: it always requires an electrical source and is not applicable in vivo.
6. Challenges and future perspectives
Since the appearance of OET in 2005, the optoelectrofluidic technologies have attracted significant press and interest. However, most of the previous studies have been focused on manipulating different materials using different optoelectrofluidic mechanisms compared to those reported by other groups. These studies are very meaningful in the point of view that the versatile applicability and flexibility of optoelectrofluidics have been successfully proven. However, such an approach, in which only the potentials and rosy expectations are leaved without demonstration of their real benefits or practical applications, may push the technology to its limit and make it quickly become unfashionable. According to Gartner's hype cycle, most technologies enter to the trough of disillusionment, during which the technology rapidly becomes unfashionable and the press abandons the topic, before reaching the plateau of productivity, during which its benefits become widely demonstrated and accepted.99 We would like to assert that the optoelectrofluidic technology is also going into the trough of disillusionment or already in that phase. To jump out the trough and to rapidly climb the slope of enlightenment up towards the plateau of productivity, more improved approaches for overcoming some challenges of the current state of the art or for showing its benefits and practical applications should be carried out.Here several big challenges of optoelectrofluidics for making it become simpler and more practical will be discussed. Firstly, a more complete theoretical model should be developed for constructing reliable and predictable systems. Some electrokinetic phenomena such as DEP, ACEO, and ET flows, which are induced by optical methods, have been well-characterized for the case of when each mechanism occurs alone or much more significantly than others. Those mechanisms, however, always occur in conjunction with others. Thus, more complex behaviour of particles and fluids are shown in practice according to the properties of the applied ac signal and the optical source. In addition, there are optical phenomena ought to be considered in addition to the electrokinetic mechanisms differently from the conventional electrokinetic microfluidic devices—i.e., optical-to-electrical or optical-to-thermal energy transfers, light absorption, reflection, and refraction, etc. The optics may make the system more complex than the conventional electrokinetic devices based on the patterned microelectrodes, in return for providing the programmability.
Secondly, new materials and schemes should be developed and utilized for higher value to cost ratio of the platform. The performance of OET is basically dependent on the media conductivity. For salty media such as blood plasma or cell culture media, the photoconductivity should be much higher than a-Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H, which has been utilized for the conventional OET device. This problem can limit the practical application of this optoelectrofluidic platform into chemistry and biology. The phototransistor-based OET device has been developed for increasing the photoconductivity of the device and applied to manipulate cells in a high-conductivity physiological buffer.30 However, the fabrication process of this device is very complicated and requires relatively high manufacturing cost. For lowering the cost and simplifying the fabrication, polymer-based OET devices have also been developed,31,32 but the photoconductivity and the wet durability are their problems awaiting solution. On the other hand, the optically induced ET vortex system does not require such a special photoconductive material like OET, but require much stronger light source for increasing local temperature of the liquid. In the case of UV-based method, the change of surface conductivity due to the UV exposure has been demonstrated only for ITO electrode. For more efficient and simpler platform, another approach such as the combination of conventional weak light source and metal electrodes is still required.
H, which has been utilized for the conventional OET device. This problem can limit the practical application of this optoelectrofluidic platform into chemistry and biology. The phototransistor-based OET device has been developed for increasing the photoconductivity of the device and applied to manipulate cells in a high-conductivity physiological buffer.30 However, the fabrication process of this device is very complicated and requires relatively high manufacturing cost. For lowering the cost and simplifying the fabrication, polymer-based OET devices have also been developed,31,32 but the photoconductivity and the wet durability are their problems awaiting solution. On the other hand, the optically induced ET vortex system does not require such a special photoconductive material like OET, but require much stronger light source for increasing local temperature of the liquid. In the case of UV-based method, the change of surface conductivity due to the UV exposure has been demonstrated only for ITO electrode. For more efficient and simpler platform, another approach such as the combination of conventional weak light source and metal electrodes is still required.
Thirdly, integration with other components such as sensors or fluidic channels should be considered. The optoelectrofluidic platform allows us to freely manipulate particles or fluids without patterned electrodes, fluidic channels, and high-power light sources. At the early stage of this technology, this capability seemed to make us omnipotent only with a slide glass-like device and a light. However, it did not take long time for the researchers to find that the manipulation within a part of a liquid droplet is not all, and to perceive the necessity of additional components for detection, collection, and bulk manipulation of the samples. In this regard, some efforts have been done to integrate other components into the optoelectrofluidic system.27,79–81 Another approach in which the optoelectrofluidic mechanisms are applied to the conventional microfluidic systems is also required.
Finally, real benefits and practical application of the optoelectrofluidic technology should be demonstrated. This challenge is the most important one for the technology progress in the future, and all the other challenges should be on the basis of this one. The optoelectrofluidic platforms have been frequently compared with other manipulation technologies such as optical tweezers and electrokinetic devices.100,101 In particular, OET allows us to manipulate more microparticles in larger area with much weaker light source than the optical tweezers, as well as to parallel control the electrokinetic behaviour of the particles in a programmable manner without patterned microelectrode array differently from the electrokinetic devices. However, these advantages of the optoelectrofluidic platforms are comparative. For some perspectives and applications, the optoelectrofluidic platforms cannot act as a complete substitute for other manipulation technologies. Optical tweezers and magnetic tweezers have been applied for measurement and analysis of physical properties of single molecules and their interactions especially in chemical and biological fields because of their high resolution, capability of 3D manipulation, and easiness for accurate force quantification.102 The current optoelectrofluidic platforms, in which complex electrokinetic mechanisms occurs in concert and only 2D manipulation with micrometre resolution is possible, are not applicable for single molecular studies. Recently, an analytical tool based on the optoelectrofluidics has also been reported.93 In the literature, the diffusion coefficient of molecules could be easily and accurately measured using an OET platform. Unfortunately, this is the only practical application of the optoelectrofluidic platforms in analytical science. The researchers have concentrated upon the manipulation-based applications such as patterning or sorting of microparticles using the optoelectrofluidic platforms as shown in Table 3. Among such those applications, however, the continuous sorting of microparticles27 could also be easily realized through the conventional microelectrode systems,88 and the performances of the optoelectrofluidic-based systems are not obviously better than those of the conventional systems. In this case, the optical components for inducing electric fields in the optoelectrofluidic platforms may become surplus compared to the conventional electrokinetic sorting systems, which require only an electrical source. Therefore, the researchers now have to try to find an answer to the question “Where is it really useful?” To answer this question, not only the function-based approach, which starts from finding what we can do with this technology for chemistry and biology, but also the purpose-based approach, which starts with considering what is required and needed for chemists or biologists, will be helpful. For example, automated or interactive separation of fertilizable oocytes without handling outside the incubator is strongly required in the fields of assisted reproductive technologies. The optoelectrofluidic separation technique would be very useful for this purpose.53 A sandwich immunoassay is also essential and powerful technique in chemistry and biology, although it requires repetitive washing steps and long incubation time. Although many microfluidic devices have been reported, they always require troublesome fluidic components, and yield large amount of dead volumes and many disposables.103–105 An optoelectrofluidic immunoassay platform, in which all the processes for sandwich immunoassay are automatically controllable by an animated image pattern in a nanolitre volume droplet without any fluidic components, has been recently proposed to overcome those limitations.106
Most of the newly developed technologies have been faded out as time goes by like a flash in the pan. Whether or not the new technology places itself as a field of study and industry depends on whether or not its real benefits and practical applications have been demonstrated and accepted to the end-users as well as the conference members or the publishers. Optoelectrofluidics has already started to take its place on various fields of study due to the efforts of many researchers. If there are continuous efforts to deal with the challenges of optoelectrofluidic technologies and to achieve its progress in chemistry and biology like this moment, it will promise better and practical applications of the optoelectrofluidics-based lab-on-a-chip systems in several analytical and biomedical fields in near future.
Acknowledgements
This research was supported by the National Research Laboratory (NRL) Program grant (R0A-2008-000-20109-0) and by the Nano/Bio Science and Technology Program grant (2008-00771) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST).References
- T. Vilkner, D. Janasek and A. Manz, Anal. Chem., 2004, 76, 3373–3386 CrossRef CAS.
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244–248 CrossRef.
- T. H. Schulte, R. L. Bardell and B. H. Weigl, Clin. Chim. Acta, 2002, 321, 1–10 CrossRef CAS.
- A. J. Tüdos, G. A. J. Besselink and R. B. M. Schasfoort, Lab Chip, 2001, 1, 83–95 RSC.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS.
- B. H. Weigl, R. L. Bardell and C. R. Cabrera, Adv. Drug Delivery Rev., 2003, 55, 349–377 CrossRef CAS.
- D. Li, Electrokinetics in microfluidics, Academic Press, UK, 2004 Search PubMed.
- E. Verpoorte, Electrophoresis, 2002, 23, 677–712 CrossRef CAS.
- D. Psaltis, S. R. Quake and C. Yang, Nature, 2006, 442, 381–386 CrossRef CAS.
- C. Monat, P. Domachuk and B. J. Eggleton, Nat. Photonics, 2007, 1, 106–114 CrossRef CAS.
- Y. Fainman, L. Lee, D. Psaltis and C. Yang, Optofluidics: Fundamentals, Devices, and Applications, McGraw Hill Professional, US, 2010.
- A. Mizuno, M. Nishioka, Y. Ohno and L. D. Dascalescu, IEEE Trans. Ind. Appl., 1995, 31, 464–468 CrossRef CAS.
- M. Nakano, H. Kurita, J. Komatsu, A. Mizuno and S. Katsura, Appl. Phys. Lett., 2006, 89, 133901 CrossRef.
- R. C. Hayward, D. A. Saville and I. A. Aksay, Nature, 2000, 404, 56–59 CrossRef CAS.
- M. Trau, D. A. Saville and I. A. Aksay, Science, 1996, 272, 706–709 CrossRef CAS.
- J. E. A. M.v. d. Meerakker, E. A. Meulenkap and M. Scholten, J. Appl. Phys., 1993, 74, 3282–3288 CrossRef.
- P. Y. Chiou, A. T. Ohta and M. C. Wu, Nature, 2005, 436, 370–372 CrossRef CAS.
- N. Manaresi, A. Romani, G. Medoro, L. Altomare, A. Leonardi, M. Tartagni and R. Guerrieri, IEEE J. Solid-State Circuits, 2003, 38, 2297–2305 CrossRef.
- H. Lee, Y. Liu, D. Ham and R. M. Westervelt, Lab Chip, 2007, 7, 331–337 RSC.
- D. G. Grier, Nature, 2003, 424, 810–816 CrossRef CAS.
- R. Schwarz, F. Wang and M. Reissner, Appl. Phys. Lett., 1993, 63, 1083–1085 CrossRef CAS.
- W. Choi, S. H. Kim, J. Jang and J.-K. Park, Microfluid. Nanofluid., 2007, 3, 217–225 CrossRef CAS.
- H. Hwang, Y.-J. Choi, W. Choi, S.-H. Kim, J. Jang and J.-K. Park, Electrophoresis, 2008, 29, 1203–1212 CrossRef CAS.
- Y.-s. Lu, Y.-p. Huang, J. Yeh and C. Lee, Opt. Quantum Electron., 2005, 37, 1385–1395 CrossRef.
- J. K. Valley, A. Jamshidi, A. T. Ohta, H. Hsan-Yin and M. C. Wu, J. Microelectromech. Syst., 2008, 17, 342–350 CrossRef CAS.
- M. Hoeb, J. O. Rädler, S. Klein, M. Stutzmann and M. S. Brandt, Biophys. J., 2007, 93, 1032–1038 CrossRef CAS.
- Y.-H. Lin and G.-B. Lee, Biosens. Bioelectron., 2008, 24, 572–578 CrossRef CAS.
- H. Hwang, Y. Oh, J. J. Kim, W. Choi, J.-K. Park, S. H. Kim and J. Jang, Appl. Phys. Lett., 2008, 92, 024108 CrossRef.
- A. T. Ohta, P. Y. Chiou, H. L. Phan, S. W. Sherwood, J. M. Yang, A. N. K. Lau, H. Y. Hsu, A. Jamshidi and M. C. Wu, IEEE J. Sel. Top. Quantum Electron., 2007, 13, 235–243 CrossRef CAS.
- H.-y. Hsu, A. T. Ohta, P.-Y. Chiou, A. Jamshidi, S. L. Neale and M. C. Wu, Lab Chip, 2010, 10, 165–172 RSC.
- W. Wang, Y.-H. Lin, R.-S. Guan, T.-C. Wen, T.-F. Guo and G.-B. Lee, Opt. Express, 2009, 17, 17603–17613 CrossRef CAS.
- S.-M. Yang, T.-M. Yu, H.-P. Huang, M.-Y. Ku, L. Hsu and C.-H. Liu, Opt. Lett., 2010, 35, 1959–1961 Search PubMed.
- H. Hwang and J.-K. Park, Anal. Chem., 2009, 81, 5865–5870 CrossRef CAS.
- S. L. Neale, M. Mazilu, J. I. B. Wilson, K. Dholakia and T. F. Krauss, Opt. Express, 2007, 15, 12619–12626 CrossRef CAS.
- A. Jamshidi, P. J. Pauzauskie, P. J. Schuck, A. T. Ohta, P. Y. Chiou, J. Chou, P. Yang and M. C. Wu, Nat. Photonics, 2008, 2, 86–89 CrossRef.
- A. Jamshidi, S. L. Neale, K. Yu, P. J. Pauzauskie, P. J. Schuck, J. K. Valley, H.-Y. Hsu, A. T. Ohta and M. C. Wu, Nano Lett., 2009, 9, 2921–2925 CrossRef CAS.
- J. N. Mehrishi and J. Bauer, Electrophoresis, 2002, 23, 1984–1994 CrossRef CAS.
- T. B. Jones, Electromechanics of particles, Cambridge University Press, New York, 1995 Search PubMed.
- A. Ramos, H. Morgan, N. G. Green and A. Castellanos, J. Colloid Interface Sci., 1999, 217, 420–422 CrossRef CAS.
- H. Hwang and J.-K. Park, Lab Chip, 2009, 9, 199–206 RSC.
- P. J. Sides, Langmuir, 2003, 19, 2745–2751 CrossRef CAS.
- J. A. Fagan, P. J. Sides and D. C. Prieve, Langmuir, 2006, 22, 9846–9852 CrossRef CAS.
- H. Hwang, J.-J. Kim and J.-K. Park, J. Phys. Chem. B, 2008, 112, 9903–9908 CrossRef CAS.
- H. Hwang, Y.-H. Park and J.-K. Park, Langmuir, 2009, 25, 6010–6014 CrossRef CAS.
- J. Kadaksham, P. Singh and N. Aubry, J. Fluids Eng., 2004, 126, 170–179 CrossRef.
- A. Ramos, H. Morgan, N. G. Green and A. Castellanos, J. Phys. D: Appl. Phys., 1998, 31, 2338–2353 CrossRef CAS.
- A. Mizuno, M. Nishioka, T. Tanizoe and S. Katsura, IEEE Trans. Ind. Appl., 1995, 31, 1452–1457 CrossRef CAS.
- S. J. Williams, A. Kumar and S. T. Wereley, Lab Chip, 2008, 8, 1879–1882 RSC.
- A. Kumar, J.-S. Kwon, S. J. Williams, N. G. Green, N. K. Yip and S. T. Wereley, Langmuir, 2010, 26, 5262–5272 CrossRef CAS.
- A. Kumar, S. J. Williams and S. T. Wereley, Microfluid. Nanofluid., 2009, 6, 637–646 CrossRef CAS.
- T. B. Jones, IEEE Eng. Med. Biol. Mag., 2003, 22, 33–42 CrossRef.
- W. Choi, S.-W. Nam, H. Hwang, S. Park and J.-K. Park, Appl. Phys. Lett., 2008, 93, 143901 CrossRef.
- H. Hwang, D.-H. Lee, W. Choi and J.-K. Park, Biomicrofluidics, 2009, 3, 014103 CrossRef.
- A. N. K. Lau, A. T. Ohta, H. L. Phan, H.-Y. Hsu, A. Jamshidi, P.-Y. Chiou and M. C. Wu, Lab Chip, 2009, 9, 2952–2957 RSC.
- A. T. Ohta, C. Pei-Yu, T. H. Han, J. C. Liao, U. Bhardwaj, E. R. B. McCabe, Y. Fuqu, S. Ren and M. C. Wu, J. Microelectromech. Syst., 2007, 16, 491–499 CrossRef.
- C. Huang, A. Chen, L. Wang, M. Guo and J. Yu, Biomed. Microdevices, 2007, 9, 335–343 CrossRef.
- K. Ratanachoo, P. R. C. Gascoyne and M. Ruchirawat, Biochim. Biophys. Acta, Biomembr., 2002, 1564, 449–458 CrossRef CAS.
- W. M. Arnold, R. K. Schmutzler, S. Al-Hasani, D. Krebs and U. Zimmermann, Biochim. Biophys. Acta, Biomembr., 1989, 979, 142–146 CrossRef CAS.
- W. Choi, J.-S. Kim, D.-H. Lee, K.-K. Lee, D.-B. Koo and J.-K. Park, Biomed. Microdevices, 2008, 10, 337–345 CrossRef.
- Y.-H. Lin, C.-M. Chang and G.-B. Lee, Opt. Express, 2009, 17, 15318–15329 CrossRef CAS.
- M. Nakano, H. Kurita, J. Komatsu, A. Mizuno and S. Katsura, Appl. Phys. Lett., 2006, 89, 133901 CrossRef.
- P.-Y. Chiou, A. T. Ohta, A. Jamshidi, H. Hsin-Yi and M. C. Wu, J. Microelectromech. Syst., 2008, 17, 525–531 CrossRef.
- S. J. Williams, A. Kumar, N. G. Green and S. T. Wereley, Nanoscale, 2009, 1, 133–137 RSC.
- M.-W. Lee, Y.-H. Lin and G.-B. Lee, Microfluid. Nanofluid., 2010, 8, 609–617 CrossRef CAS.
- P. J. Pauzauskie, A. Jamshidi, J. K. Valley, J. H. Satcher and M. C. Wu, Appl. Phys. Lett., 2009, 95, 113104 CrossRef.
- M.-C. Tien, A. T. Ohta, K. Yu, S. L. Neale and M. C. Wu, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 967–972 CrossRef CAS.
- D.-H. Lee, H. Hwang and J.-K. Park, Appl. Phys. Lett., 2009, 95, 164102 CrossRef.
- S.-H. Hung, Y.-H. Lin and G.-B. Lee, J. Micromech. Microeng., 2010, 20, 045026 CrossRef.
- S. Park, C. Pan, T. H. Wu, C. Kloss, S. Kalim, C. E. Callahan, M. Teitell and E. P. Y. Chiou, Appl. Phys. Lett., 2008, 92, 151101 CrossRef.
- S.-Y. Park, S. Kalim, C. Callahan, M. A. Teitell and E. P. Y. Chiou, Lab Chip, 2009, 9, 3228–3235 RSC.
- C. Quilliet and B. Berge, Curr. Opin. Colloid Interface Sci., 2001, 6, 34–39 CrossRef CAS.
- I. Barbulovic-Nad, H. Yang, P. S. Park and A. R. Wheeler, Lab Chip, 2008, 8, 519–526 RSC.
- R. B. Fair, Microfluid. Nanofluid., 2007, 3, 245–281 CrossRef CAS.
- A. R. Wheeler, H. Moon, C. A. Bird, R. R. O. Loo, C.-J. C. J. Kim, J. A. Loo and R. L. Garrell, Anal. Chem., 2005, 77, 534–540 CrossRef CAS.
- P. Y. Chiou, S.-Y. Park and M. C. Wu, Appl. Phys. Lett., 2008, 93, 221110 CrossRef.
- P. Y. Chiou, H. Moon, H. Toshiyoshi, C.-J. Kim and M. C. Wu, Sens. Actuators, A, 2003, 104, 222–228 CrossRef.
- H.-S. Chuang, A. Kumar and S. T. Wereley, Appl. Phys. Lett., 2008, 93, 064104 CrossRef.
- S.-Y. Park, M. A. Teitell and E. P. Y. Chiou, Lab Chip, 2010, 10, 1655 RSC.
- H. Hwang, D.-H. Lee and J.-K. Park, J. Kor. Sensors Soc., 2009, 18, 154–159 Search PubMed.
- Y.-H. Lin and G.-B. Lee, Sens. Actuators, B, 2010, 145, 854–860 CrossRef.
- G. J. Shah, A. T. Ohta, E. P. Y. Chiou, M. C. Wu and C.-J. Kim, Lab Chip, 2009, 9, 1732–1739 RSC.
- A. Kumar, H.-S. Chuang and S. T. Wereley, Langmuir, 2010, 26, 7656–7660 CrossRef CAS.
- R.-Z. Lin, C.-T. Ho, C.-H. Liu and H.-Y. Chang, Biotechnol. J., 2006, 1, 949–957 Search PubMed.
- S. J. Williams, A. Kumar, N. G. Green and S. T. Wereley, J. Micromech. Microeng., 2010, 20, 015022 CrossRef.
- W.-Y. Lin, Y.-H. Lin and G.-B. Lee, Microfluid. Nanofluid., 2010, 8, 217–229 CrossRef CAS.
- A. Y. Fu, C. Spence, A. Scherer, F. H. Arnold and S. R. Quake, Nat. Biotechnol., 1999, 17, 1109–1111 CrossRef CAS.
- J. D. Adams, U. Kim and H. T. Soh, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18165–18170 CrossRef CAS.
- X. Hu, P. H. Bessette, J. Qian, C. D. Meinhart, P. S. Daugherty and H. T. Soh, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15757–15761 CrossRef CAS.
- T. Y. Tsong, Biophys. J., 1991, 60, 297–306 CrossRef CAS.
- J. K. Valley, S. Neale, H. Y. Hsu, A. T. Ohta, A. Jamshidi and M. C. Wu, Lab Chip, 2009, 9, 1714–1720 RSC.
- J. A. Lundqvist, F. Sahlin, M. A. I. Åberg, A. Strömberg, P. S. Eriksson and O. Orwar, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 10356–10360 CrossRef CAS.
- M. Khine, A. Lau, C. Ionescu-Zanetti, J. Seo and L. P. Lee, Lab Chip, 2005, 5, 38–43 RSC.
- H. Hwang and J.-K. Park, Anal. Chem., 2009, 81, 9163–9167 CrossRef CAS.
- D. Axelrod, D. E. Koppel, J. Schlessinger, E. Elson and W. W. Webb, Biophys. J., 1976, 16, 1055–1069 CrossRef CAS.
- K. Braeckmans, L. Peeters, N. N. Sanders, S. C. D. Smedt and J. Demeester, Biophys. J., 2003, 85, 2240–2252 CrossRef CAS.
- J. Braga, J. M. P. Desterro and M. Carmo-Fonseca, Mol. Biol. Cell, 2004, 15, 4749–4760 CrossRef CAS.
- L. E. Elliot and M. Douglas, Biopolymers, 1974, 13, 1–27 CrossRef CAS.
- M. Douglas, L. E. Elliot and W. W. Watt, Biopolymers, 1974, 13, 29–61 CrossRef CAS.
- J. Fenn and M. Raskino, Mastering the Hype Cycle: How to Choose the Right Innovation at the Right Time, Harvard Business Press, 2008 Search PubMed.
- K. Dholakia, Nat. Mater., 2005, 4, 579–580 CrossRef CAS.
- J. A. Rogers, Nat. Photonics, 2008, 2, 69–70 CrossRef CAS.
- K. C. Neuman and A. Nagy, Nat. Methods, 2008, 5, 491–505 CrossRef CAS.
- X. Jiang, J. M. K. Ng, A. D. Stroock, S. K. W. Dertinger and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 5294–5295 CrossRef CAS.
- P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M. R. Tam and B. H. Weigl, Nature, 2006, 442, 412–418 CrossRef CAS.
- R. Fan, O. Vermesh, A. Srivastava, B. K. H. Yen, L. Qin, H. Ahmad, G. A. Kwong, C. C. Liu, J. Gould and L. Hood, Nat. Biotechnol., 2008, 26, 1373–1378 CrossRef CAS.
- H. Hwang, H. Chon, J. Choo and J.-K. Park, Anal. Chem., 2010, 82, 7603–7610 CrossRef CAS.
Footnote |
| † Published as part of a LOC themed issue dedicated to Korean Research. Guest Editors: Professor Je-Kyun Park and Kahp-Yang Suh. |
| This journal is © The Royal Society of Chemistry 2011 |
