A nano-needle/microtubule composite gliding on a kinesin-coated surface for target molecule transport†
Mehmet C.
Tarhan
*a,
Ryuji
Yokokawa
bc,
Céline
Bottier
d,
Dominique
Collard
d and
Hiroyuki
Fujita
a
aCenter for International Research on MicroMechatronics, Institute of Industrial Science, The University of Tokyo, 4-6-1, Komaba, Meguro-ku, Tokyo, 153-8505, Japan. E-mail: mctarhan@iis.u-tokyo.ac.jp; fujita@iis.u-tokyo.ac.jp; Fax: +81 03 5452 6250; Tel: +81 03 5452 6249
bDept. of Microengineering, Kyoto University, Yoshida-honmachi, Sakyo-ku, Kyoto, 606-8501, Japan. E-mail: ryuji@me.kyoto-u.ac.jp; Fax: +81 75 753 3559; Tel: +81 75 753 3559
cPRESTO, JST, 4-1-8, Hon-chou, Kawaguchi, Saitama, 332-0012, Japan
dLaboratory for Integrated Micro Mechatronic Systems/CNRS-IIS, The University of Tokyo, 4-6-1, Komaba, Meguro-ku, Tokyo, 153-8505, Japan. E-mail: celine@iis.u-tokyo.ac.jp; collard@iis.u-tokyo.ac.jp; Fax: +81 03 5452 6250; Tel: +81 03 5452 6249
First published on 10th November 2009
Abstract
An alternative method of micro/nano-transport has been achieved by using motor proteins. Microtubules on a kinesin-coated surface have potential to act as a nano-transport system. When microtubules are used as carriers, either cargo or cargo linkers are attached on the microtubule surface. Such cargo attachments can significantly affect kinesin motion. To deal with the difficulty caused by molecular attachment to the microtubule surface, the cargo loading and transport mechanism should be separated. In this work, we propose to use micromachined needles as cargo carriers which then can be transported on microtubules. Because of the separation of needle functionalization and transport mechanism, functionalization of the needles can proceed without any effect on the microtubule structure, significantly increasing the possible types of cargo. We have fabricated silicon needles in mass numbers using a simple and effective method and have shown that the microtubule–needle composites are transported without affecting the kinesin activity.
Introduction
Studies on motor proteins have expanded the possibilities for the development of alternative micro- and nano-scale fluidic transport systems. In contrast to other systems using pressure-driven fluid flow or electroosmotic flow, direct transport of cargo is possible without any liquid manipulation in motor protein-based devices.1 Such a transport mechanism is carried out in eukaryotic cells by proteins such as kinesin or dynein. Kinesin, a linear motor, provides motion along microtubules, rail-like structures, by hydrolyzing adenosine tri-phosphate (ATP). Microtubules, one of the major components of the cytoskeleton, are composed of polymeric structures assembled from tubulin monomers. These tubular structures are also functionally polarized providing a unidirectional motion for kinesin molecules.To build a molecular transport system based on kinesin and microtubules, motion can be obtained in two different configurations: gliding motility assay,2 where microtubules glide along a kinesin-coated glass surface, or bead motility assay,3 where particles coated with kinesin move along immobilized microtubules. A comparison of the two configurations shows that gliding assay-based transport is widely used when a long transport distance is necessary.4 For a bead assay system, the transport distance is much shorter and depends on the number of kinesin molecules involved in the motion.5 Apart from motion mechanism, several design issues should be considered to build up a practical transport system.
One of the most crucial parts of a transport system is controlling the direction of the motion. In the gliding assay configuration, several methods have attempted to achieve directional control using microfabricated tracks,6–8 fluid flow,9 electric10,11 or magnetic fields.12 All of these methods act on the minus ends of the microtubules for steering. In the bead assay configuration, the direction of motion of the kinesin is decided by microtubule polarity. To control the kinesin-coated particle motion, polarity specific alignment of microtubules must be performed by fluidic flow,13 seed extension14 and/or trapping in nano-channels15 before starting the transport process.
A practical transport system should provide different possibilities for cargo loading. Until now, in gliding assays, microtubules have been used as shuttles to carry microspheres,16 quantum dots,17 DNA18–21 and different biomolecules.22 Moreover, in a bead assay configuration, microspheres,13 quantum dots,23 oil droplets,24 lipid vesicles25 and micromachined structures26–28 have been used either as carriers or cargo. Comparing the variety of possible carriers that can be incorporated into each configuration, the bead assay configuration is superior because of the possibility of using various commercially available products or micromachined structures for selective immobilization and transport of target molecules. Despite somewhat successful efforts to stabilize microtubules with taxol or cross-linking,29 the ease with which microtubules depolymerize30 precludes them from a larger role as carriers due to the difficulty in maintaining carrier length (and thus, binding capacity) throughout the experiment. Moreover, depending on the molecule size, cargo and/or the necessary linker for cargo loading can affect kinesin motility significantly.31,32 Thus, the best strategy should be separating the cargo loading from the transport mechanism in a gliding assay-based system. One way to achieve this separation involves the use of nano/micro-particles for cargo attachment.
Several different nano/micro-particles could be used for cargo attachment. Due to the tubular geometry of microtubules, cylindrical structures might be better suited. For use in bioassays, the cylindrical structures should be obtained in large quantities. Micromachining is a fast and easy way to fabricate large amounts of cylindrical structures. Although micromachined needle-shaped cylindrical structures have been integrated with the F1-ATPase rotary motor protein,33,34 to the best of our knowledge, they have not yet been used with the gliding assay configuration until now.
In this study, we focus on the idea that isolating the cargo attachment from the transport mechanism offers stable and better-characterized carriers while maintaining the long transport distances characteristic of the gliding assay configuration without any structural manipulation of the microtubules. We have fabricated nano-needles and demonstrated a functionalization process. Finally, we have combined the needles with the gliding assay configuration and looked at the effect of attachment on the transport mechanism. A schematic view of this approach can be seen below (Fig. 1) where a microtubule–needle composite is moving along the kinesin-coated glass surface.
 | ||
| Fig. 1 Schematic view of the proposed system (not scaled). Micromachined and functionalized silicon structures were attached to the microtubules to be used as carriers in a gliding assay-based transport system. In the proposed system, transport was performed by kinesin motion along microtubules and cargo capturing was achieved by functionalized silicon needles. | ||
Materials and methods
Proteins
Kinesin 1 (Homo sapiens kinesin family member 5B (KIF5B)) and tubulin purification protocols are explained elsewhere.35–38Tubulin with a concentration of about 4 mg ml−1 was polymerized into microtubules in BRB80 buffer (80 mM PIPES–NaOH pH = 6.9, 1 mM MgCl2, 1 mM EGTA) containing MgSO4 (1 mM) and GTP (1 mM). After incubating at 37 °C for 30 min, microtubules were stabilized with paclitaxel (40 µM) and diluted 100-fold in BRB80 containing paclitaxel (20 µM). Biotinylated microtubules were polymerized by using a mixture of biotinylated and non-biotinylated tubulin (with a ratio of 1 : 3).
Needles
We used a simple but effective fabrication method to mass-produce needles (∼3 × 106 needles cm−2) on a silicon substrate without any lithography process (Fig. 2) as detailed. | ||
| Fig. 2 Schematic view of the fabrication process. (a) Silica beads (0.33 µm in diameter) were spun on a silicon wafer. (b) These beads acted as a mask for isotropic etching of the silicon wafer (DRIE etching). The diameter of the needles was defined by the size of the silica beads and the length of the needles was defined by the number of etching cycles in this step. (c) The notches used for defining the cutting point for the collection of the needles. (d) Gold was sputtered to be used in the functionalization process. | ||
Needles were obtained by micromachining a 3 inch silicon wafer. Silica beads with a diameter of 0.33 µm (Bangs Laboratories, SS02N) were prepared by a 300-fold dilution. First, the surface of the wafer was exposed to oxygen plasma in an RIE chamber (35 W, 5 min). Changing the surface of the wafer to hydrophilic was necessary for deposition by spin coating. The silica bead solution was spun on the wafer with 3000 rpm for 30 s. By spin coating, beads were distributed randomly on the wafer. Then, the silicon wafer was etched in a DRIE chamber (100 W, 8 s etching time and 5 s passivation time, 120–150 cycles). Since randomly distributed beads (Fig. 3a) acted as an etching mask, the diameter of the needles was determined by the size of the beads (Fig. 3b). Similarly, the length of the needles was determined by the number of etching cycles. In order to have an excellent profile without any scalloping effect, the etching steps were kept short. One last etching cycle (100 W, 24 s etching time, 5 s passivation time, 1 cycle) was used to obtain notches (Fig. 3c) at the base of the needles for ease of removal from the wafer. The last step was to coat the surface with 50 nm gold layer by sputtering.
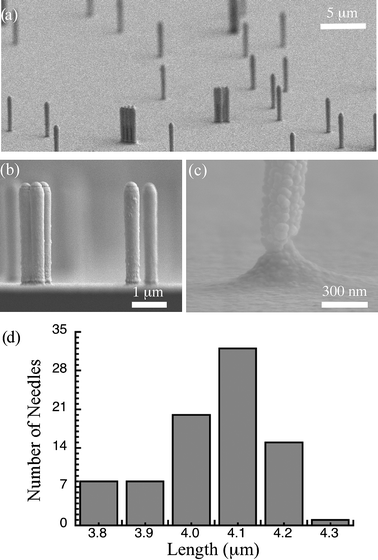 | ||
| Fig. 3 SEM images of the fabricated needles after gold sputtering. (a) A general view of the silicon wafer with fabricated needles. The random characteristics of the needle distribution due to the spin coating of silica beads can be seen clearly. (b) Cross-sectional view of the silicon wafer showing the needle profile. (c) A close-up view of a notch to be used as cutting point for the collection of needles. (d) Size distribution of needles fabricated with 150 etching cycles in DRIE chamber. | ||
Due to the DRIE process, different batches may have slightly different overall needle sizes. The distribution of needle size on a 3 inch wafer with 150 etching cycles is shown in Fig. 3d. The average needle height was measured as 4.05 ± 0.13 µm (n = 85). Needles with an aspect ratio up to 15 were easily fabricated with the detailed process.
Functionalization of needles
Functionalization of the needles is crucial for specific attachment of target molecules to the needles. Depending on the target molecules, needles can be functionalized with the necessary coatings. In this study, for demonstration purposes, we chose a needle coating of streptavidin molecules to capture biotin molecules because of their highly specific mutual affinity.Functionalization was achieved on the gold coating of the needles in three layers: self-assembled monolayer (SAM), biotin layer and streptavidin layer (Fig. 4). Fluorescent streptavidin was used to confirm the coating of the needles. Comparison of the differential interference contrast (DIC) and the fluorescence microscopy views (after the collection of the needles) shows the streptavidin coating of the needles (Fig. 5).
 | ||
| Fig. 4 Schematic view of the functionalization. First step was to form a SAM on the gold coating. Then, biotin–NHS was attached to the SAM. Finally, the functionalization was finished by adding streptavidin molecules to bind to the biotin coating. | ||
 | ||
| Fig. 5 DIC and fluorescence views of the same area in a flow cell. As the only labeled material was streptavidin, DIC and fluorescence matching shows the success of the functionalization process. White bar corresponds to a length of 10 µm. | ||
The gold-coated wafer was incubated in SAM solution (11-amino-undecanethiol hydrochloride, Djondo, 100 mM, in DMSO as stock solution, diluted in ethanol 1000-fold for use) for 60 min. Then, the wafer was washed twice with ethanol and twice with PBSPT solution (150 mM PBS (137 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4) with 0.1% polyethylene glycol (PEG) and 0.1% triton). For biotin attachment, Biotin–NHS solution (Djondo, 100 mM, in DMSO as stock solution, diluted 100-fold in PBSPT solution for use) was used. After 10 min of incubation, the wafer was washed three times with PBSPT solution. Finally, the surface was coated with a streptavidin solution (Bioscience, 0.5 mg ml−1, diluted 50-fold in PBSPT solution for use). After incubating for 10 min, the wafer was washed three times with PBSPT solution.
Collection of needles
To be used in the bioassay, the functionalized needles were removed from the silicon substrate, washed and collected in a suitable buffer for kinesin activity. The notches worked as designed during the removal process as seen in a SEM image of the silicon wafer taken after the breakage (Fig. 6). | ||
| Fig. 6 SEM image taken after ultrasonication showing the remains of the needles. The notches worked perfectly for needle removal, as all the needles were broken from the expected region. | ||
The wafer was placed upside down in a glass beaker and covered with PBSPT solution. An ultrasonic bath (UT-105HS, Sharp, Japan) was used for at least 10 min with full power to break the needles off of the wafer. Then the PBSPT solution (with broken needles inside) was put into a tube and centrifuged at 15 krpm for 10 min. The PBSPT solution inside the tube was replaced with fresh solution and centrifuged again. In order not to affect the kinesin motility with the existing surfactant, the PBSPT solution was removed completely and the collected needles were re-suspended in 100 µl of BRB80 solution (∼109 needles ml−1) to be used in the experiments.
Attachment of microtubules to needles
100-fold diluted microtubule solution was mixed with needle suspension with a ratio of 1 : 1 (v/v) and incubated for 20 min for attachment. For visualization purposes, we have used biotinylated microtubule attachment to needles. Moreover, non-biotinylated microtubule attachment to the needles was also performed.Visualization setup
For visualization of the experiments, an inverted microscope (IX-71, Olympus, Japan) with oil immersion lens (UplanApo 100x/1.35 Oil Iris Ph3, Olympus, Japan) was used with a photometrix camera (Cascade 512II, Photometrics, USA) and imaging software (MetaMorph, Molecular Devices, USA). To visualize the fluorescein label on the streptavidin an Olympus U-WIG2 filter was also used in the fluorescence setup. Differential interference contrast (DIC) microscopy was used for observing non-labeled material.Transport experiments
Transport experiments were performed in a flow cell made from two cover slips (Matsunami, Japan) connected with greased (Apiezon N6697 LA-6) thin paper slices forming a channel of about 15 µl.A kinesin solution (0.9 mg ml−1) was diluted 3-fold in caseinated BRB80 solution (0.7 mg ml−1 of casein in BRB80 solution). The diluted kinesin solution (15 µl) was injected into the flow cell and left for 3 min to allow the kinesin to bind to the surface of the cover slip as the first step. After washing with BRB80 solution (45 µl), the microtubule–needle composite solution (15 µl) was injected and left for 5 min. Finally, ATP solution (1 mM, 45 µl) was added to activate the kinesin molecules resulting in gliding microtubules with attached needles.
Results and discussion
As microtubules have a length of several micrometres, we fabricated silicon needles long enough (around 4 µm in length) to match well with a microtubule, but still short enough to keep the motion undisturbed. The diameter of the needles was kept to approximately 0.30 µm in diameter, about the same size as the diameter of the beads used for similar purposes (with a diameter of 0.20 µm,16 0.56 µm39), for good integration with microtubules (diameter of around 25 nm).Successful transport could be observed in successive photographs taken via DIC microscopy (Fig. 7). Due to the viscous drag caused by the buffer flow shown by the arrow, the direction of motion of the biotinylated microtubule–needle composite became aligned with the flow direction. This result validates the integration of the “fluid flow” method for controlling the direction of motion with the proposed transport system. We believe similar integration is possible using other steering methods based on electric10,11 and magnetic fields.12 In addition, microfabricated tracks6–8 can also be used depending on the curvature of the tracks and/or the dimensions of the needles, which can be changed by adjusting different parameters during the fabrication process.
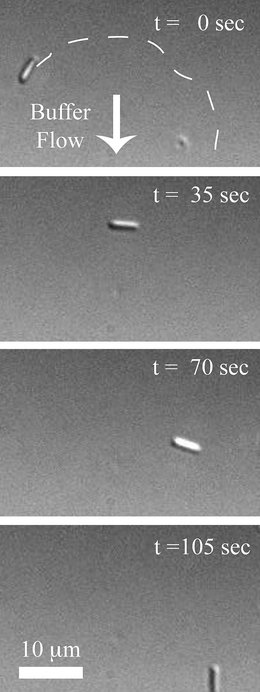 | ||
| Fig. 7 Successive photos taken via DIC microscopy showing the transport process. The approximate route of the microtubule is shown by a white dashed line in the first image. The arrow corresponds to the direction of the buffer flow. Average velocity of the shown needle–biotinylated microtubule composite was 0.35 µm s−1. | ||
Several experiments were performed to characterize the performance of our transport method. The average gliding velocity of the needle–biotinylated microtubules composite was measured to be 0.35 ± 0.07 µm s−1 (mean ± SD) in 43 events. The distribution of the average velocities in different events is shown as a histogram (Fig. 8). The result is in good agreement with data obtained in previous research where the average gliding velocities of biotinylated microtubules (without any fabricated structure attachment) were measured (0.48 ± 0.13 µm s−1,20 0.54 ± 0.14 µm s−1,31 0.37 ± 0.03 µm s−1(ref. 32)). According to the average velocity comparison and considering successful transport distances spanning hundreds of micrometres (310 µm as a recorded maximum), we conclude that integrating needles with microtubules did not have significant effects on the kinesin activity.
 | ||
| Fig. 8 Distribution of the average velocities of the biotinylated microtubule–needle composite in a gliding assay configuration. The average velocity is calculated as 0.35 ± 0.07 µm s−1 (mean ± SD) in 43 events. | ||
Although we bring together the segregated components of our system with the attachment of avidin–biotin, specific binding is not a prerequisite for constructing these composites. With the same procedure detailed above, we have formed the composites through non-specific binding of functionalized needles with non-biotinylated microtubules. An average velocity of 0.36 ± 0.07 µm s−1, similar to the biotinylated microtubule case, was achieved in 12 events. As a control experiment, the average velocity of non-biotinylated microtubules (without integrating with needles) was measured as 0.36 ± 0.04 µm s−1 in 33 events showing a perfect match with the composite case. The freedom from the constraint of specific binding between the two separate components of our composite allows for greater functionalization design possibilities.
Coating the needles with different materials allows capturing of various types of target molecules. As preparation of the needles is completely isolated from that of the microtubules, coating of the carriers could be achieved even under very harsh environments where microtubules could never survive. Integrating carrier needles and microtubules in the last step before the transport process provides complex coatings without affecting microtubule stability. This has potential for a significant increase in the variety of possible target molecules that can be transported.
Using cylindrical carriers such as needles offers better geometrical matching with microtubules and superior visualization and attachment properties when compared to spherical beads. Depending on the fabrication process, thinner needles can be obtained without facing significant visualization or attachment problems. Multiple attachment points to the microtubule provide stronger connections that maintain needle attachment even under fluid flows strong enough to remove microtubules from the surface.
Since controlling the length of microtubules is quite challenging due to the polymerization process and the instability issue, maintaining cargo attachment on microtubules throughout an experimental procedure is an important difficulty that needs to be overcome. Moreover, due to the cargo attachment to microtubules, kinesin motion might be disturbed. It was reported earlier that when the microtubule was coated with streptavidin molecules, gliding velocity along a kinesin-coated surface decreased up to 60% depending on the concentration of the streptavidin coating.31 A similar decrease in velocity was reported when DNA was transported by attachment to microtubules.32 In this method, loading the cargo on the microtubule protofilaments where kinesin can no longer move freely resulted in the reported effect in velocity. The proposed method of using intermediate silicon nano-needles not only provides more stable and uniform carriers for cargo loading but also eliminates the possible disturbance of kinesin motion by preventing any manipulation of the microtubule protofilaments. Separating the cargo loading from the transport mechanism broadens the field of possible applications by overcoming some of the difficulties associated with the simple transport scheme where microtubules are directly used as carriers.
Conclusion
Using microtubules as carriers and attaching intermediate layers of molecules for cargo loading is a choice for constructing a transport system, however, such molecular attachments on microtubules interact with kinesin and cause significant decreases in the gliding velocity. On the other hand, using intermediate carriers separates the cargo loading from the transport mechanism and thus prevents any structural changes to the microtubules that might disturb the kinesin. This separation is a key point for an advanced in vitro gliding assay-based transport system with a large diverse possibility of target molecules. To achieve the proposed system, we have fabricated silicon needles and functionalized them with streptavidin. The easy fabrication process allows mass production of the needles with the desired dimensions providing a tool for bioassay applications. The cylindrical geometry of the needles is well matched with the tubular structure of the microtubules and besides polymerization no further structural manipulation or coating was necessary for the microtubules. The needle–microtubule integration did not have any significant effect in the motility. We believe this strategy will have a significant impact on future devices by enabling the use of complex carriers for various bioengineering applications.Acknowledgements
This research was partially supported by The Ministry of Education, Culture, Sports, Science and Technology, Japan, Grant-in-Aid for Scientific Research (B), 17310082, 2005.We gratefully acknowledge Dr Agnes Tixier-Mita and Dr Edin Sarajlic for their valuable discussion about the fabrication process and Mr Mauricio Cordero for critical reading of this paper. Mehmet Cagatay Tarhan would like to thank individually the Ministry of Education, Culture, Sports, Science and Technology, Japan for providing the scholarship that helped to make this research possible.
Notes and references
- H. Hess and V. Vogel, Rev. Mol. Biotechnol., 2001, 86, 67–85 CrossRef.
- J. Howard, A. Hudspeth and R. Vale, Nature, 1989, 342, 154–158 CrossRef CAS.
- S. Block, L. Goldstein and B. Schnapp, Nature, 1990, 348, 348–352 CrossRef CAS.
- J. Clemmens, H. Hess, R. Lipscomb, Y. Hanein, K. F. Bohringer, C. M. Matzke, G. D. Bachand, B. C. Bunker and V. Vogel, Biophys. J., 1997, 19(26), 10967–10974.
- S. Klumpp and R. Lipowsky, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(48), 17284–17289 CrossRef CAS.
- Y. Hiratsuka, T. Tada, K. Oiwa, T. Kanayama and T. Uyeda, Biophys. J., 2001, 81, 1555–1561 CrossRef CAS.
- H. Hess, J. Clemmens, D. Qin, J. Howard and V. Vogel, Nano Lett., 2001, 1(5), 235–239 CrossRef CAS.
- H. Hess, J. Clemmens, C. Matzke and G. Bachand, Appl. Phys. A, 2002, 75, 309–313 CrossRef CAS.
- R. Stracke, K. Bohm, J. Burgold, H. Schacht and E. Unger, Nanotechnology, 2000, 11, 52–56 CrossRef CAS.
- T. Kim, M. Kao, E. Hasselbrink and E. Meyhofer, Nano Lett., 2007, 7(1), 211–217 CrossRef CAS.
- M. G. L. van den Heuvel, M. P. De Graaff and C. Dekker, Science, 2006, 312(5775), 910–914 CrossRef CAS.
- B. Hutchins, M. Platt, W. Hancock and M. Williams, Small, 2007, 3(1), 126–131 CrossRef CAS.
- R. Yokokawa, S. Takeuchi, T. Kon, M. Nishiura, K. Sutoh and H. Fujita, Nano Lett., 2004, 4(11), 2265–2270 CrossRef CAS.
- R. Doot, H. Hess and V. Vogel, Soft Matter, 2007, 3, 349–356 RSC.
- R. Yokokawa, Y. Yoshida, S. Takeuchi, T. Kon and H. Fujita, Nanotechnology, 2006, 17, 289–294 CrossRef CAS.
- Y. Du, Y. Hiratsuka, S. Taira, M. Eguchi and T. Uyeda, Chem. Commun., 2005, 2080–2082 RSC.
- G. D. Bachand, S. B. Rivera, A. K. Boal, J. Gaudioso, J. Liu and B. C. Bunker, Nano Lett., 2004, 4(5), 817–821 CrossRef CAS.
- S. Diez, C. Reuther, C. Dinu, R. Seidel and M. Mertig, Nano Lett., 2003, 3(9), 1251–1254 CrossRef CAS.
- S. Hiyama, T. Inoue, T. Shima, Y. Moritani and T. Suda, Small, 2008, 4(4), 410–415 CrossRef CAS.
- R. Yokokawa, J. Miwa, M. C. Tarhan, H. Fujita and M. Kasahara, Anal. Bioanal. Chem., 2008, 391(8), 2735–2743 CrossRef CAS.
- M. Raab and W. O. Hancock, Biotechnol. Bioeng., 2008, 99(4), 764–773 CrossRef CAS.
- S. Ramachandran, K. Ernst, G. Bachand and V. Vogel, Small, 2006, 2(3), 330–334 CrossRef CAS.
- G. Muthukrishnan, B. M. Hutchins, M. E. Williams and W. O. Hancock, Small, 2006, 2(5), 626–630 CrossRef CAS.
- C. Bottier, J. Fattaccioli, M. C. Tarhan, R. Yokokawa, F. O. Morin, B. J. Kim, D. Collard and H. Fujita, Lab Chip, 2009, 9, 1694–1700 RSC.
- C. Bottier, M. C. Tarhan, D. Collard, R. Yokokawa and H. Fujita, Proc. of 12th Int. Conf. on Min. Systems for Chem. & Life Sciences, San Diego, CA, 2008, pp. 871–873. Search PubMed.
- R. Yokokawa, S. Takeuchi, T. Kon, M. Nishiura, R. Ohkura, M. Edamatsu, K. Sutoh and H. Fujita, J. Microelectromech. Syst., 2004, 13(4), 612–619 CrossRef CAS.
- L. Limberis and R. Stewart, Nanotechnology, 2000, 11, 47–51 CrossRef CAS.
- L. Jia, S. Moorjani, T. Jackson and W. Hancock, Biomed. Microdevices, 2004, 6(1), 67–74 CrossRef CAS.
- A. Boal, H. Tellez, S. Rivera and N. Miller, Small, 2006, 2(6), 793–803 CrossRef CAS.
- T. Mitchison and M. Kirschner, Nature, 1984, 312, 237–242 CrossRef CAS.
- T. Korten and S. Diez, Lab Chip, 2008, 8, 1441–1447 RSC.
- S. Taira, Y. Du, Y. Hiratsuka, K. Konishi, T. Kubo, T. Uyeda, N. Yumoto and M. Kodaka, Biotechnol. Bioeng., 2006, 95(3), 553–558.
- R. Soong, G. Bachand, H. Neves and A. Olkhovets, Science, 2000, 290, 1555–1558 CrossRef CAS.
- A. R. Laine, D. Okuno, K. Tabata, A. Tixier-Mita, H. Noji and H. Fujita, Proc. of 18th Int. Conference on Micro Electro Mechanical Systems, Florida, MI, 2005, pp. 818–821 Search PubMed.
- R. Yokokawa, M. C. Tarhan, T. Kon and H. Fujita, Biotechnol. Bioeng., 2008, 101, 1–8 CrossRef CAS.
- R. D. Sloboda and J. L. Rosenbaum, Methods Enzymol., 1982, 85(B), 409–416 Search PubMed.
- R. C. Williams, Jr. and J. C. Lee, Methods Enzymol., 1982, 85(B), 376–385 Search PubMed.
- A. Hyman, D. Drechsel, D. Kellogg, S. Salser, K. Sawin, P. Steffen, L. Wordeman and T. Mitchison, Methods Enzymol., 1991, 196, 478–485 CAS.
- A. K. Boal, G. D. Bachand, S. B. Rivera and B. C. Bunker, Nanotechnology, 2006, 17, 349–354 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: In the supplementary movie, motion of functionalized nano-needles driven by gliding microtubules is shown. Direction of the microtubule transport could be changed due to the drag force induced by the buffer flow. Note that, during the transportation due to strong flow, some parts of the microtubule became detached from and reattached to the kinesin layer without affecting either the needle attachment or the transport process. See DOI: 10.1039/b913312g |
| This journal is © The Royal Society of Chemistry 2010 |
