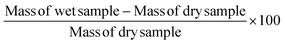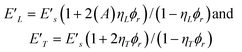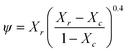DOI:
10.1039/B916130A
(Paper)
J. Mater. Chem., 2010,
20, 180-186
Bio-inspired mechanically-adaptive nanocomposites derived from cotton cellulose whiskers†
Received
5th August 2009
, Accepted 6th October 2009
First published on
5th November 2009
Abstract
A new series of biomimetic, stimuli-responsive nanocomposites, which change their mechanical properties upon exposure to physiological conditions, was investigated. The materials were produced by introducing percolating networks of cellulose whiskers isolated from cotton into poly(vinyl acetate). Below the glass-transition temperature (Tg ∼ 63 °C), the tensile storage moduli (E′) of the dry nanocomposites increased two fold, from 2 GPa for the neat polymer to 4 GPa for a nanocomposite with 16.5% v/v whiskers. The relative reinforcement was more significant above Tg, where E′ was increased nearly 40 fold, from ∼1.2 MPa to ∼45 MPa. Upon exposure to emulated physiological conditions (immersion in artificial cerebrospinal fluid at 37 °C) all nanocomposites showed a pronounced decrease in E′, for example to 5 MPa for the 16.5% v/v whisker nanocomposites with only about 28% w/w swelling. This is a significant reduction in the amount of swelling required to decrease the E′, compared to earlier material versions based on cellulose whiskers with higher surface charge density; the decreased swelling may be a considerable advantage for the intended use of these materials as adaptive substrates for intracortical electrodes and other biomedical applications.
Introduction
Materials that respond to stimuli in a selective and controlled manner are of significant interest for use in several biomedical applications like drug delivery,1 cell culture,2 and bioseparation3 as well as other applications such as sensors and actuators,4 smart clothing5etc. Electro-rheological materials6 and certain hydrogels7,8 (which rely on a sol–gel transition) and shape memory polymers9 are typical examples of stimuli responsive polymeric materials which change their physical shape or mechanical properties in response to environmental stimuli. However, while most of these mechano-responsive materials exhibit viscosity/modulus changes of several orders of magnitude, they are not very rigid (modulus in the KPa to MPa range). Examples of much stiffer materials that exhibit such morphing mechanical behavior are quite limited.
We recently developed a new family of mechanically dynamic polymer nanocomposites10 that were inspired by the stimuli-responsive dermis of sea cucumbers. These creatures have the fascinating ability to rapidly and reversibly alter the stiffness of their inner dermis in response to a threat.11 In recent studies it has been proposed that this dynamic mechanical behavior is achieved through a nanocomposite architecture, where a viscoelastic matrix is reinforced with rigid, high-aspect ratio collagen fibrils.12 The stiffness of the tissue is regulated by controlling the interactions, and therewith the stress transfer, between adjacent collagen fibrils by locally secreted proteins through either non-covalent13 or covalent14 bonds. Intrigued by this natural model, we have been investigating the possibility of creating synthetic nanocomposites that exhibit a similar architecture as well as a comparable morphing capability. In particular, we are interested in investigating the potential of such materials as adaptive intracortical electrodes,15 which are sufficiently rigid to allow for penetration of the pia mater during implantation of the device,16 but upon implantation and exposure to physiological conditions soften to more closely match the mechanical properties of the brain.
Our initial studies involved the use of cellulose nanofibers (also referred to as nanowhiskers or simply whiskers) as an alternative filler to the collagen found in the natural model. Cellulose whiskers can be obtained from a range of renewable bio-sources, including tunicates,17,18 wood,19 cotton20 and sisal.21 Our initial studies in this area have primarily focused on the use of tunicate cellulose whiskers. The whiskers obtained from these sea creatures exhibit high stiffness (tensile modulus ∼130 GPa) and dimensions on the nanometre scale (26 nm × 2.2 μm).22
Cellulose whiskers have a strong tendency for aggregation23–25 due to the high density of strongly interacting surface hydroxyl groups. If sulfuric acid is used for the hydrolysis of the bio-derived pulp, the isolated cellulose whiskers are decorated with a small number of sulfate groups.26 Good dispersion of these partially negatively charged cellulose whiskers can be achieved when the whisker–whisker hydrogen bonding interactions are “switched off” by competitive binding with a hydrogen-bond-forming solvent.10,24 Upon solvent removal, the interactions between the whiskers are “switched on” and they assemble into a percolating network.10 These strong hydrogen bonding whiskers have led to a significant enhancement in the tensile storage modulus (E′) of PEO-EPI (polyethylene oxide-co-epichlorohydrin) from ∼3.7 MPa (neat) to ∼800 MPa (19% v/v tunicate whiskers). Upon exposure to water, which acts as a chemical regulator and switches the hydrogen bonding between the whiskers within the polymer matrix “off”,10 a dramatic modulus reduction was achieved (e.g. from 800 to 20 MPa for a composite comprising 19% v/v whiskers); when these nanocomposites were dried, the original stiffness was restored, which demonstrates the reversibility of the system. However, E′ of the most rigid composition studied (800 MPa) was lower than required for the targeted use in intracortical electrodes (∼4 GPa). We therefore combined the switching mechanism with a chemically influenced thermal transition and discovered that nanocomposites based on poly(vinyl acetate) (PVAc) and cellulose whiskers display such a “dual” responsive behavior. Upon exposure to water or artificial cerebrospinal fluid (ACSF) at 37 °C, diffusion of water into the nanocomposites not only disengages the whisker network, but plasticization by water reduces the glass transition temperature (Tg) of PVAc and the nanocomposites from ∼60 to ∼20 °C, i.e. from above to below physiological temperature. This approach has afforded materials which exhibit a dramatic mechanical contrast of three orders of magnitude between the dry state at room temperature (E′ = 5.1 GPa for a nanocomposite with 16.5% v/v tunicate whiskers) and the water- or ACSF-swollen state at 37 °C (∼12 MPa).10 However, at this temperature, the water or ACSF take-up was very significant (∼70–90% w/w for 16.5% v/v tunicate whiskers). With the goals of lowering the “soft” modulus of such nanocomposites, to reduce the level of swelling, and to explore the potential of using cellulose whiskers from a more accessible bio-source than tunicates, we embarked on the exploration of stimuli responsive nanocomposites using cotton cellulose whiskers (CCW). As will be shown, CCWs exhibit properties which are suitable to achieve these goals.
Experimental
Materials
Poly(vinyl acetate) (PVAc, weight-average molecular weight, Mw = 113,000 g mol−1; density, δ = 1.19 g cm−3) was purchased from Sigma-Aldrich. All other reagents were also purchased from Sigma-Aldrich and were used without further purification. Artificial cerebrospinal fluid was prepared by dissolving the following salts in 1 L of deionized water:27 NaCl = 7.25 g, KCl = 0.22 g, NaHCO3 = 2.18 g, CaCl2·2 H2O = 0.29 g, KH2PO4 = 0.17 g, MgSO4·7 H2O = 0.25 g, D-glucose = 1.80 g.
Preparation of polymer/cotton cellulose whisker nanocomposites
Cellulose whiskers from cotton were isolated using the general procedure of Dong et al.28 with slight modifications as described in detail before.25 Lyophilized whiskers were dispersed in dimethyl formamide (DMF) at a concentration of ∼4 mg mL−1 by sonicating for 8–12 h. PVAc was dissolved in DMF at a concentration of ∼5% w/w by stirring for 4–6 h. Nanocomposites were prepared by combining the desired amounts (to yield materials containing 0–16% v/v whiskers) of the colloidal whisker dispersion and polymer solution, sonicating for 10 min and solution-casting the resulting homogeneous mixture into Teflon® petri dishes. The dishes were placed into a vacuum oven (65 °C, 15 mbar, 1 week) to evaporate the solvent and dry the resulting films, before the materials were compression-moulded between spacers in a Carver laboratory press (90 °C at 0 psi for 2 min, followed by an increase in pressure to 3000 psi for 15 min) to yield 200–300 μm thin nanocomposite films. Neat PVAc films were also prepared in a similar manner to create reference films for mechanical testing. This method has been previously reported to produce homogeneous films free of phase separation.10
Determination of charge density on cotton whiskers
Cellulose whiskers with negatively charged sulfate groups (ROSO3−) were obtained via sulfuric acid hydrolysis of cotton. The charge density was determined by conductometric titration. In short, 4 mL of a cotton whisker/water dispersion of a concentration of 20.9 mg mL−1 was added to 70 mL of deionized water and the dispersion was stirred. pH and conductivity probes were inserted into the colloidal suspension. To neutralize the acid groups, a 0.01 M KOH solution was added in steps of 50 μL and the pH and conductivity of the solution were continuously recorded to determine the endpoint. From the volume of KOH required to neutralize the solution and the amount of whiskers, the charge density was determined.
Dynamic mechanical analysis
The mechanical properties of the nanocomposites were characterized by dynamic mechanical analysis (DMA, TA instruments Model Q800). Tests were conducted in tensile mode using a temperature sweep method (23 °C to 90 °C) at a fixed frequency of 1 Hz, and amplitude of strain 15 μm. In order to determine the tensile properties of the nanocomposite films in the wet state, samples were swollen in ACSF at 37 °C for 1 week and tensile tests were conducted using a submersion clamp, which allowed us to keep the samples immersed in ACSF during testing; here a temperature sweep in the range of 23 °C to 50 °C was done. All samples were dried in vacuum at 60 °C for 16–18 h prior to DMA testing or swelling experiments (except, of course, for the analysis of swollen samples).
Swelling behavior
Prior to DMA testing of ACSF swollen samples, the degree of swelling was determined by measuring the weight of the samples pre- and post-swelling:| | 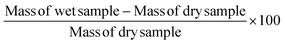 | (1) |
Results and discussion
Physical properties of cotton cellulose whiskers
Sulfuric acid hydrolysis of Whatman filter paper yields cellulose crystals with a typical diameter of ca. 10–20 nm, a length between 100–250 nm and an average aspect ratio of (∼10), which is much lower than that of tunicate cellulose (70–100). Fig. 1 shows transmission electron microscopic images of the CCWs prepared here. CCWs (tensile modulus ∼57–105 GPa29) also exhibit a lower stiffness than tunicate whiskers (tensile modulus ∼130 GPa).22 Sulfate groups on the cotton whiskers cause electrostatic repulsion between the whiskers and are important for their good dispersibility in many solvents like water, N,N-dimethyl formamide, dimethyl sulfoxide and N-methyl pyrrolidone.24,30 The density of the sulfate groups of the present CCWs was measured by conductometric titrations to be ∼31 mmol kg−1 (ESI† Fig. S1). This is significantly lower than that of tunicate cellulose whiskers (∼85 mmol kg−1) used in our previous studies; the difference can be attributed to the milder hydrolysis conditions employed for the cotton cellulose (the application of conditions normally used for the hydrolysis of tunicates leads to degradation of CCWs): the initial concentration of the aqueous cellulose pulp used for hydrolysis of cotton cellulose (∼2% w/w) was about twice that of tunicate cellulose (∼1% w/w) and the final acid/water ratio used in the hydrolysis of cotton cellulose (acid weight fraction 55%) was lower than that used in the case of tunicate cellulose (75%).
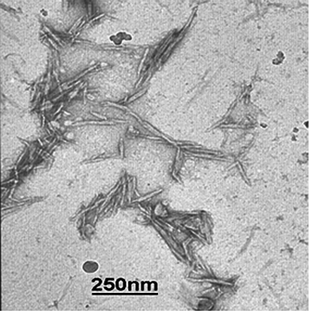 |
| | Fig. 1 Transmission electron microscopy images of cotton cellulose whiskers. The sample was produced by depositing a drop of a 0.2% w/w cotton cellulose whisker dispersion in DMF onto a carbon-coated copper grid. | |
Mechanical properties of dry PVAc/cotton cellulose whisker nanocomposites
Nanocomposite films composed of 0 to 16.5% v/v CCWs in PVAc were produced by solution-casting from DMF and subsequent compression molding (see Experimental Section for details). The thermo-mechanical properties of these materials were established by dynamic mechanical analysis (DMA). Temperature-dependent tensile storage moduli (E′) of PVAc/CCW nanocomposites in the dry state are shown in Fig. 2a. At ambient temperature (at 25 °C) the neat PVAc matrix is in a glassy state and displays an E′ of ∼2 GPa; upon increasing the temperature, the stiffness displayed a drastic reduction, to reach ∼1.2 MPa around 75–80 °C. This is related to transition to the rubbery regime upon heating the material above Tg, which is at ca. 63 °C, as seen from the DMA loss tangent (tan δ) (Fig. 2b). Incorporation of cellulose whiskers led to a modest increase in the tensile storage modulus below Tg. E′ increased from 2 GPa to 4 GPa for a nanocomposite comprising 16.5% v/v cellulose whiskers. This is typical of glassy matrices reinforced with rigid fillers and is consistent with other studies on cellulose whisker nanocomposites.31,32 The peak displayed by the tan δ vs. temperature traces of the nanocomposites (Fig. 2b) became broader upon introduction of the CCWs and the peak position slightly shifted towards higher temperatures by about 5–7 °C. Tan δ is the ratio of loss modulus to storage modulus of the material, and is indicative of its damping behavior. A peak in the tan δ vs. temperature trace reflects a loss of energy due to the relaxation processes in polymeric materials, and in the case of the present materials is related to the glass transition. The peak temperature (Tα) corresponds to the transition temperature and the intensity (Iα) is related to the magnitude of the relaxation.33 Incorporation of whiskers led to a significant reduction in peak intensity, indicating a lower magnitude of chain relaxation. This can be attributed to polymer–whisker interactions through hydrogen bonding. This effect appears also to be responsible for the fact that Tα slightly increased with increasing content of cellulose whiskers. The same trend has been previously reported, and was attributed to interactions between the matrix polymer and the polar surface of the cellulose.34 Of particular interest in these celluose whisker nanocomposites is the reinforcement that can be observed well above Tg (Fig. 2a). When incorporated in a concentration that allows for the formation of a rigid, percolating network (which relies on strong hydrogen bonding for stress transfer among the whiskers), these whiskers lead to significant reinforcement of the soft rubbery matrix. In the case of the CCW-based nanocomposites investigated here (Fig. 2a), E′ was increased from about 1.2 MPa (neat polymer) to about 45 MPa (16.5% v/v CCW) at 82 °C.
 |
| | Fig. 2 (a) Tensile storage moduli E′ of dry films of neat PVAc and PVAc/cotton cellulose whisker (CCW) nanocomposites as a function of temperature and composition. Data were acquired by DMA. (b) Loss tangent vs. temperature plots of DMA sweeps shown in (a). | |
Swelling behaviour of PVAc/cotton cellulose whisker nanocomposites
In continuation of our efforts10 to develop mechanically dynamic substrates for adaptive intracortical electrodes, we were interested in the chemo-responsive nature of the PVAc/CCW nanocomposites. Hence we determined the swelling behavior of these nanocomposites in emulated physiological conditions (immersion in artificial cerebrospinal fluid, ACSF, at 37 °C). The swelling in ACSF at 37 °C (determined according to eqn (1)) increased steadily with the whisker content (Fig. 3). This effect can be attributed to the increased hydrophilicity of the nanocomposite on account of the presence of cellulose whiskers. Previous swelling studies of PVAc/sisal whisker nanocomposites at room temperature and 98% RH demonstrated that the swelling ratio of PVAc initially increases with the addition of whiskers but becomes approximately constant at higher concentrations of whiskers (5–10% w/w).21 This is different than what we observe here. However it should be noted that our swelling temperature (37 °C), source of whiskers, solvent, and range of whisker concentration studied is much different. Interestingly, a direct comparison (similar whisker content and identical swelling conditions) of this study and our previous one with tunicate whiskers shows that the present CCW-containing materials swell much less than the analogous PVAc/tunicate whisker nanocomposites investigated before10 (data included in Fig. 3 for comparison). For example, the PVAc/CCW nanocomposite comprising 16.5% v/v whiskers displayed a degree of swelling of ∼28%, which is ∼1/3 that of a corresponding tunicate whisker nanocomposite. Apart from being derived from a different source and having different dimensions, the major difference between the tunicate and cotton cellulose whiskers used in our studies is the density of charged sulfate groups (vide supra). In view of the well-established correlation between water uptake and concentration of ionic groups in ionic polymer membranes,35 it is plausible that the very desirable, low degree of swelling displayed by the present CCW nanocomposites is related to the lower sulfate charge density of the whiskers employed.
 |
| | Fig. 3 Swelling of PVAc/cotton cellulose whisker and PVAc/tunicate whisker nanocomposites. Solvent uptake as a function of cellulose whisker volume fraction upon immersion in ACSF at 37 °C for 1 week (to equilibration). Data points represent averages (N = 3–5) ± standard deviation measurements. | |
Mechanical properties of ACSF-swollen PVAc/cotton whisker cellulose nanocomposites
Fig. 4a shows the temperature-dependent tensile storage moduli E′ of neat PVAc and PVAc/cotton cellulose whisker nanocomposite films that had been immersed in ACSF at 37 °C for 1 week (i.e. to equilibrium) before being tested in a DMA set-up that allowed the samples to be kept immersed in ACSF during the tests. The E′ dropped drastically as the temperature was increased from ambient temperature to 37 °C and beyond. At 37 °C, the physiological temperature of interest, E′ of nanocomposites with higher whisker content (12.2–16.5% v/v) was about 5 MPa, while that of neat PVAc was close to 1 MPa. Thus, compared to the dry state at room temperature (E′ ∼ 4 GPa) a modulus reduction of three orders of magnitude can be achieved with the materials studied here. As reported earlier, this significant dynamic modulus contrast is a consequence of the combined effect of thermal and chemical stimuli. Water uptake plasticises PVAc and reduces the Tg of neat PVAc and the PVAc-based nanocomposites from ∼65 to ∼21 °C (Fig. 4b); at the same time it ‘switches off’ the whisker–whisker interactions through competitive hydrogen bonding. This reasoning is further supported by the comparison of the modulus data of dry and wet nanocomposites in the context of two classical models for short fiber composites namely, the Halpin Kardos and the percolation model. The detailed theoretical framework for these models can be found elsewhere36–38 and we provide here only a brief outline and summary of their underlying assumptions. The Halpin Kardos model36 assumes that the nanocomposites are quasi-isotropic and equivalent to the superposition of four plies, where the nanofibers within each ply are parallel to one another and the directors of the plies are oriented towards each other at particular angles (−45°, 0°, 45°, and 90°). In this model the nanofiller particles are assumed to be homogeneously dispersed in a continuous matrix with no interactions between them. The modulus of the individual plies in the longitudinal and transverse directions are derived from Halpin Tsai equations,39| | 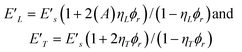 | (2) |
| where ηL = ((Elr/Es) − 1)/((Elr/Es) + 2A), and |
| ηT = ((Etr/Es) − 1)/((Etr/Es) + 2). |
and the tensile storage modulus of the nanocomposite is given by
| with U1 = 1/8(3Q11 + 3Q22 + 2Q12 + 4Q66) |
| U5 = 1/8(Q11 + Q22 − 2Q12 + 4Q66) |
where ν12 = ϕrνr + ϕsνs; G′12 = G′s(1 + ηϕr)/(1 − ηϕr); η = (G′r/G′s − 1)/(G′r/G′s + 1) and ν is the Poisson's ratio (defined above as 0.3), G′ is the shear modulus and ϕ is equal to the volume fraction of the phase (subscripts r and s refer to the rigid nanofiller and soft polymer phases, respectively). Thus, the modulus of the nanocomposites depends on the moduli and the volume fractions of nanofiller and matrix and the geometry of the filler.
 |
| | Fig. 4 (a) Tensile storage moduli E′ of ACSF-swollen films (after immersion in ACSF at 37 °C for a week) of neat PVAc and PVAc/cotton cellulose whisker (CCW) nanocomposites as a function of temperature and composition. Data were acquired by DMA. (b) Loss tangent vs. temperature plots of DMA sweeps shown in (a). | |
The percolation model of Ouali,38 derived from the parallel-series model of Takayanagi,37 assumes the filler particles do strongly interact with each other to form a network; under conditions where the matrix is too soft (i.e. above Tg) the modulus of the percolating rigid nanofiller network is assumed to be the primary factor that governs the modulus of the composite. The model expressed E′ by:
| |  | (4) |
Where
ψ is the percolating volume fraction of nanofibers that participate in the load transfer, which according to percolation theory is:
| | 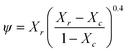 | (5) |
Where
Xr ≥
Xc and
Xc ≥ 0.7/
A (
A is the aspect ratio of the nanofibers) is the critical nanofiber volume fraction needed for percolation.
40
Fig. 5 shows the experimental data and the theoretical values predicted by these models on the basis of parameters that can be independently determined (E′s = 1.2 MPa, A = 10.5, E′r = 650 MPa,23,24E′lr = 105 GPa,29E′tr = 5 GPa, Gr = 1.77 GPa, νs = 0.3, and νr = 0.5).39 The moduli of the dry PVAc/cotton cellulose nanocomposites at 82 °C (i.e. well above Tg) follow the prediction of the percolation model quite well at higher volume fraction of whiskers, while at a whisker density of 4% v/v (i.e., below the percolation threshold), the experimental data exceed predicted values. This is consistent with previous studies that discuss the failure of the percolation model to accurately predict the modulus of nanocomposites below the percolation threshold.41 Additionally, the moduli of the corresponding ACSF-swollen nanocomposites fall well below the percolation model showing a gradually increasing trend as that of composites with non-interacting fillers. Though the modulus of the neat PVAc is virtually the same under both conditions (∼1.2 MPa), the moduli of the nanocomposites differ by up to an order of magnitude. For example at 16.5% v/v of whiskers, the E′ at 82 °C dropped from ∼45 MPa to ∼5 MPa upon swelling in ACSF. This suggests that upon exposure to a chemical stimulus, in addition to plasticization of the matrix, the hydrogen bonding interactions between whiskers are disrupted. Assuming a complete disengagement of the whisker network one would expect the moduli to fit the Halpin Kardos model. However, Fig. 5 shows that the moduli of the ACSF-swollen CCW nanocomposites are slightly higher than the prediction by the Halpin Kardos model. It is at this time not possible to assess if this difference is related to incomplete dissociation of the whisker network upon exposure to ACSF, or if the origin is the limited accuracy of the parameters fed into the model. For example, previous studies give a rather broad range of 57–105 GPa for E′lr,29 and no data for E′tr, Gr, and νr are available for cotton cellulose whiskers, and values established for tunicate whiskers39 were used instead.
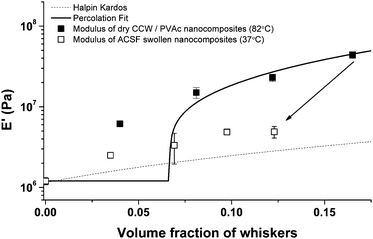 |
| | Fig. 5 Tensile storage moduli E′ of neat PVAc and PVAc/cotton cellulose whisker nanocomposites (CCW) in the dry state at 82 °C, and ACSF-swollen state (after immersion in ACSF at 37 °C for a week) at 37 °C. Lines show the values predicted by the percolation (solid) and the Halpin-Kardos (dashed) models. Data of swollen samples are at a lower volume fraction compared to their dry state due to solvent uptake. Data points represent averages (N = 3–5) ± standard deviation measurements. | |
We have previously shown that the dramatic softening of PEO-EPI/tunicate whisker nanocomposites upon exposure to water is fully reversible (vide supra); the modulus is fully restored upon drying.10 Similar experiments carried out on the PVAc/cotton whisker nanocomposites showed a similar reversible response (ESI† Fig. S2). For example, at 80 °C, above Tg, the dry nanocomposite has a modulus of 28–30 MPa that upon swelling with water drops to ca. 5 MPa. Drying the nanocomposite in a vacuum oven at 60 °C for two days results in restoration of the modulus of the film (28–30 MPa). At 37 °C, i.e. below Tg of the dry nanocomposite, a similar recovery is observed. That is, the original dry film had a modulus of 3.9 GPa which drops to 5 MPa upon exposure to an aqueous environment. Upon drying, the film's modulus returns to close to that of the original (3–3.5 GPa).
Cotton cellulose vs. tunicate cellulose whiskers
Fig. 6a compares the reinforcement offered by cotton cellulose whiskers and tunicate cellulose whiskers10 (reproduced here for comparison) for nanocomposites comprising 16.5% v/v of the respective nanofibers in a PVAc matrix. The modulus of the cotton cellulose nanocomposite below Tg is about 4 GPa, i.e., only slightly lower than that of the tunicate cellulose counterpart (E′ ∼ 5 GPa). Above Tg, the moduli differ by an order of magnitude (∼600 vs. ∼45 MPa). This difference is consistent with previous findings for nanocomposites comprising the two whisker types10,25 (cotton cellulose whiskers have been known to display a less pronounced reinforcement than tunicate cellulose whiskers) and the fact that neat, solution-cast sheets (nanopapers) of cotton cellulose whiskers display a much lower stiffness than those produced from tunicate cellulose. However, the origin of this difference is unclear at this point; the difference in aspect ratio should not be a major factor, since both materials systems investigated are significantly above the threshold for percolation. In the ACSF swollen state, however, the difference between the two systems is narrowed; at 37 °C the CCW-based PVAc nanocomposite shows a modulus of ∼5 MPa, which is only slightly lower than that (∼12 MPa) of the tunicate cellulose based nanocomposite (Fig. 6b).
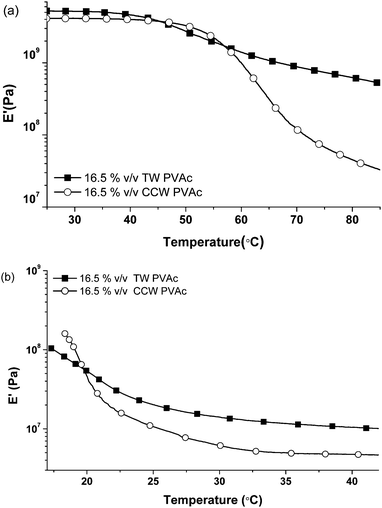 |
| | Fig. 6 Comparison of temperature-dependent tensile storage moduli E′ of PVAc nanocomposites with 16.5% v/v tunicate whiskers (TW) or cotton cellulose whiskers (CCW) in (a) dry state and (b) swollen in ACSF at 37 °C for a week. Data of tunicate whisker nanocomposites are reproduced from ref. 10 for comparison. | |
Most importantly, the overall modulus contrast of a 16.5% v/v cotton cellulose PVAc nanocomposite between the dry state at 37 °C and ACSF swollen state at 37 °C is still three orders of magnitude (4 GPa to 5 MPa) and therewith comparable to that of a similar material based on tunicate cellulose whiskers (5 GPa to 12 MPa). This is of significant importance to the targeted application as adaptive substrates for intracortical electrodes, where an initial E′ of >4 GPa is desirable to allow for the insertion of an electrode with typical dimensions into the cortex.42,43 As shown above, however, the aqueous swelling of the cotton-based materials is massively reduced, which represents a significant advantage over the tunicate-based nanocomposites.
Conclusions
In summary, nanocomposites of PVAc and cotton cellulose whiskers demonstrate a mechanically adaptive behavior in response to thermal and chemical stimuli. While the mechanical contrast in stiffness is almost similar to our earlier generation PVAc-tunicate whisker nanocomposites, the water uptake of these nanocomposites is significantly lower, presumably due to the lower surface charge density of cotton cellulose whiskers. This represents a significant advancement in our efforts to make adaptive substrates for intracortical electrodes. Moreover these nanocomposites can be produced by using cellulose whiskers isolated from cotton, which is a more readily accessible cellulose source than tunicates.
Acknowledgements
Financial support from the National Institute of Health under Grant No. R21NS053798-0, the Case School of Engineering (Ohio Innovation Incentive Fellowship to K.S.) and the Department of Veteran's Affairs Career Development Program (to J.R.C.) is gratefully acknowledged.
Notes and references
-
(a) D. Needham and M. W. Dewhirst, Adv. Drug Delivery Rev., 2001, 53, 285 CrossRef CAS;
(b) N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345 CrossRef CAS;
(c) Y. H. Kim, I. C. Kwon, Y. H. Bae and S. W. Kim, Macromolecules, 1995, 28, 939 CrossRef.
-
(a) T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Tissue Eng., 2001, 7, 141 CrossRef CAS;
(b) O. H. Kwon, A. Kikuchi, M. Yamato, Y. Sakurai and T. Okano, J. Biomed. Mater. Res., 2000, 50, 82 CAS.
- H. Lee and T. Gwan Park, Biotechnol. Prog., 1998, 14, 508 CrossRef CAS.
-
(a) R. Pelrine, R. Kornbluh, Q. B. Pei and J. Joseph, Science, 2000, 287, 836 CrossRef CAS;
(b) V. H. Ebron, Z. W. Yang, D. J. Seyer, M. E. Kozlov, J. Y. Oh, H. Xie, J. Razal, L. J. Hall, J. P. Ferraris, A. G. MacDiarmid and R. H. Baughman, Science, 2006, 311, 1580 CrossRef CAS.
- N. S. Save, M. Jassal and A. K. Agrawal, J. Ind. Text., 2005, 34, 139 Search PubMed.
-
(a)
J. D. Carlson, A. F. Sprecher and H. Conrad, in Proceedings of the Second International Conference on ER Fluids; Technomic Publishing Co. Ltd., Lancaster, PA, 1989 Search PubMed;
(b) K. Minagawa and K. Koyama, Curr. Org. Chem., 2005, 9, 1643 CrossRef CAS.
- T. Suzuki, S. Shinkai and K. Sada, Adv. Mater., 2006, 18, 1043 CrossRef CAS.
- Y. Chen and M. Yi, Radiat. Phys. Chem., 2001, 61, 65 CrossRef.
-
(a) A. Lendlein and R. Langer, Science, 2002, 296, 1673 CrossRef;
(b) A. Lendlein and S. Kelch, Angew. Chem., Int. Ed., 2002, 41, 2034 CrossRef CAS;
(c) C. Liu, H. Qin and P. T. Mather, J. Mater. Chem., 2007, 17, 1543 RSC.
-
(a) J. R. Capadona, K. Shanmuganathan, D. J. Tyler, S. J. Rowan and C. Weder, Science, 2008, 319, 1370 CrossRef CAS;
(b) K. Shanmuganathan, J. R. Capadona, S. J. Rowan and C. Weder, Progr. Polym. Sci., 2010 Search PubMed , in press.
-
(a)
T. Heinzeller and J. Nebelsick, eds. Echinoderms; Taylor & Francis Group, London, 2004 Search PubMed;
(b) T. Motokawa, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1994, 109, 613 CrossRef.
-
(a) J. A. Trotter, J. Tipper, G. Lyons-Levy, K. Chino, A. H. Heuer, Z. Liu, M. Mrksich, C. Hodneland, W. Shannon Dillmore, T. J. Koob, M. M. Koob-Emunds, K. Kadler and D. Holmes, Biochem. Soc. Trans., 2000, 28, 357 CrossRef CAS.
-
(a) J. A. Trotter and T. J. Koob, Matrix Biol., 1999, 18, 569 CrossRef CAS;
(b) G. K. Sulgit and R. E. Shadwick, J. Exp. Biol., 2000, 203, 1539 Search PubMed.
-
B. Alberts, D. Bray, J. Lewis, M. Raff, K. Roberts and J. D. Watson, Molecular Biology of The Cell 3rd Edition, New York, NY, 1994 Search PubMed.
-
(a) W. L. C. Rutten, Annu. Rev. Biomed. Eng., 2002, 4, 407 CrossRef CAS;
(b) A. B. Schwartz, Annu. Rev. Neurosci., 2004, 27, 487 CrossRef CAS.
- D. H. Szarowski, M. D. Andersen, S. Retterer, A. J. Spence, M. Isaacson, H. G. Craighead, J. N. Turner and W. Shain, Brain Res., 2003, 983, 23 CrossRef CAS.
- P. S. Belton, S. F. Tanner, N. Cartier and H. Chanzy, Macromolecules, 1989, 22, 1615 CrossRef CAS.
- M. De Souza Lima and R. Borsali, Macromol. Rapid Commun., 2004, 25, 771 CrossRef.
- R. T. Woodhams, G. Thomas and D. K. Rodgers, Polym. Eng. Sci., 1984, 24, 1166 CrossRef CAS.
- S. J. Eichhorn, C. A. Baillie, N. Zafeiropoulos, L. Y. Mwaikambo, M. P. Ansell, A. Dufresne, K. M. Entwistle, P. J. Herrera-Franco, G. C. Escamilla, L. Groom, M. Hughes, C. Hill, T. G. Rials and P. M. Wild, J. Mater. Sci., 2001, 36, 2107 CrossRef CAS.
- N. Rodriguez, W. Thielemans and A. Dufresne, Cellulose, 2006, 13, 261 CrossRef.
- A. Sturcova, J. R. Davies and S. J. Eichhorn, Biomacromolecules, 2005, 6, 1055 CrossRef CAS.
- J. R. Capadona, O. van den Berg, L. Capadona, M. Schroeter, D. Tyler, S. J. Rowan and C. Weder, Nat. Nanotechnol., 2007, 2, 765 CrossRef CAS.
- O. van den Berg, J. R. Capadona and C. Weder, Biomacromolecules, 2007, 8, 1353 CrossRef CAS.
- J. R. Capadona, K. Shanmuganathan, S. Trittschuh, S. Seidel, S. J. Rowan and C. Weder, Biomacromolecules, 2009, 10, 712 CrossRef CAS.
- A. Dufresne, M. B. Kellerhals and B. Witholt, Macromolecules, 1999, 32, 7396 CrossRef CAS.
-
http://www.alzet.com/products/cfs_prep.php (08/04/2009).
- X. M. Dong, J. F. Revol and D. G. Grey, Cellulose, 1998, 5, 19 CrossRef CAS.
- R. Rusli and S. J. Eichhorn, Appl. Phys. Lett., 2008, 93, 033111 CrossRef.
-
(a) D. Bondeson, A. Mathew and K. Oksman, Cellulose, 2006, 13, 171 CrossRef CAS;
(b) S. M. A. S. Azizi, F. Alloin, J. Y. Sanchez, N. El Kissi and A. Dufresne, Macromolecules, 2004, 37, 1386 CrossRef;
(c) N. E. Marcovich, M. L. Auad, N. E. Bellesi, S. R. Nutt and M. I. Aranguren, J. Mater. Res., 2006, 21, 870 CrossRef CAS;
(d)
A. Tubak, F. Snyder and K. Sandberg, United States Patent Number 4378381, 1983.
- V. Favier, H. Chanzy and J. Y. Cavaillé, Macromolecules, 1995, 28, 6365 CrossRef CAS.
- N. Ljungberg, J.-Y. Cavaillé and L. Heux, Polymer, 2006, 47, 6285 CrossRef CAS.
- A. Dufresne, Compos. Interfaces, 2003, 10, 369 Search PubMed.
- M. Matos Ruiz, J. Y. Cavaillé, A. Dufresne, J. F. Gérard and C. Graillat, Compos. Interfaces, 2000, 7(2), 117 Search PubMed.
-
T. S Zhao, K. D. Kreuer and T. V. Nguyen, eds. Advances in Fuel Cells: Elsevier publishers ( 2007) pp. 235–326 Search PubMed.
- J. C. Halpin and J. L. Kardos, J. Appl. Phys., 1972, 43, 2235 CrossRef CAS.
- M. Takayanagi, S. Uemura and S. Minami, J. Polym. Sci. Part C, 1964, 5, 113 Search PubMed.
- N. Ouali, J. Y. Cavaillé and J. Pérez, Elastic Plast. Rubber Comp. Process Appl., 1991, 16, 55 Search PubMed.
- P. Hajji, J.-Y. Cavaillé, V. Favier, C. Gauthier and G. Vigier, Polym. Compos., 1996, 17, 612 Search PubMed.
- V. Favier, R. Dendievel, G. Canova, J. Y. Cavaillé and P. Gilormini, Acta Mater., 1997, 45, 1557 CrossRef CAS.
- A. Morin and A. Dufresne, Macromolecules, 2002, 35, 2190 CrossRef CAS.
- K. Najafi and J. F. Hetke, IEEE Trans. Biomed. Eng., 1990, 37, 474 CrossRef CAS.
-
A. Hess, J. Dunning, J. Harris, J. R. Capadona, K. Shanmuganathan, S. J. Rowan, C. Weder, D. J. Tyler and C. A. Zorman, Proceedings of the 15th International Conference on Solid-state Sensors, Actuators and Microsystems, IEEE Transducers 2009, CO, USA Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here.