Application of calcium phosphate nanoparticles in biomedicine
M.
Epple
*a,
K.
Ganesan
a,
R.
Heumann
b,
J.
Klesing
a,
A.
Kovtun
a,
S.
Neumann
b and
V.
Sokolova
a
aInorganic Chemistry and Center for Nanointegration Duisburg-Essen (CeNIDE), University of Duisburg-Essen, Universitaetsstr. 5-7, 45117, Essen, Germany
bFaculty of Chemistry and Biochemistry, University of Bochum, Universitaetsstrasse 150, 44780, Bochum, Germany
First published on 1st October 2009
Abstract
Calcium phosphate has excellent biocompatibility due to its chemical similarity to human hard tissue (bone and teeth). In nanoparticulate dispersed form, it can be used as a carrier in biological systems, e.g. to transfer nucleic acids or drugs. If such nanoparticles are suitably functionalized with fluorescing dyes, they can also be used for imaging or for photodynamic therapy.
 M. Epple | Matthias Epple studied Chemistry at the Technical University of Braunschweig. After post-doctoral stays at the University of Washington (Seattle) and the Royal Institution (London) he went to the Universities of Hamburg, Bochum, and Duisburg-Essen where he now holds a chair in Inorganic Chemistry. |
 R. Heumann | Rolf Heumann studied Microbiology in Munich and performed his thesis on the regulation of protein expression after cell fusions at the Max-Planck Institute in Martinsried, Germany. After receiving his PhD in 1979 he continued his postdoctoral research in Martinsried, Department for Neurochemistry, until 1991. Since then he has held a chair in Biochemistry at Ruhr-University Bochum. |
Introduction
Nanomedicine is an emerging field of biomedical research.1 Calcium phosphate nanoparticles have gained increasing interest in medicine because of their high biocompatibility and good biodegradability which is due to the fact that calcium phosphate is the inorganic mineral of human bone and teeth.2–5 Therefore, they complement typical nanoparticulate systems which are used in medicine, e.g. polymers, metallic nanoparticles (like gold), iron oxide nanoparticles (like magnetite) and quantum dots (like CdS). The preparation of calcium phosphate nanoparticles can be conveniently carried out from aqueous solutions, provided that a suitable functionalization agent is used, e.g. a polymer or a charged adsorbing molecule. In this article, we highlight a number of applications where calcium phosphate nanoparticles were successfully applied in biological systems. However, here we do not consider nanocrystalline calcium phosphate ceramics (bulk systems) but only dispersed systems (colloids).Calcium phosphate nanoparticles for transfection
The process of DNA entry through the plasma membrane all the way to the nucleus into the cells is called transfection. The DNA is then read out by the cell, and the biosynthesis of the encoded protein is turned on. Due to the fact that naked DNA cannot enter the cell due to its negative charge, a suitable delivery vehicle is required.6 The introduction of desirable genetic sequences into mammalian cells can be accomplished by viral, polymeric or liposomal agents, or by inorganic nanoparticles, e.g. gold, magnetite, and calcium phosphate. However, an optimal carrier for nucleic acids is still missing.7–14 Calcium phosphate is promising in this respect because of its unequivocal biocompatibility and biodegradability, and also because it is not subject to microbiological degradation like organic or polymeric carrier systems.The standard calcium phosphate transfection method, first introduced by Graham and van der Eb in 1973,15 is still used in biochemistry. It involves a straightforward in-situ precipitation of calcium phosphate/DNA aggregates. The main parameters such as reagent concentration, pH, temperature, way of mixing, and precipitation time are decisive for the efficiency of cell transfection and sometimes difficult to control. Chowdhury et al. prepared calcium phosphate nanoparticles with the addition of magnesium, achieving an inhibition of the particle growth and a higher transfection efficiency.16 The treatment of human mesenchymal stem cells with functionalized calcium phosphate nanoparticles enhanced the osteoblastic differentiation as Gonzalez-McQuire et al. have shown.17 A number of systems have been developed which use calcium phosphate nanoparticles for the delivery of nucleic acids (see the reviews by Maitra,7 Xu,18 Cai,19 and Sokolova13). Maitra proposed that calcium ions promote the transfer of DNA into the cell nucleus, therefore calcium may also be beneficial as a carrier due to its chemical composition.7 To obtain good colloidal stability, systems which involve calcium phosphate combined with polymers have been developed, e.g. with block copolymers20 or polysaccharides.21
DNA can be protected against intracellular attack by nucleases if it is enclosed within a calcium phosphate nanoparticle with an additional shell of calcium phosphate. This leads to a considerable increase in the transfection efficiency compared to single-shell calcium phosphate nanoparticles with a core of calcium phosphate and a shell of DNA (CaP-DNA). Triple shell nanoparticles (CaP-DNA-CaP-DNA) with a diameter of about 100 nm have been prepared.22,23
The pathway of DNA-carrying calcium phosphate nanoparticles in cells was shown by labelling them with red-fluorescing tetramethylrhodamin isothiocyanate/bovine serum albumin (TRITC/BSA). The penetration of the cell wall and the adsorption on the nuclear membrane were followed by confocal laser scanning microscopy, as well as the expression of enhanced green fluorescing protein (eGFP) after successful transfection (Fig. 1).24
 | ||
| Fig. 1 Transmission light microscopy (A) and fluorescence microscopy (B) of eGFP-transfected T24 cells. The DNA-carrying nanoparticles were labelled with red-fluorescing TRITC-BSA and can be seen on the nuclear membrane 48 h after transfection (arrow). | ||
Calcium phosphate nanoparticles for gene silencing
Acquired or inherited diseases may be effectively treated by inhibition of the protein synthesis using gene silencing techniques such as RNA interference (RNAi).25 In this cellular process, the expression of a gene can be sequence-specifically inhibited by applying double-stranded RNA (dsRNA). RNAi was originally discovered by Fire and Mello who introduced dsRNA into C. elegans and observed a reduced expression of the homologous gene. In 2006, Fire and Mello received the Nobel Prize for Medicine or Physiology for this discovery.26,27 Inside the cell, long dsRNA, e.g. short hairpin RNA (shRNA),28,29 is processed by the endonuclease Dicer into short fragments of about 21 nucleotides which are called small interfering RNAs (siRNAs). The siRNA is incorporated into the RNA-induced silencing complex (RISC) which is guided by the antisense strand of the siRNA to the complementary target messenger RNA (mRNA). Finally the mRNA is endonucleolytically cleaved so that no target mRNA is available any longer for further protein synthesis (Fig. 2A).30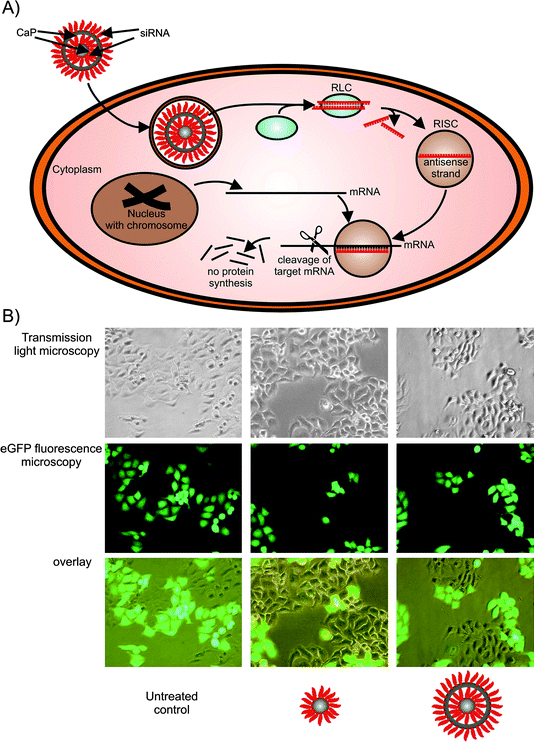 | ||
| Fig. 2 Calcium phosphate nanoparticles as tool for gene silencing. A) siRNA-functionalised calcium phosphate nanoparticles (triple-shell) are taken up by endocytosis. The siRNA escapes from the endosome, is recognized by the RISC loading complex (RLC) and the antisense strand guides the RNA-induced silencing complex (RISC) to its target mRNA. Finally, the target mRNA is cleaved and consequently the protein synthesis of this mRNA transcript is stopped. B) Single- as well as triple-shell siRNA calcium phosphate nanoparticles were used to silence the eGFP expression of stably transfected HeLa-eGFP cells (magnification 200× in all cases). | ||
Like DNA, siRNA alone cannot permeate the cell membrane due to its negative charge.6 Like with DNA transfection, the delivery strategies can be grouped into the nonviral delivery of synthetic siRNA and the delivery of shRNA-expressing DNA constructs by viral delivery systems. Examples of nonviral delivery systems for siRNA are lipofection, microinjection, and inorganic nanoparticles. There has been intensive work on developing biocompatible delivery approaches for a therapeutic application of siRNA.6,31 In addition, calcium phosphate nanoparticles were discussed (among other kinds of nanoparticles) as carriers for nucleic acids for genetic vaccination.32,33
As described above we have previously demonstrated the suitability of calcium phosphate nanoparticles for cellular DNA delivery, and we have also extended this system to siRNA delivery.34 Functionalized monodisperse colloids of calcium phosphate nanoparticles were successfully used, and there was no influence of the sequence of single-stranded or double-stranded oligonucleotides (25 nucleobases) on the colloidal stability. The particle size was in the range of about 100 nm, i.e. well-suited for cellular uptake. Human cervix epithelial cells (HeLa) which were genetically modified to stably express enhanced green fluorescent protein (eGFP) were used as a model system. We applied siRNA-functionalized calcium phosphate nanoparticles on these HeLa-eGFP cells in order to silence the eGFP expression which is quite easy to recognize by fluorescence microscopy (Fig. 2B). The gene silencing efficiency for single-shell particles (CaP-siRNA) was 27 ± 12% whereas triple-shell particles (CaP-siRNA-CaP-siRNA) achieved 48 ± 16%. Like with the DNA transfection experiments, the increased silencing efficiency of triple-shell particles can be explained by the protection of the inner siRNA layer from enzymatic degradation. These siRNA nanoparticles kept their biological activity for several weeks of storage, thereby enabling convenient handling procedures.
Calcium phosphate nanoparticles for drug delivery
In general, drug delivery is a promising application of nanocarriers. In this case the nanoparticles can be used to simultaneously achieve different goals: drug delivery, imaging, and therapeutic application of the solid carrier itself.35 The composition of the nanoparticles strongly depends on the target and can be divided into five main components (Fig. 3):35–37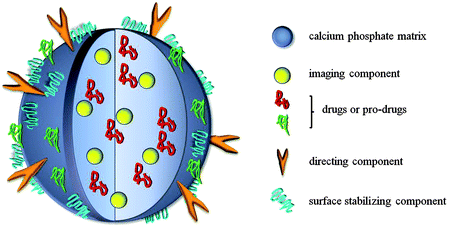 | ||
| Fig. 3 Generalized schematic setup of a calcium phosphate nanoparticle for both imaging and drug delivery. | ||
1. The matrix (or core) of the nanoparticle which often acts only as a carrier (e.g. with liposomes), but may also have therapeutic properties itself (e.g. magnetic nanoparticles for tumour hyperthermia therapy);38
2. One or several drugs or pro-drugs as active compounds which can be of different chemical nature, e.g. peptide, proteins, oligonucleotides;
3. A directing component which enables specific delivery of the particle to the targeted cell or tissue (e.g. antibodies or their fragments or other molecules which enhance the receptor-mediated endocytosis of the particle);
4. Surface-active components which ensure colloidal stability of the particle and can furthermore facilitate its penetration through the vessel epithelium or cell membrane (e.g. polymers, surfactants, charged molecules);
5. Imaging components that enable the visualization of a tumour or tracking of the nanoparticles. These may be present in the core or the shell of the nanoparticle.
In addition, particles can carry further components such as tumour-specific cleavable linkages. The desired properties of such nanoparticles strongly depend on their application and target tissue. A most important parameter is the particle size. Particles which are smaller than 100 nm are hardly recognized by the immune system and can be easily taken up by cells. On the other hand, they are big enough to escape renal filtration, thus providing longer circulating half-life and enhanced passive targeting of tumour tissues.39 If the targeted cells are phagocytes, a micrometer size of the particles is preferable.31 Krishnamachari and Salem reviewed the activation of dendritic cells by oligonucleotides: the uptake of oligonucleotides leads to the maturation of dendritic cells and better presentation of otherwise undetectable antigen leading to an efficient immune response.31 Other important parameters for delivery systems are the half-life time in the circulation, the stability in blood and the surface charge for penetration through the blood vessel epithelium and targeted cellular membrane. In addition, the biocompatibility and biodegradability of all components must be taken into account.
As drug carriers, calcium phosphate nanoparticles have some advantageous properties. They are dissolved at low pH (around 4), e.g. in lysosomes after the cellular intake40 or in the environment of solid tumours, thereby releasing incorporated drugs or biomolecules. Their size can easily be controlled by stabilizing agents, such as polymers or nucleic acids. The nanoparticles can be made to fluoresce by the incorporation of lanthanide ions,41,42 and they can also act as carriers for different drugs, e.g. insulin,43 cisplatin,44 or ceramide.45 Liu et al. have shown by in vivo experiments that calcium phosphate nanoparticles, carrying a suicide gene, can treat nasopharyngeal carcinoma.46
As an example, we discuss the application of calcium phosphate nanoparticles in photodynamic therapy (PDT). Photodynamic therapy is an emerging method in the treatment of tumours and bacterial biofilms. By this method, a light-sensitive species is brought into the malignant tissue and irradiated with a laser source.47 This irradiation leads to an electronic excitation of the photoactive dye, followed by an energy transfer to dissolved triplet oxygen which is transformed into singlet oxygen. Singlet oxygen is the active species which destroys malignant cells or bacteria.48 The hydrophobic character of the common photoactive dyes is a drawback with respect to the administration and also causes pain to the patient due to the injection of an alcoholic dispersion.49 Therefore, current research is seeking water-based formulations. Typical drug-delivery systems are liposomes, hydrophilic drug-loaded polymer complexes, polymer-based micellar systems and lipoproteins. An inorganic nanoparticle consisting of calcium phosphate can be surface-functionalized to form a stable colloid which can be easily achieved with suitable polymers.50,51 Calcium phosphate nanoparticles can be stabilized in aqueous media with different polyelectrolytes which can incorporate the photoactive dye (Fig. 4).52 It is also possible to prepare nanocapsules based on calcium phosphate nanoparticles which are coated with alternating layers of cationic and anionic polyelectrolytes (layer-by-layer method). The calcium phosphate core can then be extracted by a combination of acidification, dialysis, and ion exchange.53
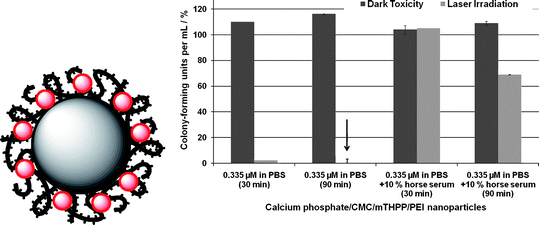 | ||
| Fig. 4 Left: A polymer-stabilized calcium phosphate particle incorporating a photoactive dye (red). Right: Photodynamic activity of calcium phosphate nanoparticles, coated with an inner layer of carboxymethylcellulose (CMC) incorporating 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin (mTHPP) and an outer layer of poly(ethyleneimine) (PEI), against the gram-negative bacterium Pseudomonas aeruginosa. The particles were dispersed either in pure phosphate buffered saline solution (PBS) or in PBS supplemented with 10% horse serum. Shown are the dark toxicities of the nanoparticles and the toxicity under laser irradiation. The incubation time with the nanoparticles before irradiation was either 30 or 90 min. As control, pure PBS was used (100% of colony-forming units; CFU). The photosensitizer mTHPP alone is not active against this bacterial strain. | ||
For the preparation we used methylene blue and 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin (mTHPP) as model photosensitizers and different polyelectrolytes, e.g. carboxymethylcellulose, poly(ethyleneimine), poly(styrene sulfonate) and polyallylamine-hydrochloride. By variation of the polymer it is possible to adjust the charge of the nanoparticle and to regulate the cell membrane penetration. The formulations were tested against different cell lines and bacteria.52 Against the bacterial strain S. aureus, positively as well as negatively charged mTHPP-doped particles showed good killing (note that “killing” is defined as the difference between dark toxicity and the toxicity under laser irradiation). In the case of the gram-negative bacterium P. aeruginosa, positively charged particles loaded with mTHPP showed good results (Fig. 4). However, a general problem of the in vivo application of nanoparticles is obvious from these results: administration in the purely inorganic medium PBS gave excellent results but the addition of the protein-containing horse serum strongly decreased the activity. This is probably due to the fact that the nanoparticles agglomerate in the presence of proteins and that their surface is also coated by proteins. This obviously strongly reduces the uptake by bacteria and the photodynamic activity.
Fluorescing calcium phosphate nanoparticles for imaging
Imaging of tissues or intracellular structures is an important issue in medicine.35,54–56 Calcium phosphate nanoparticles can be used as fluorescing probes after doping with lanthanides41,42,57–59 or surface-functionalization with organic dyes.52,60–63Small amounts of doped lanthanide ions in calcium phosphate can determine the fluorescence efficiency within narrow emission band widths (Fig. 5). Different colours were achieved by doping with different lanthanide ions, e.g. europium-doped calcium phosphate nanoparticles emit red light and terbium-doped particles emit green light.42 However, the fluorescence of lanthanide ions requires some degree of crystallinity, i.e. it is very weak in amorphous nanoparticles, and a compromise must be found between small particle size and sufficient crystallinity.
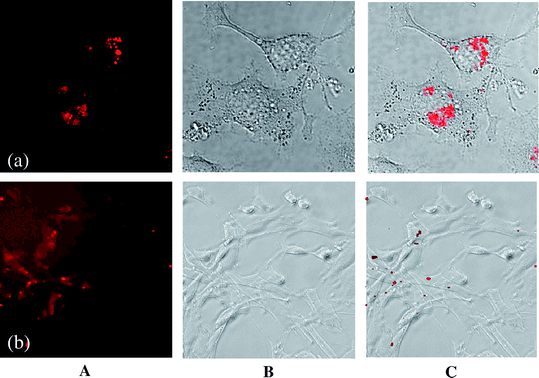 | ||
| Fig. 5 Confocal laser scanning micrographs (A), optical micrographs (transmission mode) (B) and overlay (C) of both micrographs of europium-doped42 (a) and porphyrin-functionalized61 calcium phosphate nanoparticles (b) in cell culture. (a) T-HUVEC endothelial cells; (b) NIH 3T3 fibroblasts. | ||
Another option is the incorporation of fluorescing molecules like porphyrins into the coating layers, similar to the above experiments in photodynamic therapy.62 Antinoglu et al. have prepared calcium phosphate nanoparticles for in vivo imaging of breast cancer.60 We have prepared a porphyrin with four phosphate groups that was able to bind to the surface of calcium phosphate nanoparticles and to keep the particles in dispersion due to electrostatic repulsion.61 The molecule acted both as stabilizing agent and as fluorescing molecule. The biocompatibility of such porphyrin-functionalized particles was confirmed with NIH 3T3 fibroblast cells, and the particles were also easily taken up by cells without any adverse effects (Fig. 5). Therefore, these biocompatible nanoparticles can be used for tracking and detecting the particles in the cell because of their high efficiency of fluorescence. They may also be used for the delivery of the fluorescing dye into the cell.
Conclusion and outlook
The use of calcium phosphate nanoparticles as carriers of compounds into cells is possible if they are suitably stabilized in colloidal form by charged molecules (including oligonucleotides) or polymers (including DNA and polyelectrolytes). In general, they possess high biocompatibility. With a size of about 100 nm, they are able to penetrate the outer membrane of cells and bacteria. The uptake of calcium phosphate nanoparticles into cells can be monitored if they are made fluorescing, either by doping the inorganic core or by adding fluorescing dyes. Typically, they are dissolved in the acidic lysosomes inside the cell which may lead to a harmful increase in the intracellular calcium level.64 For unfunctionalized nanoparticles, i.e. added to cell culture as a solid agglomerate, cell toxicity for smooth muscle cells was observed which was ascribed to the increased intracellular calcium level.40 However, we never observed adverse effects when the nanoparticles were present in functionalized colloidal form,23,34,42,52,61,65,66 probably because the number of ingested particles was smaller in this case and the calcium was rapidly excreted. The only exception were macrophages where colloidal particles were also lethal, possibly due to these cells' tendency to take up large amounts of any kind of particle.52 In contrast to metallic nanoparticles, semiconductor quantum dots, carbon nanotubes, and many polymeric systems, the fate of calcium phosphate nanoparticles in the body is predictable, and adverse effects are not expected.A fundamental insight into the nature of surface-functionalized nanoparticles is still missing, despite the application of modern methods.4,19,51,67,68 This is an unsolved problem in nanoparticle research as recently pointed out by Grainger and Castner.69 For in vivo applications, there is the general problem of the change of the dispersion medium from aqueous dispersion over inorganic physiological solutions (like PBS) to cell culture media (containing also proteins) and finally to blood or tissue. If the ion strength of the solution is increased, electrostatic repulsion between the particles decreases and agglomeration can occur. If proteins are present, they will probably adsorb on the particle surface and change their charge, hydrophilicity and surface chemistry. Thus, a functionalized particle in the bloodstream or in a tissue will behave differently than in pure water. This problem has to be overcome for a final clinical application. Finally, the practical questions of sterilization and storage have to be solved.
In conclusion, there are still many open questions concerning the clinical application of nanoparticles in general. As calcium phosphates are highly biocompatible, can be easily functionalized, and have a predictable fate in the body (i.e. dissolution), they are expected to play a major role in the future of nanomedicine. However, the problem of regulatory issues should not be underestimated because the biological properties of nanoparticles often completely differ from the corresponding bulk materials, and little is still known about the fundamental properties of nanoparticles.69,70 If the particles are modified, e.g. in the form of a composite with polymers or fluorescing molecules, the physico-chemical situation becomes even more complicated, and the biological outcome becomes less predictable. For any new developments in which calcium phosphate is combined with other compounds, it will be necessary to consider the biocompatibility of any new component.
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for financial support of this project.References
- K. Riehemann, S. W. Schneider, T. A. Luger, B. Godin, M. Ferrari and H. Fuchs, Angew. Chem., Int. Ed., 2009, 48, 872 CrossRef CAS.
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130 CrossRef CAS.
- M. Epple and E. Baeuerlein (Ed.), Biomineralisation: Medical and Clinical Aspects, Wiley-VCH, Weinheim, 2007 Search PubMed.
- C. Rey, C. Combes, C. Drouet, H. Sfihi and A. Barroug, Mater. Sci. Eng., C, 2007, 27, 198 CrossRef CAS.
- M. Vallet-Regi, Dalton Trans., 2006, 5211 RSC.
- D. Reischl and A. Zimmer, Nanomedicine, 2009, 5, 8.
- A. Maitra, Expert Rev. Mol. Diagn., 2005, 5, 893 Search PubMed.
- I. R. Gilmore, S. P. Fox, A. J. Hollins and S. Akhtar, Curr. Drug Delivery, 2006, 3, 147 Search PubMed.
- K. Kodama, Y. Katayama, Y. Shoji and H. Nakashima, Curr. Med. Chem., 2006, 13, 2155 CrossRef CAS.
- S. E. McNeil and Y. Perrie, Expert Opin. Ther. Pat., 2006, 16, 1371 Search PubMed.
- C. A. Jewell and D. M. Lynn, Adv. Drug Delivery Rev., 2008, 60, 979 CrossRef CAS.
- M. Morille, C. Passirani, A. Vonarbourg, A. Clavreul and J. P. Benoit, Biomaterials, 2008, 29, 3477 CrossRef CAS.
- V. Sokolova and M. Epple, Angew. Chem., Int. Ed., 2008, 47, 1382 CrossRef CAS.
- L. Hu, Z. Mao and C. Gao, J. Mater. Chem., 2009, 19, 3108 RSC.
- F. L. Graham and A. J. van der Eb, Virology, 1973, 52, 456 CrossRef CAS.
- E. H. Chowdhury, M. Kunou, M. Nagaoka, A. K. Kundu, T. Hoshiba and T. Akaike, Gene, 2004, 341, 77 CrossRef CAS.
- R. Gonzalez-McQuire, D. W. Green, K. A. Partridge, R. O. C. Oreffo, S. Mann and S. A. Davis, Adv. Mater., 2007, 19, 2236 CrossRef CAS.
- Z. P. Xu, Q. H. Zeng, G. Q. Lu and A. B. Yu, Chem. Eng. Sci., 2006, 61, 1027 CrossRef CAS.
- Y. Cai and R. Tang, J. Mater. Chem., 2008, 18, 3775 RSC.
- Y. Kakizawa, S. Furukawa, A. Ishii and K. Kataoka, J. Controlled Release, 2006, 111, 368 CrossRef CAS.
- D. W. Green, S. Mann and R. O. C. Oreffo, Soft Matter, 2006, 2, 732 RSC.
- V. Sokolova, O. Prymak, W. Meyer-Zaika, H. Cölfen, H. Rehage, A. Shukla and M. Epple, Materialwiss. Werkstofftech., 2006, 37, 441 Search PubMed.
- V. V. Sokolova, I. Radtke, R. Heumann and M. Epple, Biomaterials, 2006, 27, 3147 CrossRef CAS.
- V. Sokolova, A. Kovtun, R. Heumann and M. Epple, JBIC, J. Biol. Inorg. Chem., 2007, 12, 174 CrossRef CAS.
- J. Kurreck, Angew. Chem., Int. Ed., 2009, 48, 1378 CrossRef CAS.
- A. Z. Fire, Angew. Chem., Int. Ed., 2007, 46, 6966 CrossRef CAS.
- C. C. Mello, Angew. Chem., Int. Ed., 2007, 46, 6985 CrossRef CAS.
- T. R. Brummelkamp, R. Bernards and R. Agami, Cancer Cell, 2002, 2, 243 CrossRef CAS.
- A. Vermeulen, L. Behlen, A. Reynolds, A. Wolfson, W. S. Marshall, J. Karpilow and A. Khvorova, RNA-a Publication of the RNA Society, 2005, 11, 674 Search PubMed.
- S. M. Elbashir, J. Harborth, W. Lendeckel, A. Yalcin, K. Weber and T. Tuschl, Nature, 2001, 411, 494 CrossRef CAS.
- Y. Krishnamachari and A. K. Salem, Adv. Drug Delivery Rev., 2009, 61, 205 CrossRef CAS.
- Q. He, A. R. Mitchell, S. L. Johnson, C. Wagner-Bartak, T. Morcol and S. J. D. Bell, Clin. Diagn. Lab. Immunol., 2000, 7, 899 CAS.
- Z. R. Cui and R. J. Mumper, Crit. Rev. Ther. Drug Carrier Syst., 2003, 20, 103 CrossRef CAS.
- V. Sokolova, A. Kovtun, O. Prymak, W. Meyer-Zaika, E. A. Kubareva, E. A. Romanova, T. S. Oretskaya, R. Heumann and M. Epple, J. Mater. Chem., 2007, 17, 721 RSC.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889 CrossRef CAS.
- R. Sinha, G. J. Kim, S. Nie and D. M. Shin, Mol. Cancer Ther., 2006, 5, 1909 CrossRef CAS.
- P. R. Gil and W. J. Parak, ACS Nano, 2008, 2, 2200 CrossRef CAS.
- V. I. Shubayev, T. R. Pisanic and S. Jin, Adv. Drug Delivery Rev., 2009, 61, 467 CrossRef CAS.
- F. Yuan, Semin. Radiat. Oncol., 1998, 8, 164 CAS.
- A. E. Ewence, M. Bootman, H. L. Roderick, J. N. Skepper, G. McCarthy, M. Epple, M. Neumann, C. M. Shanahan and D. Proudfoot, Circ. Res., 2008, 103, e28 CrossRef CAS.
- A. Doat, F. Pelle, N. Gardant and A. Lebugle, J. Solid State Chem., 2004, 177, 1179 CrossRef CAS.
- S. Padilla Mondejar, A. Kovtun and M. Epple, J. Mater. Chem., 2007, 17, 4153 RSC.
- R. Ramachandran, W. Paul and C. P. Sharma, J. Biomed. Mater. Res., Part B, 2009, 88b, 41 CrossRef CAS.
- X. Cheng and L. Kuhn, Int. J. Nanomed., 2007, 2, 667 CAS.
- M. Kester, Y. Heakal, T. Fox, A. Sharma, G. P. Robertson, T. T. Morgan, E. I. Altinoglu, A. Tabakovic, M. R. Parette, S. M. Rouse, V. Ruiz-Velasco and J. H. Adair, Nano Lett., 2008, 8, 4116 CrossRef CAS.
- T. Liu, A. Tang, G. Y. Zhang, Y. X. Chen, J. Y. Zhang, S. S. Peng and Z. M. Cai, Cancer Biother. Radiopharm., 2005, 20, 141 CrossRef CAS.
- R. Bonnett, P. Charlesworth, B. D. Djelal, S. Foley, D. J. McGarvey and T. G. Truscott, J. Chem. Soc., Perkin Trans. 2, 1999, 325 RSC.
- R. Bonnett, Chemical aspects of photodynamic therapy, ed. D. Phillips, Gordon and Breach, London, 2000 Search PubMed.
- Y. N. Konan, R. Gruny and E. Allemann, J. Photochem. Photobiol., B, 2002, 66, 89 CrossRef CAS.
- H. Urch, S. Franzka, D. Dahlhaus, N. Hartmann, E. Hasselbrink and M. Epple, J. Mater. Chem., 2006, 16, 1798 RSC.
- H. Urch, M. Vallet-Regi, L. Ruiz, J. M. Gonzalez-Calbet and M. Epple, J. Mater. Chem., 2009, 19, 2166 RSC.
- J. Schwiertz, A. Wiehe, S. Graefe, B. Gitter and M. Epple, Biomaterials, 2009, 30, 3324 CrossRef CAS.
- J. Schwiertz, W. Meyer-Zaika, L. Ruiz-Gonzalez, J. M. Gonzales-Calbet, M. Vallet-Regi and M. Epple, J. Mater. Chem., 2008, 18, 3831 RSC.
- A. P. Alivisatos, W. W. Gu and C. Larabell, Annu. Rev. Biomed. Eng., 2005, 7, 55 CrossRef CAS.
- Y. Fukumori and H. Ichikawa, Adv. Powder Technol., 2006, 17, 1 Search PubMed.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578 CrossRef CAS.
- J. Y. Chane-Ching, A. Lebugle, I. Rousselot, A. Pourpoint and F. Pellé, J. Mater. Chem., 2007, 17, 2904 RSC.
- A. Lebugle, F. Pellé, C. Charvillat, I. Rousselot and J. Y. Chane-Ching, Chem. Commun., 2006, 606 RSC.
- F. Wang, W. B. Tan, Y. Zhang, X. P. Fan and M. Q. Wang, Nanotechnology, 2006, 17, R1 CrossRef.
- E. I. Antinoglu, T. J. Russin, J. M. Kaiser, B. M. Barth, P. C. Eklund, M. Kester and J. H. Adair, ACS Nano, 2008, 2, 2075 CrossRef CAS.
- K. Ganesan, A. Kovtun, S. Neumann, R. Heumann and M. Epple, J. Mater. Chem., 2008, 18, 3655 RSC.
- T. T. Morgan, H. S. Muddana, E. I. Altinoglu, S. M. Rouse, A. Tabakovic, T. Tabouillot, T. J. Russin, S. S. Shanmugavelandy, P. J. Butler, P. C. Eklund, J. K. Yun, M. Kester and J. H. Adair, Nano Lett., 2008, 8, 4108 CrossRef CAS.
- H. S. Muddana, T. T. Morgan, J. H. Adair and P. J. Butler, Nano Lett., 2009, 9, 1559 CrossRef CAS.
- M. P. Mattson and S. L. Chan, Nat. Cell Biol., 2003, 5, 1041 CrossRef CAS.
- M. Wiemann, D. Bingmann, S. Franzka, N. Hartmann, H. Urch and M. Epple, Adv. Eng. Mater., 2007, 9, 1077 CrossRef.
- T. Welzel, I. Radtke, W. Meyer-Zaika, R. Heumann and M. Epple, J. Mater. Chem., 2004, 14, 2213 RSC.
- C. Jaeger, T. Welzel, W. Meyer-Zaika and M. Epple, Magn. Reson. Chem., 2006, 44, 573 CrossRef.
- C. Rey, C. Combes, C. Drouet and M. J. Glimcher, Osteoporosis Int., 2009, 20, 1013 Search PubMed.
- D. W. Grainger and D. G. Castner, Adv. Mater., 2008, 20, 867 CrossRef CAS.
- S. J. Choi, J. M. Oha and J. H. Choy, J. Mater. Chem., 2008, 18, 615 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
