 Open Access Article
Open Access ArticleSustainable selective oxidations using ceria-based materials
Jurriaan Beckers and Gadi Rothenberg*
Van’t Hoff Institute for Molecular Sciences, University of Amsterdam, Nieuwe Achtergracht 166, 1018, WV, Amsterdam, The Netherlands. E-mail: G.Rothenberg@uva.nl; Web: http://www.science.uva.nl/~gadi Fax: +31 20 525 5604
First published on 9th April 2010
Abstract
This Perspective covers sustainable oxidation processes using doped cerias, ceria-supported catalysts and ceria-based mixed oxides. Firstly, we consider the general properties of ceria-based catalysts. We outline the advantages of the ceria redox cycle, and explain the dynamic behaviour of these catalysts in the presence of metal additives and dopants. We then review three types of catalytic oxidation processes: preferential CO oxidation (PROX), oxidative dehydrogenation and the selective oxidations of hydrocarbons and inorganics. The preferential oxidation of CO from hydrogen-rich mixtures is interesting for fuel cell applications. Copper-ceria catalysts, where the copper species are well dispersed and interact strongly with the ceria surface, show good selectivity and activity. The oxidative dehydrogenation of small alkanes is another important potential application. To avoid mixing oxygen and hydrocarbons at high temperatures, one can run the dehydrogenation over a conventional Pt/Sn catalyst while burning the hydrogen formed using the ceria lattice oxygen. This safer redox process allows separate tuning of the dehydrogenation and hydrogen combustion stages. A combinatorial screening showed good results for ceria doped with Pb, Cr or Bi, amongst others. Finally, we also discuss the catalytic selective oxidation of various hydrocarbons, oxygenates and inorganic molecules (H2S, H2 and NH3). The goal here is either product valorisation or waste stream purification. Ceria-based materials show promise in a variety of such selective oxidations.
 Jurriaan Beckers | Jurriaan Beckers (Reimerswaal, 1972) obtained his bachelor's degree at the Hogeschool Zeeland in Vlissingen, after which he worked in the research departments of several companies and governmental institutes. In 2009, he obtained his PhD at the University of Amsterdam. Dr Beckers is now working as a senior technologist at Avantium, a research and technology company based in Amsterdam that focuses on high throughput catalysis research. |
 Gadi Rothenberg | Gadi Rothenberg (Jerusalem, 1967) is Professor and Chair of heterogeneous catalysis and sustainable chemistry at the University of Amsterdam, and teaches courses on catalysis, thermodynamics and scientific writing. He has published over 100 papers in peer-reviewed journals and discovered two catalysts, for which he received the Marie Curie Excellence Award in 2004 and the Paul Rylander Award in 2006. Rothenberg also invented a method for monitoring pollutants in water, and co-founded the companies Sorbisense and Yellow Diesel. In 2007, he was voted teacher of the year by the chemistry students. He is married, owns no car, and has a black belt in Shaolin Kung-Fu. |
1. Introduction
Cerium was discovered in 1803 by Jöns Jakob Berzelius and Wilhelm Hisinger in Sweden, and Martin Heinrich Klaproth in Germany.1 It was named after the dwarf planet Ceres, discovered in 1801, that was, in turn, named after the Roman goddess of agriculture (particularly the growth of cereals).2 A light rare-earth element, Ce is the main component of the minerals Bastnasite (USA, China), Monazite (Australia) and Loparite (Russia).3 Cerium or cerium oxide (“ceria”) is used in various applications, such as removing free oxygen and sulfur from the iron casting melts, as a polishing agent for glass and as a glass decolourant.3,4 Due to their good ionic conduction, ceria-based materials are also applied as electrolytes in solid oxide fuel cells.5 Since the 1980s, the most successful industrial application of ceria has been as an oxygen storage material in automotive three-way catalytic converters (TWCs).6-8 In 2003, TWC sales accounted for one quarter of the global catalyst market.9 The success of ceria in TWCs is due to its temperature stability and the facile Ce3+⇆ Ce4+ + e− redox cycle.10 In the catalytic converter, this aids both hydrocarbon combustion in the fuel-rich mode and NO reduction in the fuel-lean mode. The application of ceria-based materials in TWCs has been reviewed by several authors.9,11,12 An excellent review on the physical and (electro-) chemical properties of ceria-based oxides was published by Mogensen et al. in 2001.13 Trovarelli and co-workers did a lot of work on the (redox) chemistry and catalysis of ceria-based materials,10,14,15 publishing a book on the subject in 2002.3Since 2000, much research has been devoted to alternative power sources and alternative fuels. Interestingly, here, ceria-based materials have also gained attention. But, contrary to TWCs, recent papers have focused on selective oxidation applications rather than full combustion. Many of these deal with the selective oxidation of CO from hydrogen (preferential oxidation, PROX), often using copper-ceria to “clean up” the hydrogen used in fuel cells (this is essential as CO poisons the Pt catalyst at low temperatures). Ceria-based oxides are also used in the oxidative dehydrogenation (ODH) of ethane and propane, as well as for the selective oxidations of other hydrocarbons and inorganics.
Recent reviews cover the use of ceria in TWCs and in syngas production.14,16,17 We therefore focus on the application of ceria-based materials in selective oxidation over the period 1998–2008. This mini-review centres on the catalytic behaviour of ceria-based materials, rather than characterisation. Note that ceria can act as a support for “active metals”, but at low concentrations these metals also dope in the fluorite lattice, forming “solid solutions”. Importantly, the ceria redox cycle often leads to a strong metal–support interaction (SMSI), which can affect the catalysis. We thus start by explaining this interaction and outline some basic properties relevant to catalysis, before discussing the role of ceria in selective oxidations.
2. General considerations, properties and pitfalls
There are various routes for synthesising ceria,3 but the simplest is by calcining cerium nitrate, Ce(NO3)3.18 This salt melts at about 65 °C, followed by dehydration and, from about 200 °C onwards, nitrate decomposition.19,20 A temperature of 400 °C is sufficient to form the ceria fluorite structure.20 High surface area cerias can be obtained by using sol–gel- or surfactant-assisted synthesis, yielding 125–230 m2 g−1.21–23 The sintering behaviour of ceria depends on the atmosphere (see Fig. 1), so when used under reducing conditions, it can still sinter, even below its calcination temperature. | ||
| Fig. 1 The BET surface area of plain ceria heated under various conditions.24 All data pertain to the same batch of ceria. | ||
Ceria can be used as a support for active metals, but metals can also be doped into the ceria lattice. Doping atoms into the lattice allows tuning of the oxygen conduction, electronic conduction, and with them the catalytic properties.13,25 The distinction between an active metal supported on ceria or doped into the lattice is often unclear, especially at elevated temperatures and/or in the presence of reducing gases. The ceria redox cycle can lead to a strong metal–support interaction.26–28 Phase segregation and/or changes in phase composition can occur for ceria-based solid solutions and mixed oxides.29 Thus, the actual active site often changes during catalysis. For example, when nickel supported on ceria is reduced at 750 °C, the nickel crystallites can spread over the reduced support (see Scheme 1).26 Conversely, at too-high doping levels or temperatures, surface enrichment or phase segregation can occur.30–33 Confusingly, the spreading of nickel crystallites over the ceria surface, in the case of a ceria support, and surface enrichment, in the case of nickel-doped ceria, can lead to similar surface structures. These effects also complicate catalyst characterisation. Lattice doping can be seen by using XRD, EXAFS or EPR.31,34–37 This does not exclude, however, that the surface where catalysis takes place may be dopant enriched. Surface sensitive techniques such as LEIS and XPS can detect surface enrichment, and in case of XPS, oxidation states.32 Their signal, however, is still the average of the entire catalyst surface, which can complicate the analysis when several types of surface species are present.
 | ||
| Scheme 1 A cartoon showing the spreading of nickel supported on ceria upon reduction at 750 °C. Re-drawn from ref. 26. | ||
Another type of SMSI is so-called “decoration” by ceria. This is seen upon reduction to 600–700 °C. The supported metal crystals stay intact, but are decorated with a layer of ceria, shielding the metal surface and thereby affecting the catalysis (see Fig. 2).27 Note that bulk techniques such as X-ray diffraction cannot observe decoration effects.
3. Preferential CO oxidation (PROX)
Electrochemical energy can be obtained from hydrogen by polymer electrolyte (or proton exchange) membrane fuel cells (PEMFC).38 The hydrogen generally comes from the steam reforming or partial oxidation of light hydrocarbons, followed by a water gas shift reaction.39 The effluents of these processes contain >100 ppm of CO, which poisons the fuel cell anodes. Because of this, catalytic preferential CO oxidation from hydrogen-rich gas mixtures has gained a lot of attention.38 It can be achieved by supported noble metal catalysts, such as Pt, Pd or Rh on alumina, or Au on iron oxide.38 Indeed, commercial systems containing Pt are available, but these are expensive.38 Alternatively, copper supported on ceria40–44 and copper-ceria mixed oxides or copper doped cerias have been studied.45–56 Several groups have compared these catalyst types.57–60Gamarra et al. found higher activities for both CO and H2 oxidation when using a mixed oxide catalyst instead of copper on ceria. The higher activity of the mixed oxide, however, generally meant a lower selectivity.57 This was ascribed to a higher copper dispersion and easier oxygen vacancy formation of the mixed oxide, which gives a high activity for both CO and hydrogen oxidation. Characterisation using DRIFTS and XANES showed that the H2 oxidation occurred after reduction of the copper to Cu+, indicating that the active sites are partially reduced dispersed copper species.61 This was also proposed by Tada et al.54 and Snytnikov et al.58 At temperatures >150 °C, the mixed oxide showed the highest selectivity. Testing 30 catalysts, Tada et al. show that high selectivities could be obtained by using both supported and mixed oxide copper-ceria catalysts.54 The highest activities were found, however, using a mixed oxide catalyst. Avgouropoulos et al. have also compared the effect of the synthesis method on the catalytic properties of copper-ceria catalysts.59 Their CO PROX data, in the presence of water and CO2, showed that the highest activity, selectivity and stability were achieved when using mixed oxides. The superior performance of these catalysts was ascribed to well dispersed copper species that strongly interacted with the ceria surface.60,62
Studies on different supports showed that the activity of copper on ceria, and of copper-ceria mixed oxides, was much higher than of copper on alumina or silica, plain copper oxide, or plain ceria.55,63 To increase dispersion, alumina was also added to copper-ceria catalysts,64,65 but a direct comparison showed that whilst the activity remained high, the selectivity was lower.66 Replacing either the copper or the ceria by other metal (oxides) resulted in lower activities.47,51,67
Several authors have studied the effect of adding a third metal to copper-ceria mixed oxides. Iron, nickel or tin can increase the activity and selectivity of copper-ceria catalysts, although the CO oxidation occurs over copper species.42,56,68 Doping copper-ceria with Pt can increase the activity, but no beneficial effect on the selectivity was observed when doping with Pt, Ru, Pd, Ni or Co.69,70 Chen et al. found increased activity upon adding zirconia, but only at low concentration (10 mol%).63
Although the above reports show that copper-ceria-based catalysts can give high CO PROX activities and selectivities, important parameters for practical application are long term stability (in feeds containing H2O and CO2), fast CO conversion and, of course, a low CO effluent concentration. These data are often missing. The CO effluent concentration, for example, is seldom reported.70–72 Indeed, this is zero at full CO conversion, but at the typically used feed concentration of 1% v/v, a conversion of 99% yields a CO effluent concentration of 100 ppm, which is at the top of the acceptable 10–100 ppm range for fuel cell feeds (and still much too high for Pt anodes). Stability data is given in some publications, but not always in H2O and CO2-containing gas feed.64,72,73 The stability tests with H2O and CO2-containing gas feed showed little drop in conversion when performed at full CO conversion (copper-ceria52,74 or copper on tin doped ceria catalysts66,68). The selectivity was also not affected. Kim et al., however, did find a significant decrease in activity of a copper-ceria mixed oxide catalyst running at 60% CO conversion.72 The activity was completely restored, however, by heating for 3 h in an inert gas, possibly due to the accumulation of carbonate species.
4. Oxidative dehydrogenation (ODH)
The demand for small alkenes is high. Worldwide propene demand, for example, is expected to rise to 80 million tonnes in 2010.75–77 The main routes to propene are steam cracking, fluid catalytic cracking and catalytic dehydrogenation. The latter enables the on-demand production of alkenes but is equilibrium limited.78 Oxidative dehydrogenation (ODH), where oxygen is added to the gas feed, allows exothermic, on-demand alkene production.78 Furthermore, the added oxygen limits coking. It can, however, result in the over-oxidation of hydrocarbons to CO and CO2.79 This is a big challenge, since the alkene product is more reactive than the alkane starting material. Thus, specific ODH catalysts have been developed, and until now, (supported) vanadium or molybdenum oxides have held sway.78 Ethane ODH gives comparable yields to those of steam cracking, but propane ODH yields are still far from being attractive to industry. In both cases, little has been reported on catalyst lifetime.78 In the search for better ODH catalysts, various ceria-based materials have also been investigated. The results are summarised in the following sections.4.1 Oxidative dehydrogenation of ethane
Table 1 gives the catalyst composition and catalytic performance of ceria-based materials in ethane ODH. Solid solutions or mixed oxides containing metal M and ceria are denoted as “Ce–M–O”. Metal M supported on ceria is denoted as “M/CeO2”. The catalysts generally give a high selectivity at low conversion, and lower selectivities at high conversion. We have incorporated the data of the highest selectivity, highest activity and highest overall performance in the table, where available. Fig. 3 shows an activity vs. selectivity plot.| Catalyst | Catalyst composition | Concentration of added metal | Alkane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2a O2a | Space velocity/ ml g−1 h−1 | T/°C | Ethane conversion (%) | Selectivity towards ethene (%) | Ref.e |
|---|---|---|---|---|---|---|---|---|
a CO2 where applicable.b Steam was added at a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H6 = 1 C2H6 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.c The same performance was seen at 510 and 590 °C.d This is the value obtained after pre-treating the catalyst at 750 °C under the reaction conditions. The fresh catalyst has a selectivity of ∼55%. The increase in selectivity is irreversible.e Some of the data is derived from figures, rather than tables, in these references. 1 molar ratio.c The same performance was seen at 510 and 590 °C.d This is the value obtained after pre-treating the catalyst at 750 °C under the reaction conditions. The fresh catalyst has a selectivity of ∼55%. The increase in selectivity is irreversible.e Some of the data is derived from figures, rather than tables, in these references. | ||||||||
| 1 | Sr/CeO2 | 10 mol% | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar) 1 (molar) | 10200 | 700 | 18 | 56b | 84 |
| 2 | 800 | 50 | 88b | |||||
| 3 | SrCl2/CeO2 | 30 mol% | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 6000 | 660 | 73 | 69 | 13 |
| 4 | V/CeO2 | 3 wt% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | 90000 | 510 | 1 | 69 | 86–88 |
| 5 | 3 wt% | 590 | 9 | 38 | ||||
| 6 | 1 wt% | 590 | 19 | 20 | ||||
| 7 | CeO2 ref. | 550c | 66 | 4 | ||||
| Using CO2 instead of oxygen: | ||||||||
| 8 | Ce–Ca–O | 10 mol% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CO2) 2 (CO2) | 12000 | 650 | 3 | 98d | 82 |
| 9 | 750 | 25 | 90 | |||||
| 10 | CeO2 ref. | 750 | 41 | 71 | ||||
| Non-ceria-based reference catalyst: | ||||||||
| 11 | Mo–V–Te–Nb | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17 0.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17 0.17 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 | 100 | 400 | 87 | 84 | 89 |
 | ||
| Fig. 3 Activity and selectivity in ethane ODH. The labels show the type of metal added to the ceria and the corresponding catalyst number in Table 1. An Mo–V–Te–Nb mixed oxide catalyst (11) is shown for reference. | ||
The data show that the best catalysts, achieving the highest selectivity and activity, are calcium-doped 9, supported strontium 2 and 3, and plain ceria 10. Interestingly, most of these catalysts were tested under special conditions: steam was added in the case of 2, and the ODH was performed with CO2 instead of oxygen in the cases of 9 and 10.80,81 The ODH of these catalysts was performed at rather high temperatures (≥750 °C), higher than the temperature needed for (endothermic) catalytic dehydrogenation.82 Vanadium-containing catalysts 4–6 ran below 600 °C, but also had lower activities and selectivities. Supported 3 had a rather high selectivity and activity at 660 °C, running without added steam or CO2.83 However, their performance fell short compared to the best reference catalyst (11), a V- and Mo-containing mixed oxide that also operates at a lower temperature (400 °C), and without steam and/or CO2 (see Table 1 and Fig. 3). Note that far less research has been performed on ceria-based materials. No data is available, for example, for molybdenum-containing cerias, although the data on Ce–V–O shows that using components of the benchmark reference catalyst does not guarantee good performance.
4.2 Oxidative dehydrogenation of propane
The success of nickel-containing catalysts in propane and isobutane ODH inspired the testing of nickel-ceria catalysts in propane ODH (see Fig. 4).90,91 The group of Barbaux showed that this allows lower reaction temperatures compared to non-ceria-based nickel catalysts (300 °C).90 When comparing ceria-nickel mixed oxides with nickel on ceria, they found that the latter gave higher selectivity but lower conversion (compare 12 with 13 and 14 in Table 2). Nickel-containing mixed oxides gave higher yields than those with either Cr, Co, Cu or Zn (catalysts 15, 15a and 15b).92 As was the case for ethane ODH, the supported vanadium catalysts performed worse than other ceria-based mixed oxides (catalysts 16, 17 and 17a; Table 2).91 | ||
| Fig. 4 Activity and selectivity in propane ODH. The labels show the type of metal added to the ceria and the catalyst number. A supported vanadia catalyst (22) is shown for reference. | ||
| Catalyst | Catalyst composition | Concentration of added metal | Alkane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2 | Alkane, O2 conc. (%v/v) | Space velocity/ml g−1 h−1 | T/°C | Propane conversion (%) | Selectivity towards propene (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a These are atomic ratios.b Reference measurements were performed on ceria and nickel oxide. Ceria: 3% conversion, 2% selectivity (300 °C); 10% conversion, 6% selectivity (400 °C, note: quite close to catalyst 21). NiO 10% conversion, 17%, selectivity (350 °C).c Ni outperforms similar catalysts containing Cr, Co, Cu or Zn.d These are catalyst 15 tested at higher temperatures.e This is catalyst 17 tested at a higher temperature.f The concentration is in kPa instead of %v/v.g At this oxygen pressure, the values for plain ceria are: 7% conversion, 10% selectivity.h This is the GHSV/h−1.i MCF stands for mesocellular silica foams. | |||||||||
| 12 | Ni–K/CeO2 | Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 1, K Ce = 1, K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 0.05a Ni = 0.05a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 | 4, 8 | 545 | 300 | 8 | 72 | 90 |
| 13 | Ce–Ni–O | Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 0.5a Ce = 0.5a | 15 | 58 | |||||
| 14 | Ce–Ni–O | Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 1a Ce = 1a | 19 | 60 | |||||
| 15 | Ce–Ni–Ob,c | Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 0.5a Ce = 0.5a | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 | 5, 15 | 30000 | 200 | 2 | 50 | 92 |
| 15ad | 300 | 25 | 12 | ||||||
| 15bd | 375 | 62 | 10 | ||||||
| 16 | V/CeO2 | 12 wt% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 | 5, 15 | 5000 | 300 | 2 | 85 | 91 |
| 17 | 6 wt% | 300 | 14 | 34 | |||||
| 17ae | 6 wt% | 400 | 24 | 20 | |||||
| 18 | CeO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 14, 13f | 3600 | 450 | 17 | 5 | 93 | |
| 19 | CeO2+TCM | 17% v/v TCM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 14, 13f | 23 | 52 | |||
| 20 | CeO2+TCM | 17% v/v TCM | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 14, 4f | 17 | 70g | |||
| 21 | Cs2O/CeO2-2CeF3 | 3 wt% | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 | — | 6000h | 500 | 41 | 81 | 94 |
| Non-ceria-based reference catalyst: | |||||||||
| 22 | V/MCFi | 4.2 wt% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 10, 10 | 72000 | 550 | 31 | 84 | 95 |
Contrary to ethane ODH, propane ODH is performed at temperatures well below that of catalytic propane dehydrogenation (∼600 °C, albeit at a rather low conversion). No data is available on other oxidants, such as CO2, but the selectivity of plain ceria increases substantially upon adding trichloromethane (18–20).93 It is worthwhile investigating the effect of halogen addition on catalyst performance in propane ODH via a more practically applicable route, such as using supported metal-halogens, as was done for ethane ODH.85 Indeed, high selectivity and activity, rivalling those of the non ceria-based reference catalyst 22, are obtained when using catalyst 21, caesium oxide on ceria modified with CeF3.94
4.3 Oxidative dehydrogenation of other hydrocarbons
Besides ethane and propane, ceria-based materials have been applied in isobutane and ethylbenzene ODH (see Table 3). High selectivity and conversion at temperatures lower than those at which commercial catalysts are used were obtained for ethylbenzene ODH over plain ceria 23, using N2O as the oxidant.96 The high activity was attributed to a high concentration of Ce4+–O−–Ce3+ defect sites. Both doped and supported chromium-ceria catalysts were applied in isobutane ODH (see Fig. 5).97,98 The chromium-containing catalysts showed better results than plain ceria. The activity of chromium supported on ceria is higher than that of plain chromium oxide and chromium-ceria mixed oxide (both at 270 and 300 °C). Conversely, the selectivity of the chromium on ceria is somewhat lower compared to the mixed oxide and plain Cr2O3. Note that well dispersed Cr6+–Ox, and not Cr2O3, was proposed as the active site.98| Catalyst | Catalyst composition | Concentration of added metal | Reactant | Space velocity/ml g−1 h−1 | T/°C | Alkane conversion (%) | Selectivity towards alkene (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
a N2O was used instead of O2. No data on reactant concentrations was given.b The isobutane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio was 1 O2 ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 6.5% v/v.c These are catalysts 24, 25, 26 and 27, respectively, tested at higher temperature.d These are (bulk) atomic ratios. 1 at 6.5% v/v.c These are catalysts 24, 25, 26 and 27, respectively, tested at higher temperature.d These are (bulk) atomic ratios. | ||||||||
| 23a | CeO2 | Ethylbenzene | — | 325 | 45 | 94 | 96 | |
| 24 | Cr/CeO2 | 5–40 Cr atoms nm−2 | Isobutaneb | — | 270 | 10 | 54 | 97 |
| 24ac | 300 | 20 | 36 | |||||
| 25 | Ce–Cr–O | Cr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 0.2–1.8d Ce = 0.2–1.8d | 270 | 5 | 60 | |||
| 25ac | Cr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce = 0.2–1.8d Ce = 0.2–1.8d | 300 | 10 | 45 | ||||
| 26 | CeO2 | 270 | 5 | 20 | ||||
| 26ac | 300 | 10 | 20 | |||||
| 27 | Cr2O3 | 270 | 5 | 68 | ||||
| 27ac | 300 | 15 | 40 |
 | ||
| Fig. 5 Isobutane ODH over chromium-ceria catalysts at 270 (○) and 300 (●) °C. Plain ceria and Cr2O3 are added for reference. | ||
4.4 Combined dehydrogenation and selective hydrogen combustion
Another type of ODH has been industrially implemented. Here, the dehydrogenation is performed over a conventional dehydrogenation catalyst, and a second catalyst is added to selectively combust part of the hydrogen formed. The process may therefore be viewed as a “two-step ODH” (Scheme 2). Selective hydrogen combustion (SHC) generates heat and shifts the equilibrium to the products side, yielding the same benefits as “conventional” ODH. The use of two catalysts and/or two reactors, however, allows separate tuning of the dehydrogenation and hydrogen oxidation reactions, and the advantage of this over conventional ODH is proven by the industrial implementation of the process. For example, the steam active reforming (STAR) oxydehydrogenation process is implemented in two plants for the ODH of isobutane to MTBE.99 Styrene monomer advanced reheat technology (SMART) is in operation in five plants for the ODH of ethylbenzene to styrene.100,101 These processes use a co-fed approach, where small amounts of oxygen are added to the gas feed (Scheme 3, left). The mixing of gaseous oxygen with hydrogen and hydrocarbons at elevated temperatures is, however, a safety risk, which is avoided in redox-mode (Scheme 3, right). Here, no gaseous oxygen is added, but the lattice oxygen of the selective hydrogen combustion catalyst is used. High hydrogen combustion selectivities can be obtained for several supported oxides (e.g. Sb2O4, In2O3 and Bi2O3).102–105 These are, however, unstable under high temperature redox cycling. Conversely, ceria is stable under redox cycling and has a good oxygen storage capacity. The selectivity of plain ceria is low, but doping with metals such as Pb, Cr or Bi can overcome both the problems of low selectivity and low stability.18,106–108 | ||
| Scheme 2 The catalytic cycle for redox mode oxidative dehydrogenation using ceria as a solid oxygen reservoir. | ||
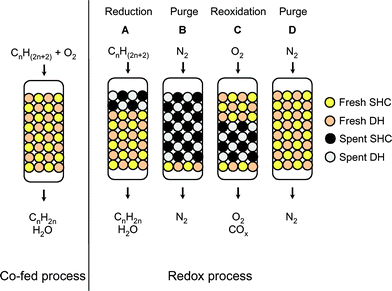 | ||
| Scheme 3 The co-fed (left) and redox (right) oxidative dehydrogenation process. After dehydrogenation step A, the bed is flushed with nitrogen (B) and the catalyst regenerated through reoxidation (C). This burns coke from the dehydrogenation catalyst and restores the lattice oxygen of the SHC catalyst. After another nitrogen flush (D), the reactor is ready for the next redox cycle. | ||
5. Various other selective oxidations
Besides the applications described in the previous sections, ceria-based materials have been used in various selective oxidations of hydrocarbons, oxygenates and inorganic molecules. An overview of this work is presented in Table 4. The common goal of these selective oxidations is product valorisation. The exceptions are catalytic wet air oxidation (CWAO, entries 10, 11, 14 and 15), which is used for waste water treatment, and sulfur oxidation (entry 12), which is used to recover sulfur from process gas.| Entry | Catalyst | Catalyst compositiona | Reactants | Desired products | Feed composition (% v/v)b | T/°C | Ref. |
|---|---|---|---|---|---|---|---|
| a Solid solutions or mixed oxides containing metal M and ceria are denoted as “Ce–M–O”. Metal M supported on ceria is denoted as “M/CeO2”.b In volume percent, unless stated otherwise. The last digit is rounded-off for clarity.c This is the molar ratio.d The feed concentration was also varied.e The aldehydes used were n-heptanal, dihydrocyclocitral, cinnamaldehyde and 4-iso-propyl-benzaldehyde. | |||||||
| Hydrocarbons | |||||||
| 1 | 28 | BaF2/CeO2 | Methane | Ethane, ethene | CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 3 O2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c | 800 | 94 |
| 2 | 29–35 | (Li, Na, K, Mg, Ca, Sr, or Ba)/CeO2 | Methane | Ethane, ethene | CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 1 CO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c,d 1c,d | 780–900 | 124 |
| 3 | 36 | Mo/CeO2 | Isobutene | Methacrolein | i-C4H8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 8 N2 = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 | 400 | 125 |
| 4 | 37 | Ce–Mo–O | Toluene | Benzaldehyde | C7H6O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) air air | 400 | 126 |
| Benzoic acid | |||||||
| Oxygenates | |||||||
| 5 | 38 | Re/CeO2 | Methanol | Methylfornate | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 6 He = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 84 84 | 240 | 127 |
| 6 | 39, 40 | Pt/CeO2, Rh/CeO2 | Ethanol | Ethanal | EtOH/O2/N2 EtOH : O2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 | 200–800 | 128 |
| 7 | 41 | Ce–Au–O | Benzylalcohol | Benzylaldehyde | Solvent free, 2 bar pure O2 | 100 | 117 |
| Octanol | Octanal | ||||||
| Geraniol | Citral | ||||||
| 8 | 42 | Au/CeO2 | Propanol | Propionate | 1.5 M alcohol in water, 1 bar pure O2 | 60 | 129 |
| Propylene glycol | Lactate | ||||||
| Glycerol | Glycerate | ||||||
| 9 | 43 | Au/CeO2 | Various aldehydese | Corresponding acids | Aldehyde/acetonitrile/air | 50–65 | 116 |
| 10 | 44–47 | (Ru, Pd, Pt or Ir)/CeO2 | Stearic acid | Acetic acid | Aqueous solution, 1–10 bar, O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) stearic acid = 0.1–16c stearic acid = 0.1–16c | 160–230 | 119 |
| 11 | 48–50 | (Ru, Pd or Pt)/CeO2 | Phenol | CO2 | 5 g L−1 (aq) | 160, 200 | 118 |
| Carboxylic acids | 20 bar O2 | ||||||
| Inorganic molecules | |||||||
| 12 | 51 | Ce–V–O | H2S | S | 1% H2S in He O2/H2S = 0–2.7 | 200–300 | 120 |
| 13 | 52 | Pd/CeO2 | H2 | H2O2 | 0.02 M H2SO4, 2.8% H2 in O2 | 22–27 | 122,123,130 |
| 14 | 53–55 | (Ru, Pd or Pt)/CeO2 | NH3 | N2 | 50 mmol L−1 (aq) | 160, 200 | 118 |
| 20 bar O2 | |||||||
| Various | |||||||
| 15 | 56–58 | (Ru, Pd or Pt)/CeO2 | Aniline | CO2, N2 | 5 g L−1 (aq) | 160, 200 | 118 |
| 20 bar O2 |
Reactions with hydrocarbons involve coupling into larger molecules (entries 1 and 2, oxidative coupling of methane, OCM) and aldehyde formation (entry 3 and 4). Using ceria-based materials for OCM was studied by several groups, with either O2109–111 or CO2112,113 (a reference of each is given in Table 4). Using O2, plain ceria mainly combusted the methane, but the C2 yield increased when adding BaF2.94 Similar results were obtained when supporting BaF2 on Pr6O11 or Tb4O7 supports. Comparing Li, Na, K, Mg, Ca, Sr and Ba supported on ceria, Wang et al. found that Ca/CeO2 was the best catalyst for OCM, using CO2 as the oxidising agent.114,115
Reactions with oxygenates include alcohol esterification (entry 5), aldehyde formation (entry 6 and 7) and acid formation from alcohols (entry 8) or aldehydes (entry 9). Gold is often used (entries 7, 8 and 9). Gold supported on ceria shows better performance in the selective oxidation of aliphatic and aromatic aldehydes compared to gold on iron oxide, Au/TiO2 or Pt/C/Bi-based catalysts.116 Catalyst activity was correlated to the gold and ceria particle size. Starting from alcohols, using a gold-ceria mixed oxide catalyst primarily yielded the corresponding aldehydes.117 Gold on a more acidic support, such as iron oxide, yielded carboxylic acids that esterify the alcohol feed.
Besides gold, other metals, such as rhenium, platinum and rhodium, can be added to ceria to achieve the selective oxidation of oxygenates (entries 5 and 6). Oxidising methanol to methyl formate, for example, can be performed with high selectivity using rhenium (entry 5). The reaction is most efficient when a monolayer of rhenium is supported on the ceria. In the partial oxidation of ethanol (entry 6), the added metal plays a prominent role in the product distribution. Using platinum primarily yields acetyl species, which can be oxidised into acetate species or decomposed into CH4, H2, CO and CO2. Using rhodium, however, yields a relatively stable cyclic intermediate, which decomposes into CO, CHx and Cx at higher temperatures, and can then be oxidised into CO2.
Entries 10, 11, 14 and 15 in Table 4 concern the CWAO of carboxylic acids, phenol and nitrogen-containing molecules.118,119 This process is applied in waste water treatment. Ideally, complete combustion into CO2 and H2O, with N2 formation, occurs. Alternatively, more easily biodegradable products can form, such as small carboxylic acids. Indeed, using Pt/CeO2 favours the full combustion of stearic acid, whilst Ru/CeO2 has a higher affinity for C–C bond splitting, followed by oxidation into acetic acid.119 Barbier Jr. et al. showed that Ru/CeO2 is unselective in oxidising ammonia into N2, but that adding a small amount of Pd enhanced both activity and selectivity.118 Interestingly, Ru dispersion was higher on low surface area ceria (see Fig. 6). This is because there was more room on the larger ceria crystals for supporting and stabilising small Ru crystals (Fig. 6, right). High surface area ceria consists of smaller ceria crystals that cannot accommodate the Ru particles (Fig. 6, left).
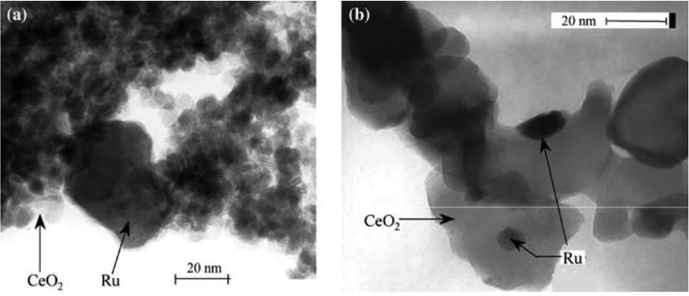 | ||
| Fig. 6 A TEM image of Ru supported on high surface area ceria (∼7 nm ceria crystallites, 160 m2 g−1, left) and low surface area ceria (∼25 nm ceria crystallites, 40 m2 g−1, right). Reproduced with permission of the author.118 | ||
Due to their high toxicity, the removal of nitrogen-containing aromatic compounds, such as aniline, is an important step in waste water purification. A comparison of Ru, Pd and Pt on ceria showed (entry 15) that Ru/CeO2 is very selective towards CO2. Besides the metal dispersion, the activity was correlated to oxygen transfer from the ceria towards the Ru. Concerning their selectivity and activity towards the formation of molecular nitrogen, however, both Pd and Pt outperform Ru.
Besides the conversion of organic compounds, the selective oxidation of H2S, H2 and NH3 by ceria-based catalysts were studied (the NH3 oxidation was described above). Yasyerli et al. showed that cerium-vanadium mixed oxides can selectively convert H2S to elemental sulfur (entry 12), and that both the selectivity and stability are higher than plain ceria.120 Recently, they also studied the application of cerium-manganese mixed oxides as high temperature H2S sorbents.121 Choudhary et al. showed that palladium supported on ceria can selectively oxidise H2 into H2O2, given that an oxidising agent such as perchloric acid is present.122 The activity of ceria supported palladium was higher compared to thoria or gallia supports, but carbon and alumina supports were even better. Chloride or bromide anions can further improve yield and selectivity.123
Acknowledgements
We thank Dr O. Touret (Rhodia) for permission to use Fig. 1, Prof. A. Caballero (University of Sevilla) for permission to use Scheme 1, Prof. J. M. Pintado and Dr J. J. Calvino (University of Cádiz) for permission to use Fig. 2 and Prof. J. Barbier Jr. (University of Poitiers) for permission to use Fig. 6.References
- J. Emsley, The Elements, Oxford University Press, Oxford, 1989 Search PubMed
.
- G. Houtzager, Geïllustreerde Griekse Mythologie Encyclopedie, Rebo productions b.v., Lisse, 2003 Search PubMed
.
- A. Trovarelli, Catalysis by ceria and related materials, Imperial College Press, London, 2002 Search PubMed
.
- T. C. Schutt, Ceram. Bull., 1972, 51, 155
.
- B. Zhu and M. D. Mat, Int. J. Electrochem. Sci., 2006, 1, 383 Search PubMed
.
- S. Bernal, G. Blanco, J. J. Calvino, J. A. P. Omil and J. M. Pintado, J. Alloys Compd., 2006, 408–412, 496 CrossRef CAS
.
- J. Kašpar, P. Fornasiero and N. Hickey, Catal. Today, 2003, 77, 419 CrossRef CAS
.
- R. Di Monte and J. Kašpar, Top. Catal., 2004, 28, 47 CrossRef
.
- J. Kašpar and P. Fornasiero, J. Solid State Chem., 2003, 171, 19 CrossRef CAS
.
- A. Trovarelli, C. de Leitenburg, M. Boaro and G. Dolcetti, Catal. Today, 1999, 50, 353 CrossRef CAS
.
- J. Kašpar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285 CrossRef CAS
.
- M. Boaro, M. Vicario, C. de Leitenburg, G. Dolcetti and A. Trovarelli, Catal. Today, 2003, 77, 407 CrossRef CAS
.
- M. Mogensen, N. M. Sammes and G. A. Tompsett, Solid State Ionics, 2000, 129, 63 CrossRef CAS
.
- E. Aneggi, M. Boaro, C. de Leitenburg, G. Dolcetti and A. Trovarelli, J. Alloys Compd., 2006, 408–412, 1096 CrossRef CAS
.
- E. Aneggi, C. de Leitenburg, G. Dolcetti and A. Trovarelli, Catal. Today, 2006, 114, 40 CrossRef
.
- A. P. E. York, T. C. Xiao, M. L. H. Green and J. B. Claridge, Catal. Rev. - Sci. Eng., 2007, 49, 511 CAS
.
- T. V. Choudhary and V. R. Choudhary, Angew. Chem., Int. Ed., 2008, 47, 1828 CrossRef CAS
.
- J. Beckers, F. Clerc, J. H. Blank and G. Rothenberg, Adv. Synth. Catal., 2008, 350, 2237 CrossRef CAS
.
- P. F. Campbell, M. H. Ortner and C. J. Anderson, Anal. Chem., 1961, 33, 58 CrossRef CAS
.
- B. Zhu, X. G. Liu, M. T. Sun, S. J. Ji and J. C. Sun, Solid State Sci., 2003, 5, 1127 CrossRef CAS
.
- S. H. Overbury, D. R. Huntley, D. R. Mullins and G. N. Glavee, Catal. Lett., 1998, 51, 133 CrossRef CAS
.
- D. Terribile, A. Trovarelli, J. Llorca, C. de Leitenburg and G. Dolcetti, Catal. Today, 1998, 43, 79 CrossRef CAS
.
- D. Terribile, A. Trovarelli, J. Llorca, C. de Leitenburg and G. Dolcetti, J. Catal., 1998, 178, 299 CrossRef CAS
.
- V. Perrichon, A. Laachir, S. Abouarnadasse, O. Touret and G. Blanchard, Appl. Catal., A, 1995, 129, 69 CrossRef CAS
.
- A. E. C. Palmqvist, M. Wirde, U. Gelius and M. Muhammed, Nanostruct. Mater., 1999, 11, 995 CrossRef
.
- V. M. Gonzalez-DelaCruz, J. P. Holgado, R. Pereñíguez and A. Caballero, J. Catal., 2008, 257, 307 CrossRef CAS
.
- S. Bernal, J. J. Calvino, M. A. Cauqui, J. M. Gatica, C. Larese, J. A. P. Omil and J. M. Pintado, Catal. Today, 1999, 50, 175 CrossRef CAS
.
- J. Beckers and G. Rothenberg, Dalton Trans., 2008, 6573 RSC
.
- X. C. Zheng, X. L. Zhang, X. Y. Wang, S. R. Wang and S. H. Wu, Appl. Catal., A, 2005, 295, 142 CrossRef CAS
.
- M. Jobbágy, F. Mariño, B. Schöbrod, G. Baronetti and M. Laborde, Chem. Mater., 2006, 18, 1945 CrossRef CAS
.
- P. Bera, K. R. Priolkar, P. R. Sarode, M. S. Hegde, S. Emura, R. Kumashiro and N. P. Lalla, Chem. Mater., 2002, 14, 3591 CrossRef CAS
.
- P. J. Scanlon, R. A. M. Bink, F. P. F. van Berkel, G. M. Christie, L. J. van IJzendoorn, H. H. Brongersma and R. G. van Welzenis, Solid State Ionics, 1998, 112, 123 CrossRef CAS
.
- A. Y. C. Tschöpe, Mater. Sci. Eng., A, 1995, 204, 267 CrossRef
.
- T. S. Zhang, J. Ma, L. H. Luo and S. H. Chan, J. Alloys Compd., 2006, 422, 46 CrossRef CAS
.
- S. Hočevar, U. O. Krašovec, B. Orel, A. S. Aricó and H. Kim, Appl. Catal., B, 2000, 28, 113 CrossRef CAS
.
- C. Lamonier, A. Bennani, A. D'Huysser, A. Aboukais and G. Wrobel, J. Chem. Soc., Faraday Trans., 1996, 92, 131 RSC
.
- B. Murugan, A. V. Ramaswamy, D. Srinivas, C. S. Gopinath and V. Ramaswamy, Chem. Mater., 2005, 17, 3983 CrossRef CAS
.
- E. D. Park, D. Lee and H. C. Lee, Catal. Today, 2009, 139, 280 CrossRef CAS
.
- Y. Liu, Q. Fu and M. Flytzani-Stephanopoulos, Catal. Today, 2004, 93–95, 241 CrossRef CAS
.
- A. Gómez-Cortés, Y. Márquez, J. Arenas-Alatorre and G. Díaz, Catal. Today, 2008, 133–135, 743 CrossRef CAS
.
- A. Martínez-Arias, A. B. Hungría, G. Munuera and D. Gamarra, Appl. Catal., B, 2006, 65, 207 CrossRef CAS
.
- A. A. Firsova, A. N. Il'ichev, T. I. Khomenko, L. V. Gorobinskii, Y. V. Maksimov, I. P. Suzdalev and V. N. Korchak, Kinet. Catal., 2007, 48, 282 CrossRef CAS
.
- A. A. Firsova, T. I. Khomenko, A. N. Il'ichev and V. N. Korchak, Kinet. Catal., 2008, 49, 682 CrossRef CAS
.
- K. Y. Kim, J. Han, S. W. Nam, T. H. Lim and H. I. Lee, Catal. Today, 2008, 131, 431 CrossRef CAS
.
- G. Sedmak, S. Hočevar and J. Levec, J. Catal., 2003, 213, 135 CrossRef
.
- F. Mariño, G. Baronetti, M. Laborde, N. Bion, A. Le Valant, F. Epron and D. Duprez, Int. J. Hydrogen Energy, 2008, 33, 1345 CrossRef CAS
.
- N. F. P. Ribeiro, M. M. V. M. Souza and M. Schmal, J. Power Sources, 2008, 179, 329 CrossRef CAS
.
- C. R. Jung, A. Kundu, S. W. Nam and H. I. Lee, Appl. Catal., B, 2008, 84, 426 CrossRef CAS
.
- H. B. Zou, X. F. Dong and W. M. Lin, Appl. Surf. Sci., 2006, 253, 2893 CrossRef CAS
.
- C. M. Bae, J. B. Ko and D. H. Kim, Catal. Commun., 2005, 6, 507 CrossRef CAS
.
- G. Marbán and A. B. Fuertes, Appl. Catal., B, 2005, 57, 43 CrossRef
.
- G. Avgouropoulos and T. Ioannides, Appl. Catal., A, 2003, 244, 155 CrossRef CAS
.
- Z. G. Liu, J. H. Chen, R. X. Zhou and X. M. Zheng, Catal. Lett., 2008, 123, 102 CrossRef CAS
.
- M. Tada, R. Bal, X. D. Mu, R. Coquet, S. Namba and Y. Iwasawa, Chem. Commun., 2007, 4689 RSC
.
- M. F. Luo, J. M. Ma, J. Q. Lu, Y. P. Song and Y. J. Wang, J. Catal., 2007, 246, 52 CrossRef CAS
.
- K. Sirichalprasert, A. Luengnaruemitchai and S. Pongstabodee, Int. J. Hydrogen Energy, 2007, 32, 915 CrossRef
.
- D. Gamarra, G. Munuera, A. B. Hungría, M. Fernández-García, J. C. Conesa, P. A. Midgley, X. Q. Wang, J. C. Hanson, J. A. Rodríguez and A. Martínez-Arias, J. Phys. Chem. C, 2007, 111, 11026 CrossRef CAS
.
- P. V. Snytnikov, A. I. Stadnichenko, G. L. Semin, V. D. Belyaev, A. I. Boronin and V. A. Sobyanin, Kinet. Catal., 2007, 48, 448 CrossRef CAS
.
- G. Avgouropoulos, T. Ioannides and H. Matralis, Appl. Catal., B, 2005, 56, 87 CrossRef CAS
.
- X. F. Dong, H. B. Zou and W. M. Lin, Int. J. Hydrogen Energy, 2006, 31, 2337 CrossRef CAS
.
- D. Gamarra, C. Belver, M. Fernández-Garcia and A. Martínez-Arias, J. Am. Chem. Soc., 2007, 129, 12064 CrossRef CAS
.
- Z. G. Liu, R. X. Zhou and X. M. Zheng, J. Mol. Catal. A: Chem., 2007, 267, 137 CrossRef CAS
.
- Y. Z. Chen, B. J. Liaw and H. C. Chen, Int. J. Hydrogen Energy, 2006, 31, 427 CrossRef CAS
.
- P. K. Cheekatamarla, W. S. Epling and A. M. Lane, J. Power Sources, 2005, 147, 178 CrossRef CAS
.
- E. Moretti, M. Lenarda, L. Storaro, A. Talon, R. Frattini, S. Polizzi, E. Rodríguez-Castellón and A. Jiménez-López, Appl. Catal., B, 2007, 72, 149 CrossRef CAS
.
- Y. Z. Chen, B. J. Liaw, J. M. Wang and C. T. Huang, Int. J. Hydrogen Energy, 2008, 33, 2389 CrossRef CAS
.
- P. Ratnasamy, D. Srinivas, C. V. V. Satyanarayana, P. Manikandan, R. S. S. Kumaran, M. Sachin and V. N. Shetti, J. Catal., 2004, 221, 455 CrossRef CAS
.
- Y. Z. Chen, B. J. Liaw and C. W. Huang, Appl. Catal., A, 2006, 302, 168 CrossRef CAS
.
- E. Y. Ko, E. D. Park, K. W. Seo, H. C. Lee, D. Lee and S. Kim, Catal. Today, 2006, 116, 377 CrossRef CAS
.
- C. R. Jung, A. Kundu, S. W. Nam and H. I. Lee, Appl. Catal., A, 2007, 331, 112 CrossRef CAS
.
- P. V. Snytnikov, A. I. Stadnichenko, G. L. Semin, V. D. Belyaev, A. I. Boronin and V. A. Sobyanin, Kinet. Catal., 2007, 48, 439 CrossRef CAS
.
- D. H. Kim and J. E. Cha, Catal. Lett., 2003, 86, 107 CrossRef CAS
.
- J. B. Wang, S. C. Lin and T. J. Huang, Appl. Catal., A, 2002, 232, 107 CrossRef CAS
.
- G. Avgouropoulos, T. Ioannides, C. Papadopoulou, J. Batista, S. Hočevar and H. K. Matralis, Catal. Today, 2002, 75, 157 CrossRef CAS
.
- J. Plotkin and E. Glatzer, Eur. Chem. News, 2005, 82, 20 Search PubMed
.
- N. Alperowicz, Chem. Week, 2007, 169, 27 Search PubMed
.
- N. Alperowicz, Chem. Week, 2006, 168, 17 Search PubMed
.
- F. Cavani, N. Ballarini and A. Cericola, Catal. Today, 2007, 127, 113 CrossRef CAS
.
- R. K. Grasselli, Top. Catal., 2002, 21, 79 CrossRef CAS
.
- R. X. Valenzuela, G. Bueno, A. Solbes, F. Sapiña, E. Martínez and V. C. Corberán, Top. Catal., 2001, 15, 181 CrossRef CAS
.
- M. Guío, J. Prieto and V. C. Corberán, Catal. Today, 2006, 112, 148 CrossRef CAS
.
- V. C. Corberán, Catal. Today, 2005, 99, 33 CrossRef CAS
.
- Note that the increased activity, as compared to the Sr-containing catalysts 1 and 2, could originate from the higher Sr loading (30 mol% instead of 10 mol% for catalysts 1 and 2).
- V. R. Choudhary, S. A. R. Mulla and V. H. Rane, J. Chem. Technol. Biotechnol., 1998, 71, 167 CrossRef CAS
.
- H. X. Dai, C. F. Ng and C. T. Au, J. Catal., 2001, 199, 177 CrossRef CAS
.
- M. V. Martínez-Huerta, J. M. Coronado, M. Fernández-García, A. Iglesias-Juez, G. Deo, J. L. G. Fierro and M. A. Bañares, J. Catal., 2004, 225, 240 CrossRef
.
- M. V. Martínez-Huerta, G. Deo, J. L. G. Fierro and M. A. Bañares, J. Phys. Chem. C, 2007, 111, 18708 CrossRef CAS
.
- M. V. Martínez-Huerta, G. Deo, J. L. G. Fierro and M. A. Bañares, J. Phys. Chem. C, 2008, 112, 11441 CrossRef CAS
.
- P. Botella, E. García-González, A. Dejoz, J. M. L. Nieto, M. I. Vázquez and J. González-Calbet, J. Catal., 2004, 225, 428 CrossRef CAS
.
- P. Boizumault-Moriceau, A. Pennequin, B. Grzybowska and Y. Barbaux, Appl. Catal., A, 2003, 245, 55 CrossRef CAS
.
- W. Daniell, A. Ponchel, S. Kuba, F. Anderle, T. Weingand, D. H. Gregory and H. Knözinger, Top. Catal., 2002, 20, 65 CrossRef CAS
.
- L. Jalowiecki-Duhamel, A. Ponchel, C. Lamonier, A. D'Huysser and Y. Barbaux, Langmuir, 2001, 17, 1511 CrossRef CAS
.
- S. Sugiyama, Y. Iizuka, E. Nitta, H. Hayashi and J. B. Moffat, J. Catal., 2000, 189, 233 CrossRef CAS
.
- H. L. Wan, X. P. Zhou, W. Z. Weng, R. Q. Long, Z. S. Chao, W. D. Zhang, M. S. Chen, J. Z. Luo and S. Q. Zhou, Catal. Today, 1999, 51, 161 CrossRef CAS
.
- Y. M. Liu, W. L. Feng, T. C. Li, H. Y. He, W. L. Dai, W. Huang, Y. Cao and K. N. Fan, J. Catal., 2006, 239, 125 CrossRef CAS
.
- B. Murugan and A. V. Ramaswamy, J. Am. Chem. Soc., 2007, 129, 3062 CrossRef CAS
.
- P. Moriceau, B. Grzybowska, Y. Barbaux, G. Wrobel and G. Hecquet, Appl. Catal., A, 1998, 168, 269 CrossRef CAS
.
- P. Moriceau, B. Grzybowska, L. Gengembre and Y. Barbaux, Appl. Catal., A, 2000, 199, 73 CrossRef CAS
.
- www.uhde.eu.
- F. M. Dautzenberg, Catal. Rev. Sci. Eng., 2004, 46, 335 CrossRef CAS
.
- F. M. Dautzenberg and P. J. Angevine, Catal. Today, 2004, 93–95, 3 CrossRef CAS
.
- R. K. Grasselli, D. L. Stern and J. G. Tsikoyiannis, Appl. Catal., A, 1999, 189, 9 CrossRef CAS
.
- L. M. Van Der Zande, E. A. de Graaf and G. Rothenberg, Adv. Synth. Catal., 2002, 344, 884 CrossRef CAS
.
- E. A. de Graaf, A. Andreini, E. J. M. Hensen and A. Bliek, Appl. Catal., A, 2004, 262, 201 CrossRef CAS
.
- E. A. de Graaf, G. Zwanenburg, G. Rothenberg and A. Bliek, Org. Process Res. Dev., 2005, 9, 397 Search PubMed
.
- G. Rothenberg, E. A. de Graaf and A. Bliek, Angew. Chem., Int. Ed., 2003, 42, 3366 CrossRef CAS
.
- J. H. Blank, J. Beckers, P. F. Collignon, F. Clerc and G. Rothenberg, Chem.–Eur. J., 2007, 13, 5121 CrossRef CAS
.
- J. H. Blank, J. Beckers, P. F. Collignon and G. Rothenberg, ChemPhysChem, 2007, 8, 2490 CrossRef CAS
.
- X. L. Zhang, C. S. M. Lee, D. M. P. Mingos and D. O. Hayward, Appl. Catal., A, 2003, 249, 151 CrossRef CAS
.
- A. G. Dedov, A. S. Loktev, I. I. Moiseev, A. Aboukais, J. F. Lamonier and I. N. Filimonov, Appl. Catal. A: Gen., 2003, 245, 209 CAS
.
- D. Wolf, M. Heber, W. Grünert and M. Muhler, J. Catal., 2001, 199, 92 CrossRef CAS
.
- Istadi and N. A. S. Amin, Fuel Process. Technol., 2006, 87, 449 CrossRef
.
- Y. He, B. Yang and G. Cheng, Catal. Today, 2004, 98, 595 CrossRef CAS
.
- The C2-yields remain below 5%, however, when using CO2.
- Y. Wang, Y. Takahashi and Y. Ohtsuka, J. Catal., 1999, 186, 160 CrossRef CAS
.
- A. Corma and M. E. Domine, Chem. Commun., 2005, 4042 RSC
.
- D. I. Enache, D. W. Knight and G. J. Hutchings, Catal. Lett., 2005, 103, 43 CrossRef CAS
.
- J. Barbier Jr., L. Oliviero, B. Renard and D. Duprez, Top. Catal., 2005, 33, 77 CrossRef
.
- B. Renard, J. Barbier Jr., D. Duprez and S. Durecu, Appl. Catal., B, 2005, 55, 1 CrossRef CAS
.
- S. Yasyerli, G. Dogu and T. Dogu, Catal. Today, 2006, 117, 271 CrossRef CAS
.
- S. Yasyerli, Chem. Eng. Process., 2008, 47, 577 CrossRef CAS
.
- V. R. Choudhary, A. G. Gaikwad and S. D. Sansare, Catal. Lett., 2002, 83, 235 CrossRef CAS
.
- V. R. Choudhary and C. Samanta, J. Catal., 2006, 238, 28 CrossRef CAS
.
- Y. Wang, Y. Takahashi and Y. Ohtsuka, Appl. Catal., A, 1998, 172, L203 CrossRef CAS
.
- F. De Smet, P. Ruiz, B. Delmon and M. Devillers, J. Phys. Chem. B, 2001, 105, 12355 CrossRef CAS
.
- M. W. Xue, X. D. Gu, J. P. Chen, H. L. Zhang and J. Y. Shen, Thermochim. Acta, 2005, 434, 50 CrossRef CAS
.
- J. L. Liu, E. S. Zhan, W. J. Cai, J. Li and W. J. Shen, Catal. Lett., 2008, 120, 274 CrossRef CAS
.
- A. M. Silva, L. O. O. Costa, A. P. M. G. Barandas, L. E. P. Borges, L. V. Mattos and F. B. Noronha, Catal. Today, 2008, 133–135, 755 CrossRef CAS
.
- S. Demirel, P. Kern, M. Lucas and P. Claus, Catal. Today, 2007, 122, 292 CrossRef CAS
.
- V. R. Choudhary, C. Samanta and T. V. Choudhary, Appl. Catal., A, 2006, 308, 128 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2010 |

