A simple approach to reduce the environmental impact of olefin metathesis reactions: a green and renewable solvent compared to solvent-free reactions
Manuela Kniesea and Michael A. R. Meier*ab
aUniversity of Applied Sciences Oldenburg/Ostfriesland/Wilhelmshaven, Constantiaplatz 4, 26723, Emden, Germany
bUniversity of Potsdam, Institute of Chemistry, Karl-Liebknecht-Str. 24–25, 14476, Golm, Germany. E-mail: michael.meier@uni-potsdam.de; Web: www.meier-michael.com
First published on 5th November 2009
Abstract
Two widely-studied olefin metathesis reactions, the cross-metathesis of allyl benzene with cis-1,4-diacetoxy-2-butene and the ring closing metathesis of diethyl diallylmalonate, were studied under environmentally more benign reaction conditions. All studied catalysts allowed these reactions to be performed under bulk conditions, thus avoiding large amounts of solvent waste. Moreover, methyl decanoate, a fatty acid-derived, and thus renewable and non-toxic, solvent, was shown to be an environmentally friendly alternative solvent to the usually applied dichloromethane. Most interestingly, only the reactions performed under bulk conditions allowed a 25-fold catalyst loading reduction, thus offering the “greenest” alternative of the investigated reaction conditions.
Introduction
Since the development and continuous improvement of well defined, highly active and functional group-tolerant ruthenium based catalysts,1–11 olefin metathesis has found manifold possible applications in organic and polymer synthesis.12–16More recently, many methods for the reduction of the environmental impact of olefin metathesis reactions were reported in the literature. For instance, supported catalysts that facilitate catalyst removal and recycling were often reported.17 Similarly, procedures for the removal of homogenous catalysts from the reaction mixture are being developed.17 However, since the catalyst is not the only parameter that can influence the environmental impact of a reaction, many reports are available that describe olefin metathesis in alternative solvents. Most often, homogeneously-catalyzed metathesis reactions are carried out in dichloromethane (and, less frequently, in aromatic solvents) at high dilutions. Current investigations focus on the use of ionic liquids,18,19 water,20 other environmentally friendly solvents,21 and supercritical fluids22,23 for olefin metathesis reactions. All of these investigations claim the use of less toxic solvents and/or an easier recycling of the catalyst as environmental advantages. However, it should be mentioned here that a certain solvent should not generally be considered as “green”, since all process parameters have to be carefully evaluated and compared (e.g. by using environmental factors) to each other before such a statement can be made.
Although the use of ruthenium based metathesis initiators under bulk conditions is a standard procedure for acyclic diene metathesis (ADMET) polymerization and, in some cases, for ring-opening metathesis polymerization (ROMP), very little has been reported on olefin metathesis reactions to yield defined low molecular weight organic compounds under solvent-free conditions. In 2002, Grubbs et al. reported a solvent free cross-metathesis procedure for the synthesis of trisubstituted olefins.24 Practically, a non-dimerizing cross-metathesis reaction partner, isobutylene or 2-methyl-2-butene in this case, was used in excess and acted as solvent for these reactions, allowing a 5-fold catalyst loading reduction (from 5 to 1%). Along the same lines, we observed that low catalyst loadings of 0.1–1% were sufficient for the quite challenging cross-metathesis reactions of allyl chloride25 and methyl acrylate26 with fatty acid derivatives under bulk conditions to yield renewable monomers for step-growth polymerizations. Here, an excess of allyl chloride or methyl acrylate also acted practically as the solvent, but it is important to note that these reactions did not proceed well when performed in dichloromethane or other organic solvents. Moreover, a solvent-free ring-closing metathesis procedure under microwave conditions with 1% of catalyst that provided quantitative conversions in many cases was recently reported.27 Despite these promising observations, no detailed studies on the performance of ruthenium-based metathesis initiators under bulk conditions is available.
With the aim of reducing the environmental impact of the reaction conditions commonly applied in olefin metathesis reactions, we investigated the use of a fatty acid-derived (and thus renewable) solvent, as well as solvent-free conditions, in detail for two commonly-studied metathesis reactions with three frequently-studied highly active second generation catalysts (see Fig. 1). The studied reactions were the cross-metathesis (CM) of allyl benzene with cis-1,4-diacetoxy-2-butene and the ring closing metathesis (RCM) of diethyl diallylmalonate (see Fig. 2), since both reactions were suggested by Grubbs et al. as standard reactions for the evaluation of a new catalyst.28 However, here we used these widely-studied reactions to evaluate and compare more environmentally friendly reaction conditions.
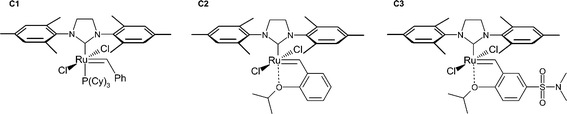 | ||
| Fig. 1 Investigated second generation catalysts. | ||
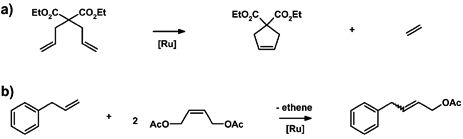 | ||
| Fig. 2 Investigated metathesis reactions: (a) ring-closing metathesis of diethyl diallylmalonate; (b) cross-metathesis of allyl benzene with cis-1,4-diacetoxy-2-butene. | ||
Results and discussion
We studied the CM of allyl benzene with cis-1,4-diacetoxy-2-butene and the RCM of diethyl diallylmalonate under bulk conditions and in a renewable and non-toxic solvent with the aim of reducing the environmental impact of metathesis reactions. Our findings were then compared to the conventionally-applied reaction conditions, e.g. reaction in the toxic solvent dichloromethane at high dilution.In the case of the ring closing metathesis of diethyl diallylmalonate, Grubbs et al. suggested performing the reaction at a concentration of 0.1 mol L−1 in dichloromethane (DCM) with a catalyst loading of 1% at 30 °C.28 These conditions were suggested to test and evaluate new catalysts. We used these conditions as a starting point and as a reference for our target to minimize the environmental impact of olefin metathesis reactions. Thus, we performed this reaction in methyl decanoate (MD) and in bulk under otherwise unchanged conditions with catalysts C1–C3.
All reactions were performed in triplicate and the averaged results of these investigations are provided in Table 1. The reproducibility of these reactions was good (<3% standard deviation). Only the reactions performed in MD showed somewhat larger deviations (up to 5%). It is obvious that all investigated catalysts were able to catalyze the reaction, as expected and reported many times in the literature. The standard conditions at high dilution in DCM with 1% catalyst loading resulted in almost full conversion for all catalysts. MD gives somewhat poorer results for all catalysts and at all catalyst loadings, if compared to the reactions in DCM. Therefore, MD is generally suitable as a solvent for this RCM reaction, but somewhat less than DCM, if high conversions are desired. Nevertheless, the choice of MD can offer significant environmental benefits. More interestingly, with the exception of C1, the solvent-free conditions also show full conversions and thus offer an environmentally friendly alternative to the reactions performed in solvent.
| Catalyst | Catalyst loading/mol%a | Solventb | C/%c |
|---|---|---|---|
| a Amount of catalyst in mol% relative to diethyldiallyl malonate.b DCM: dichloromethane ([diethyldiallyl malonate] = 0.1 mol L−1); MD: methyl decanoate ([diethyldiallyl malonate] = 0.1 mol L−1); none: reaction performed in bulk ([diethyldiallyl malonate] = 4.136 mol L−1).c Conversion of diethyldiallyl malonate (by GC-MS). | |||
| C1 | 1.0 | DCM | 97.8 |
| C1 | 1.0 | MD | 79.3 |
| C1 | 1.0 | none | 88.1 |
| C2 | 1.0 | DCM | 95.9 |
| C2 | 1.0 | MD | 66.4 |
| C2 | 1.0 | none | 99.5 |
| C3 | 1.0 | DCM | 99.6 |
| C3 | 1.0 | MD | 71.5 |
| C3 | 1.0 | none | 99.4 |
In order to further reduce the environmental impact of these reactions, we investigated a 5-fold reduction in catalyst loading to 0.2 mol% (Table 2). We were pleased to still observe good to excellent results under these conditions in all solvent systems. Once more, the reactions performed in MD showed somewhat poorer results for all catalysts. However, the solvent-free reactions provided an advantage here, since the observed conversions remained high and were higher than in DCM in all cases.
| Catalyst | Catalyst loading/mol%a | Solventb | C/%c |
|---|---|---|---|
| a Amount of catalyst in mol% relative to diethyldiallyl malonate.b DCM: dichloromethane ([diethyldiallyl malonate] = 0.1 mol L−1); MD: methyl decanoate ([diethyldiallyl malonate] = 0.1 mol L−1); none: reaction performed in bulk ([diethyldiallyl malonate] = 4.136 mol L−1).c Conversion of diethyldiallyl malonate (by GC-MS). | |||
| C1 | 0.2 | DCM | 76.4 |
| C1 | 0.2 | MD | 60.8 |
| C1 | 0.2 | none | 81.5 |
| C2 | 0.2 | DCM | 86.5 |
| C2 | 0.04 | DCM | 62.4 |
| C2 | 0.2 | MD | 49.9 |
| C2 | 0.2 | none | 99.4 |
| C2 | 0.04 | none | 15.9 |
| C3 | 0.2 | DCM | 94.9 |
| C3 | 0.04 | DCM | 76.5 |
| C3 | 0.2 | MD | 66.0 |
| C3 | 0.2 | none | 99.6 |
| C3 | 0.04 | none | 97.0 |
Since diethyl diallylmalonate is an α,ω-diene one might expect ADMET as a side reaction, especially under these highly concentrated conditions. However, we did not observe the formation of oligomers or even polymers by GPC, ruling out this side reaction. Therefore, the RCM reactions with 0.2% of C2 and C3 avoid 46 L of toxic DCM per kg of obtained product without loss of catalyst activity. Inspired by these findings, we tried to reduce the loading of C2 and C3 even further to 0.04 mol%. C2 was somewhat less active under these conditions than C3. Generally, this catalyst loading reduction resulted in decreased conversions, but C3 remained highly active under bulk conditions. This is a very interesting and promising result, since not only does it save 46 L of DCM per kg of product, but also the amount of potentially toxic and expensive catalyst can be reduced at least 25-fold, compared to the classic literature conditions. Quite interestingly, the bulk reactions with low catalyst loadings showed a better reproducibility than the corresponding reactions in solvent, which is an additional advantage.
In order to evaluate if the above findings are only applicable to the investigated RCM reaction, or if they can be transferred to other metathesis reactions, we investigated the cross-metathesis of allyl benzene with cis-1,4-diacetoxy-2-butene in a similar set of experiments (see Table 3). Again, for these reactions, we used the reaction conditions suggested by Grubbs et al. as the starting point for our investigations: catalyst concentration of 2.5 mol%, 0.2 M allyl benzene in dichloromethane, 2 equivalents of cis-1,4-diacetoxy-2-butene, 25 °C.28 In order to be able to compare the obtained results to the RCM reaction described above, we changed the concentration of allyl benzene to 0.1 M and left all other parameters unchanged. Starting from these conditions, we tested methyl decanoate and solventless reactions in a similar way as described above (Table 3). Under all reaction conditions, the observed formation of the allyl benzene self-metathesis product was below 1% and thus we will neglect this side-reaction in further discussions. The reproducibility was also good for these reactions (< 3% standard deviation). In contrast to the RCM reaction described above, MD is a very suitable solvent for this CM reaction for all catalysts and provides nearly full conversions of ally benzene, similar to the reactions performed in DCM and in bulk. Thus, either an environmentally friendly and renewable solvent can be used for this reaction, or solvent-free conditions that avoid solvent waste completely may be chosen without losing catalyst activity.
| Catalyst | Catalyst loading/mol%a | Solventb | C/%c |
|---|---|---|---|
| a Amount of catalyst in mol% relative to allyl benzene.b DCM: dichloromethane ([allyl benzene] = 0.1 mol L−1); MD: methyl decanoate ([allyl benzene] = 0.1 mol L−1); none: reaction performed in bulk ([allyl benzene] = 2.216 mol L−1).c Conversion of allyl benzene (by GC) | |||
| C1 | 2.5 | DCM | 86.6 |
| C1 | 2.5 | MD | 94.8 |
| C1 | 2.5 | none | 85.8 |
| C2 | 2.5 | DCM | 90.6 |
| C2 | 2.5 | MD | 91.2 |
| C2 | 2.5 | none | 90.6 |
| C3 | 2.5 | DCM | 91.7 |
| C3 | 2.5 | MD | 89.7 |
| C3 | 2.5 | none | 90.2 |
Similar to the RCM reaction described above, a reduction in catalyst loadings led to decreased conversions (Table 4). This effect was more pronounced for solvent reactions than for the bulk reactions. Moreover, MD gives good results for all catalysts and all catalyst loadings, comparable to, and sometimes better than, the corresponding reactions in DCM. However, the solvent-free reaction conditions show the highest conversions for all catalysts and at all catalyst loadings. A further 5-fold reduction in catalyst loading was then attempted with all systems that provided conversions higher than 80% with a catalyst loading of 0.5%. These results are also presented in Table 4. A 25-fold reduction in catalyst loading while still maintaining high conversions was only possible under bulk conditions. These results clearly show that the most environmentally benign reaction conditions are once more the solvent-free conditions.
| Catalyst | Catalyst loading/mol%a | Solventb | C/%c |
|---|---|---|---|
| a amount of catalyst in mol% relative to allyl benzene;b DCM: dichloromethane (c[allyl benzene] = 0.1 mol L−1); MD: methyl decanoate (c[allyl benzene] = 0.1 mol L−1); none: reaction performed in bulk (c[allyl benzene] = 2.216 mol L−1);c Conversion of allyl benzene in % (by GC) | |||
| C1 | 0.5 | DCM | 63.6 |
| C1 | 0.5 | MD | 78.6 |
| C1 | 0.5 | none | 81.9 |
| C1 | 0.1 | none | 54.8 |
| C2 | 0.5 | DCM | 87.7 |
| C2 | 0.1 | DCM | 14.9 |
| C2 | 0.5 | MD | 72.2 |
| C2 | 0.5 | none | 88.4 |
| C2 | 0.1 | none | 84.7 |
| C3 | 0.5 | DCM | 71.7 |
| C3 | 0.5 | MD | 79.7 |
| C3 | 0.1 | MD | 35.9 |
| C3 | 0.5 | none | 89.4 |
| C3 | 0.1 | none | 81.8 |
The E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio of the formed product was ∼9
Z ratio of the formed product was ∼9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, quite independent of the reaction conditions. The only exceptions were the reactions at high dilution and low catalyst loading in solvent, where the E
1, quite independent of the reaction conditions. The only exceptions were the reactions at high dilution and low catalyst loading in solvent, where the E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio was ∼4
Z ratio was ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These observations are probably due to a deactivation of the catalyst, which is also in accordance with the low observed conversions under these reaction conditions (data not presented in Table 4). If the catalyst is deactivated, it cannot perform secondary metathesis reactions, which would equilibrate the product to the more stable E isomer.28 Moreover, the E
1. These observations are probably due to a deactivation of the catalyst, which is also in accordance with the low observed conversions under these reaction conditions (data not presented in Table 4). If the catalyst is deactivated, it cannot perform secondary metathesis reactions, which would equilibrate the product to the more stable E isomer.28 Moreover, the E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio of the bulk reactions with C2 and C3 was again ∼9
Z ratio of the bulk reactions with C2 and C3 was again ∼9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is in agreement with the high observed conversions for these reactions and, thus, the remaining high catalyst activity.
1, which is in agreement with the high observed conversions for these reactions and, thus, the remaining high catalyst activity.
In summary, solvent-free conditions for this metathesis reaction also allowed for a straightforward 25-fold reduction of the amount of catalysts C2 and C3. Moreover, MD was even better suited for this reaction than DCM and can provide a green alternative reaction medium, in case a solvent is required for the reaction. Furthermore, the solvent-free reactions showed significantly higher reproducibility, even at very low catalyst loadings.
Conclusions
The conclusions of these investigations are manifold. Most importantly, metathesis reactions in methyl decanoate and solvent-free metathesis reactions offer environmentally friendly alternatives to the commonly applied solvent dichloromethane. This finding seems to be of a general nature, but this has to be confirmed with different metathesis reactions in the future. In particular, the solvent free reaction conditions offer the most sustainable alternative by avoiding large amounts of solvent waste without losing catalyst activity. Along the same lines, only the bulk conditions allowed a straighforward 25-fold reduction of the amount of metathesis catalyst for both investigated metathesis reactions. This is not only interesting in terms of green chemistry, because large amounts of potentially toxic transition metal can be avoided, but also in terms of process economics, because the catalyst is by far the most expensive component of these reactions.Experimental
Materials
Decanoic acid (Cognis, Edenor C10, 98%), methanol (VWR, 99%), sulfuric acid (Fluka, 95-97%), allylbenzene (Aldrich, 98%), cis-1,4-diacetoxy-2-butene (Aldrich, 95%), diethyl diallylmalonate (Aldrich, 98%), dichloromethane (Riedel-de Häen, 99.8%), tetradecane (Fluka, ≥99%), decane (Fluka, ≥99.8%), ethyl vinyl ether (Aldrich, 99%), tetrahydrofuran (Sigma, ≥99%), (1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(o-isopropoxyphenyl-methylene)ruthenium (C2, Aldrich), benzylidene[1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(tricyclohexylphosphine)ruthenium (C1, Aldrich), and 1,3-bis-(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene[2-(i-propoxy)-5-(N,N-dimethylaminosulfonyl)phenyl]methyleneruthenium(II)dichloride (C3, ABCR, 96%) were used as received. Methyl decanoate was prepared by esterification with methanol from the corresponding decanoic acid according to standard laboratory procedures.1H NMR spectra were recorded in CDCl3 on a Bruker AVANCE DPX spectrometer operating at 300 (75.5) MHz. Chemical shifts (δ) are reported in parts per million relative to the internal standard tetramethylsilane (TMS, δ = 0.00 ppm).
Analytical GC characterization of reaction mixtures was carried out with a Shimadzu GC-2010 equipped with a fused silica capillary column (Stabilwax®, 30 m × 0.25 mm × 0.25 μm, Restek), using flame ionization detection. The oven temperature program was: initial temperature 50 °C, hold for 5 min, ramp at 10 °C min−1 to 250 °C, hold for 5 min (total analysis time: 30 min, cross-metathesis of allylbenzene with cis-1,4-diacetoxy-2-butene). Measurements were performed in the split–split mode (split ratio 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using hydrogen as the carrier gas (linear velocity of 31.4 cm s−1 at 220 °C).
1) using hydrogen as the carrier gas (linear velocity of 31.4 cm s−1 at 220 °C).
GC-MS (EI) chromatograms were recorded using a VARIAN 3900 GC instrument with a capillary column FactorFour™ VF-5ms (30 m × 0.25 mm × 0.25 μm, Varian) and a Saturn 2100T ion trap mass detector. Scans were performed from 40 to 650 m/z at rate of 1.0 scan s−1. The oven temperature program was: initial temperature 95 °C, hold for 1 min, ramp at 10 °C min−1 to 200 °C, hold for 3 min (total analysis time: 14.5 min, ring-closing metathesis of diethyl diallylmalonate). The injector transfer line temperature was set to 250 °C. Measurements were performed in the splitless and split–split modes (split ratio 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using helium as the carrier gas (flow rate 1.0 ml min−1).
1) using helium as the carrier gas (flow rate 1.0 ml min−1).
Mass spectra (ESI) were recorded on a VARIAN 500-MS ion trap mass spectrometer with the TurboDDS™ option installed. Samples were introduced by direct infusion with a syringe pump. Nitrogen served both as the nebulizer gas and the drying gas. Helium served as the cooling gas for the ion trap and collision gas for MSn. Nitrogen was generated by a nitrogen generator Nitrox from Domnick Hunter.
Gel permeation chromatograms were measured on a SEC system LC-20A from Shimadzu equipped with a SIL-20A autosampler, PLgel 5 μm MIXED-D column (Polymer Laboratories, 300 mm × 7.5 mm), and a RID-10A refractive index detector in THF (flow rate 1 ml min−1) at 50 °C. All determinations of molar mass were performed relative to poly(methyl-methacrylate) standards (Polymer Standards Service, Mp 102–981.000 Da).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and dichloromethane or methyl decanoate was added in different amounts. Some experiments were also performed in bulk, without the addition of solvent. Reactions were carried out in parallel using a carousel reaction station™ RR98072 (Radleys Discovery Technologies, UK) under stirring at 25 °C. The solid catalyst (C1–C3) was then added to the solution, in the quantities of 0.1, 0.5 and 2.5 mol% of the educt allylbenzene. All reactions were carried out without a nitrogen atmosphere. Samples were taken periodically and quenched with an excess of ethyl vinyl ether in order to stop the metathesis reaction. Tetradecane was used as an internal standard in the samples with solvent and decane as external standards in the solvent-free samples for GC analysis. After this procedure, a conversion and composition analysis was performed by GC. For isolation of the cross-metathesis product, this procedure was performed with 1.175 ml (1.05 g, 0.0089 mol) allylbenzene, 2.828 ml (3.05 g, 0.018 mol) cis-1,4-diacetoxy-2-butene and 0.1627 g (2.5 mol %) of C3. The reaction mixture was stirred magnetically at 25 °C. After 25 h reaction time the compound was purified by column chromatography on silica with a mixture of hexane and diethyl ether (1
2 and dichloromethane or methyl decanoate was added in different amounts. Some experiments were also performed in bulk, without the addition of solvent. Reactions were carried out in parallel using a carousel reaction station™ RR98072 (Radleys Discovery Technologies, UK) under stirring at 25 °C. The solid catalyst (C1–C3) was then added to the solution, in the quantities of 0.1, 0.5 and 2.5 mol% of the educt allylbenzene. All reactions were carried out without a nitrogen atmosphere. Samples were taken periodically and quenched with an excess of ethyl vinyl ether in order to stop the metathesis reaction. Tetradecane was used as an internal standard in the samples with solvent and decane as external standards in the solvent-free samples for GC analysis. After this procedure, a conversion and composition analysis was performed by GC. For isolation of the cross-metathesis product, this procedure was performed with 1.175 ml (1.05 g, 0.0089 mol) allylbenzene, 2.828 ml (3.05 g, 0.018 mol) cis-1,4-diacetoxy-2-butene and 0.1627 g (2.5 mol %) of C3. The reaction mixture was stirred magnetically at 25 °C. After 25 h reaction time the compound was purified by column chromatography on silica with a mixture of hexane and diethyl ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent.
10) as eluent.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 4.54 (d, 2H, –CH2–O–CO–), 3.39 (d, 2H, C6H5–CH2–), 2.05 (s, 3H, –O–CO–CH3).
CH–), 4.54 (d, 2H, –CH2–O–CO–), 3.39 (d, 2H, C6H5–CH2–), 2.05 (s, 3H, –O–CO–CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluate.
1) as eluate.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–), 1.26 (t, 6H, 2×–CO–O–CH2–CH3).
CH–CH2–), 1.26 (t, 6H, 2×–CO–O–CH2–CH3).Acknowledgements
This project was partially financed by the German Federal Ministry of Food, Agriculture and Consumer Protection (represented by the Fachagentur nachwachsende Rohstoffe; FKZ 22026905). The authors thank the University of Oldenburg (Germany) for access to NMR facilities.Notes and references
- S. T. Nguyen, L. K. Johnson, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1992, 114, 3974–3975 CrossRef CAS.
- P. Schwab, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1996, 118, 100–110 CrossRef CAS.
- P. Schwab, M. B. France, J. W. Ziller and R. H. Grubbs, Angew. Chem., Int. Ed. Engl., 1995, 34, 2039–2041 CrossRef CAS.
- T. Weskamp, W. C. Schattenmann, M. Spiegler and W. A. Herrmann, Angew. Chem., Int. Ed., 1998, 37, 2490–2493 CrossRef CAS.
- J. Huang, E. D. Stevens, S. P. Nolan and J. L. Peterson, J. Am. Chem. Soc., 1999, 121, 2674–2678 CrossRef CAS.
- J. Huang, H.-J. Schanz, E. D. Stevens and S. P. Nolan, Organometallics, 1999, 18, 5375–5380 CrossRef CAS.
- L. Ackermann, A. Fürstner, T. Weskamp, F. J. Kohl and W. A. Herrmann, Tetrahedron Lett., 1999, 40, 4787–4790 CrossRef CAS.
- T. Weskamp, F. J. Kohl, W. Hieringer, D. Gleich and W. A. Herrmann, Angew. Chem., Int. Ed., 1999, 38, 2416–2419 CrossRef CAS.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953–956 CrossRef CAS.
- M. Scholl, T. M. Trnka, J. P. Morgan and R. H. Grubbs, Tetrahedron Lett., 1999, 40, 2247–2250 CrossRef CAS.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS.
- A. Fürstner, Angew. Chem., Int. Ed., 2000, 39, 3012–3043 CrossRef CAS.
- S. J. Connon and S. Blechert, Angew. Chem., Int. Ed., 2003, 42, 1900–1923 CrossRef.
- B. M. Novak, W. Risse and R. H. Grubbs, Adv. Polym. Sci., 1992, 102, 47–72 CAS.
- C. Slugovc, Macromol. Rapid Commun., 2004, 25, 1283–1297 CrossRef CAS.
- T. W. Baughman and K. B. Wagener, Adv. Polym. Sci., 2005, 176, 1–42 CAS.
- H. Clavier, K. Grela, A. Kirschning, M. Mauduit and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 6786–6801 CrossRef CAS.
- D. Sémeril, H. Olivier-Bourbigou, C. Bruneau and P. H. Dixneuf, Chem. Commun., 2002, 146–147 RSC.
- Q. Yao and Y. Zhang, Angew. Chem., Int. Ed., 2003, 42, 3395–3398 CrossRef CAS.
- D. Burtscher and K. Grela, Angew. Chem., Int. Ed., 2009, 48, 442–454 CrossRef CAS.
- X. Miao, C. Fischmeister, C. Bruneau and P. H. Dixneuf, ChemSusChem, 2008, 1, 813–816 CrossRef CAS.
- A. Fürstner, L. Ackermann, K. Beck, H. Hori, D. Koch, K. Langemann, M. Liebl, C. Six and W. Leitner, J. Am. Chem. Soc., 2001, 123, 9000–9006 CrossRef CAS.
- F. Michalek, D. Mädge, J. Rühe and W. Bannwarth, Eur. J. Org. Chem., 2006, 577–581 CrossRef CAS.
- A. K. Chatterjee, D. P. Sanders and R. H. Grubbs, Org. Lett., 2002, 4, 1939–1942 CrossRef CAS.
- T. Jacobs, A. Rybak and M. A. R. Meier, Appl. Catal., A, 2009, 353, 32–35 CrossRef CAS.
- A. Rybak and M. A. R. Meier, Green Chem., 2007, 9, 1356–1361 RSC.
- G. V. Thanh and A. Loupy, Tetrahedron Lett., 2003, 44, 9091–9094 CrossRef CAS.
- T. Ritter, A. Hejl, A. G. Wenzel, T. W. Funk and R. H. Grubbs, Organometallics, 2006, 25, 5740–5745 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
