An efficient approach to homocoupling of terminal alkynes: Solvent-free synthesis of 1,3-diynes using catalytic Cu(II) and base†
Dong Wang, Jihui Li, Na Li, Tingting Gao, Sihua Hou and Baohua Chen*
Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, Lanzhou, 730000, P. R. China; State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: chbh@lzu.edu.cn
First published on 30th October 2009
Abstract
We report an environmentally friendly, efficient method for transforming terminal acetylenes into 1,3-diynes based on catalytic amounts of a Cu(II) salt and base under solvent-free conditions. The developed process conforms to the principles of ‘green’ chemistry and addresses the shortage of such methods for the synthesis of 1,3-diynes. The reaction is quite general and results in good yields. Interestingly, the system also allows the synthesis of unsymmetric 1,3-diynes by cross-coupling of two different terminal alkynes. Finally, the catalyst can also be recycled.
Introduction
One of the challenges facing chemists this century is to develop new transformations that are not only efficient, selective, and high-yielding but that are also environmentally benign.1 During the last decade, the topic of ‘green’ chemistry has received increasing attention.1,2‘Green’ chemistry aims at the total elimination (or at least the minimization) of waste, and the implementation of sustainable processes.1a The utilization of nontoxic chemicals, renewable materials and solvent-free conditions are the key issues in a green synthetic strategy. In the present work, we describe a green approach towards the synthesis of conjugate 1,3-diyne.Conjugate 1,3-diyne derivatives are very important materials in the fields of biology and materials science, because they can be converted into various structural entities, especially substituted heterocyclic compounds.3 Traditional methods for the synthesis of 1,3-diynes include Glaser oxidative dimerization of terminal alkynes,4a various improved Glaser oxidative homocoupling reactions of terminal alkynes,4b–e and Sonogashira coupling.4f The catalyst system used commonly is the Pd,5 which involves Cu(I) salts as co-catalyst. Although Pd catalysts play a crucial role because of their mild, efficient and selective properties, they are expensive and often require phosphine or amine reagents.5e,5g,6 To address this, several groups have reported homocoupling reactions of terminal alkynes using a Pd-free catalytic system.7 For example, D. F. Li et al. described the reaction in the presence of CuI/I2,8 and H. F. Jiang et al. reported the Cu(II)-promoted oxidative homocoupling reaction of terminal alkynes in supercritical carbon dioxide.9 These Pd-free catalytic systems are efficient and economic, but their imperfections include the requirements of stoichiometric amounts of amine reagents, high pressure, high temperature, utilization of a co-catalyst and an oxygen atmosphere.5h,7b,7c,10 Moreover, it should be pointed out that the classical syntheses of conjugate 1,3-diynes (including the Pd-catalyzed and the Pd-free systems) generally involve organic solvents such as methanol, acetone, pyridine, methyl cellosolve (2-methoxyethanol) and toluene. Use of organic solvents is environmentally unfriendly, and so it is highly desirable to develop more environmentally friendly and economic methodologies for synthesizing conjugate 1,3-diynes. To achieve this, we report an environmentally friendly, economic, efficient and simple solvent-free system that allows the homocoupling reactions of terminal alkynes based on catalytic amounts of CuCl2 and triethylamine at 60 °C in air. The method is also useful for the synthesis of unsymmetric 1,3-diynes by cross-coupling of two different terminal alkynes. The results are summarized below.
Results and discussion
In order to identify the optimal reaction conditions, phenylacetylene was chosen as a test substrate. In this preliminary experiment, the homocoupling of phenylacetylene was carried out in various solvents, with CuCl2 (3 mol%) as a catalyst and Et3N (3 mol%) as a base, in air at 60 °C for 6 h (Table 1). It was found that the reaction proceeded perfectly in toluene or benzene (Table 1, entries 1 and 2), but that the yields decreased when the reaction was carried out in other solvents (Table 1, entries 3–11). However, the reaction surprisingly proceeded in excellent yields (>96%) under solvent-free conditions (Table 1, entry 12). The pure product was purified by column chromatography or reduced-pressure distillation. Considering our goal of an economic and environmentally friendly reaction, these solvent-free conditions are clearly the most favorable.
| |||
|---|---|---|---|
| Entry | Catalyst | Solvent | Yield (%)b |
| a The reaction was carried out using 1a (1 mmol) and Et3N (0.03 mmol) in the presence of CuCl2 (0.03 mmol) in the solvent (2 ml) at 60 °C in air.b Isolated yields after column chromatography. | |||
| 1 | CuCl2 | Toluene | 99 |
| 2 | CuCl2 | Benzene | 98 |
| 3 | CuCl2 | Water | 3 |
| 4 | CuCl2 | Methanol | 14 |
| 5 | CuCl2 | THF | 16 |
| 6 | CuCl2 | Acetonitrile | 12 |
| 7 | CuCl2 | Ethanol | 8 |
| 8 | CuCl2 | DMF | 40 |
| 9 | CuCl2 | DMSO | 42 |
| 10 | CuCl2 | Dioxane | 72 |
| 11 | CuCl2 | Acetone | 25 |
| 12 | CuCl2 | None | >96 |
Then we examined the influence of the catalysts on the yields. The reaction did not occur without a catalyst (Table 2, entry 1), and CuCl2 was more efficient than other Cu(0), Cu(I) and Cu(II) catalysts (Table 2, entries 2–12). It was noted that Cu(OH)x/TiO2 produced the desired product in 65% yield in the absence of base at 100 °C under an oxygen atmosphere (entry 2).
| ||
|---|---|---|
| Entry | Catalyst | Yield (%)b |
| a The reaction was carried out using 1a (1 mmol) and Et3N (0.03 mmol) in the presence of catalyst (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography.c Cu(OH)x/TiO2 (Cu = 5 mol%). | ||
| 1 | None | 0 |
| 2 | Cu(OH)x/TiO2c | 65 |
| 3 | CuCl | 16 |
| 4 | CuBr2 | 8 |
| 5 | Cu(OAc)2·H2O | 7 |
| 6 | CuI | Trace |
| 7 | Cu(OTf)2 | 7 |
| 8 | Cu | 4 |
| 9 | Cu(PPh)3Cl | 2 |
| 10 | CuCl2·2H2O | 49 |
| 11 | Cu(PPh3)2NO3 | 15 |
| 12 | Cu(acac)2 | Trace |
In the third set of experiments, we performed the reaction with various bases under solvent-free conditions in air (Table 3). As can be seen, the reaction could not be performed without a base (Table 3, entry 1). Organic bases including primary amines, secondary amines and tertiary amines were more effective than inorganic bases, with triethylamine being the best. There was also a good yield with n-butylamine as base (Table 3, entry 5). Therefore, the optimal solvent-free system for this reaction involves Et3N (3 mol%) and CuCl2 (3 mol%).
| ||
|---|---|---|
| Entry | Base | Yield (%)b |
| a The reaction was carried out using 1a (1 mmol) and base (0.03 mmol) in the presence of CuCl2 (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography.c TMEDA = tetramethylethylenediamine. | ||
| 1 | None | 0 |
| 2 | Triethylamine | >96 |
| 3 | Diethylamine | 69 |
| 4 | Pyridine | 12 |
| 5 | n-butylamine | 90 |
| 6 | K2CO3 | 24 |
| 7 | KOtBu | 10 |
| 8 | Piperidine | 38 |
| 9 | Diisopropylamine | 35 |
| 10 | tert-Butylamine | 18 |
| 11 | NaOH | 14 |
| 12 | TMEDA | 70c |
| 13 | K3PO4·3H2O | 7 |
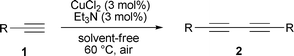
Encouraged by the efficiency of the reaction protocol described above, we investigated the substrate scope. A variety of terminal alkynes including aromatic and aliphatic acetylenes were tested under the optimized conditions. The results show that the solvent-free CuCl2-catalyzed homocoupling reaction tolerates a variety of functional groups. As shown in Table 4, the catalytic oxidative homocoupling of phenylacetylenes 1a–h, which contain electron-donating as well as electron-withdrawing substituents, proceeded readily to afford the corresponding diyne derivatives 2a–h in 50–99% yields (entries 1–8). The reaction of the heteroatom-containing alkyne 1i also proceeded efficiently (entry 9), but the desired product of the heteroatom-containing alkyne 1j was isolated in 70% yield, due to the partial carbonization of the substrate (entry 10). When aliphatic acetylenes were used, the yields were somewhat lower (entries 11 and 12). Alkynes based on propargylic alcohols also gave the corresponding diynes, but the yields were low (entries 13 and 14). Interestingly, we successfully extended the procedure to higher-boiling alkynes such as ferrocenylacetylene, and the yield of the obtained product being 99% (entry 15). In addition, the crude products of symmetric 1,3-diynes can be purified by reduced pressure distillation (except 1,4-diferrocenylbuta-1,3-diyne 2o) – reduced pressure distillation is simpler and more environmentally friendly than column chromatography.
| ||||
|---|---|---|---|---|
| Entry | R | Time/h | Product | Yield (%)b |
a The reaction was carried out using RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (1 mmol) and Et3N (0.03 mmol) in the presence of CuCl2 (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography.c The reaction was carried out using 1o (1 mmol) and Et3N (2 mmol) in the presence of CuCl2 (0.03 mmol) at 100 °C in air for 10 h. CH (1 mmol) and Et3N (0.03 mmol) in the presence of CuCl2 (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography.c The reaction was carried out using 1o (1 mmol) and Et3N (2 mmol) in the presence of CuCl2 (0.03 mmol) at 100 °C in air for 10 h. | ||||
| 1 | Phenyl (1a) | 6 | 2a | >96 |
| 2 | 4-CH3OC6H4 (1b) | 6 | 2b | 99 |
| 3 | 3-CH3C6H4 (1c) | 6 | 2c | >98 |
| 4 | 4-FC6H4 (1d) | 6 | 2d | 80 |
| 5 | Naphthalen-1-yl (1e) | 6 | 2e | 88 |
| 6 | 2-ClC6H4 (1f) | 6 | 2f | 85 |
| 7 | 3-NH2C6H4 (1g) | 4 | 2g | 50 |
| 8 | 4-n-C5H11OC6H4 (1h) | 6 | 2h | >98 |
| 9 | Thiophen-3-yl (1i) | 6 | 2i | 90 |
| 10 | Pyridine-2-yl (1j) | 4 | 2j | 70 |
| 11 | BrCH2 (1k) | 6 | 2k | 60 |
| 12 | n-C4H9 (1l) | 6 | 2l | 75 |
| 13 | HOC(CH3)2 (1m) | 6 | 2m | 40 |
| 14 | HOCH2 (1n) | 6 | 2n | 45 |
| 15 | Ferrocenyl (1o) | 10 | 2o | 99c |
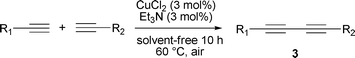
The cross-coupling of two different terminal alkynes was also investigated in the catalytic system by using an excess of one of the terminal alkyne substrates. As shown in Table 5, unsymmetric 1,3-diynes were produced in 32–72% yield. Phenylacetylene 1a successfully cross-coupled with some alkynes including alkoxy alkynes (entries 1 and 4), propargylic alcohols (entries 2 and 5); p-methoxyphenylacetylene 1b could also be cross-coupled with aromatic acetylenes (entries 1 and 3) and a heteroatom-containing alkyne (entry 6), although the yields were somewhat lower. The reaction between 1l and 1a (or 1b) only gives trace product (entries 7 and 8). It should be noted that the crude products of unsymmetric 1,3-diynes only can be purified by column chromatography – reduced pressure distillation does not work for these compounds.
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield (%)b |
a The reaction was carried out using R1C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (0.5 mmol), R2C CH (0.5 mmol), R2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (3 mmol) and Et3N (0.03 mmol) in the presence of CuCl2 (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography. CH (3 mmol) and Et3N (0.03 mmol) in the presence of CuCl2 (0.03 mmol) at 60 °C in air.b Isolated yields after column chromatography. | ||||
| 1 | 4-CH3OC6H4 | C6H5 | 3a | 70 |
| 2 | HOCH2 | C6H5 | 3b | 35 |
| 3 | 4-CH3OC6H4 | 3-CH3C6H4 | 3c | 55 |
| 4 | 4-n-C5H11OC6H4 | C6H5 | 3d | 72 |
| 5 | HOC(CH3)2 | C6H5 | 3e | 32 |
| 6 | 4-CH3OC6H4 | Thiophen-3-yl | 3f | 56 |
| 7 | n-C4H9 | C6H5 | 3g | Trace |
| 8 | n-C4H9 | 4-CH3OC6H4 | 3h | Trace |
Finally, we examined the recovery and reuse of CuCl2. The catalyst can be recovered by filtration, acidification, and drying under vacuum, and can then be reused. After five recycles, the activity of the recovered catalyst decreased slightly to 85%. The average catalyst recovery was about 80% (Table 6).
Conclusion
In conclusion, we have developed an environmentally friendly, economical and efficient method for transforming terminal acetylenes into 1,3-diynes under solvent-free conditions with catalytic amounts of CuCl2 and triethylamine. The procedure is suitable for many substrates, and various conjugate 1,3-diynes can be produced conveniently with excellent yields. This catalytic system can also be used for the synthesis of unsymmetrical 1,3-diynes, although the yields are not perfect. The catalyst can also be recycled five times with only a moderate reduction in activity, making it acceptable for industrial-scale production.Experimental
General
Solvents were dried and degassed by standard methods, and all alkynes are readily available. Flash column chromatography was performed using silica gel (300–400 mesh). Analytical thin-layer chromatography was performed using glass plates pre-coated with 200–400 mesh silica gel impregnated with a fluorescent indicator (254 nm).Typical experimental procedure for the homocoupling reaction under solvent-free conditions
Typical reaction procedure: to a mixture of CuCl2 (3.0 mol%) and Et3N (3.0 mol%), phenylacetylene (1.0 mmol) was added. The mixture was stirred at 60 °C under air for 6 h.Acknowledgements
We are grateful to the project sponsored by the Scientific Research Foundation for the State Education Ministry (No. 107108) and the Project of the National Science Foundation of P. R. China (No. J0730425).References
- (a) P. T. Anastas and T. C. Williamson, Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes, Oxford Science Publications, New York, 1998 Search PubMed; (b) J. M. DeSimone, Science, 2002, 297, 799–803 CrossRef CAS; (c) S. J. Jeon, H. M. Li and P. J. Walsh, J. Am. Chem. Soc., 2005, 127, 16416–16425 CrossRef CAS.
- (a) M. Poliakoff and P. T. Anastas, Nature, 2001, 413, 257 CrossRef CAS; (b) A. S. Matlack, Introduction to Green Chemistry, Marcel Dekker, Inc., New York, 2001 Search PubMed; (c) R. A. Cross and B. Kalra, Science, 2002, 297, 803–807 CrossRef CAS.
- (a) F. Bohlmann, T. Burkhardt and C. Zdero, Naturally Occurring Acetylenes, Academic Press, London, 1973 Search PubMed; (b) L. Hansen and P. M. Boll, Phytochemistry, 1986, 25, 285 CrossRef CAS; (c) H. Matsunaga, M. Katano, H. Yamamoto, H. Fujito, M. Mori and K. Takata, Chem. Pharm. Bull., 1990, 38, 3480 CAS.
- (a) C. Glaser, Ber. Dtsch. Chem. Ges., 1869, 2, 422 CrossRef; (b) A. S. Hay, J. Org. Chem., 1962, 27, 3320 CrossRef CAS; (c) E. Valenti, M. A. Pericas and F. Serratosa, J. Am. Chem. Soc., 1990, 112, 7405 CrossRef CAS; (d) P. Cadiot and W. Chodkiewicz, in Chemistry of Acetylene, H. G. Viehe, Marcel Dekker, New York, 1969, p. 597 Search PubMed; (e) G. Eglington and R. Galbraith, J. Chem. Soc., 1959, 889 RSC; (f) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef.
- (a) M. Vlassa, I. Ciocan-Tarta, F. Margineanu and I. Oprean, Tetrahedron, 1996, 52, 1337 CrossRef; (b) Q. Liu and D. J. Burton, Tetrahedron Lett., 1997, 38, 4371 CrossRef CAS; (c) A. Lei, M. Srivastava and X. Zhang, J. Org. Chem., 2002, 67, 1969 CrossRef CAS; (d) I. J. S. Fairlamb, P. S. B. auerlein, L. R. Marrison and J. M. Dickinson, Chem. Commun., 2003, 632 RSC; (e) A. S. Batsanov, J. C. Collings, I. J. S. Fairlamb, J. P. Holland, J. A. K. Howard, Z. Y. Lin, T. B. Marder, A. C. Parsons, R. M. Ward and J. Zhu, J. Org. Chem., 2005, 70, 703 CrossRef CAS; (f) J. H. Li, Y. Liang and X. D. Zhang, Tetrahedron, 2005, 61, 1903 CrossRef CAS; (g) J. H. Li, Y. Liang and Y. X. Xia, J. Org. Chem., 2005, 70, 4393 CrossRef CAS; (h) M. Shi and H. X. Qian, Appl. Organomet. Chem., 2006, 20, 771 CrossRef CAS.
- (a) P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632 CrossRef CAS; (b) V. D. Subhash, S. Dong and P. H. Lee, J. Org. Chem., 2003, 68, 7085 CrossRef CAS.
- (a) C. Z. Bing and Z. Xuan, Appl. Organomet. Chem., 2007, 21, 345 CrossRef; (b) K. Keigo, Y. Syuhei, K. Miyuki, Y. Kazuya and M. Noritaka, Angew. Chem., Int. Ed., 2008, 47, 2407–2410 CrossRef CAS; (c) J. S. Yadav, B. V. S. Reddy, K. Bhaskar Reddy, K. U. Gayathri and A. R. Prasad, Tetrahedron Lett., 2003, 44, 6493 CrossRef CAS; (d) A. Sharifi, M. Mirzaei and M. R. N. Jamal, Monatsh. Chem., 2006, 137, 213 CrossRef CAS; (e) G. W. Kabalka, L. Wang and R. M. Pagni, Synlett, 2001, 108–110 CAS.
- D. F. Li, K. Yin, J. Li and X. S. Jia, Tetrahedron Lett., 2008, 49, 5918 CrossRef CAS.
- (a) J. H. Li and H. F. Jiang, Chem. Commun., 1999, 2369 RSC; (b) H. F. Jiang, J. Y. Tang, A. Z. Wang, G. H. Deng and S. R. Yang, Synthesis, 2006, 1155 CrossRef CAS.
- T. Oishi, T. Katayama, K. Yamaguchi and N. Mizuno, Chem. Eur. J., 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental information. See DOI: 10.1039/b917448f |
| This journal is © The Royal Society of Chemistry 2010 |