Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption
Ren-Hau
Lai
a,
Michael J.
Miller
ab and
Elizabeth
Jeffery
*ab
aDivision of Nutritional Sciences, University of Illinois, 905 S Goodwin Ave, Urbana, IL 61801, USA
bFood Science and Human Nutrition, University of Illinois, 905 S Goodwin Ave, Urbana, IL 61801, USA. E-mail: ejeffery@illinoi.edu
First published on 22nd October 2010
Abstract
In the absence of the plant enzyme myrosinase, such as in cooked broccoli, glucoraphanin is considered to be hydrolyzed by bacteria in the lower gut to produce the bioactive isothiocyanate sulforaphane. Simulated digestion using US Pharmacopeia methods caused no loss of glucoraphanin, confirming that glucoraphanin is not destroyed by digestive enzymes during passage through the digestive tract and is able to reach the rat cecum intact. Introduction of glucoraphanin (150 μmol/kg BW) directly into the cecum resulted in appearance of isothiocyanates in the mesenteric plasma by 120 min. In contrast, introduction of sulforaphane (150 μmol/kg BW) directly into the cecum resulted in the appearance of isothiocyanates in the mesenteric plasma within 15 min. Plasma levels remained constant for over an hour. Anaerobic incubation ex vivo of cecal microbiota from male F344 rats with glucoraphanin resulted in very low levels of the hydrolytic metabolite erucin nitrile, showing that hydrolysis of glucosinolates is carried out by cecal microbiota, but metabolism ex vivo by microbiota did not reflect not reflect metabolism in situ. These data are the first to report direct evidence of hydrolysis of glucoraphanin to sulforaphane in the cecum of rats and to show that sulforaphane is able to cross the cecal enterocyte for systemic absorption.
Introduction
In epidemiological studies, vegetable intake has been shown to reduce cancer risk, including cancer of the colon, lung, breast, and prostate.1,2 In particular, cruciferous vegetables may provide greater protective benefit than many other vegetables or fruits.3,4 Broccoli, Brassica oleracea L., is well known as providing a high content of fiber, minerals and vitamins, all of which may aid in reducing carcinogenesis. Broccoli is rich in antioxidants, including ascorbic acid, tocopherols, carotenoids, and flavonoids, which may also play important roles in chemoprotection.5,6 But in addition to these components shared with many other plant foods, crucifers contain glucosinolates, which are considered to play the major role in reduction of cancer risk by crucifers.7Glucoraphanin (GRP), 4-methylsufinylbutyl glucosinolate, is a major glucosinolate in broccoli and precursor to the bioactive isothiocyanate sulforaphane (SF). The hydrolysis of glucosinolates requires the enzyme myrosinase (EC 3.2.1.147), a thioglucoside glucohydrolase, which is endogenous to the plant and physically separated from glucosinolates.8 However, once raw broccoli undergoes tissue disruption from chewing, the myrosinase gains access to GRP and catalyzes hydrolysis within the gastrointestinal tract.
Upon cooking of broccoli, myrosinase is heat inactivated, yet low levels of SF metabolites appear in urine following ingestion of cooked crucifers, suggesting that hydrolysis has occurred.9 Data supporting a role for microbiota include the lack of glucosinolate hydrolysis products in urine of germ-free rats10 and in urine of subjects undergoing bowel cleansing together with antibiotic treatment11 suggesting that rats and humans lack a thioglucoside glucohyrodrolase and that the gut microbiota are required for GRP conversion to SF. These conclusions are supported by studies of purified GRP metabolism in rats.12
Absorption of SF across the upper intestinal wall (jejunum) has been evaluated in a clinical study where a tube was passed through the stomach to the jejunum and a broccoli soup containing myrosinase was introduced. Sulforaphane and a SF-glutathione conjugate (SF-GSH) were later effluxed into the perfusate collected from the jejunum.13 There are no studies directly evaluating GRP or SF metabolism and absorption in the lower gastrointestinal tract (cecum or colon), although this is implied by the appearance of urinary SF metabolites following oral administration of purified GRP to rats.14 Furthermore, there are reports that the rat colon exhibits induced levels of quinone reductase following feeding with purified GRP.15
Our long-term goal is to enhance SF bioavailability, by improving SF formation by gut microbiota. However, it is not yet clear by what mechanism and to what extent GRP is hydrolyzed and/or SF absorbed in the lower gut; both steps are required for systemic bioavailability. It therefore becomes critical to study the relationship between GRP, microbiota and the gastrointestinal tract. The objectives of this study were 1) to evaluate simulated digestion of GRP in the upper gastrointestinal tract; 2) to determine if GRP is hydrolyzed by rat cecal microbiota ex-vivo; 3) to determine if GRP is hydrolyzed in the rat cecum in situ and if the hydrolysis product(s) are absorbed across the cecal wall.
Materials and methods
Materials
Semi-purified GRP (30.4% pure) was prepared from Premium Crop broccoli seed (Brassica oleracea L.) as previous described.14 Sulforaphane and SF nitrile standards were purified from broccoli seed as previously described.16 Erucin standard was purchased from LKT laboratories (St. Paul, MN). Both MRS and RCM media were purchased from Beckton Dickenson (Franklin Lakes, NJ). Dichloromethane and HPLC grade methanol was purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).Simulated upper gut digestion of glucoraphanin
The simulation model of upper gastrointestinal enzymatic digestion was modified from the methods laid out in the US Pharmacopeia.17 Briefly, the major enzymes, salivary α-amylase, gastric pepsin, and intestinal pancreatin, were tested sequentially and in triplicate. First, 0.5 mM GRP was incubated with α-amylase (100 U/mL) at 37 °C for 3 min to simulate oral digestion. After withdrawing a 450 μL sample for analysis, pepsin (0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/v, pepsin:GRP) and concentrated HCl (to bring down the pH to 2.0) were added and incubated at 37 °C for 2 h. After 2 h, a 450 μL sample was collected for analysis. The remainder was added to a mixture of 0.8 mg/L pancreatin (8-fold USP specifications) with 25 g/L bile salts mixture (Sigma-Aldrich), and the pH was titrated to 7.5 with 0.5 N NaHCO3. The incubation time of the simulated intestinal digestion was 2 h. Samples collected from each step of the simulation were hydrolyzed in excess myrosinase to form SF, which was estimated by GC as previously described.16
1 w/v, pepsin:GRP) and concentrated HCl (to bring down the pH to 2.0) were added and incubated at 37 °C for 2 h. After 2 h, a 450 μL sample was collected for analysis. The remainder was added to a mixture of 0.8 mg/L pancreatin (8-fold USP specifications) with 25 g/L bile salts mixture (Sigma-Aldrich), and the pH was titrated to 7.5 with 0.5 N NaHCO3. The incubation time of the simulated intestinal digestion was 2 h. Samples collected from each step of the simulation were hydrolyzed in excess myrosinase to form SF, which was estimated by GC as previously described.16
Animals and treatments
Male F344 rats from Harlan Inc. (Indianapolis, IN) were housed individually in hanging wire bottom cages under controlled conditions (12 h light-dark cycle, 22 °C, and 60% humidity). They were fed AIN93G diet and tap water ad libitum throughout the study. Food was available 24 h a day and provided fresh daily. Animal use was approved by the Animal Care and Use Committee of the University of Illinois. Animals were acclimated for three days, during the last two days of which all animals received saline daily by gavage.Six male F344 rats, weighing 235 ± 4.1 g (mean ± SD) were used for in situ cecal metabolism. Rats were anesthetized using ketamine: xylazine (87 mg/mL: 13 mg/mL; 1.0 mL/kg body weight). Anesthesia was maintained with ketamine, 30% of the starting dose, given hourly as necessary. The surgical procedure was modified from Johnson et al.18 Briefly, the lower gastrointestinal tract was exposed by a midline incision. The gut was closed by surgical thread ties at the cecal-colonic junction to prevent flow from the cecum to the colon. Sulforaphane or GRP (150 μmol/kg body weight) was injected into the cecum through the ileum, which was immediately closed at the ileo-cecal junction, using surgical thread. A catheter was installed into a mesenteric vein 2–3 cm from the cecum (20G x 2′′, Exelint Inc., Los Angeles, CA). Before the installation, catheters and syringes were flushed with heparinized saline (25 U/mL) to prevent clotting. Blood samples (approximate 400 μL) were collected at 15, 30, 45, 60 min from the animals administered SF and at 30, 60, 90, 120, 150 min from the animals administered GRP. During surgery, a heating pad was used to maintain body temperature, and saline-soaked gauze was placed over the exposed gut to maintain hydration. Blood samples were placed on ice until centrifugation at 1000 x g at 4 °C for 12 min to separate the plasma (Eppendorf Centrifuge 5415D; Westbury, NY). The plasma was then immediately collected and frozen at −80 °C until analysis. Total isothiocyanates (isothiocyanates and their glutathione metabolites) were measured by a modification of the cyclocondensation assay,19 as previously described.20
For hydrolysis ex vivo, rats were administrated either saline (control group, n = 6) or 150 μmol GRP/kg body weight by gavage daily for 4 days (GRP pretreated group, n = 6), since pretreatment might stimulate metabolism. The rats were fasted for 16 h prior to termination and killed 24 h after the last treatment. The abdominal cavity was opened by a midline incision to expose the gastrointestinal tract and the cecum was ligated and excised proximally and distally to the ileal and colonic ties, respectively, to maintain anaerobic conditions. Ceca were immediately transported in an air-tight bag at 37 °C to an anaerobic chamber (Coy, Grass Lake MI; 90% N2, 5% H2 and 5% CO2). Inside the anaerobic chamber, cecal contents were removed and diluted with sterile distilled water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v). After mixing well, 100 μL samples were diluted into 10 mL medium (MRS, RCM, or 0.5 mM phosphate buffer pH 6.8; see below for choice of media) and divided into 5 aliquots for anaerobic incubation at 37 °C for 0, 3, 6, 12, or 24 h. After measuring the pH, incubations were stopped, and microbiota were removed by centrifugation (6000 x g for 10 min) at 4 °C followed by filtration, using a 0.2 μm syringe filter, and stored at −80 °C until analysis of GRP, isothiocyanate and nitrile.
10 (w/v). After mixing well, 100 μL samples were diluted into 10 mL medium (MRS, RCM, or 0.5 mM phosphate buffer pH 6.8; see below for choice of media) and divided into 5 aliquots for anaerobic incubation at 37 °C for 0, 3, 6, 12, or 24 h. After measuring the pH, incubations were stopped, and microbiota were removed by centrifugation (6000 x g for 10 min) at 4 °C followed by filtration, using a 0.2 μm syringe filter, and stored at −80 °C until analysis of GRP, isothiocyanate and nitrile.
Choice of media for glucosinolate metabolism by cecal microbiota
In order to support growth of rat cecal bacterial strains with the greatest potential for GRP hydrolysis, we performed a search for bacterial genes most similar to myrosinase from white mustard (thioglucoside glucohydrolase, EC3.2.1.147, Sinapis alba). Using the Basic Local Alignment Search Tool (BLAST), we probed the bacterial protein database archived at the National Center for Biotechnology Information, against the known amino acid sequence of myrosinase.21 An integrated framework analysis for identification and characterization of taxonomic and phenotypic variation of enzymes (CHISEL) was then used to identify which bacteria contained the best matches to white mustard myrosinase.Statistical analysis
For the incubation of cecal microbiota ex vivo, significant differences (p ≤ 0.05) in metabolism between microbiota from rats gavaged with saline (control group) and with GRP (pretreated group) were determined by Student's t-test using SAS software (SAS, Inc., Cary, NC) at each time. For the cecal metabolism of GRP in situ, samples from each time point were compared to zero time, and a p-value ≤ 0.05 was accepted as significantly different.Results
Simulated gastrointestinal digestion of glucoraphanin
No digestive enzymes had any effect on GRP in vitro. The recovery of GRP following simulated oral/ amylase digestion (100.5 ± 2.5%), gastric/HCl-pepsin digestion (100.7 ± 0.7%), and intestinal/pancreatin-bile digestion (101.3 ± 1.2%) were not different from the original dose (Fig. 1).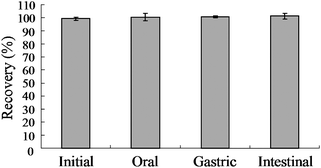 | ||
| Fig. 1 Effect of digestive enzymes on glucoraphanin recovery. Glucoraphanin (0.5 mM) was incubated with amylase, HCl/pepsin then pancreatin/bile salts and samples taken to determine oral, gastric and intestinal recovery of glucoraphanin, respectively. Mean ± SE, n = 3. | ||
Cecal metabolism of glucoraphanin in situ
In order to determine if SF can be absorbed from the cecum, we injected purified SF directly into the cecal lumen and measured mesenteric plasma levels of total isothiocyanate (free isothiocyanate plus glutathione metabolites). Total isothiocyanate increased from 0 to 105 μM by 15 min, the first time point (Fig. 2). Blood samples were collected every 15 min for the next 45 min, and total isothiocyanate levels were not statistically different from the level at 15 min. Although only one animal survived surgery past 60 min, this last plasma sample contained a total isothiocyanate level very similar to the concentration at 15 min. Plasma total isothiocyanate levels in rats administered GRP (150 μmol/kg body weight) showed a very different pattern. Mesenteric plasma total isothiocyanate levels did not rise significantly until 120 min (Fig. 3). At 120 min, plasma total isothiocyanate levels rose to 9.1 ± 1.8 μM, less than 10% of the value following SF administration. Only one animal remained alive until 150 min after GRP administration, and plasma levels were 10.9 μM, very similar to the 120 min value.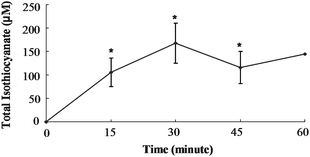 | ||
| Fig. 2 Total isothiocyanate equivalents in mesenteric plasma of rats treated with Sulforaphane. Rats were dosed with 150 μmol SF/kg BW directly into the cecum. Values were compared to the zero time value using the Student's t-test. The asterisk indicates significantly different from 0 h (p ≤ 0.05). Mean ± SE, n = 3 (n = 1 at 60 min). | ||
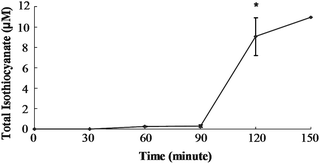 | ||
| Fig. 3 Total isothiocyanate equivalents in mesenteric plasma of rats treated with Glucoraphanin. Rats were dosed with 150 μmol GRP/kg BW directly into the cecum. Values were compared to the zero time value using the Student's t-test. The asterisk indicates significantly different from 0 h (p ≤ 0.05). Mean ± SE, n = 3 (n = 1 at 150 min). | ||
Choice of media for support of glucosinolate metabolism by cecal microbiota
CHISEL analysis identified 7 taxonomy-specific clusters for thioglucoside glucohydrolase from domains in either Eukarya or Bacteria (Table 1). The two most similar models SF001079-4-B-Firmicutes (phylum which includes Lactobacillus, Clostridium and many other genera) and SF001079-5-B-Lactobacillales, (order which includes Lactobacillus species), include bacterial species abundant in the human gastrointestinal tract. The specific proteins identified from these two models are members of the glycoside hydrolase family 1.22 Activities associated with the glycoside hydrolase family 1 include beta-glucosidase, beta-galactosidase, 6-phospho-beta-galactosidase, 6-phospho-beta-glucosidase, lactase-phlorizin hydrolase, and beta-mannosidase activities. From these data, we chose to perform microbial metabolism ex-vivo using media that support lactobacilli and clostridia growth for GRP metabolism.| Model | Description | Score | E-Value |
|---|---|---|---|
| SF001079_2_E_Brassicaceae | 3.2.1.147 thioglucosidase (Eukaryotic version) | 1447.8 | 0 |
| SF001079_3_E_Magnoliophyta | 3.2.1.21 beta-glucosidase (Eukaryotic version) | 663.8 | 1.1e−198 |
| SF001079_4_B_Firmicutes | 3.2.1.21 beta-glucosidase (Bacterial version) | 329.6 | 4.3e−98 |
| SF001079_5_B_Lactobacillales | 3.2.1.86 6-phospho-beta-glucosidase (Bacterial version) | 127.0 | 4.3e−37 |
| SF001079_5_B_Gammaproteobacteria | 3.2.1.86 6-phospho-beta-glucosidase (Bacterial version) | 96.3 | 7.6e−28 |
| SF001079_6_B_Bacilli | 3.2.1.85 6-phospho-beta-galactosidase (Bacterial version) | 91.0 | 2.9e−26 |
| SF001079_1_B_Bacilli | 3.2.1.21 beta-glucosidase (Bacterial version) | 23.3 | 6.9e−06 |
Glucoraphanin metabolism by cecal microbiota ex-vivo
Body weight and food intake of GRP-pretreated animals were not different from the control, saline gavaged rats (data not shown). Cecal microbiota were transferred anaerobically, as described in methods, and incubated in an anaerobic chamber with GRP (0.5 mM) in MRS medium (supports growth of lactobacilli and others) or RCM medium (supports growth of clostridia, bifidobacteria and others). The pH was recorded at the end of the incubation (Table 2). The pH, an indicator of microbial growth, dropped to 4.0 in MRS and to 4.7 in RCM medium by 24 h.| pH | Glucoraphanin (mM) | |||
|---|---|---|---|---|
| Control | GRP Pretreated | Control | GRP Pretreated | |
| a Glucoraphanin content remaining at termination of metabolism was significantly different in cecal samples from glucoraphanin-pretreated rats compared to samples from control rats, (p ≤ 0.05). | ||||
| MRS | ||||
| 0h | 6.30 ± 0.06 | 6.33 ± 0.07 | 0.49 ± 0.01 | 0.46 ± 0.01 |
| 3h | 5.39 ± 0.21 | 5.46 ± 0.26 | 0.42 ± 0.05 | 0.37 ± 0.03 |
| 6h | 4.55 ± 0.25 | 4.65 ± 0.27 | 0.26 ± 0.04 | 0.21 ± 0.02 |
| 12h | 4.08 ± 0.08 | 4.12 ± 0.09 | 0.28 ± 0.04 | 0.19 ± 0.03a |
| 24h | 4.01 ± 0.03 | 4.03 ± 0.05 | 0.30 ± 0.05 | 0.21 ± 0.03a |
| RCM | ||||
| 0h | 6.27 ± 0.12 | 6.36 ± 0.12 | 0.50 ± 0.00 | 0.48 ± 0.00 |
| 3h | 5.31 ± 0.18 | 5.31 ± 0.18 | 0.38 ± 0.10 | 0.23 ± 0.11 |
| 6h | 4.81 ± 0.18 | 4.82 ± 0.18 | 0.17 ± 0.04 | 0.08 ± 0.05 |
| 12h | 4.64 ± 0.09 | 4.66 ± 0.09 | 0.07 ± 0.02 | 0.03 ± 0.01a |
| 24h | 4.71 ± 0.07 | 4.72 ± 0.08 | 0.00 ± 0.00 | 0.02 ± 0.02 |
| Buffered | ||||
| 0h | 6.89 ± 0.01 | 6.88 ± 0.02 | 0.48 ± 0.01 | 0.44 ± 0.00 |
| 3h | 6.76 ± 0.02 | 6.76 ± 0.01 | 0.29 ± 0.05 | 0.16 ± 0.08a |
| 6h | 6.75 ± 0.01 | 6.75 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 12h | 6.74 ± 0.01 | 6.75 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 24h | 6.75 ± 0.01 | 6.76 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
In the MRS medium, GRP hydrolysis in microbiota from the GRP-pretreated rats was slightly but significantly greater than from the control group at both 12 and 24 h (p ≤ 0.05; Table 2), suggesting that there was some change in the microbial population toward improved metabolism of GRP. A similar pattern was seen in GRP metabolism in microbiota grown in RCM medium. However, the effect of GRP pre-treating was smaller than that in MRS medium and only significant at 12 h (Table 2). Sulforaphane was found as product in only one cecal incubation in MRS medium, and in no RCM incubations. The SF-positive incubation was from a control animal, and the level of SF in that sample was 0.16 mM at 3 h, decreasing to 0.07 mM by 24 h. In addition, a small peak identified as erucin nitrile was found in all samples, increasing over time in the MRS medium, but remaining very small (data not shown). We have previously identified erucin nitrile by GC/MS, but have not developed quantification and no erucin nitrile standard is commercially available.12
Nitriles are alternative products to isothiocyanates, but they lack bioactivity.16 Because a low pH environment supports nitrile formation during myrosinase-dependent metabolism of glucosinolates, we considered that metabolism in buffer, to maintain neutral pH, might support greater isothiocyanate formation. Using phosphate buffer (pH 6.8) as the medium, the pH was stable throughout the 24 h incubation (Table 2). The microbiota from GRP-pretreated rats were significantly more able (p ≤ 0.05) to support GRP disappearance (metabolism) at 3 h, compared to GRP metabolism in microbiota from control rats (Table 2). At 24 h, almost 100% of the GRP was metabolized by the microbiota from both the GRP-pretreated and the control rats. However, even with the pH controlled, erucin nitrile was the only detectable hydrolysis product.
Discussion
Digestive enzymes from the upper gastrointestinal tract did not destroy GRP. Not all glucosinolates respond similarly, since progoitrin and sinigrin, unsaturated aliphatic glucosinolates, are reported to be lost during digestion. A study feeding pigs rapeseed showed that only 60% of intact glucosinolates (mostly progoitrin) reached the colon, measured by examining the ileal digesta.23 Another study found that sinigrin was either absorbed or destroyed during passage through the rat gastrointestinal tract, so that total AITC in colon and cecum could only account for 55% of the dose administrated.24 However, simulated digestion is far less complex than an animal feeding study and a variety of factors could affect recovery in vivo, such as absorption of intact glucosinolates.12 Reports of effects of digestive enzymes on specific glucosinolates in vitro also suggest that individual glucosinolates are differently affected.25,26 Our data are the first to indicate that GRP is not degraded by upper gastrointestinal digestive enzymes in vitroThe doses of GRP and SF chosen to study cecal metabolism in situ approximated an oral dose of 60 mg GRP/kg body weight, which we have shown is effective (colonic quinone reductase was upregulated) but not toxic to the cecum.14,15 When SF was introduced into the cecum, SF/ isothiocyanates were found in the mesenteric blood within 15 min. Most broccoli feeding and SF dosing studies report peripheral blood levels and cannot be compared to the present study.27,28 In a study that sampled mesenteric blood during constant infusion of SF (10 μM) through a portion of the ileum of Sprague-Dawley rats, comparably early appearance of SF and metabolites in plasma was reported.29 That study estimated SF and glutathione metabolites separately, by LC-MS/MS, and found that most SF was conjugated rather than free, showing that SF metabolism had occurred in the enterocyte during absorption.29 The mechanism of SF absorption at the enterocyte could be passive or mediated by transporters, such as multi-drug resistance associated proteins (MRP) or P-glycoprotein.30 Sulforaphane has been shown to upregulate MRP-1 and -2 in human cells, suggesting that pretreating with broccoli/SF can alter SF uptake.31,32 Active transport was also suggested by Petri and colleagues, who found SF-GSH efflux into the jejunum of humans given a broccoli soup by feeding tube.13 Athough our data are consistent with an active transport system at the cecum, with mesenteric plasma total isothiocyanates constant for an hour or more, there are insufficient data to determine if transport is active or passive (Fig. 2). Although the analysis we used does not allow us to differentiate between SF, other isothiocyanates and glutathione metabolites of isothiocyanates, it was chosen because it captures total SF and SF metabolites and because it is sufficiently sensitive to be used with plasma samples of less than 200 μL.33
The environment inside the cecum is delicate, and the microbiota are sensitive to even slight changes, including nutrients, temperature or oxygen tension. Specialized media have been developed to support optimal growth of specific bacteria, although there is no reliable method to provide nutrient support of the entire mixed population of microbiota within the cecum. A comparison of bacterial enzymes to the amino acid sequence of white mustard myrosinase suggested that lactobacilli and clostridia might be most likely to hydrolyze glucosinolates (Table 1). MRS medium is widely used to support the growth of lactobacilli,34 whereas RCM is used to support growth of clostridia and bifidobacteria, both of which are abundant gut microorganisms.35,36 A GRP dose (0.5mM) was almost completely metabolized in RCM by 12 h, whereas almost 50% remained in the MRS medium, even at 24 h, indicating a possible key role for clostridia or bifidobacteria in GRP hydrolysis. Unexpectedly, the 0.5 mM phosphate buffer pH 6.8 “control” medium supported the most robust GRP metabolism, depleting the GRP to less than 5% by 6 h, possibly suggesting the importance of pH maintenance for activity of a bacterial thiohydrolase, and thus for metabolism of GRP.
Pretreating rats with GRP resulted in only slight, although significant, improvement in GRP metabolism in both MRS and RCM media, suggesting that four days' treatment with GRP was unable to greatly alter the microbial profile toward one with improved GRP metabolism. In a study where subjects ate 14 g crucifers/kg body weight/day for 14 days, there were measurable changes in the profile of microbiota.37 Cruciferous vegetables typically consist of ∼40% of dry weight as fiber which could have altered the profile.37 Future studies comparing metabolism of GRP in digesta from rats administered broccoli and GRP may be able to distinguish between effects of GRP and broccoli fiber on GRP metabolism.
Hydrolysis of glucoraphanin was supported by microbiota, although the only hydrolysis product present in all incubations was erucin nitrile (Fig. 4). The isothiocyanate erucin, the reduced form of SF, has similar bioactivity to SF.38 Reduction of SF to erucin occurs following i.p. administration of SF.39 Erucin nitrile could be formed by reduction of SF nitrile in the anaerobic environment of the cecum or by hydrolysis of glucoerucin (Fig. 4). When rats were dosed with GRP, the reduced glucosinolate glucoerucin was found in urine.12 Also, incubation of GRP with E. coli resulted in glucoerucin appearance concomitantly with GRP disappearance; at the completion of 24 h, the major hydrolytic product was erucin nitrile (data not shown). Therefore, it is possible that GRP was reduced to glucoerucin and hydrolyzed to erucin nitrile.
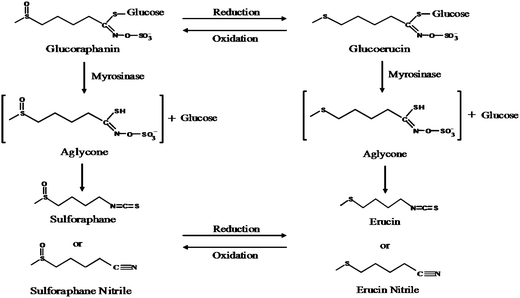 | ||
| Fig. 4 Scheme of possible routes for glucoraphanin hydrolysis to form erucin nitrile. | ||
One cecal preparation (from a control rat) displayed more rapid metabolism of GRP than other preparations, regardless of the medium used. Whereas erucin nitrile was formed in all media, incubation in MRS medium also resulted in SF formation, accounting for ∼50% of the GRP disappearance at 3 h. However, much of the SF was lost by 24 h. When SF was incubated in MRS medium for 24 h, even without lactobacilli, there was a loss of almost 50% of the SF during this time (data not shown). A similar lack of stability has previously been reported for allyl isothiocyanate in MRS medium.40 However, when SF was incubated at room temperature in buffer, recovery was 100% at 24 h (data not shown). Since SF was not lost from the buffered incubations, it appears unlikely that the cause for lack of SF appearance in most incubations was SF degradation. Nitrile is less labile than isothiocyanates, decreasing less than 20% during 48 h incubation, providing possible rationale for our finding nitrile in our incubations.41 Overall, results from incubation of GRP with microbiota ex vivo show that microbiota in the gastrointestinal tract have the ability to hydrolyze GRP but, as with other reports of in vitro studies of glucosinolate metabolism by microbiota, these studies did not show abundant isothiocyanate formation. Furthermore, metabolism ex vivo did not reflect metabolism in situ, where SF was formed.
Conclusion
This study provides direct evidence that GRP was hydrolyzed by F344 rat cecal microbiota, both in situ and ex vivo. It is known that the unstable intermediate formed during glucosinolate hydrolysis can undergo non-enzymatic rearrangement to a nitrile, particularly in an environment of low pH when ferrous iron is present (Fig. 4). This may explain why nitrile is the main hydrolytic product in MRS and RCM incubations, particularly later in the incubation when bacterial growth and metabolism have lowered the pH. This is the first study to show direct evidence of GRP hydrolysis to bioactive SF and SF absorption across the cecal wall of rats.Acknowledgements
Thanks to H. Gaskins and G. Rivera for support in running the database searches. This work was supported by a grant from USDA/ AFRI, NIFA 2009-02961.References
- K. A. Steinmetz and J. D. Potter, J. Am. Diet. Assoc., 1996, 96, 1027–1039 CrossRef CAS.
- D. T. Verhoeven, R. A. Goldbohm, G. van Poppel, H. Verhagen and P. A. van den Brandt, Cancer Epidemiol. Biomarkers Prev., 1996, 5, 733–748 CAS.
- J. H. Cohen, A. R. Kristal and J. L. Stanford, J. Natl. Cancer Inst., 2000, 92, 61–68 CrossRef CAS.
- J. V. Higdon, B. Delage, D. E. Williams and R. H. Dashwood, Pharmacol. Res., 2007, 55, 224–236 CrossRef CAS.
- A. C. Kurilich, G. J. Tsau, A. Brown, L. Howard, B. P. Klein, E. H. Jeffery, M. Kushad, M. A. Wallig and J. A. Juvik, J. Agric. Food Chem., 1999, 47, 1576–1581 CrossRef CAS.
- G. W. Plumb, K. R. Price, M. J. Rhodes and G. Williamson, Free Radical Res., 1997, 27, 429–435 CrossRef CAS.
- A. S. Keck and J. W. Finley, Integr. Cancer Ther., 2004, 3, 5–12 Search PubMed.
- A. M. Bones and J. T. Rossiter, Phytochemistry, 2006, 67, 1053–1067 CrossRef CAS.
- C. C. Conaway, S. M. Getahun, L. L. Liebes, D. J. Pusateri, D. K. Topham, M. Botero-Omary and F. L. Chung, Nutr. Cancer, 2000, 38, 168–178 Search PubMed.
- G. Rouzaud, S. Rabot, B. Ratcliffe and A. J. Duncan, Br. J. Nutr., 2003, 90, 395–404 CrossRef CAS.
- T. A. Shapiro, J. W. Fahey, K. L. Wade, K. K. Stephenson and P. Talalay, Cancer Epidemiol. Biomarkers Prev., 1998, 7, 1091–1100 CAS.
- R. M. Bheemreddy and E. H. Jeffery, J. Agric. Food Chem., 2007, 55, 2861–2866 CrossRef CAS.
- N. Petri, C. Tannergren, B. Holst, F. A. Mellon, Y. Bao, G. W. Plumb, J. Bacon, K. A. O'Leary, P. A. Kroon, L. Knutson, P. Forsell, T. Eriksson, H. Lennernas and G. Williamson, Drug Metab. Dispos., 2003, 31, 805–813 CrossRef CAS.
- R. H. Lai, A. S. Keck, M. A. Wallig, L. G. West and E. H. Jeffery, Food Chem. Toxicol., 2008, 46, 195–202 CrossRef CAS.
- N. Zhu, M. Soendergaard, E. H. Jeffery and R. H. Lai, J. Agric. Food Chem., 2010, 58, 1558–1563 CrossRef CAS.
- N. V. Matusheski and E. H. Jeffery, J. Agric. Food Chem., 2001, 49, 5743–5749 CrossRef CAS.
- United States Pharmacopeial Convention Inc., The United States Pharmacopeia, United States Pharmacopeial Convention Inc., Rockville, MD, 2000 Search PubMed.
- B. M. Johnson, W. Chen, R. T. Borchardt, W. N. Charman and C. J. Porter, J. Pharmacol. Exp. Ther., 2003, 305, 151–158 CrossRef CAS.
- Y. Zhang, K. L. Wade, T. Prestera and P. Talalay, Anal. Biochem., 1996, 239, 160–167 CrossRef CAS.
- J. Cramer and E. H. Jeffery, Nutrition and Cancer, submitted Search PubMed.
- L. Rask, E. Andreasson, B. Ekbom, S. Eriksson, B. Pontoppidan and J. Meijer, Plant Mol. Biol., 2000, 42, 93–113 CrossRef CAS.
- B. Henrissat, Biochem. J., 1991, 280(Pt 2), 309–316 CAS.
- I. Maskell, University of Newcastle upon Tyne, 1990 Search PubMed.
- L. Elfoul, S. Rabot, N. Khelifa, A. Quinsac, A. Duguay and A. Rimbault, FEMS Microbiol. Lett., 2001, 197, 99–103 CrossRef CAS.
- I. Maskell and R. Smithard, Br. J. Nutr., 1994, 72, 455–466 CAS.
- H. G. M. Tiedink, C. E. Malingre, L. W. van Broekhoven, W. M. F. Jongen and G. R. Fenwick, J. Agric. Food Chem., 1991, 39, 922–966 CrossRef CAS.
- L. Ye, A. T. Dinkova-Kostova, K. L. Wade, Y. Zhang, T. A. Shapiro and P. Talalay, Clin. Chim. Acta, 2002, 316, 43–53 CrossRef CAS.
- A. V. Gasper, A. Al-Janobi, J. A. Smith, J. R. Bacon, P. Fortun, C. Atherton, M. A. Taylor, C. J. Hawkey, D. A. Barrett and R. F. Mithen, Am. J. Clin. Nutr., 2005, 82, 1283–1291 CAS.
- S. Agrawal, B. Winnik, B. Buckley, L. Mi, F. L. Chung and T. J. Cook, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2006, 840, 99–107 CrossRef CAS.
- B. Holst and G. Williamson, Nat. Prod. Rep., 2004, 21, 425–447 RSC.
- J. R. Bacon, G. W. Plumb, A. F. Howie, G. J. Beckett, W. Wang and Y. Bao, J. Agric. Food Chem., 2007, 55, 1170–1176 CrossRef CAS.
- K. E. Harris and E. H. Jeffery, J. Nutr. Biochem., 2007, 19, 246–254.
- Y. Zhang, C. G. Cho, G. H. Posner and P. Talalay, Anal. Biochem., 1992, 205, 100–107 CAS.
- Y. Sawatari, T. Hirano and A. Yokota, J. Gen. Appl. Microbiol., 2006, 52, 349–356 CrossRef CAS.
- S. Kajiwara, H. Gandhi and Z. Ustunol, J. Food Prot., 2002, 65, 214–218 CAS.
- S. C. Long, P. C. Arango and J. D. Plummer, Can. J. Microbiol., 2005, 51, 413–422 CrossRef CAS.
- F. Li, M. A. Hullar, Y. Schwarz and J. W. Lampe, J. Nutr., 2009, 139, 1685–1691 CrossRef CAS.
- R. Munday and C. M. Munday, J. Agric. Food Chem., 2004, 52, 1867–1871 CrossRef CAS.
- K. Kassahun, M. Davis, P. Hu, B. Martin and T. Baillie, Chem. Res. Toxicol., 1997, 10, 1228–1233 CrossRef CAS.
- B. Combourieu, L. Elfoul, A. M. Delort and S. Rabot, Drug Metab. Dispos., 2001, 29, 1440–1445 CAS.
- D. L. Cheng, K. Hashimoto and Y. Uda, Food Chem. Toxicol., 2004, 42, 351–357 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
