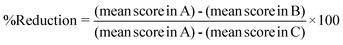Modulation of doxorubicin-induced genotoxicity by squalene in Balb/c mice
Bhilwade Hari
Narayan
,
Naoto
Tatewaki
,
Vijayasree Vayalanellore
Giridharan
,
Hiroshi
Nishida
and
Tetsuya
Konishi
*
Niigata University of Pharmacy & Applied Life Sciences, Department of Functional and Analytical Food Sciences, Higashijima 738-1, Niigata, 956-8603, Japan. E-mail: Konishi@nupals.ac.jp
First published on 14th October 2010
Abstract
The present study aims to evaluate the protective effect of squalene against the genotoxicity of the chemotherapeutic agent doxorubicin (Dox) using two genotoxicity assays, the micronucleus assay and the comet assay. Different groups of mice were fed squalene at the doses of 1 and 4 mmol g−1 body weight (100 or 400 μl as squalene oil) either at 4 h before or 1 h after Dox (20 mg kg−1) treatment. 24 h after the Dox treatment, bone marrow erythrocytes were evaluated for the incidence of micronuclei, and the induced DNA strand breaks were examined in heart tissue by the alkaline comet assay. As expected, Dox significantly induced micronuclei in polychromatic (immature) erythrocytes, as well as in total erythrocytes. The frequency of Dox-induced micronucleated erythrocytes was significantly reduced in the mice treated with squalene both before and after Dox administration. Squalene itself obviously did not induce any micronuclei in bone marrow erythrocytes. The comet assay also demonstrated a significant increase in DNA damage, especially DNA single strand breaks in the Dox-treated group of mice as compared to the control. The Dox-induced DNA damage was also effectively reduced by squalene when it was administered either before or after the Dox treatment. Squalene did not induce any significant DNA damage by itself. Compared to the pre-treatment of squalene, post treatment gave rise to more effective prevention against Dox-induced DNA damage. The data suggest that the complimentary use of squalene with Dox will be beneficial to reduce the adverse effect of Dox in cancer chemotherapy, such as the increased incidence of undesirable mutagenic side effects.
1. Introduction
Human beings are exposed to a variety of physical, chemical and biological stressors that can induce genetic transformations. This is a substantive factor leading to chronic diseases, such as arteriosclerosis, heart diseases and cancers, which are the main causes of human death.1 All of these disorders more or less associate with genomic disturbances, and thus the search for antimutagenic compounds and the evaluation of their mechanism of action deserves special attention because of their possible significance in the protection of human health.2Short-term mutagenicity assays have been effectively used for screening mutagens and potential carcinogens in human environments. The same methodologies are applicable for the identification of antimutagens or anticarcinogens. The micronucleus assay, a well known cytogenetic mutagenicity test, is one of these methods and has been proven suitable for such studies.3,4 The frequency of micronuclei is a reliable measure of both chromosome loss and breakage, and thus it is unique compared to other cytogenetic tests. However, this test can only be conducted in rapidly dividing cells, and thus mainly measures chromosomal damage induced in the bone marrow, thereby providing a limited assessment of the genotoxic potential of chemicals. The alkaline comet assay is, on the other hand, an in vivo genotoxicity assay to complement the in vivo micronucleus assay, and is gaining the status of a standard in testing and regulatory agencies, since it can detect a broad spectrum of DNA damage, including DNA single and double strand breaks, base damage, alkali-labile DNA adducts, and other DNA lesions associated with diverse reactive oxygen species in virtually any tissue.5–7 The comet assay is recommended as a follow-up to a negative or equivocal in vivo micronucleus assay result, and also as a means to measure genotoxicity in target tissues other than bone marrow.8,9
In the present study, we focus our attention on the protective effect of squalene as a food factor against Doxorubicin (Dox) genotoxicity. Dox is an anthracycline anticancer drug that is commonly used for the treatment of many types of human cancers such as solid tumors, leukemia, soft tissue sarcoma and breast cancer.10 However, its clinical usefulness is restricted due to the toxicity frequently observed in cardiac tissue.11 The cumulative administration of Dox leads to abnormal cardiac function such as electrocardiographic changes, congestive heart failure and cardiomyopathy.12,13 The cause of Dox cardiotoxicity is multifactorial, but the major cause of Dox-induced cardiotoxicity can be attributed to the formation of reactive oxygen species (ROS), leading to myocyte apoptosis.14,15 The prevention of Dox-induced cardiotoxicity is the primary request so as to exploit the full therapeutic potential of Dox in cancer chemotherapy.
Squalene is a physiological compound known to be a key precursor of steroid biosynthesis in organisms and is a natural product belonging to the terpenoid family. It occurs widely in nature from vegetable oil to fish oil. For example, common human dietary fat and oil contain approximately 0.002– 0.03% on average, but its content in olive oil is rather higher (0.2–0.7%). It is also known that squalene is highly concentrated in the liver oil of the blue shark.16,17 The anticarcinogenic potential of squalene was proposed a long time ago,18,19 but only limited studies have been reported to support its anticarcinogenic function. For example, Newmark20 and also Rao et al.17 reported that squalene shows preventive activities against the action of several carcinogens. On the other hand, Senthilkumar et al.21 demonstrated the protective role of squalene in the tissue defence system of rats towards cyclophosphamide-induced toxicity, in that they observed increased levels of antioxidant enzymes, such as SOD, and non-enzyme antioxidants, such as reduced glutathione, in heart tissue. Furthermore it is reported that the prior administration of squalene prevents isoprenaline-induced adverse changes in plasma and heart tissue by inhibiting lipid peroxidation and exerting an antioxidant effect by maintaining the level of non-enzymatic free radical scavengers such as reduced glutathione at near normal.22 It is therefore suggested that squalene is an attractive food factor for targeting integrative or complementary applications in cancer chemotherapy.
The aim of the present study is to evaluate the protective potential of squalene against the genotoxicity of chemotherapeutic agent Dox.
2. Materials and methods
Dox was obtained from Kyowa Hakko Kogyo Co., Ltd., Tokyo, Japan and was dissolved in phosphate buffered saline (PBS). The Dox dose (20 mg kg−1) was determined based on its effectiveness at inducing micronuclei.4 Squalene (>99% purity) was a generous gift from Nissei Marin Technology Co., Ltd., Tokyo, Japan.2.1. Animals
Male Balb/c mice (8–10 weeks, 22 ± 1.5 g body weight) were adapted in the animal house for a period of 1 week before the experiments under controlled temperature (21 °C) and humidity (50 ± 10%) conditions with a 12 h light–dark cycle. Water and diet were given ad libitum. Animal experiments were carried out according to the guidelines of the Niigata University of Pharmacy and Life Science Animal Ethics Committee.2.2. Experimental design
Squalene is an oil with a specific density of 0.867, and thus 100 and 400 μl volumes were directly given orally to mice by intubation (1 and 4 mmol g−1 body weight, respectively). Mice were randomly divided into 9 groups (n = 4). Group I served as the control and were treated intraperitoneally with 400 μl PBS. Group II received 400 μl of mineral oil as a squalene reference. Groups III and IV were orally administered squalene at doses of 100 and 400 μl, respectively. Group V animals intraperitoneally received Dox dissolved in PBS at a dose of 20 mg kg−1. Group VI and VII animals were orally administered 100 and 400 μl of squalene, and then Dox was received 4 h after the squalene administration. Group VIII and IX animals were treated with Dox following squalene administration at doses of 100 and 400 μl, respectively, 1 h after Dox treatment. All animals were sacrificed 24 h after Dox treatment, and bone marrow and heart tissues were removed for the micronucleus test and the comet assay, respectively.2.3. Micronucleus assay
The bone marrow micronucleus assay was performed according to protocol described by Schmid23 with a slight modification, as reported previously by Bhilwade et al.24 In brief, mice were sacrificed 24 h after Dox treatment by cervical dislocation. Both femur bones were removed clear from the adhering tissues. The marrow was flushed in a 5 ml eppendorff tube containing fetal bovine serum and centrifuged at 1500 rpm for 5 min. The cell pellet was mixed thoroughly, bone marrow smears were made on a glass slide, and they were stained in May–Gruenwald and Giemsa as follows: 5 min in undiluted May–Gruenwald (0.25% in methanol), 3 min in diluted May–Gruenwald solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, May–Gruenwald
1, May–Gruenwald![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water), rinsed in distilled water three times and then stained with diluted Giemsa (1
distilled water), rinsed in distilled water three times and then stained with diluted Giemsa (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 of the Giemsa stock
6 of the Giemsa stock![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) distilled water) for 10 min following thorough washing with distilled water. The slides were dried, cleared for 5 min in xylene and mounted in DPX. Two slides were made from each animal. Coded slides were scored for the incidence of micronucleated polychromatic erythrocytes (Mn-PCEs) and micronucleated normochromatic erythrocytes (Mn-NCEs) at ×100 magnification under oil. (Fig. 1). Approximately 2000 PCEs with or without micronuclei and a corresponding number of NCEs were analyzed per animal.
distilled water) for 10 min following thorough washing with distilled water. The slides were dried, cleared for 5 min in xylene and mounted in DPX. Two slides were made from each animal. Coded slides were scored for the incidence of micronucleated polychromatic erythrocytes (Mn-PCEs) and micronucleated normochromatic erythrocytes (Mn-NCEs) at ×100 magnification under oil. (Fig. 1). Approximately 2000 PCEs with or without micronuclei and a corresponding number of NCEs were analyzed per animal.
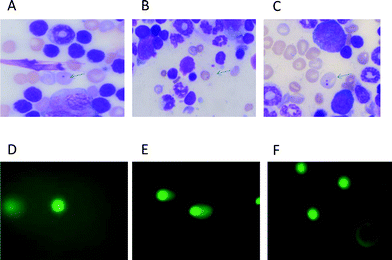 | ||
| Fig. 1 Photomicrographs showing mouse bone marrow erythrocytes stained with May–Gruenwald–Giemsa (A–C) and comets from heart cells stained with SYBR green-II. (D–F). A: micronucleus in polychromatic (immature) erythrocyte, bluish in colour. B: micronucleus in normochromatic (matured) erythrocytes, golden yellow in colour. C: polychromatic erythrocytes containing two micronuclei. D: comet in a normal cell. E: comet in a doxorubicin treated cell. F: comet in a cell of mice treated with Dox following squalene oral administration of 400 μl 1 h after Dox. | ||
2.4. Comet assay
The comet assay was carried out by the method described by Chaubey et al.,6 which is based on the original work of Singh et al.25 Briefly, heart tissue was removed immediately after sacrificing the mice and washed with chilled PBS. The tissue was dissociated into cells using a cell dissociation sieve-tissue grinder kit under ice cold conditions and processed for the comet assay. Approximately 1 × 105 cells were mixed with 1.5 ml of 0.8% low melting agarose solution prepared in 0.9% saline at 38 °C, and poured onto fully frosted microscope slides. After solidification, the slides were kept in lysis buffer (2.5 M NaCl, 100 mM Na2-EDTA, and 10% DMSO and 0.1% Triton X-100) for 1 h at 4 °C. After lyses, the slides were set in a horizontal electrophoresis apparatus and kept in alkaline buffer (30 mM NaOH, 1 mM Na2-EDTA, pH 13.0) at room temperature for 20 min to unwind the DNA strands. Electrophoresis was carried out for 30 min at 25 V, 300 mA using a power supply. After electrophoresis, the slides were washed gently in a neutralizing buffer (0.4 M Tris-HCl, pH 7.5) to remove the alkaline buffer and detergent, and stored on wet tissue paper in a closed plastic box at 4 °C until observation. The slides were stained with SYBR green II, and at least 50 cells were captured per slide at ×20 magnification using a fluorescence microscope (Olympus (BH2-RFCA), Japan) equipped with green light excitation with a 590 nm barrier filter. (Fig. 1). The comet images were analyzed by the digital imaging software “CASP”. Two parameters, tail moment (TM) and tail length (TL), were extended from the images directly. TM is the product of the tail length and the percentage DNA in the tail.26The percentage reduction of micronucleated erythrocytes in the micronucleus assay and the DNA damage in the comet assay were calculated by the formula below to evaluate the anti-genotoxicity potential of squalene according to a method reported elsewhere.27
Where A is the group of cells from Dox-treated mice (positive control), B is the group of cells from squalene plus Dox-treated mice and C is the control.
2.5. Statistical analysis
All the data are expressed as mean ± SD. Statistical analysis of the data was carried out by one-way ANOVA using the Tukey–Kramer test. The differences were considered significant at the 95% confidence limit.3. Results
3.1. Micronucleus assay
The genotoxicity of squalene was first examined using mineral oil as a reference. The induction of micronuclei observed in both groups treated with 100 and 400 μl squalene, respectively, was slightly higher than that observed in the buffer control, although the differences were not statistically significant. Furthermore, the level was the same as that of the mineral oil reference. It was thus concluded that squalene itself, even in a 400 μl dose, does not have any mutagenic effects.The frequencies of Mn-PCEs, Mn-NCEs and total micronucleated erythrocytes (Mn-Es) in the bone marrow of mice treated with squalene alone or in combination with Dox are summarized in Table 1. As expected, the frequency of Mn-PCEs and total Mn-Es increased significantly (p < 0.01) in animals receiving Dox. Both pre-treatment with squalene 4 h before and post treatment 1 h after the Dox treatment, respectively, showed a significant reduction (p < 0.01) in the frequency of Mn-PCEs and Mn-Es when compared to the group treated with only Dox. It was revealed that the frequency of both Mn-PCEs and Mn-Es were more markedly reduced when squalene was given after the Dox treatment (post-treatment) than before (pre-treatment). The percentage reduction of Mn-Es in the squalene pre-treated group was 44.72 and 33.23% at 100 and 400 μl, respectively, while in the post-treated group it was 56.69 and 59.53%, respectively. Similarly, the percentage reduction of Mn-PCEs was 52.90 and 44.58% at 100 and 400 μl in the squalene pre-treated groups, respectively, and 61.71 and 69.47% in the post-treated groups (Table 2). The PCEs/NCEs ratio, which reflects the proliferation rate of bone marrow, was significantly decreased (p < 0.05) in the Dox-treated group as compared to the control and squalene-treated groups. The PCEs/NCEs ratio in the group treated with a combination of a higher dose of squalene and Dox was observed to be significantly higher (p < 0.05) as compared to the Dox-treated group (Table 1).
| Treatment | ‰Mn-Es | ‰Mn-PCEs | ‰Mn-NCEs | PCEs/NCEs ratio |
|---|---|---|---|---|
| a Numbers within parenthesis are actual numbers of micronucleated cells/total erythrocytes scored. b p < 0.01, significantly different from the control. c p < 0.05. d p < 0.01, significantly different from the Dox group. | ||||
| Control | 1.71 ± 0.19 | 2.08 ± 0.22 | 0.85 ± 0.45 | 0.97 ± 0.02 |
| (28/16336) | (21/8063) | (7/8273) | ||
| Mineral oil | 2.01 ± 0.51 | 2.98 ± 0.42 | 1.08 ± 0.61 | 0.97 ± 0.01 |
| (33/16369) | (24/8053) | (9/8316) | ||
| Squalene 100 μl | 2.25 ± 0.65 | 3.34 ± 0.74 | 1.20 ± 0.63 | 0.97 ± 0.01 |
| (37/16437) | (27/8083) | (10/8354) | ||
| Squalene 400 μl | 2.18 ± 0.46 | 3.21 ± 0.63 | 1.19 ± 0.63 | 0.96 ± 0.02 |
| (36/16500) | (26/8099) | (10/8401) | ||
| Dox 20 mg kg−1 | 8.06 ± 1.93b | 16.39 ± 4.26b | 1.33 ± 0.37 | 0.89 ± 0.04b |
| (144/17073) | (132/8052) | (12/9021) | ||
| Squalene 100 μl (4 h) + Dox | 5.22 ± 1.97c | 8.82 ± 2.89d | 1.86 ± 1.36 | 0.93 ± 0.03 |
| (87/16652) | (71/8045) | (16/8607) | ||
| Squalene 400 μl (4 h) + Dox | 5.95 ± 1.90 | 10.01 ± 3.74d | 2.11 ± 0.57 | 0.95 ± 0.01c |
| (99/16629) | (81/8091) | (18/8538) | ||
| Dox (1 h) + squalene 100 μl | 4.46 ± 0.37d | 7.56 ± 0.82d | 1.60 ± 0.29 | 0.92 ± 0.02 |
| (75/16812) | (61/8066) | (14/8746) | ||
| Dox (1 h) + squalene 400 μl | 4.28 ± 0.54d | 6.45 ± 0.79d | 2.23 ± 0.45 | 0.95 ± 0.01c |
| (71/16579) | (52/8056) | (19/8523) |
| Group | Micronucleus assay | Comet assay | ||
|---|---|---|---|---|
| Mn-Es | Mn-PCEs | Tail moment | Tail length | |
| Pre-treatment 4 h (squalene 100 μl) | 44.72 | 52.90 | 51.08 | 38.25 |
| Pre-treatment 4 h (squalene 400 μl) | 33.23 | 44.58 | 78.35 | 58.37 |
| Post-treatment 1 h (squalene 100 μl) | 56.69 | 61.71 | 82.26 | 55.46 |
| Post-treatment 1 h (squalene 400 μl) | 59.53 | 69.47 | 85.41 | 55.40 |
3.2. Comet assay
Studies were carried out to evaluate cellular DNA damage with or without squalene administration in Dox-treated mice. The values of TM and TL are shown in Fig. 2. As is evident from the figure, the values of TM and TL increased significantly in mice treated with Dox at a dose of 20 mg kg−1 compared to cells from untreated control mice. Cells from the mice administered with squalene either 4 h before or 1 h after Dox treatment showed significant inhibition (p < 0.01) of DNA damage measured in terms of TM and TL. The results also indicate that the inhibition of Dox-induced DNA damage by squalene was relatively more efficient in the post-treated group compared to the pre-treated group (Table 2). Although squalene treatment alone showed a little DNA damage, it was not a significant difference from that of the control.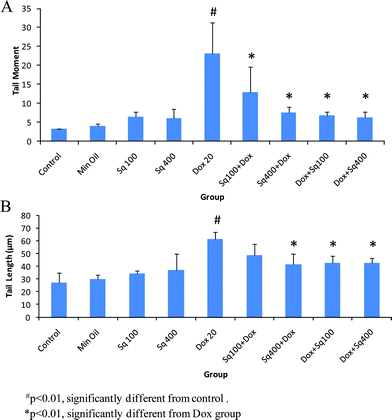 | ||
| Fig. 2 Single cell gel electrophoresis analysis of DNA damage in mouse heart cells treated with squalene and Dox. A: Tail moment (TM), B: Tail length (TL). | ||
4. Discussion
In the present study, both the micronucleus assay and the comet assay were cooperatively used to study the modulatory effect of squalene against Dox-induced genotoxicity at a chromosomal level as well as a DNA level. The results clearly show that squalene efficiently prevents Dox-induced DNA damage and protects heart tissue. These two methods cover different aspects of genotoxic events. Essentially, the micronucleus assay detects chromosomal damage that persists for at least one mitotic cycle, while the comet assay identifies repairable DNA damage or alkali-labile sites.28 Some of the DNA lesions, such as single and double strand breaks, and adduct formation, led to permanent fetal damage, and the micronucleus test is an excellent means of evaluating any permanent damage in genetic material. On the other hand, the comet assay is a very sensitive method for measuring DNA strand breaks at a single cell level and thus is sensitive to acute DNA lesions. Moreover, it was recently shown that the comet assay detected nearly 90% of carcinogens that were negative or equivocal in the micronucleus assay. Therefore, a combination of the micronucleus assay and the comet assay is recommended for the broad assessment of in vivo genotoxic potential.29,30 This protocol allows evaluation of two distinct genotoxicity end points in the same animal. The National Toxicological Program (NTP) is presently using this combined protocol as part of its efforts to evaluate the genotoxicity of substances of public health concern.31As shown in Table 1 and Table 2, when we evaluated the frequency of micronuclei (as genetic marker) in PCEs, NCEs and the total erythrocytes (PCEs and NCEs) in bone marrow using the micronucleus assay, and the extent of DNA damage in heart tissue by the comet assay, the numbers of micronucleated erythrocytes increased by Dox treatment was markedly reduced in mice administered with squalene, either before or after the Dox treatment. In the same way, the Dox-mediated DNA strand breaks were inhibited in mice pre- and post-administered with squalene.
The present study is thus the first in vivo demonstration of the modulation effect of squalene on Dox-induced chromosomal or DNA damage in mice assessed by the two genotoxicity evaluation methods mentioned above. Regarding the genotoxicity of squalene itself, however, Fan et al.32 have reported that no induction of sister chromatid exchanges and micronuclei occurs in squalene-treated Chinese hamster ovary-K1 cells.
Dox is an antineoplastic agent in the anthracyclin antibiotic family that is used widely in the treatment of human cancer. The drug is metabolically activated to a free radical form and interacts with molecular oxygen to generate superoxide radicals.33 The superoxide radicals can react with hydrogen peroxide to form highly reactive hydroxyl radicals through the iron-catalyzed Haber–Weiss reaction. Secondarily-derived hydroxyl radicals can cause protein and DNA damage and initiate lipid peroxidation.34 Thomas et al.35 showed that DNA damage is an early event in Dox-induced cardiac myocyte death in the H9c2 cardiac cell line derived from embryonic rat heart. It is thus highly likely that the Dox-mediated induction of micronuclei and DNA damage observed here is due to the ROS including free OH radicals produced by Dox.
It has been reported that squalene is an antioxidant molecule having a high scavenging activity towards ROS, especially towards singlet oxygen.36,37 Although it is not yet clear how the singlet oxygen is involved in Dox-induced DNA lesion formation, its antioxidant property is likely to be associated with the mechanism underlying the protective effect of squalene against Dox-induced mutagenicity and DNA or chromosomal damage. This idea is supported by our present observation that the squalene administered after Dox treatment gave effective protection against the production of DNA damage in both micronuclei and the comet assay, and the effect was also dependent on the dose of squalene. Since squalene is rapidly metabolized after intake,38 post-administration will be more effective at maintaining the plasma and tissue levels, and thus a higher ROS scavenging activity of squalene is implicated in the case of post-administration than pre-administration.
It is also important to note that squalene itself did not increase the frequency of micronucleated erythrocytes in mouse bone marrow, even with 400 μl administration (4 mmol g−1 body weight), indicating that squalene has essentially no genotoxicity, because it is well known that certain molecules with a high ROS scavenging potential in vitro, such as catecholamines, show cytotoxicity rather than oxidative stress prevention.39
Furthermore, our experiments suggest that treatment with squalene is effective for reducing the mutagenic effect of Dox, both in heart tissue and bone marrow, even at 100 μl or less, because the inhibitory action of squalene is almost saturated above 100 μl administration in the comet and micronucleus assay results.
On the other hand, in the several experimental models,32,40 squalene was demonstrated to detoxify the adverse effects of diverse chemicals such as hexachlorobiphenyl, hexachlorobenzene, arsenic, theophylline, phenobarbital and strychnine.41 These compounds are not strong antioxidants by themselves, but their cell toxicity is associated with oxidative stress.42–44 Therefore, this also supports the idea that the primary mechanism involved in the antigenotoxic effect of squalene is expected to be an antioxidant effect. However, as De Flora and Ramel45 suggested, multiple mechanisms for antimutagen action and the possible involvement of mechanisms other than the direct scavenging of ROS cannot be excluded, such as through the modulation of antioxidant enzymes, damage repair or the metabolic inactivation of Dox. These effects might be reflected in the present observation that the dose dependency of squalene action was more clearly shown in the pre-treated group, both in the micronucleus assay and the comet assay. Moreover, the present study revealed that in the micronucleus assay, the PCEs/NCEs ratio, which reflects the cytotoxicity in the erythropoietic system, was reduced in the Dox-treated group as compared to the control and squalene groups. However, the ratio became higher in the group treated with a combination of Dox and squalene. This is consistent with the observation by Das et al. that squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth.46 Therefore, a further study is needed to confirm these mechanisms.
In summary, the combination of both assays in the present work proved to be adequate and useful for the evaluation of the genotoxicity of Dox. Both assays evidenced the protective function of squalene against Dox-induced genotoxicity in bone marrow and heart tissue. The extent of DNA damage was measured qualitatively by the comet assay. On the other hand, the micronucleus assay measured chromosomal damage and provided evidence of cytotoxicity. Although the comet assay is observed to be the more sensitive method, the micronucleus assay was also informative, and its usefulness should be considered for the evaluation of genotoxicity. The results obtained by these studies indicate that squalene as a food factor might be an effective antimutagen and might be applicable for the reduction of the adverse effect of Dox in complementary or integrative cancer chemotherapy.
Acknowledgements
We thank Nissei Marin Technology Co., Ltd., Tokyo, Japan for supporting our squalene research project. We are also grateful to Kyowa Hakko Kirin Co., Ltd., Japan for providing doxorubicin.References
- S. De Flora, A. Izzotti and K. Randerath, et al., DNA adducts and chronic degenerative diseases. Pathogenetic relevance and implications in preventive medicine, Mutat. Res., Rev. Genet. Toxicol., 1996, 366, 197–238 Search PubMed.
- M. D. Waters, A. L. Brady, H. F. Stack and H. E. Brockman, Antimutagenicity profiles for some model compounds, Mutat. Res., Rev. Genet. Toxicol., 1990, 238, 57–85 Search PubMed.
- Y. Kuroda, A. K. Jain, H. Tezuka and T. Kada, Antimutagenicity in cultured mammalian cells, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1992, 267, 201–209 CrossRef CAS.
- F. M. Andrade, L. C. Fernandes de Almeida, R. A. Furtado, W. R. Cunha and D. C. Tavares, Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2008, 657, 150–154 CrossRef.
- P. Fortini, G. Raspaglio, M. Falchi and E. Dogliotti, Analysis of DNA alkylation damage and repair in mammalian cells by the Comet assay, Mutagenesis, 1996, 11, 169–175 CrossRef CAS.
- R. C. Chaubey, H. N. Bhilwade, R. Rajagopalan and S. V. Bannur, Gamma ray induced DNA damage in human and mouse leucocytes measured by SCGE-Pro: a software developed for automated image analysis and data processing for Comet assay., Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2001, 490, 187–197 CrossRef CAS.
- C. M. Gedik and A. Collins, Establishing the background level of base oxidation in human lymphocyte DNA: results of an inter laboratory validation study, FASEB J., 2005, 19, 82–84 CAS.
- D. Blakey, S. M. Galloway, D. J. Kirkland and J. T. MacGregor, Regulatory aspects of genotoxicity testing: from hazard identification to risk assessment, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2008, 657, 84–90 CrossRef CAS.
- D. A. Estmond, A. Hartwig, D. Anderson, W. A. Anwar, M. C. Cimino, I. Dobrev, G. R. Douglas, T. Nohmi, D. H. Phillips and C. Vickers, Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS harmonized scheme, Mutagenesis, 2009, 24, 341–349 CrossRef.
- R. H. Blum and S. K. Carter, Adriamycin: a new anticancer drug with significant clinical activity, Ann. Interm. Med., 1974, 80, 249 Search PubMed.
- S. Zhon, C. M. Palmeira and K. B. Wallace, Doxorubicin-induced persistent oxidative stress to cardiac myocytes, Toxicol. Lett., 2001, 121, 151–157 CrossRef.
- L. N. Lenaz and J. A. Page, Cardiotoxicity of adriamycin and related anthracyclines, Cancer Treat. Rev., 1976, 3, 111–120 CrossRef CAS.
- K. A. Wouters, L. C. Kremer, T. L. miller, E. H. Herman and S. E. Lipshultz, Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies, Br. J. Haematol., 2005, 131, 561–578 CrossRef CAS.
- M. S. Horenstein, R. S. Vander Heide and T. J. L'Ecuyer, Molecular basis of anthracycline-induced cardiotoxicity and its prevention, Mol. Genet. Metab., 2000, 71, 436–444 CrossRef CAS.
- H. A. Hanaa, M. Fathia, A. E. Gamal and H. D. Senot, Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin induced cardiotoxicity, Bioorg. Med. Chem., 2005, 13, 1847 CrossRef.
- McCance and Widdowson's The composition of food (revised by Paul and Southgate), Elsevier, Oxford, 1976 Search PubMed.
- V. C. Rao, H. L. Newmark and S. B. Reddy, Chemopreventive effect of squalene on colon cancer, Carcinogenesis, 1998, 19(2), 287–290 CrossRef CAS.
- T. Yamaguchi, M. Nakagawa, K. Hidaka, T. Yoshida, T. Sasaki and S. Akiyama, et al., Potentiation by squalene of antitumor effect of 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-1-(2-chloroethy)-nitrosourea in a murine tumor system, Jpn. J. Cancer Res., 1985, 76, 1021–1026 CAS.
- T. Ikikawa, M. Umeji, T. Manabe, S. Yanoma, K. Orinoda and H. Mizunuma, et al., Studies on antitumor activity of squalene and its related compounds (in Japanese), J. Pharm. Soc. Jpn., 1986, 106, 578–582.
- H. L. Newmark, Squalene, olive oil and cancer risk: A review and hypothesis, Cancer Epidemiology, Biomarkers & Prevention, 1997, 6, 1101–1103 Search PubMed.
- S. Senthilkumar, T. Devaki, B. M. Manohar and M. S. Babu, Effect of squalene on cyclophosphamide-induced toxicity, Clin. Chim. Acta, 2006, 364, 335–342 CrossRef CAS.
- K. H. S. Farvin, S. H. S. Kumar, R. Anandan, S. Mathew, T. V. Sankar and Nair P. G. Viswanathan, Supplimentation of squalene attenuates experimentally induced myocardial infarction in rats, Food Chem., 2007, 105, 1390–1395 CrossRef CAS.
- W. Schmid, Chemical mutagen testing on in vivo somatic mammalian cell, Agents Actions, 1973, 3, 77–85 Search PubMed.
- H. N. Bhilwade, R. C. Chaubey and P. S. Chauhan, Gamma ray induced bone marrow micronucleated erythrocytes in seven strains of mouse, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2004, 560, 19–26 CrossRef CAS.
- N. P. Singh, M. T. McCoy and R. R. Tice, A simple technique for quantitation of low levels of DNA damage in individual cells, Exp. Cell Res., 1988, 175, 184–191 CrossRef CAS.
- D. K. Chandrasekharan, T. V. Kagiya and C. K. Nair, Radiation protection by 6-palmitoyl ascorbic acid-2-glucoside: studies on DNA damage in vitro, ex vivo, in vivo and oxidative stress in vivo, J. Radiat. Res., 2009, 50, 203–212 Search PubMed.
- S. J. Mara, M. Bisarro dos Reis, J. Rodrigues, L. Campaner dos Santos, W. Vilegas, E. A. Varanda, A. L. Dokkedal and I. M. S. Colus, In vivo assessment of DNA damage and protective effects of extracts from Miconia species using the comet assay and micronucleus test, Mutagenesis, 2008, 23(6), 501–507 CrossRef.
- Y. Zhong, S. L. Feng, Y. Luo, G. D. Zhang and Z. M. Kong, Evaluating the genotoxocity of surface water of Yangzhong city using the Vicia faba micronucleus test and the comet assay, Bull. Environ. Contam. Toxicol., 2001, 67, 217–224 CAS.
- D. Kirkland and G. Speit, Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens III. Appropriate follow-up testing in vivo, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2008, 654, 114–132 CrossRef CAS.
- S. Pfuhler, S. Albertini, R. Fautz, B. Herbold, S. Madle, D. Utesch and A. Poth, Genetic toxicity assessment: employing the best science for human safety evaluation part IV: Recommendation of a working group of the Gesellschaft fuer Umweltmutaionsforchung (GUM) for a simple and straight forward approach to genotoxicity testing, Toxicol. Sci., 2007, 97, 237–240 CrossRef CAS.
- R. Leslie, C. Hobbs, W. Caspary and K. L. Witt, Dose-response assessment of four genotoxic chemicals in a combined mouse and rat micronucleus (MN) and comet assay protocol, J. Toxicol. Sci., 2010, 35(2), 149–162 Search PubMed.
- S.-r. Fan, I. C. Ho, F. L. Yeoh, C. J. Lin and T. C. Lee, Squalene inhibits sodium arsenite-induced sister chromatid exchanges and micronuclei in Chinese hamster ovary-k1 cells, Mutat. Res., Genet. Toxicol., 1996, 368, 165–169 Search PubMed.
- G. Powis, Free radical formation by antitumor quinines, Free Radical Biol. Med., 1989, 6, 63–101 CrossRef CAS.
- B. Halliwell, Reactive oxygen species in living system: source, biochemistry, and role in human disease, Am. J. Med., 1991, 30, 14–22.
- T. L'Ecuyer, S. Sanjeev, R. Thomas, R. Novak, L. Das, Wendy Campbell and Richard Vander Heide, DNA damage is an early event in doxorubicin-induced cardiac myocyte death, Am. J. Physiol.: Heart Circ. Physiol., 2006, 291, H1273–1280 CrossRef CAS.
- D. Saint-Legar, A. Bague, E. Cohen and M. Chivot, Possible role for squalene in the pathogenesis of acne. I. In vitro study of squalene oxidation, Br. J. Dermatol., 1986, 114, 535–542 CrossRef.
- Y. Kohno, Y. Egawa and S. Itoh, Kinetic study of quenching reaction of singlet oxygene and scavenging reaction of free radical by squalene in n-butanol, Biochem. Biophys. Acta, 1995, 1257, 52–56.
- Z.-R. Huang, Y.-K. Lin and J.-Y. Fang, Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology, Molecules, 2009, 14, 540–554 Search PubMed.
- W. C. Lin, P. S. Tsai and C. J. Huang, Catecholamines enhancement of inducible nitric oxide synthase-induced nitric oxide biosynthesis involves CAT-1 and CAT-2A, Anesth. Analg. (N. Y.), 2005, 101, 226–232 Search PubMed.
- H. Kamimura, N. Koga, K. Ogari and H. Yoshimura, Enhanced elimation of theophylline, Phenobarbital and strychnine from the bodies of rats and mice by squalene treatment, J. Pharmacobiol. Dyn., 1992, 15, 215–221 Search PubMed.
- N. Dhandapani, B. Ganesan, R. Anandan, R. Jeyakumar, D. Rajaparabhu and R. Anbin Ezhilan, Synergistic effects of squalene and polyunsaturated fatty acid concentrate on lipid peroxidation and antioxidant status in isoprenaline-induced myocardial infarction in rats, Afr, J. Biotechnol., 2007, 6(8), 1021–1027 Search PubMed.
- D. Srivastava, R. B. Subramanian, D. Madamwar and S. J. Flora, Protective effects of selenium, calcium, and magnesium against arsenic-induced oxidative stress in male rats, Arh. Hig. Rada. Toksikol., 2010, 61(2), 153–9 Search PubMed.
- Y. F. Li, N. Shi, H. Y. Li, Y. S. Liu, M. Sun and F. Y. Hu, Toxicity and oxidative stress on rats by hexachlorobenzene (article in Chinese), Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 2006, 24(10), 601–4 Search PubMed.
- N. A. Santos, W. S. Medina, N. M. Martins, M. A. Rodrigues, C. Curti and A. C. Santos, Involvement of oxidative stress in the hepatotoxicity induced by aromatic antiepileptic drugs, Toxicol. in Vitro, 2008, 22(8), 1820–4 CrossRef CAS.
- S. De Flora and C. Ramel, Mechanisms of inhibitors of mutagenesis and carcinogenesis: classification and overview, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1988, 202, 285–306 CrossRef CAS.
- B. Das, R. Antoon, R. Tsuchida, S. Lotfi, O. Morozova, W. Farhat, D. Malkin, G. Koren, H. Yeger and S. Baruchel, Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth, Neoplasia, 2008, 10(10), 1105–1119 CAS.
| This journal is © The Royal Society of Chemistry 2010 |