Interfacial design of protein-stabilized emulsions for optimal delivery of nutrients
Amir
Malaki Nik
a,
Amanda J.
Wright
b and
Milena
Corredig
*a
aDepartment of Food Science, University of Guelph, Guelph, Ontario, Canada. E-mail: mcorredi@uoguelph.ca; Fax: (+519) 824-6631; Tel: (+519) 824-4120 ext 56101
bDepartment of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada, N1G 2W1
First published on 5th October 2010
Abstract
Proteins are often used as ingredients in food emulsions, as their amphiphilic structures provide electrostatic and steric stabilization. Significant attention has recently been directed at understanding how the composition and structure of oil–water interfaces change during digestion and how these can be manipulated to enhance the delivery of nutrients contained within the oil droplets. These efforts have necessitated the development of more sophisticated in vitro digestion models of greater physiological relevance and increased efforts in research to identify the role of the various digestive parameters on interfacial dynamics. The changes occurring at the oil–water interface will affect the adsorption of gastro-intestinal lipases and, ultimately, affect lipid digestion. The composition of a protein-stabilized oil droplet changes continuously during digestion, because of proteolysis and the formation of peptides with different affinities for the interface. In addition, natural bio-surfactants such as phospholipids and bile salts, other surface- active molecules present in foods, and the products of lipolysis (i.e. mono and diglycerides, lysophospholipids), all compete for access to the interface, and contribute to the dynamic changes occurring on the surface of the oil droplets. A better understanding of how to tailor the composition of oil droplet surfaces in food emulsions will aid in optimizing lipid digestion and, as a result, delivery of lipophilic nutrients. This review focuses on the physico-chemical changes occurring in protein-stabilized oil-in-water emulsions during gastric and small intestine digestion, and on how interfacial engineering could lead to differences in fatty acid release and the potential bioavailability of lipophilic molecules.
Introduction
Proteins are a major component in foods, not only because of their nutritional properties but also their various processing functionalities (gelling, emulsifying, foaming, etc.). Knowledge of their digestive metabolism has, primarily, been obtained from in vivo studies based on nitrogen or amino acid balances, or from in vitro experiments using relatively simple proteolytic cocktails. More recently, however, there has been increased interest in understanding the breakdown of food proteins during digestion, as clear evidence has emerged that the food matrix plays an important role in the bioavailability of nutrients. This has necessitated the development and use of increasingly sophisticated in vitro digestion models, resulting in a better understanding of the molecular details occurring during transit of food in the gastro-intestinal (GI) tract1–3 and will enable better microstructural engineering of foods for optimal delivery of bioactives and nutrients.Proteins encode in their sequence a number of bioactive peptides with recognized effects on human health. These include, for example, peptides with iron-binding capacity, immune-stimulation, antimicrobial, angiotensin I-converting enzyme inhibition, or opioid activities.4 The ability of these peptides to act depends on their release during digestion, which, in turn, depends on the changes occurring to the protein structure, as this influences the protein's susceptibility to specific enzymes. Proteins in foods form structural hierarchies, and these structures affect the proteolytic patterns during digestion. Differences in protein digestion depending on processing history and interaction with other molecules present in foods have relevance in terms of the potential allergenicity of protein-based products.1 For example, it was recently demonstrated, using in vitro gastro-intestinal models, that heat5 or high pressure6,7 treatments can improve the digestibility of β-lactoglobulin (β-LG). Furthermore, the kinetics of β-LG hydrolysis are also significantly altered when the protein is adsorbed at an oil–water interface.8,9 Thus, understanding the biophysical mechanisms related to protein digestion is important to modulate peptide bioactivity. For example, it would be desirable to design protein structures that are predictably digested to form specific peptides during digestion. However, this requires more research into the complex interplay between foods and the digestive environment.
There are also implications of protein digestion in terms of the release and absorption of molecules contained within protein-based food matrices. For instance, differences in the proteins present at an emulsion interface lead to changes in the physico-chemical characteristics of the emulsion droplets, including size, charge, and aggregation state. All of these characteristics contribute to the stability of an emulsion, and change the accessibility of the interface to important hydrolytic enzymes such as the proteases and lipases. A number of studies have been published on the role played by the surface area and interfacial composition on the ability and rate of lipase to digest lipids in oil droplets.10–13 Indeed, it has been demonstrated that the postprandial plasma triglycerol response is affected by the size of emulsion droplets.14 In addition, gastric emptying rates are affected by the physical state of an emulsion (i.e. stable, aggregated, creamed).15 Since lipolysis is a prerequisite for the release and absorption of molecules contained within the oil droplets of an emulsion, research to address the relationships between protein digestion, interfacial changes, lipolysis, and bioavailability is warranted.
Most advances in elucidating digestion mechanisms have been made using model systems, including protein-stabilized oil-in-water emulsions. Such research is laying the foundation for the design of more complex matrices and mixed food systems. It is also identifying where opportunities may exist to engineer structures that allow for slowing down digestion or tailoring the delivery of bioactive molecules to particular sections of the human GI tract. However, studies with relatively simple systems have also clearly illustrated the complex and very intricate dynamics occurring during digestion. In this regard, the specifics of protein digestion, particularly under physiological conditions, require more careful consideration and study. Given the complexity of the interactions at and competition for the interface, it is critical that attention be directed specifically at understanding protein digestion, in light of the presence of bio-surfactants and other ingredients present in foods. This review aims to provide an overview of recent knowledge on the digestion of protein-stabilized emulsions with an emphasis on how protein-stabilized interfaces can be designed to modulate lipolysis and the release of molecules encapsulated within oil droplets.
Physico-chemical properties of protein-stabilized emulsions
During homogenization of oil in an aqueous solution, the size of the oil droplets is dramatically reduced, improving their physical stability. Water-soluble surfactants quickly adsorb onto the interface and decrease the surface tension, contributing to the stability of the droplets to flocculation or coalescence. Due to their amphiphilic nature, proteins are often used in food emulsions. Because of their interfacial adsorption, they unfold, to different extents and expose their hydrophilic portions to the aqueous phase, while their hydrophobic moieties favor the oil phase. During homogenization, flexible proteins spread to cover the maximum possible interfacial area. However, if the concentration is not sufficient to cover the entire surface, the emulsion droplets will coalesce. The difference in density between the oil and the aqueous phase leads to creaming of the oil droplets, and the rate of creaming will depend on the square of the size of the droplets as well as the viscosity of the continuous phase. This emphasizes the importance of an efficient homogenization process and proper formulation.The conformation proteins adopt at an interface depends on their state of minimum conformational energy. Either as individual molecules or as aggregates, proteins create a thick and charged layer at the interface, causing steric and charge repulsion when the droplets come into close contact with one another. Emulsion stability is affected by the strength and type of interactions occurring between droplets and these depend on the structure, concentration, and composition of the adsorbed layer. Milk proteins are amongst the most employed emulsifiers in foods. Their changes at the interface have been well studied16,17 and highlight the various interfacial behaviours of proteins. Monomeric and globular proteins (such as β-LG, whey protein isolate, or lysozyme) cover interfaces with surface loads of about 2–3 mg m−2 and partially unfold and rearrange upon adsorption.17–19 On the other hand, more flexible proteins, such as monomeric caseins, adsorb readily at the interface forming loops (with part of the molecules adsorbed and other parts protruding in the water phase) and trains (where a portion is at the interface while the other protrudes in the aqueous phase) with hydrophilic moieties protruding into the water phase, forming thicker hydrodynamic layers of a few nanometres in size (8–10 nm). These proteins spread at the interface very efficiently, with very low surface coverage (less than 1 to 3 mg m−2). In systems containing cysteine residues, disulfide cross-linking can also occur at the interface, creating more elastic and brittle surfaces, as for example, in the case of whey proteins.20 When present in abundance, globular proteins can form multilayers,18 while supramolecular assemblies of proteins such as casein micelles, calcium caseinate or soy proteins instead adsorb as aggregates. In this case, a much higher concentration is needed to form stable emulsions, the proteins form thicker interfaces, and the surface coverage exceeds 10–20 mg m−2.21,22 Therefore, protein-stabilized interfaces differ in terms of protein loads, conformations, and supramolecular assemblies.
Oil-in-water emulsions are thermodynamically unstable and can destabilize in several ways, including through physical mechanisms related to the droplets' colloidal properties and by chemical means (such as oxidation or hydrolysis). The type of destabilization depends on the properties of the interfacial layer,16 and the influence of interfacial composition on oil-in-water emulsion stability has been well described.16,17 Upon collision, oil droplets may flocculate. This means they associate with one another, but still retain their identity. The behaviour of the oil droplets now resembles that of one oil droplet of bigger size, corresponding to the size of the floc. Flocculation can be reversible or irreversible.16 When the surfactant (for example a protein aggregate) is shared between two droplets, bridging flocculation occurs. This is often observed when polymers with a tendency to interact with the protein adsorbed at the interface are present in the continuous phase. For example, charged emulsion droplets will interact with oppositely charged polysaccharides, forming bridged flocs. When sufficient interacting biopolymer is present in the aqueous phase, the emulsion droplets may become completely covered by a secondary charged layer that re-stabilizes the system. On the other hand, when a non-adsorbing polymer is present in the continuous phase and it does not interact with the oil droplet surface, an osmotic pressure gradient develops in the proximity of the oil droplets. This drives the oil droplets closer to one another, ultimately causing the formation of flocs. This type of flocculation, called depletion flocculation, is reversible and the flocs will disrupt upon dilution.
As the stability of an emulsion is determined greatly by the properties of the adsorbed layer, environmental conditions affecting the protein structure will ultimately affect the emulsion's physico-chemical properties. Changes in pH and the presence of ions, for example, can have a substantial impact on the charge and hydration of the interfacial layer, ultimately affecting the stability of the droplets upon collision. The presence of other polymers or surface-active molecules in the continuous phase is also important in determining the type of interface and stability of oil droplets. For example, polymers that only interact at certain pH and ionic strengths may not adsorb or desorb from the interface, ultimately causing destabilization of an emulsion, as described above. Also, hydrophilic polysaccharides can form a thick secondary layer at the interface, driven by electrostatic interactions and further stabilization of the interface may occur, decreasing the accessibility of proteases and lipase.23,24 In addition, the presence of other surface-active molecules can cause competitive displacement or co-adsorption at the interface, ultimately modifying the properties of the oil droplets. Products of lipolysis, such as the mono- and diglycerides as well as bio-surfactants such as bile salts and phospholipids will cause desorption of the original protein at the interface. It has also been shown that phospholipids can interact with proteins in solution as well as on the surface of the oil droplets, thus changing the quality of the interface and affecting the accessibility of proteases to the substrate.25,26 Despite their inherent instability, a solid understanding of the molecular interactions occurring enables formulation of emulsions for optimal stability during processing and storage. While many studies have considered the effect of protein composition on the stability aspects of emulsions, relatively little is understood in terms of the changes in interfacial quality of the protein layer and the associated physicochemical changes occurring during gastrointestinal digestion.
Physico-chemical changes of protein-stabilized emulsions during gastrointestinal transit
Structure modifications of emulsion-based foods initiate in the oral cavity. Although the residence time is relatively short (i.e. 5–20 s in the case of beverage emulsions), changes in droplet structure in the mouth can be very significant, particularly as they relate to flavor release and sensory perception. The physical changes which occur during oral processing are non covalent in nature and depend on the droplets' surface charge.27,28 They are the result of mixing with the salivary components, changes in environmental conditions (such as temperature, pH, and ionic strength), physical contact with the oral surfaces, and the frictional forces present.29 The presence of mucins (i.e. negatively charged glycoproteins) and an ionic environment were recently cited as the most important factors affecting emulsion stability in the mouth. Mucins induce reversible droplet flocculation through a depletion mechanism in the case of weakly negatively charged or neutral oil droplets (β-LG or Tween 20-stabilized emulsions at pH 6.7). In contrast, with positively charged oil droplets (lysozyme or lactoferrin-stabilized emulsions), the mucins form electrostatic complexes, causing bridging flocculation.28–30 The presence of ions at certain concentration promotes emulsion destabilization as well. Indeed parotid saliva causes emulsion aggregation, depending on the initial charge of the emulsion.31 Aggregation in the mouth results in changes in viscosity and different sensory qualities. For example, bridging flocculation between salivary proteins and lysozyme-stabilized oil droplets leads to the perception that an emulsion is astringent.32Once an emulsion reaches the stomach, the droplets are diluted and exposed to acidic conditions. Shear forces also result in mixing with other food ingredients and the proteins undergo substantial hydrolysis by pepsin. After the stomach, the emulsion droplets are processed in the duodenum where lipolytic and proteolytic enzymes are present, together with inorganic salts and bio-surfactants. As previously mentioned, the bile salts and phospholipids both affect protein digestibility as they have high interfacial activity and compete for the interface. Phospholipids can also form complexes, both in solution and at the interface. Of great importance are the interfacially active molecules produced during digestion, as they also compete for adsorption at the interface, or form supramolecular structures (i.e. micelles, vesicles, mixed complexes) in solution. The net result of these physico-chemical changes is that the state of an emulsion interface constantly changes during GI transit. Since the composition of the oil–water interface affects the ability of the lipolytic enzymes (i.e. pancreatic lipase and colipase) to adsorb, there are consequences for lipid digestion and the release of bioactives contained within the oil droplets. Fig. 1 represents schematically the physical changes occurring during GI transit, as well as the exchanges occurring at the oil–water interface.
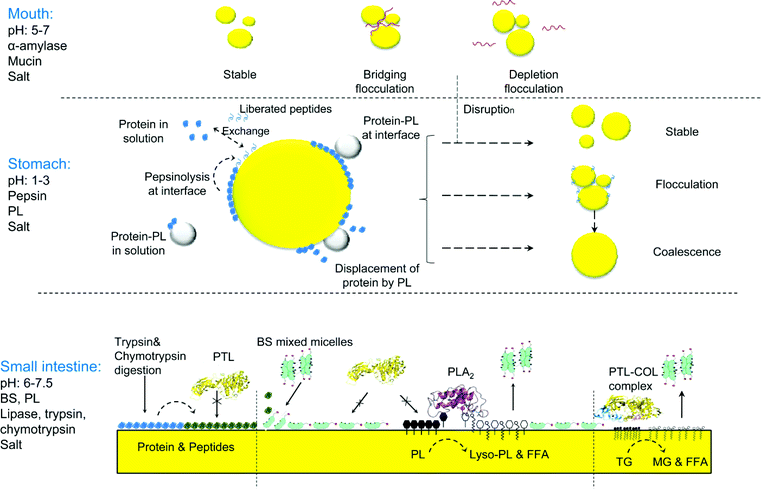 | ||
| Fig. 1 Schematic diagram of the physico-chemical changes occurring in emulsion droplets during transit through the gastrointestinal tract. Interfacial composition changes occur in the stomach and the small intestine. The proteolytic activity of pepsin results in compositional changes at the interface, and different types of droplet destabilization will occur, depending on the type of interface formed. Phospholipids can form aggregates with the proteins in solution or can bind or displace proteins at the interface. In the small intestine and after a drastic change in pH further hydrolysis of the interfacial layer by pancreatic proteases also alters the interfacial composition. Furthermore, competition for the interface occurs between pancreatic lipase, colipase, bile salts and phospholipids, as well as the products of hydrolysis. The contribution of phospholipase A2, which hydrolyzes phospholipids to lysophospholipids and free fatty acids, is also important. Overall, accessibility of pancreatic lipase to the oil–water interface lead to triglyceride hydrolysis into fatty acids and monoglycerides. The product of lipolysis will be removed from the interface by incorporation into bile salt micelles. | ||
Proteolysis occurs at neutral pH in the small intestine through the action of pancreatic proteases, including trypsin, chymotrypsin and membrane peptidases. Proteins have very different susceptibility to proteases, and the profile of peptides liberated during digestion is affected by a number of factors. For example, α-lactalbumin (α-LA) and β-casein (β-Cas) in solution are very susceptible to pepsinolysis.8,9,33 In contrast, native β-LG in solution shows resistance to pepsinolysis at low pH 5,34; However, during in vitro duodenal incubation, in the presence of trypsin and chymotrypsin, β-LG is completely hydrolyzed within 30 min.35 Changes in protein hydrolysis patterns are of particular significance in terms of protein allergenicity and the role played by processing and molecular interactions. As previously mentioned, other components such as salts and phospholipids can strongly affect the structure and accessibility of a protein to proteolytic enzymes. For example, the supramolecular structures formed in the GI tract between proteins and phospholipids could modify protein digestion patterns.
Of utmost importance are the changes in protein conformation induced by adsorption on the oil water interface, as they affect protein digestion. Emulsification was recently reported to increase the susceptibility of β-LG and β-Cas to in vitro pepsinolysis.8 Digestibility of β-Cas at the interface was doubled compared to that of the protein in solution, with the formation of one major peptide of about 6 kDa. A 60% increase in pepsinolysis of β-LG was also reported when this protein was adsorbed at the oil–water interface.8 Moreover, there were consequences of the newly formed peptides in terms of interfacial properties. Using drop tensiometry, increases in surface tension were observed during pepsinolysis of β-Cas adsorbed at the interface.8Fig. 2 illustrates the differences between the gastric digestion of proteins in solution compared with the same proteins adsorbed at an interface. In particular, Fig. 2 shows the changes in the polypeptide patterns of solubilized versus interfacial α-LA (Fig. 2A) and β-LG (Fig. 2B) during exposure to simulated gastric conditions. When a 10% oil-in-water emulsion stabilized with α-LA was subjected to pepsinolysis, the adsorbed protein showed a higher level of resistance to pepsinolysis compared with the same protein in solution. The conformational changes occurring in α-LA at the interface resulted in a very limited hydrolysis, with only a few sites accessible to pepsin.9 In contrast, when a 10% oil-in-water emulsion was stabilized with β-LG, the protein was fully hydrolyzed under in vitro gastric conditions, with no residual protein observed after 1 h of digestion (Fig. 2).
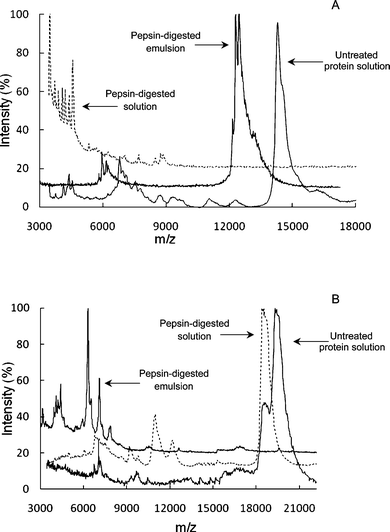 | ||
| Fig. 2 MALDI-TOF mass spectra as reported by Malaki Nik et al. (2010) for α-lactalbumin (A) and β-lactoglobulin (B) solutions and the cream phases of 10% oil-in-water emulsions after pepsin digestion, expressed as m/z (where z = 1). Untreated protein solutions are also shown. Surface adsorption significantly altered the hydrolysis pattern of the proteins. While native α-lactalbumin was fully hydrolyzed by pepsin, adsorbed α-lactalbumin still showed a peak at about 13 kDa after digestion (A). In the case of β-lactoglobulin, while the protein was resistant to hydrolysis in solution, it was fully hydrolyzed when adsorbed at the interface, with a major peak at about 6 kDa (B). | ||
Protein concentration can also affect the polypeptide patterns resulting from in vitro digestion of emulsions.9 When 10% oil-in-water emulsions prepared with 0.5 and 1.5% whey protein isolate (WPI) are exposed to in vitro gastric digestion, exchanges occur between the interfacially adsorbed and the unadsorbed proteins. These exchanges, in turn, affect the susceptibility of the proteins to hydrolysis. Interestingly, regardless of protein concentration (i.e. if the protein is all at the interface or present partly unadsorbed in solution), subsequent incubation of the whey protein-stabilized emulsions in simulated duodenal fluids lead to rapid hydrolysis of both β-LG and α-LA by trypsin and chymotrypsin.9 The same authors also showed that heat treatment of the emulsions before digestion also lead to an increase in the extent of gastric hydrolysis, especially for the unadsorbed protein fraction. Again, these observations stress the importance of emulsion formulation and processing history to ensure delivery of nutrients during digestion.
The composition of the interface determines the physical properties of the emulsion droplets and their state of aggregation. A number of studies have demonstrated that droplet stability during in vitro digestion is strongly dependent on emulsifier type.8,10,36 For example, the stability of WPI-stabilized emulsions during in vitro digestion is significantly lower than that of emulsions stabilized with Tween 20, a low molecular weight emulsifier. Substantial flocculation and coalescence has been shown in β-Cas-stabilized emulsions subjected to simulated gastric condition containing pepsin.8 Of interest, subsequent incubation of this emulsion with duodenal fluids resulted in re-emulsification of the oil droplets and disruption of the flocs.
Proteolysis and the newly formed peptides weaken the steric and electrostatic forces involved in emulsion droplet stabilization. In another example, WPI-stabilized emulsions (0.5% protein, 10% oil) showed extensive aggregation during incubation with simulated gastric fluids containing pepsin. Although the oil droplets were stable against the low pH and ionic strengths associated with the gastric environment, pepsinolysis of the interfacial layer caused destabilization.37 The instability was dependent on protein concentration: during exposure to gastric conditions, 1.5% WPI emulsions were relatively more stable than those prepared with 0.5% WPI. The presence of intact proteins, along with a higher concentration of surface-active peptides liberated during pepsinolysis, may have played a role in stabilizing the system.
Of critical importance to the interfacial dynamics during digestion are the bile salts (BS). BS are strong anionic detergents, consisting of rigid steroid backbones and short aliphatic side chains.38 They are present as mixed micelles in the aqueous phase, but rapidly adsorb at the oil–water interface, decreasing the interfacial tension. They can either partly or fully displace other surface-active molecules from an interface.10,27,39,40 Phospholipids (PL), mainly phosphatidylcholine (PC) are another major constituent of biliary fluids, also secreted by the gastric and duodenal mucosa. PL, alone or in combination with BS, play a major role in modulating gastro-duodenal proteolysis.3 For example, it was recently reported that the presence of PC protected β-LG against trypsin and chymotrypsin hydrolysis. However, this effect seemed to depend on the ratio of PL to protein, with greater protection afforded when a higher proportion of PC was present.26,35
BS promote the digestion of dietary lipids by aiding in re-emulsification and contributing to pancreatic lipase adsorption at the interface. However, they also promote absorption of the products of lipid digestion by solubilizing them in mixed micelles.38 Moreover, the presence of BS can modulate proteolysis by trypsin and chymotrypsin, as was recently demonstrated for β-LG, bovine serum albumin, and myoglobin in solution.41 This behaviour may be a result of BS increasing the accessibility of the proteolytic enzymes by altering the protein structure. BS can also penetrate and disrupt β-LG films, thereby displacing the proteins from both air–water and oil–water interfaces42 and facilitating pancreatic protease activity.9
The above discussion highlights the complex and dynamic interplay between foods and the digestive environment and underscores the challenges in adequately mimicking digestion using in vitro models. Most reports on the in vitro changes in protein-covered oil droplet stability during digestion are specific to only the mouth, stomach, or small intestine. However, surface-active molecules, BS and PL, as well as the products of lipid digestion, play a major role in altering the composition at the interface, by competing for adsorption and by forming mixed micelles in the aqueous phase. In addition, colipase is recognized for its role in overcoming the inhibition of pancreatic lipase by BS, and phospholipase A2 for its role in cleaving the sn-2 position of PL. This co-factor (colipase) and enzyme also contribute to interfacial changes. Despite the roles played by all these constituents during digestion, studies of protein-stabilized emulsions typically do not include the full range of the digestion co-factors or utilize concentrations, which are expected to approximate physiological relevance. Of course, the challenges in doing so are substantial and this is a criticism of in vitro digestion modelling in general.
The influence of protein digestion on protein-stabilized oil droplet stability, in the context of exposure to gastric and small intestinal conditions, is clearly illustrated in Fig. 3, where 10% oil in water emulsions prepared with 1.5% soy protein isolate was first incubated with simulated gastric fluids containing pepsin, and then subjected to simulated duodenal fluids containing pancreatic proteolytic enzymes as well as pancreatic lipase, and bio-surfactants (PL and BS) as described elsewhere.37
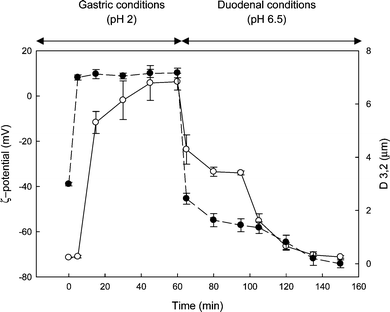 | ||
| Fig. 3 Changes in ζ-potential (●, left hand axis) and average droplet diameter (D3,2) (○, right hand axis), for a 10% oil in water emulsion stabilized by 1.5% soy protein isolate. The emulsion was first incubated with simulated gastric fluids containing pepsin, and then subjected to simulated duodenal and bile fluids containing lipase, phospholipase A2, bile salts and phospholipids as described elsewhere.37 Data are the average of three independent experiments and bars represent standard deviation. | ||
The average droplet diameter and surface charge (ζ-potential) of the initial emulsion were 180 nm and −40 mV, respectively. Incubation with gastric fluids caused a significant increase in particle size and a shift in ζ-potential to a positive value of about +10 mV. Immediately after the addition of intestinal fluids, the emulsion droplet size decreased because of the disruption of bridged flocs. Further proteolysis of the interfacial film by the pancreatic proteases (trypsin and chymotrypsin), interfacial adsorption of BS, and the release of highly surface-active lipolysis products (mainly monoglycerides), all contributed to a further decrease in the mean droplet diameter. The presence of BS along with the change in pH caused a rapid decrease in the charge, reaching a plateau at about −70 mV.
Impact of interfacial composition on lipid hydrolysis
The hydrolysis of dietary lipids begins in the stomach with partial hydrolysis of triglycerides (TG) by the pre-duodenal (gastric and lingual) lipases. In healthy adults and under physiological conditions, only 10–30% of lipid digestion occurs in the gastric phase, but this facilitates the subsequent hydrolysis by pancreatic lipases.43 Lipid hydrolysis continues in the upper small intestine through the action of pancreatic triglyceride lipase (PTL), the main lipid digestion enzyme. PTL catalyzes TG breakdown at the oil–water interface and its activity is critically determined by the quality of the interface. In solution, PTL's active site is covered by an amino acid lid that makes the protein hydrophilic. However, in the presence of an interface, the hydrophobic environment induces opening of the lid, exposing a small (i.e. ∼ 5 nm) hydrophobic region that anchors the enzyme on the surface of the emulsion droplet.13 While BS can inhibit adsorption of PTL at the interface, this effect is overcome through the formation of a PTL-colipase complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio), which ensures displacement of BS and optimal binding of PTL at the interface.
1 ratio), which ensures displacement of BS and optimal binding of PTL at the interface.
Interfacial composition is a key factor in determining the rate of lipid hydrolysis,11,12 and compounds that bind to or interact with the interface have the potential to alter PTL activity. Therefore, the impact of original emulsifier on PTL activity depends on its stability against enzymatic degradation, its ability to control the stability of the emulsion droplets, and its ability to remain at the interface when in competition with other interfacially active molecules present during digestion. The rate of lypolysis is higher for protein-stabilized emulsions compared with emulsions stabilized with phospholipids or low molecular weight surfactants (i.e. Tween 20).10,24,44 However, the type of protein present affects PTL activity. For example, non-covalent inhibition of PTL activity (i.e. when the inhibitor does not bind to the enzyme, but affects its activity) has been observed by certain proteins, including bovine serum albumin (BSA), β-LG, and soy proteins, because PTL access to the surface is hindered.40 Importantly, the inhibition observed is overcome in the presence of BS, as they penetrate and disrupt the interfacial protein films.42
Inhibition of PTL activity has also been reported in the presence of dietary fiber (pectin and/or chitosan).24,45 For example, in vitro PTL activity decreases when lipid droplets are coated with the cationic biopolymer chitosan.24 However, an in vivo study showed that chitosan did not affect the rate of lipolysis during digestion in rats.46 This may be in part explained by in vitro studies with gum Arabic, where the activity of human PTL depends heavily on the presence and concentration of BS.47,48 Research on the effects of mixed interfaces on lipid digestion is in its infancy; however, it is clear that using physiologically relevant in vitro systems it is possible to derive information on the mechanisms related to competition at interfaces. It is important to note that when polysaccharides are present with proteins at the interface, interactions are electrostatic in nature, and the dynamics of adsorption and digestion will change depending on the pH and the ionic conditions.
Since PL are present in the GI tract and contained within many food products, their consideration and inclusion in in vitro models is warranted. The presence of PL, either in the form of vesicles or mixed micelles, also can inhibit PTL interfacial adsorption through steric hindrance by the phospholipids' head groups, but this can be overcome by the action of pancreatic phospholipase A2 (PLA2).49 The products of phospholipase hydrolysis (free fatty acids and lyso-phospholipids) tend to dissolve in the mixed micelles and leave the interface. Incubation of soybean oil (10%) emulsion stabilized with 0.5% WPI in simulated duodenal fluids containing PLA2 led to a significantly higher percentage of lipid hydrolysis than in the absence of this enzyme.37
Fig. 4 illustrates the impact of interfacial protein type and concentration on digestion of emulsion droplets stabilized with WPI (0.5 and 1.5%) or soy protein isolate (SPI) (1.5%). These emulsions were incubated in a two-step in vitro digestion model containing BS, PL, colipase, PTL and PLA2, as described elsewhere.37 Production of free fatty acids (FFA) increased rapidly in all the emulsions within the first 20 min of duodenal incubation, and no lag phase was observed. The final plateau differed between the emulsions with the most extensive lipid hydrolysis observed in the SPI- versus the WPI-stabilized emulsions. In addition, the emulsions prepared with 1.5% WPI had a lower FFA maximum compared to the same emulsion prepared with 0.5% WPI. This clearly shows that both composition and concentration of the proteins initially present in an emulsion affect not only the emulsion stability during GI transit, but also the extent and kinetics of lipid hydrolysis.
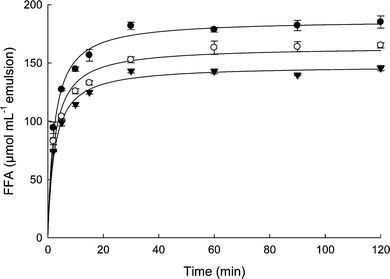 | ||
| Fig. 4 Amount of free fatty acids liberated from emulsion droplets stabilized with 0.5% whey protein isolate (○), 1.5% whey protein isolate (▼), and 1.5% soy protein isolate (●) subjected to two-step in vitro digestion model (gastric and duodenal) containing bile salts, phospholipids, colipase, phospholipase A2 along with digestive enzymes.37 Data are the average of three independent experiments and bars represent standard deviation. | ||
Micellar solubilization and the release of lipophilic bioactives
The bioavailability of lipophilic micronutrients and bioactives can be highly variable and depends on many dietary and physiological factors. It has long been acknowledged that the solubilization of these molecules in the aqueous, micellar phase of the digestate is an important and perhaps limiting step, in their absorption. Inclusion in the micellar phase allows lipophilic molecules within the intestinal lumen to approach the intestinal epithelium for absorption. Therefore, micellization is now routinely quantified in in vitro studies and is considered to be an estimate of so-called ‘bioaccessibility’.50–52 Bioaccessibility depends on a molecule's chemical structure, the physicochemical properties of the carrier, the extent of lipolysis, and the actual incorporation in the micelles.53 Studies of bioaccessibility enable a better understanding of bioavailability. For example, carotenoids are known to be more bioavailable when solubilized in oil droplets, compared to when they are present as crystals in vegetable tissues.54 This has been attributed to an easier transfer to the micellar phase in the case of the oil-solubilized molecules.BS, especially, play a fundamental role in formation of the micellar phase during digestion. The presence of micelles is also of great importance in the self-regulation of PTL activity by sn-2 monoglycerides.12 It is important to note that the micellar phase is not homogeneous, but consists of a series of colloidal structures, including multilamellar and unilamellar vesicles, mixed micelles, and micelles.50,55 The type of micellar structures formed is highly dependent on the concentration of the individual species involved (i.e. BS, PL, cholesterol, monoglycerides, fatty acids, etc.). Therefore, lipolysis, which itself depends on the interface, influences the micellization capacity.
Fig. 5 shows how in vitro modelling can be used to investigate lipolysis in protein-stabilized emulsions and the relationship with bioaccessibility. The lipophilic bioactive molecule β-carotene (BC) is shown as an example. BC was solubilized in 10% oil, 0.5% WPI emulsion and exposed to simulated gastric and duodenal conditions, as previously described.37 The micellization of BC was markedly influenced by the extent of lipid hydrolysis. A positive correlation (r2 0.95) was observed between the extent of lipolysis and transfer of the carotenoid into the aqueous micellar phase. However, comparing the experimental data with a theoretical relation between lipolysis and micellization, the results suggest that the extent of lipolysis was greater than the BC micellization. For example, at 70% lipid hydrolysis, only 50% of BC was incorporated in the aqueous phase, suggesting that BC tended to remain in the oil phase. This agrees with earlier observations that the degree of hydrophobicity of a molecule affects its bioavailability. Xanthophylls, for example, tend to be more bioavailable than carotenes.56 As they are more hydrophilic than carotenes, the xanthophylls are postulated to locate closer to the surface of an oil droplet and to more readily partition into the micelles.54,57
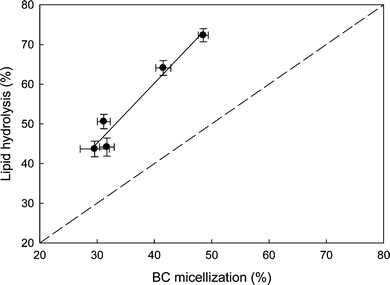 | ||
Fig. 5 Relation between lipid hydrolysis and incorporation of β-carotene (BC), as a model of lipophilic bioactives, in the aqueous micellar phase during digestion of a 10% oil and 0.5% whey protein isolate stabilized emulsion. Broken line represents a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 relationship between % lipid hydrolysis and % micellization of BC. Data are the average of three independent experiments and bars represent standard deviation. 1 relationship between % lipid hydrolysis and % micellization of BC. Data are the average of three independent experiments and bars represent standard deviation. | ||
Conclusions
Food manufacturers have learnt how to manipulate the interfacial composition of emulsions to achieve stability system during processing and storage. In contrast, our understanding of how to manipulate protein-based emulsions for desired outcomes in the GI tract is still in its infancy. Not only protein type, but also protein concentration of an initial emulsion plays an important role in the release of fatty acids and, as a consequence, bioaccessibility of bioactive components. Furthermore, consideration of how the presence of BS and PL, as well as the products of pepsinolysis and lipolysis, affect the accessibility of the digestive enzymes, is critical to understanding the changes occurring to protein-stabilized emulsions during GI transit, at the molecular level.Recent studies have stressed the need for physiologically relevant in vitro models, especially given the complexity of the biochemical and biophysical changes and the interactions that occur between the emulsion constituents and the GI environment. Different results are observed, in terms of emulsion proteolysis and lipolysis, depending on the absence or presence of the various digestive co-factors. Once a better understanding exists in terms of the fundamental molecular interactions and changes occurring during GI transit, it will be possible to develop strategies for optimal lipid digestion and bioavailability of lipophilic nutrients.
References
- M. Wickham, R. Faulks and C. Mills, Mol. Nutr. Food Res., 2009, 53, 952–958 CrossRef CAS.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS.
- A. Mackie and A. Macierzanka, Curr. Opin. Colloid Interface Sci., 2010, 15, 102–108 CrossRef CAS.
- A. Pihlanto-Leppala, Trends Food Sci. Technol., 2001, 11, 347–356.
- I. M. Reddy, N. K. D. Kella and J. E. Kinsella, J. Agric. Food Chem., 1988, 36, 737–741 CrossRef CAS.
- R. Chicon, I. Lopez-Exposito, J. Belloque, E. Alonso and R. Lopez-Fandino, J. Allergy Clin. Immunol., 2008, 121, S249 CrossRef.
- M. Zeece, T. Huppertz and A. Kelly, Innovative Food Sci. Emerging Technol., 2008, 9, 62–69 CrossRef CAS.
- A. Macierzanka, A. I. Sancho, E. N. C. Mills, N. M. Rigby and A. R. Mackie, Soft Matter, 2009, 5, 538–550 RSC.
- A. Malaki Nik, A. J. Wright and M. Corredig, J. Colloid Interface Sci., 2010, 344, 372–381 CrossRef.
- S. Mun, E. A. Decker and D. J. McClements, Food Res. Int., 2007, 40, 770–781 CrossRef CAS.
- P. Reis, K. Holmberg, H. Watzke, M. E. Leser and R. Miller, Adv. Colloid Interface Sci., 2009, 147–148, 237–250 CrossRef CAS.
- P. Reis, H. Watzke, M. Leser, K. Holmberg and R. Miller, Biophys. Chem., 2010, 147, 93–103 CrossRef CAS.
- P. Reis, R. Miller, J. Kragel, M. Leser, V. B. Fainerman, H. Watzke and K. Holmberg, Langmuir, 2008, 24, 6812–6819 CrossRef CAS.
- M. Armand, B. Pasquier, M. Andre, P. Borel, M. Senft, J. Peyrot, J. Salducci, H. Portugal, V. Jaussan and D. La, Am. J. Clin. Nutr., 1999, 70, 1096–1106 CAS.
- M. Golding and T. J. Wooster, Curr. Opin. Colloid Interface Sci., 2010, 15, 90–101 CrossRef CAS.
- D. G. Dalgleish, Trends Food Sci. Technol., 1997, 8, 1–6 CrossRef CAS.
- E. Dickinson, Colloids Surf., B, 1999, 15, 161–176 CrossRef CAS.
- J. Hunt and D. G. Dalgleish, Food Hydrocolloids, 1994, 8, 175–182 CAS.
- M. Corredig and D. G. Dalgleish, Colloids Surf., B, 1995, 4, 411–422 CrossRef CAS.
- E. Dickinson and Y. Matsumura, Int. J. Biol. Macromol., 1991, 13, 26–30 CrossRef CAS.
- S. R. Euston and R. L. Hirst, Int. Dairy J., 1999, 9, 693–701 CrossRef CAS.
- M. Keerati-u-rai and M. Corredig, J. Agric. Food Chem., 2010, 58, 9171–9180 CAS.
- E. Dickinson and J. D. James, Food Hydrocolloids, 2000, 14, 365–376 CrossRef CAS.
- S. Mun, E. A. Decker, Y. Park, J. Weiss and D. J. McClements, Food Biophys., 2006, 1, 21–29 CrossRef.
- Y. Fang and D. G. Dalgleish, J. Agric. Food Chem., 1996, 44, 59–64 CrossRef CAS.
- M. Wickham, M. Garrood, J. Leney, P. D. G. Wilson and A. Fillery-Travis, J. Lipid Res., 1998, 39, 623–632 CAS.
- E. Silletti, M. H. Vingerhoeds, W. Norde and G. A. van Aken, Food Hydrocolloids, 2007, 21, 596–606 CrossRef CAS.
- A. Sarkar, K. K. T. Goh and H. Singh, Food Hydrocolloids, 2009, 23, 1270–1278 CrossRef CAS.
- G. A. van Aken, M. H. Vingerhoeds and E. H. A. de Hoog, Curr. Opin. Colloid Interface Sci., 2007, 12, 251–262 CrossRef CAS.
- E. Silletti, M. H. Vingerhoeds, W. Norde and G. A. van Aken, J. Colloid Interface Sci., 2007, 313, 485–493 CrossRef CAS.
- M. H. Vingerhoeds, T. B. J. Blijdenstein, F. D. Zoet and G. A. van Aken, Food Hydrocolloids, 2005, 19, 915–922 CrossRef CAS.
- M. H. Vingerhoeds, E. Silletti, J. De Groot, R. G. Schipper and G. A. van Aken, Food Hydrocolloids, 2009, 23, 773–785 CrossRef CAS.
- F. J. Moreno, A. R. Mackie and E. N. C. Mills, J. Agric. Food Chem., 2005, 53, 9810–9816 CrossRef CAS.
- M. Dalgalarrondo, E. Dufour, J. M. Chobert, C. Bertrandharb and T. Haertle, Int. Dairy J., 1995, 5, 1–14 CrossRef CAS.
- G. Mandalari, A. M. Mackie, N. M. Rigby, M. S. J. Wickham and E. N. C. Mills, Mol. Nut. Food Res., 2009, 53, S131–S139 Search PubMed.
- A. Sarkar, D. S. Horne and H. Singh, Food Hydrocolloids, 2010, 24, 142–151 CrossRef CAS.
- A. Malaki Nik, M. Corredig and A. J. Wright, Food Digestion, 2010 Search PubMed , in press.
- S. Mukhopadhyay and U. Maitra, Curr. Sci., 2004, 87, 1666–1683.
- G. Fave, T. C. Coste and M. Armand, Cell. Mol. Biol., 2004, 50, 815–831 Search PubMed.
- M. G. Ivanova, I. Panaiotov, A. G. Bois, Y. Gargouri and R. Verger, J. Colloid Interface Sci., 1990, 136, 363–374 CrossRef CAS.
- J. Gass, H. Vora, A. F. Hofmann, G. M. Gray and C. Khosla, Gastroenterology, 2007, 133, 16–23 CrossRef CAS.
- J. Maldonado-Valderrama, N. C. Woodward, A. P. Gunning, M. J. Ridout, F. A. Husband, A. R. Mackie, V. J. Morris and P. J. Wilde, Langmuir, 2008, 24, 6759–6767 CrossRef CAS.
- H. L. Mu and C. E. Hoy, Prog. Lipid Res., 2004, 43, 105–133 CrossRef CAS.
- D. J. McClements, E. A. Decker, Y. Park and J. Weiss, Food Biophys., 2008, 3, 219–228 CrossRef.
- M. Beysseriat, E. A. Decker and D. J. McClements, Food Hydrocolloids, 2006, 20, 800–809 CrossRef CAS.
- G. Y. Park, S. Mun, Y. Park, S. Rhee, E. A. Decker, J. Weiss, D. J. McClements and Y. Park, Food Chem., 2007, 104, 761–767 CrossRef CAS.
- A. Tiss, F. Carriere, I. Douchet, S. Patkar, A. Svedsen and R. Verger, Colloids Surf., B, 2002, 26, 135–145 CrossRef CAS.
- A. Tiss, F. Carriere and R. Verger, Anal. Biochem., 2001, 294, 36–43 CrossRef CAS.
- B. Borgstrom, Gastroent., 1980, 78, 954–962 Search PubMed.
- C. J. H. Porter and W. N. Charman, Adv. Drug Delivery Rev., 2001, 50, S127–S147 CrossRef CAS.
- A. Rube, S. Klein and K. Mader, Pharm. Res., 2006, 23, 2024–2029 CrossRef.
- C. H. M. Versantvoort, A. G. Oomen, E. van de Kamp, C. J. M. Rompelberg and A. J. A. M. Sips, Food Chem. Toxicol., 2005, 43, 31–40 CrossRef CAS.
- T. Sugawara, M. Kushiro, H. Zhang, E. Nara, H. Ono and A. Nagao, J. Nutr., 2001, 131, 2921–2927 CAS.
- G. T. Rich, A. L. Bailey, R. M. Faulks, M. L. Parker, M. S. J. Wickham and A. Fillery-Travis, Lipids, 2003, 38, 933–945 CrossRef CAS.
- C. J. H. Porter, A. M. Kaukonen, A. Taillardat-Bertschinger, B. J. Boyd, J. M. O'Connor, G. A. Edwards and W. N. Charman, J. Pharm. Sci., 2004, 93, 1110–1121 CrossRef CAS.
- K. H. het Hof, I. A. Brower, C. E. West, E. Haddeman, R. P. M. Steegers-Theunissen, M. van Dussldorp, J. A. Weststrate, T. K. A. B. Eskes and J. G. A. J. Hautvast, Amer. J. Clin. Nut., 1999, 70, 261–268 Search PubMed.
- D. A. Garrett, M. L. Failla and R. J. Sarama, J. Agric. Food Chem., 1999, 47, 4301–4309 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
